IA-Body Composition CT at T12 in Idiopathic Pulmonary Fibrosis: Diagnosing Sarcopenia and Correlating with Other Morphofunctional Assessment Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting Study
2.2. Anthropometric and Body Composition Measurements
2.2.1. Bioelectrical Impedance Vector Analysis
2.2.2. Nutritional Ultrasound® [27]
2.2.3. Functional Assessment
2.2.4. Computed Tomography at T12 Level by FocusedON® [49,50]
2.2.5. Assessment of Malnutrition and Sarcopenia
2.2.6. Statistical Analysis
3. Results
3.1. Sarcopenia Criteria (EWGSOP2)
3.2. Body Composition Parameters by T12-CT by Sarcopenia Criteria
3.3. Predictive Values to Diagnose Sarcopenia at T12 Computed Tomography Level
3.4. Correlation Analysis between Muscle Measures: CT, BIVA, NU and Functional Test (HGS)
3.5. Kaplan–Meier Survival Curve in Idiopathic Pulmonary Fibrosis Patients Categorized by Muscle Mass Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef]
- Faverio, P.; Fumagalli, A.; Conti, S.; Madotto, F.; Bini, F.; Harari, S.; Mondoni, M.; Oggionni, T.; Barisione, E.; Ceruti, P.; et al. Sarcopenia in idiopathic pulmonary fibrosis: A prospective study exploring prevalence, associated factors and diagnostic approach. Respir. Res. 2022, 23, 228. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Ohkubo, H.; Nakano, A.; Mori, Y.; Fukumitsu, K.; Fukuda, S.; Kanemitsu, Y.; Uemura, T.; Tajiri, T.; Maeno, K.; et al. Frequency and impact on clinical outcomes of sarcopenia in patients with idiopathic pulmonary fibrosis. Chron. Respir. Dis. 2022, 19, 147997312211172. [Google Scholar] [CrossRef]
- Iwanami, Y.; Ebihara, K.; Nakao, K.; Kubo, R.; Miyagi, M.; Nakamura, Y.; Sakamoto, S.; Kishi, K.; Okuni, I.; Ebihara, S. Impact of Controlling Nutritional Status Score on Mortality in Elderly Patients with Idiopathic Pulmonary Fibrosis. J. Clin. Med. 2024, 13, 2825. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Gilliland, J.; O’Connor, C.; Seabrook, J.A.; Mura, M.; Madill, J. Fat-Free Mass Index Controlled for Age and Sex and Malnutrition Are Predictors of Survival in Interstitial Lung Disease. Respiration 2021, 100, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Kanjrawi, A.A.; Mathers, L.; Webster, S.; Corte, T.J.; Carey, S. Nutritional status and quality of life in interstitial lung disease: A prospective cohort study. BMC Pulm. Med. 2021, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.; John, M.; Pedersen, P.; Raguso, C.; Cicoira, M.; Dardai, E.; Laviano, A.; Ponikowski, P.; Schols, A.; Becker, H.; et al. ESPEN Guidelines on Enteral Nutrition: Cardiology and Pulmonology. Clin. Nutr. 2006, 25, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Faverio, P.; Bocchino, M.; Caminati, A.; Fumagalli, A.; Gasbarra, M.; Iovino, P.; Petruzzi, A.; Scalfi, L.; Sebastiani, A.; Stanziola, A.A.; et al. Nutrition in Patients with Idiopathic Pulmonary Fibrosis: Critical Issues Analysis and Future Research Directions. Nutrients 2020, 12, 1131. [Google Scholar] [CrossRef]
- Kreuter, M.; Ehlers-Tenenbaum, S.; Palmowski, K.; Bruhwyler, J.; Oltmanns, U.; Muley, T.; Heussel, C.P.; Warth, A.; Kolb, M.; Herth, F.J.F. Impact of Comorbidities on Mortality in Patients with Idiopathic Pulmonary Fibrosis. PLoS ONE 2016, 11, e0151425. [Google Scholar] [CrossRef]
- Zheng, Q.; Cox, I.A.; Campbell, J.A.; Xia, Q.; Otahal, P.; de Graaff, B.; Corte, T.J.; Teoh, A.K.Y.; Walters, E.H.; Palmer, A.J. Mortality and survival in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. ERJ Open Res. 2022, 8, 00591–2021. [Google Scholar] [CrossRef]
