The Association between Fluoride and Bone Mineral Density in US Children and Adolescents: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participant
2.2. Measurement of Fluoride
2.3. Examination of BMD
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
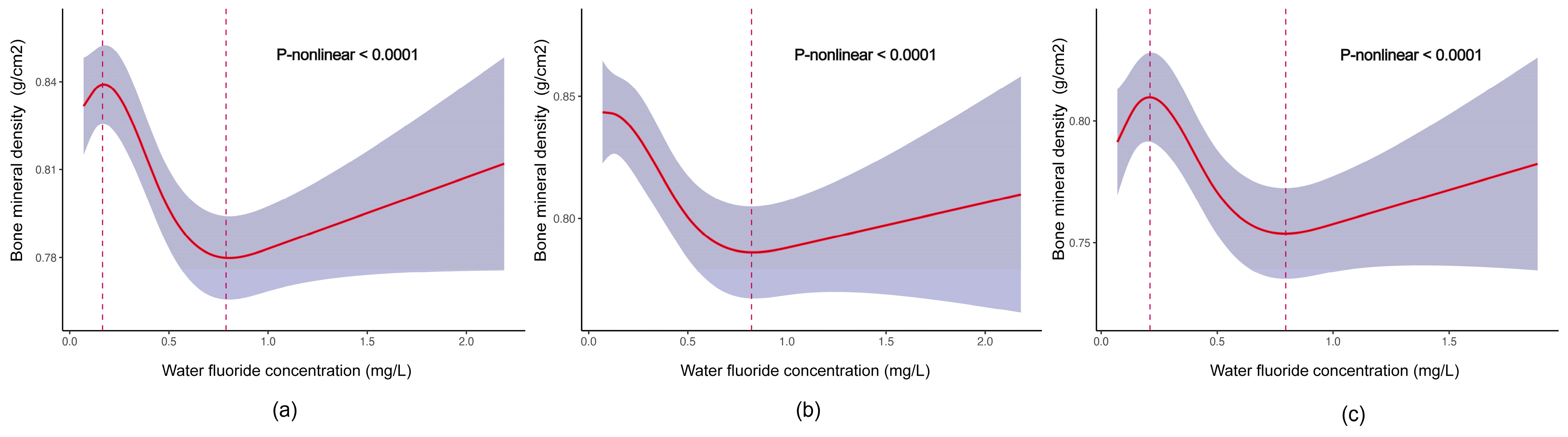
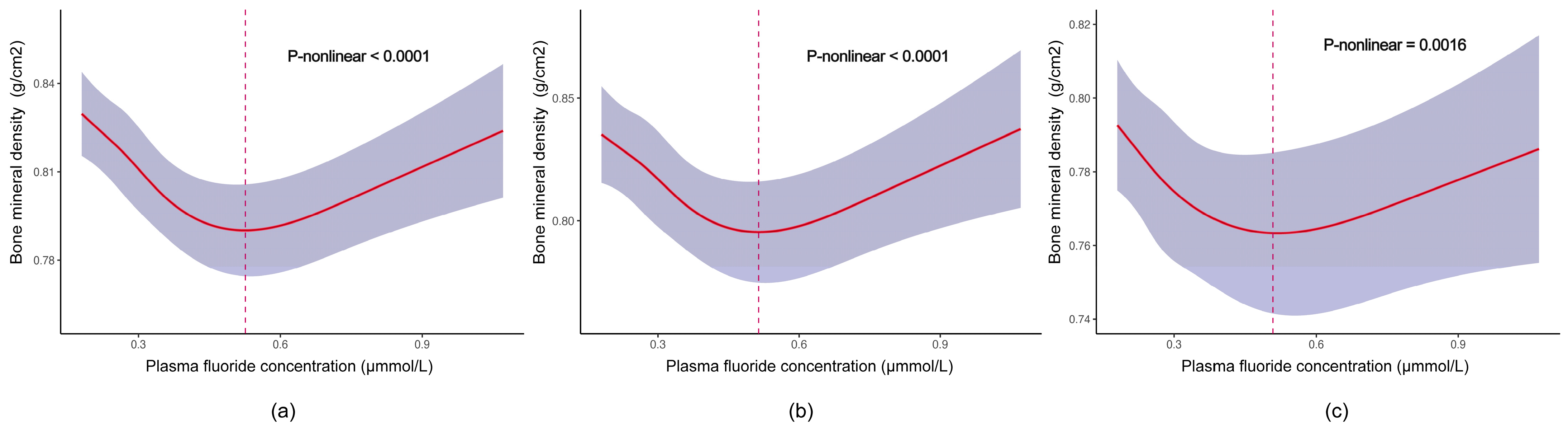
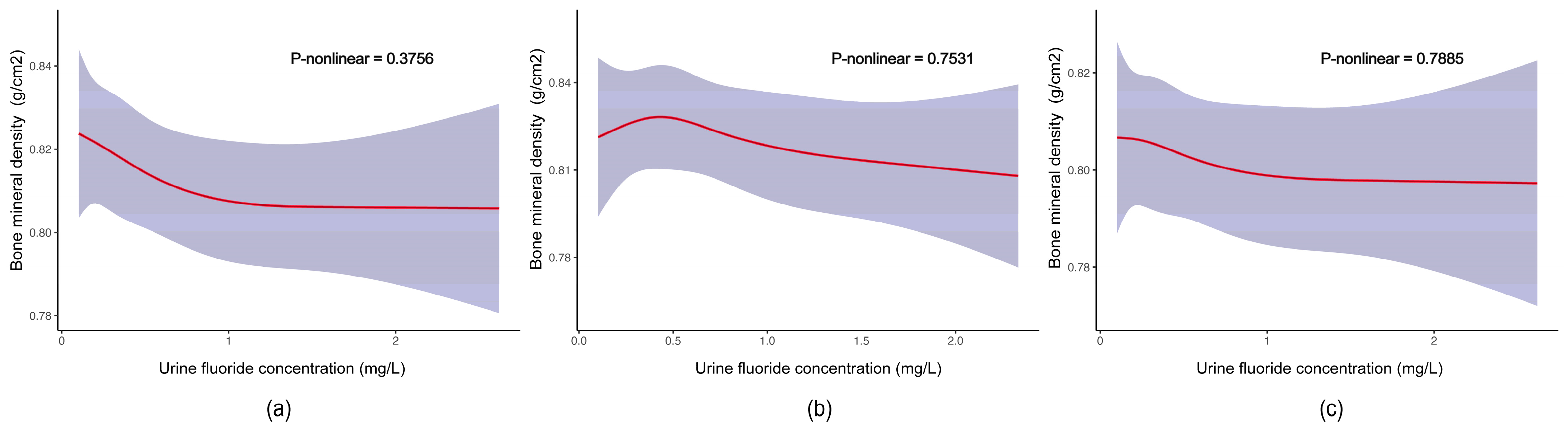
3.2. Associations between Fluoride Exposure and TBLH BMD among US Children and Adolescents
3.3. Correlation between Concentrations of Different Fluoride Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ammann, P.; Rizzoli, R. Bone strength and its determinants. Osteoporos. Int. 2003, 14 (Suppl. S3), S13–S18. [Google Scholar] [CrossRef]
- Mäkitie, O. Causes, mechanisms and management of paediatric osteoporosis. Nat. Rev. Rheumatol. 2013, 9, 465–475. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.X.; Liu, C.; Mustieles, V.; Pan, X.F.; Zhang, Y.; Messerlian, C. Exposure to Trihalomethanes and Bone Mineral Density in US Adolescents: A Cross-Sectional Study (NHANES). Environ. Sci. Technol. 2023, 57, 21616–21626. [Google Scholar] [CrossRef]
- Madhuchani, D.; Seneviratne, S.N.; Ward, L.M. Bone health in childhood and adolescence: An overview on dual-energy X-ray absorptiometry scanning, fracture surveillance and bisphosphonate therapy for low-middle-income countries. Front. Endocrinol. 2023, 14, 1082413. [Google Scholar] [CrossRef]