- Çinkooğlu, A.; Bayraktaroğlu, S.; Ufuk, F.; Unat, Ö.S.; Köse, T.; Savaş, R.; Bishop, N.M. Reduced CT-derived erector spinae muscle area: A poor prognostic factor for short- and long-term outcomes in idiopathic pulmonary fibrosis patients. Clin. Radiol. 2023, 78, 904–911. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Herault, A.; Lévêque, E.; Draye-Carbonnier, S.; Decazes, P.; Zduniak, A.; Modzelewski, R.; Libraire, J.; Achamrah, N.; Ménard, A.-L.; Lenain, P.; et al. High prevalence of pre-existing sarcopenia in critically ill patients with hematologic malignancies admitted to the intensive care unit for sepsis or septic shock. Clin. Nutr. ESPEN 2023, 55, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Aro, R.; Mäkäräinen-Uhlbäck, E.; Ämmälä, N.; Rautio, T.; Ohtonen, P.; Saarnio, J.; Meriläinen, S. The impact of sarcopenia and myoste-atosis on postoperative outcomes and 5-year survival in curatively operated colorectal cancer patients—A retrospective register study. Eur. J. Surg. Oncol. 2020, 46, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, P.M.; Manini, T.; Patel, S.M.; Newman, A.; Travison, T.; Kiel, D.P.; Santanasto, A.J.; Xue, Q.; Shardell, M.; Duchowny, K.; et al. Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. J. Am. Geriatr. Soc. 2020, 68, 1429–1437. [Google Scholar] [CrossRef]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef]
- Sridhar, M.; Bodduluri, S.; O’hare, L.; Blumhoff, S.; Lara, M.d.P.A.; de Andrade, J.A.; Kim, Y.-I.; Luckhardt, T.; McDonald, M.; Kulkarni, T. Association of musculoskeletal involvement with lung function and mortality in patients with idiopathic pulmonary fibrosis. Respir. Res. 2024, 25, 81. [Google Scholar] [CrossRef] [PubMed]
- Jouneau, S.; Rousseau, C.; Lederlin, M.; Lescoat, A.; Kerjouan, M.; Chauvin, P.; Luque-Paz, D.; Guillot, S.; Oger, E.; Vernhet, L.; et al. Malnutrition and decreased food intake at diagnosis are associated with hospitalization and mortality of idiopathic pulmonary fibrosis patients. Clin. Nutr. 2022, 41, 1335–1342. [Google Scholar] [CrossRef]
- Mochizuka, Y.; Suzuki, Y.; Kono, M.; Hasegawa, H.; Hashimoto, D.; Yokomura, K.; Inoue, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; et al. Geriatric Nutritional Risk Index is a predictor of tolerability of antifibrotic therapy and mortality risk in patients with idiopathic pulmonary fibrosis. Respirology 2023, 28, 775–783. [Google Scholar] [CrossRef]
- Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; Crivelli, A.; Evans, D.; Gramlich, L.; Fuchs-Tarlovsky, V.; Keller, H.; Llido, L.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- De Benedetto, F.; Marinari, S.; De Blasio, F. Phase angle in assessment and monitoring treatment of individuals with respiratory disease. Rev. Endocr. Metab. Disord. 2023, 24, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, K.; Sato, S.; Fuseya, Y.; Hasegawa, K.; Uemasu, K.; Sato, A.; Oguma, T.; Hirai, T.; Mishima, M.; Muro, S. Quantitative Assessment of Erector Spinae Muscles in Patients with Chronic Obstructive Pulmonary Disease. Novel Chest Computed Tomography–derived Index for Prognosis. Ann. Am. Thorac. Soc. 2016, 13, 334–341. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshimura, K.; Enomoto, Y.; Yasui, H.; Hozumi, H.; Karayama, M.; Furuhashi, K.; Enomoto, N.; Fujisawa, T.; Nakamura, Y.; et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci. Rep. 2018, 8, 14074. [Google Scholar] [CrossRef] [PubMed]
- García Almeida, J.M.; García García, C.; Vegas Aguilar, I.M.; Bellido Castañeda, V.; Bellido Guerrero, D. Morphofunctional As-sessment of Patient Nutritional Status: A Global Approach. Nutrición Hospitalaria [Internet]. 2021. Available online: https://www.nutricionhospitalaria.org/articles/03378/show (accessed on 22 June 2024).
- García-Almeida, J.M.; García-García, C.; Ballesteros-Pomar, M.D.; Olveira, G.; Lopez-Gomez, J.J.; Bellido, V.; Bretón Lesmes, I.; Burgos, R.; Sanz-Paris, A.; Matia-Martin, P.; et al. Expert Consensus on Morphofunctional Assessment in Disease-Related Malnutrition. Grade Review and Delphi Study. Nutrients 2023, 15, 612. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Bellido Guerrero, D.; Bretón Lesmes, I.; Tinahones Madueño, F.J.; et al. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70, 74–84. [Google Scholar] [CrossRef]
- Fernández-Jiménez, R.; Cesar, E.C.; García, A.S.; Hernández, F.E.; Vegas-Aguilar, I.M.; Amaya-Campos, M.d.M.; Cornejo-Pareja, I.; Guirado-Peláez, P.; Simón-Frapolli, V.; Murri, M.; et al. Rectus Femoris Cross-Sectional Area and Phase Angle as Predictors of 12-Month Mortality in Idiopathic Pulmonary Fibrosis Patients. Nutrients 2023, 15, 4473. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quan-tification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Hong, J.H.; Hong, H.; Choi, Y.R.; Kim, D.H.; Kim, J.Y.; Yoon, J.H.; Yoon, S.H. CT analysis of thoracolumbar body composition for esti-mating whole-body composition. Insights Imaging 2023, 14, 69. [Google Scholar] [CrossRef]
- Tewari, N.; Awad, S.; Macdonald, I.A.; Lobo, D.N. A comparison of three methods to assess body composition. Nutrition 2018, 47, 1–5. [Google Scholar] [CrossRef]
- Clement, D.; Brown, S.; Leerdam, M.V.; Tesselaar, M.; Ramage, J.; Srirajaskanthan, R. Sarcopenia and Neuroendocrine Neoplasms. Curr. Oncol. Rep. 2024, 26, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Joffe, L.; Shen, W.; Shadid, G.; Jin, Z.; Ladas, E.J. Skeletal muscle and adipose tissue changes in the first phase of treatment of pediatric solid tumors. Cancer Med. 2021, 10, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Arayne, A.A.; Gartrell, R.; Qiao, J.; Baird, P.N.; Yeung, M.C.J. Comparison of CT derived body composition at the thoracic T4 and T12 with lumbar L3 vertebral levels and their utility in patients with rectal cancer. BMC Cancer 2023, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Brath, M.S.G.; Sahakyan, M.; Mark, E.B.; Frøkjær, J.B.; Rasmussen, H.H.; Østergaard, L.R.; Weinreich, U.M. Association between thoracic and third lumbar CT-derived muscle mass and density in Caucasian patients without chronic disease: A proof-of-concept study. Eur. Radiol. Exp. 2023, 7, 26. [Google Scholar] [CrossRef]