- Liu, H.; Bao, M.; Liu, M.; Deng, F.; Wen, X.; Wan, P.; Lin, X.; Dong, G.; Li, Z.; Han, J. The Association between Serum Copper and Bone Mineral Density among Adolescents Aged 12 to 19 in the United States. Nutrients 2024, 16, 453. [Google Scholar] [CrossRef]
- Li, T.; Xie, Y.; Wang, L.; Huang, G.; Cheng, Y.; Hou, D.; Liu, W.; Zhang, T.; Liu, J. The Association between Lead Exposure and Bone Mineral Density in Childhood and Adolescence: Results from NHANES 1999-2006 and 2011-2018. Nutrients 2022, 14, 1523. [Google Scholar] [CrossRef]
- Solanki, Y.S.; Agarwal, M.; Gupta, A.B.; Gupta, S.; Shukla, P. Fluoride occurrences, health problems, detection, and remediation methods for drinking water: A comprehensive review. Sci. Total Environ. 2022, 807, 150601. [Google Scholar] [CrossRef]
- Mesdaghinia, A.; Vaghefi, K.A.; Montazeri, A.; Mohebbi, M.R.; Saeedi, R. Monitoring of fluoride in groundwater resources of Iran. Bull. Environ. Contam. Toxicol. 2010, 84, 432–437. [Google Scholar] [CrossRef]
- Veneri, F.; Vinceti, M.; Generali, L.; Giannone, M.E.; Mazzoleni, E.; Birnbaum, L.S.; Consolo, U.; Filippini, T. Fluoride exposure and cognitive neurodevelopment: Systematic review and dose-response meta-analysis. Environ. Res. 2023, 221, 115239. [Google Scholar] [CrossRef]
- Veneri, F.; Iamandii, I.; Vinceti, M.; Birnbaum, L.S.; Generali, L.; Consolo, U.; Filippini, T. Fluoride Exposure and Skeletal Fluorosis: A Systematic Review and Dose-response Meta-analysis. Curr. Environ. Health Rep. 2023, 10, 417–441. [Google Scholar] [CrossRef]