- Molwitz, I.; Ozga, A.K.; Gerdes, L.; Ungerer, A.; Köhler, D.; Ristow, I.; Leiderer, M.; Adam, G.; Yamamura, J. Prediction of abdominal CT body composition param-eters by thoracic measurements as a new approach to detect sarcopenia in a COVID-19 cohort. Sci. Rep. 2022, 12, 6443. [Google Scholar] [CrossRef]
- Nemec, U.; Heidinger, B.; Sokas, C.; Chu, L.; Eisenberg, R.L. Diagnosing Sarcopenia on Thoracic Computed Tomography. Acad. Radiol. 2017, 24, 1154–1161. [Google Scholar] [CrossRef]
- Awano, N.; Inomata, M.; Kuse, N.; Tone, M.; Yoshimura, H.; Jo, T.; Takada, K.; Sugimoto, C.; Tanaka, T.; Sumikawa, H.; et al. Quantitative computed tomography measures of skeletal muscle mass in patients with idiopathic pulmonary fibrosis according to a multidisciplinary discussion diagnosis: A retrospective nationwide study in Japan. Respir. Investig. 2020, 58, 91–101. [Google Scholar] [CrossRef]
- Nakano, A.; Ohkubo, H.; Taniguchi, H.; Kondoh, Y.; Matsuda, T.; Yagi, M.; Furukawa, T.; Kanemitsu, Y.; Niimi, A. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci. Rep. 2020, 10, 2312. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Piccoli, A.; Nigrelli, S.; Caberlotto, A.; Bottazzo, S.; Rossi, B.; Pillon, L.; Maggiore, Q. Bivariate normal values of the bioelectrical imped-ance vector in adult and elderly populations. Am. J. Clin. Nutr. 1995, 61, 269–270. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [PubMed]
- De Palo, T.; Messina, G.; Edefonti, A.; Perfumo, F.; Pisanello, L.; Peruzzi, L.; Di Iorio, B.; Mignozzi, M.; Vienna, A.; Conti, G.; et al. Normal values of the bioelectrical impedance vector in childhood and puberty. Nutrition 2000, 16, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, T.R.; Wazlawik, E.; Bastos, J.L.; Nakazora, L.M.; Scheunemann, L. Standardized phase angle indicates nutritional sta-tus in hospitalized preoperative patients. Nutr. Res. 2010, 30, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.; Stobäus, N.; Gonzalez, M.C.; Schulzke, J.D.; Pirlich, M. Hand grip strength: Outcome predictor and marker of nutri-tional status. Clin. Nutr. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardio-vascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank par-ticipants. BMJ 2018, 361, k1651. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Mesquita, R.; Wilke, S.; Smid, E.D.; Janssen, D.J.; Franssen, F.M.; Probst, V.S.; Wouters, E.F.; Muris, J.W.; Pitta, F.; Spruit, A.M. Measurement properties of the Timed Up & Go test in patients with COPD. Chronic Respir. Dis. 2016, 13, 344–352. [Google Scholar] [CrossRef]
- Palmas, F.; Ciudin, A.; Guerra, R.; Eiroa, D.; Espinet, C.; Roson, N.; Burgos, R.; Simó, R. Comparison of computed tomography and dual-energy X-ray absorptiometry in the evaluation of body composition in patients with obesity. Front. Endocrinol. 2023, 14, 1161116. [Google Scholar] [CrossRef]
- Soria-Utrilla, V.; Sánchez-Torralvo, F.J.; Palmas-Candia, F.X.; Fernández-Jiménez, R.; Mucarzel-Suarez-Arana, F.; Guirado-Peláez, P.; Olveira, G.; García-Almeida, J.M.; Burgos-Peláez, R. AI-Assisted Body Composition Assessment Using CT Imaging in Colorectal Cancer Patients: Predictive Capacity for Sarcopenia and Malnutrition Diagnosis. Nutrients 2024, 16, 1869. [Google Scholar] [CrossRef]
- Cho, Y.H.; Do, K.H.; Chae, E.J.; Choi, S.H.; Jo, K.W.; Lee, S.O.; Hong, S.B. Association of Chest CT-Based Quantitative Measures of Muscle and Fat with Post-Lung Transplant Survival and Morbidity: A Single Institutional Retrospective Cohort Study in Korean Population. Korean J. Radiol. 2019, 20, 522. [Google Scholar] [CrossRef]
- Oh, J.K.; Ahn, M.I.; Kim, H.L.; Park, S.H.; Shin, E. Retrodiaphragmatic portion of the lung: How deep is the posterior costophrenic sulcus on posteroanterior chest radiography? Clin. Radiol. 2009, 64, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Jalaber, C.; Lemerre-Poincloux, J.; Jouneau, S.; Rousseau, C.; Dolou, B.; Rouag, E.; Lescoat, A.; Luque-Paz, D.; Lucas, C.; Vernhet, L.; et al. Usefulness of Body Composition CT Analysis in Patients with Idiopathic Pulmonary Fibrosis: A Pilot Study. Acad. Radiol. 2021, 29, S191–S201. [Google Scholar] [CrossRef] [PubMed]
- Vegas-Aguilar, I.M.; Guirado-Peláez, P.; Fernández-Jiménez, R.; Boughanem, H.; Tinahones, F.J.; Garcia-Almeida, J.M. Exploratory Assessment of Nutritional Evaluation Tools as Predictors of Complications and Sarcopenia in Patients with Colorectal Cancer. Cancers 2023, 15, 847. [Google Scholar] [CrossRef]
- García-García, C.; Vegas-Aguilar, I.M.; Rioja-Vázquez, R.; Cornejo-Pareja, I.; Tinahones, F.J.; García-Almeida, J.M. Rectus Femoris Muscle and Phase Angle as Prognostic Factor for 12-Month Mortality in a Longitudinal Cohort of Patients with Cancer (AnyVida Trial). Nutrients 2023, 15, 522. [Google Scholar] [CrossRef] [PubMed]
- Simón-Frapolli, V.J.; Vegas-Aguilar, I.M.; Fernández-Jiménez, R.; Cornejo-Pareja, I.M.; Sánchez-García, A.M.; Martínez-López, P.; Nuevo-Ortega, P.; Reina-Artacho, C.; Estecha-Foncea, M.A.; Gómez-González, A.M.; et al. Phase angle and rectus femoris cross-sectional area as predictors of severe malnutrition and their relationship with compli-cations in outpatients with post-critical SARS-CoV2 disease. Front. Nutr. 2023, 10, 1218266. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, S.; Pan, B.; Xie, W.; Meng, J. Ectopic and visceral fat deposition in aging, obesity, and idiopathic pulmonary fibrosis: An interconnected role. Lipids Health Dis. 2023, 22, 201. [Google Scholar] [CrossRef]
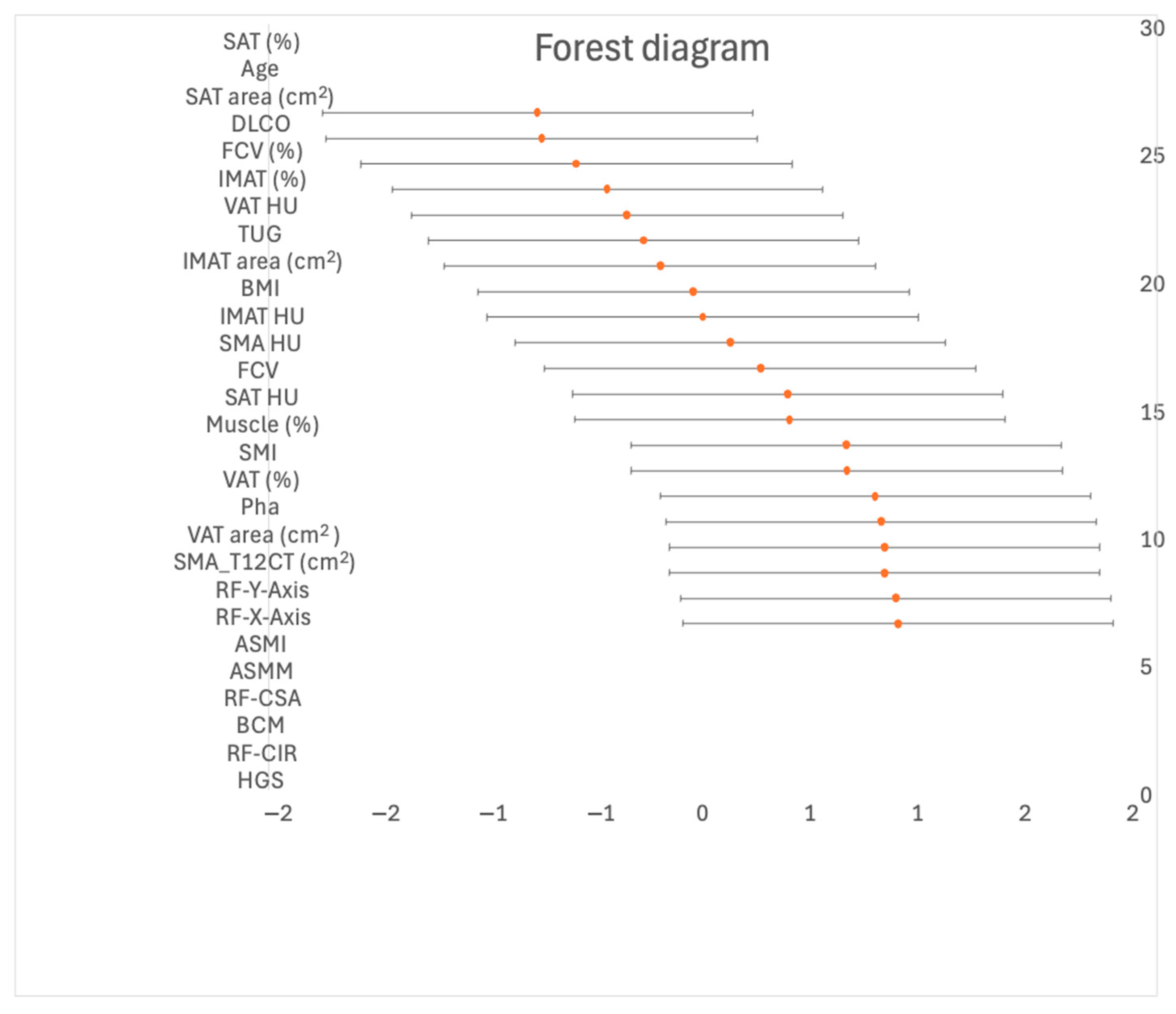


| All N = 60 | Non-Sarcopenic n = 48 | Sarcopenic n = 12 | Effect Size (Hedge’s) | 95% IC (Lower) | 95% IC (Upper) | p Value | |
|---|---|---|---|---|---|---|---|
| Demographic and anthropometric variables | |||||||
| Age (years) | 70.9 ± 7.8 | 69.7 ± 7.4 | 75.3 (8.1) | −0.751 | −13.951 | −0.0978 | 0.024 |
| Gender (male) | 52.0 (85.2%) | 46.0 (76.7%) | 5.0 (8.3%) | <0.001 | |||
| BMI (kg/m2) | 27.7 ± 3.7 | 27.8 ± 3.7 | 27.8 (3.8) | 0.0153 | −0.6141 | 0.6449 | 0.969 |
| Respiratory variables | |||||||
| DLCO (%) | 51.2 ± 17.7 | 48.3 ± 16.0 | 62.2 (19.9) | −0.57 | −12.511 | 0.1181 | 0.021 |
| FCV (L) | 2638.0 (772.0) | 2708.0 ± 742.0 | 2322 (840.0) | 0.411 | −0.2332 | 10.562 | 0.126 |
| FCV (%) | 65.1 ± 15.4 | 63.0 ± 14.9 | 68.8 (17.1) | −0.428 | −10.705 | 0.2164 | 0.315 |
| Functional measurement | |||||||
| HGS (kg) | 33.4 ± 10.2 | 36.7 ± 8.7 | 20.8 (4.4) | 1.849 | 10.967 | 25.834 | <0.001 |
| TUG | 8.31 ± 5.94 | 8.1 ± 6.2 | 9.1 (5.1) | −0,181 | −0.8353 | 0.4753 | 0.632 |
| BIVA | |||||||
| Pha | 4.8 ± 0.7 | 4.9 ± 0.7 | 4.5 ± 0.7 | 0,847 | 0.1882 | 14.973 | 0.130 |
| BCM | 26.3 ± 5.18 | 27.3 ± 5.1 | 22.3 ± 3.2 | 1.267 | 0.5784 | 19.466 | <0.05 |
| ASMM (kg) | 20.6 ± 3.2 | 21.2 ± 3.2 | 17.8 ± 1.1 | 1.210 | 0.5245 | 18.821 | 0.014 |
| ASMI (kg/m2) | 7.2 ± 0.7 | 7.3 ± 0.8 | 6.6 ± 0.4 | 1.158 | 0.4778 | 18.268 | 0.139 |
| NU | |||||||
| RF-CSA (cm2) | 3.3 ± 1.0 | 3.6 ± 1.1 | 2.5 ± 0.5 | 1.240 | 0.5502 | 19.181 | <0.001 |
| RF-Y-axis (cm) | 1.1 ± 0.2 | 1.2 ± 0.3 | 1.0 ± 0.2 | 1.428 | 0.7215 | 21.225 | 0.022 |
| RF-X-axis (cm) | 3.4 ± 0.4 | 3.5 ± 0.4 | 3.1 ± 0.5 | 0.926 | 0.2603 | 15.815 | 0.002 |
| RF-CIR (cm) | 8.16 ± 1.0 | 8.4 ± 0.9 | 7.1 ± 0.9 | 1.428 | 0.7215 | 21.225 | <0.001 |
| Malnutrition after follow-up (GLIM) (%) | <0.05 | ||||||
| Non malnutrition | 42.6% | 31.7% | 11.7% | ||||
| Moderate malnutrition | 31.1% | 30.0% | 0.0% | ||||
| Severe malnutrition | 26.2% | 18.3% | 8.3% | ||||
| Mortality (%) | <0.05 | ||||||
| No | 70% | 50.0% | 20.0% | ||||
| Yes | 30% | 30.0% | 0.0% |
| N = 60 | p | ||
|---|---|---|---|
| Handgrip strength (kg) | |||
| Total | Mean ± SD | 33.4 ± 10.2 | <0.001 |
| Men | Mean ± SD | 35.3 ± 8.9 | |
| Women | Mean ± SD | 22.7 ± 10.6 | |
| Low handgrip strength | Mean (%) | 19 (31.7%) | |
| ASMM (kg) | |||
| Total | Mean ± SD | 20.6 ± 3.2 | 0.014 |
| Men | Mean ± SD | 21.0 ± 3.1 | |
| Women | Mean ± SD | 18.2 ± 2.6 | |
| Low ASMM | Mean (%) | 30 (49.2%) | 0.009 |
| ASMI (kg/talla) | |||
| Total | Mean ± SD | 7.2 ± 0.8 | 0.139 |
| Men | Mean ± SD | 7.3 ± 0.8 | |
| Women | Mean ± SD | 6.8 ± 0.4 | |
| Low ASMI | Mean (%) | 28 (45.9%) | 0.182 |
| Total low muscle massbb (low ASMI or ASMM) | Mean (%) | 32 (56.1%) | |
| Sarcopenia(Low HGS and Los muscle mass) | Mean (%) | 12 (20%) |
| T12-CT Parameters | All (N = 60) | Non Sarcopenic (n = 48) | Sarcopenic (n = 12) | Effect Size (Hedge’s) | 95% IC (Lower) | 95% IC (Upper) | p |
|---|---|---|---|---|---|---|---|
| SMA_T12CT (cm2) | 75 ± 21.8 | 78.8 ± 22.3 | 60.6 ± 12.9 | 0.8604 | 0.2084 | 15.040 | 0.009 |
| Muscle (%) | 9.5 ± 2.1 | 9.9 ± 2.2 | 8.4 ± 1.7 | 0.6837 | 0.041 | 13.204 | 0.036 |
| Muscle (HU) | 39.0 ± 7.2 | 39.5 ± 7.6 | 37.4 ± 5.6 | 0.2841 | −0.3445 | 0.9103 | 0.376 |
| SMI_T12CT (cm2/m2) | 26.2 ± 6.9 | 27.2 ± 7.1 | 22.6 ± 4.8 | 0.6862 | 0.0433 | 13.217 | 0.035 |
| IMAT area (cm2) | 14.9 ± 6.8 | 14.8 ± 6.9 | 15.0 ± 6.7 | −0.0264 | −0.6501 | 0.5983 | 0.934 |
| IMAT (%) | 1.87 ± 0.7 | 1.8 ± 0.7 | 2.1 ± 0.8 | −0.3363 | −0.9622 | 0.2933 | 0.296 |
| IMAT (HU) | −63.9 ± 5.4 | −63.7 ± 5.5 | −64.5 ± 5.4 | 0.1443 | −0.4822 | 0.7684 | 0.652 |
| VAT area (cm2) | 177.0 ± 81.6 | 191.5 ± 84.8 | 123.5 ± 36.3 | 0.8604 | 0.2084 | 15.040 | 0.009 |
| VAT (%) | 22.2 ± 8.4 | 23.6 ± 8.7 | 16.9 ± 4.2 | 0.8176 | 0.1681 | 14.592 | 0.013 |
| VAT (HU) | −97.7 ± 6.3 | −98.0 ± 6.0 | −96.3 ± 7.7 | −0.2584 | −0.8838 | 0.3694 | 0.420 |
| SAT area (cm2) | 119 ± 56.6 | 111.6 ± 48.3 | 152.4 ± 77.3 | −0.2584 | −0.8838 | 0.3694 | 0.025 |
| SAT (%) | 15 ± 6.5 | 13.6 ± 4.8 | 20.8 ± 9.6 | −11.875 | −18.491 | −0.5142 | <0.001 |
| SAT HU | −98.5 ± 9.7 | −97.6 ± 9.2 | −101.7 ± 11.5 | 0.4207 | −0.2115 | 10.497 | 0.192 |
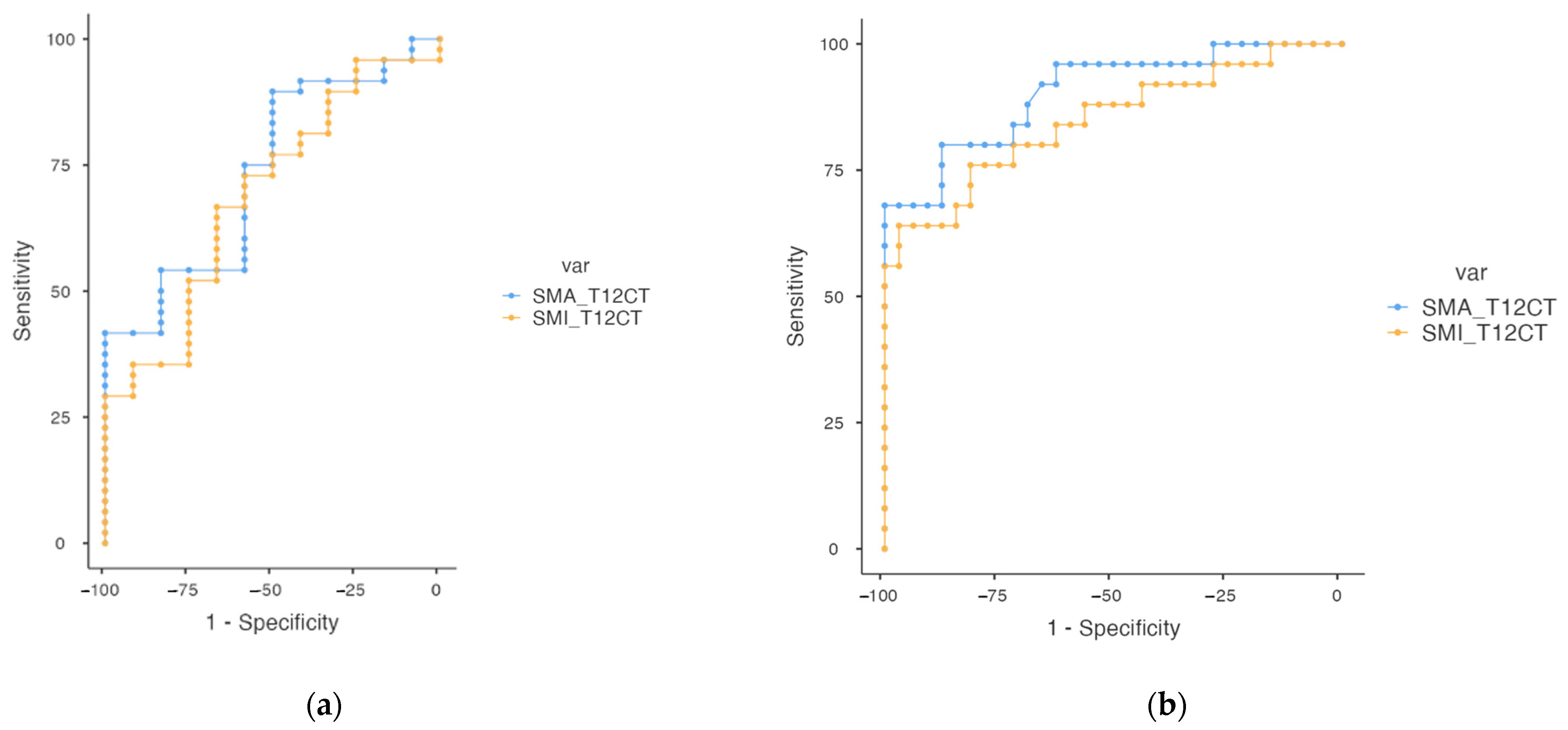
| Variables | Cut-Off | AUC | Sensitivity | Specificity | Youden’s Index | p | |
|---|---|---|---|---|---|---|---|
| Sarcopenia | SMA_T12CT | 77.4 | 0.734 | 41.7% | 100% | 0.417 | <0.05 |
| SMI_T12CT | 24.5 | 0.689 | 66.7% | 66.7% | 0.333 | <0.05 | |
| Low muscle mass | SMA_T12CT | 80.5 | 0.904 | 68.0% | 100.0% | 0.680 | <0.05 |
| SMI_T12CT | 28.8 | 0.848 | 64.0% | 96.8% | 0.609 | <0.05 |
| BCM | ASMM | ASMI | RF-CSA | HGS | Muscle_Area_T12 | |
|---|---|---|---|---|---|---|
| BCM | — | |||||
| ASMM | 0.864 *** | — | ||||
| ASMI | 0.810 *** | 0.825 *** | — | |||
| RF-CSA | 0.637 *** | 0.575 *** | 0.679 *** | — | ||
| HGS | 0.560 *** | 0.592 *** | 0.441 *** | 0.497 *** | — | |
| SMA_T12CT | 0.785 *** | 0.761 *** | 0.786 *** | 0.616 *** | 0.465 *** | — |
| SMA_perc_T12 | 0.591 *** | 0.478 *** | 0.562 *** | 0.528 *** | 0.373 ** | 0.831 *** |
| SMI_T12CT | 0.681 *** | 0.589 *** | 0.775 *** | 0.599 *** | 0.350 ** | 0.956 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Jiménez, R.; Sanmartín-Sánchez, A.; Cabrera-César, E.; Espíldora-Hernández, F.; Vegas-Aguilar, I.; Amaya-Campos, M.d.M.; Palmas-Candia, F.X.; Claro-Brandner, M.; Olivares-Alcolea, J.; Simón-Frapolli, V.J.; et al. IA-Body Composition CT at T12 in Idiopathic Pulmonary Fibrosis: Diagnosing Sarcopenia and Correlating with Other Morphofunctional Assessment Techniques. Nutrients 2024, 16, 2885. https://doi.org/10.3390/nu16172885
Fernández-Jiménez R, Sanmartín-Sánchez A, Cabrera-César E, Espíldora-Hernández F, Vegas-Aguilar I, Amaya-Campos MdM, Palmas-Candia FX, Claro-Brandner M, Olivares-Alcolea J, Simón-Frapolli VJ, et al. IA-Body Composition CT at T12 in Idiopathic Pulmonary Fibrosis: Diagnosing Sarcopenia and Correlating with Other Morphofunctional Assessment Techniques. Nutrients. 2024; 16(17):2885. https://doi.org/10.3390/nu16172885
Chicago/Turabian StyleFernández-Jiménez, Rocío, Alicia Sanmartín-Sánchez, Eva Cabrera-César, Francisco Espíldora-Hernández, Isabel Vegas-Aguilar, María del Mar Amaya-Campos, Fiorella Ximena Palmas-Candia, María Claro-Brandner, Josefina Olivares-Alcolea, Víctor José Simón-Frapolli, and et al. 2024. "IA-Body Composition CT at T12 in Idiopathic Pulmonary Fibrosis: Diagnosing Sarcopenia and Correlating with Other Morphofunctional Assessment Techniques" Nutrients 16, no. 17: 2885. https://doi.org/10.3390/nu16172885
APA StyleFernández-Jiménez, R., Sanmartín-Sánchez, A., Cabrera-César, E., Espíldora-Hernández, F., Vegas-Aguilar, I., Amaya-Campos, M. d. M., Palmas-Candia, F. X., Claro-Brandner, M., Olivares-Alcolea, J., Simón-Frapolli, V. J., Cornejo-Pareja, I., Guirado-Peláez, P., Vidal-Suárez, Á., Sánchez-García, A., Murri, M., Garrido-Sánchez, L., Tinahones, F. J., Velasco-Garrido, J. L., & García-Almeida, J. M. (2024). IA-Body Composition CT at T12 in Idiopathic Pulmonary Fibrosis: Diagnosing Sarcopenia and Correlating with Other Morphofunctional Assessment Techniques. Nutrients, 16(17), 2885. https://doi.org/10.3390/nu16172885