- US Department of Health and Human Services Federal Panel on Community Water Fluoridation. U.S. Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public. Health Rep. 2015, 130, 318–331. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, X.; He, Y.; Zhang, J.; Huang, H.; Bian, W.; Chilufya, M.M.; Zhao, Y.; Han, J. Progress of Signaling Pathways, Stress Pathways and Epigenetics in the Pathogenesis of Skeletal Fluorosis. Int. J. Mol. Sci. 2021, 22, 11932. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, D.; Wei, W. Necessity to Pay Attention to the Effects of Low Fluoride on Human Health: An Overview of Skeletal and Non-skeletal Damages in Epidemiologic Investigations and Laboratory Studies. Biol. Trace Elem. Res. 2023, 201, 1627–1638. [Google Scholar] [CrossRef]
- Helte, E.; Donat Vargas, C.; Kippler, M.; Wolk, A.; Michaëlsson, K.; Åkesson, A. Fluoride in Drinking Water, Diet, and Urine in Relation to Bone Mineral Density and Fracture Incidence in Postmenopausal Women. Environ. Health Perspect. 2021, 129, 47005. [Google Scholar] [CrossRef]
- Ba, Y.; Feng, Z.; Fu, X.; Chen, R.; Jiao, X.; Du, Y.; Liu, X.; Huang, H.; Yu, F.; Zhou, G. Mediation of mitochondrial DNA copy number and oxidative stress in fluoride-related bone mineral density alteration in Chinese farmers. Environ. Geochem. Health 2024, 46, 184. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Xu, K.; Zhang, L.; Zhang, Y.; He, T.; Sun, R.; Huang, H.; Zhu, J.; Zhang, Y.; et al. Association between low-to-moderate fluoride exposure and bone mineral density in Chinese adults: Non-negligible role of RUNX2 promoter methylation. Ecotoxicol. Environ. Saf. 2020, 203, 111031. [Google Scholar] [CrossRef]
- Lan, C.F.; Lin, I.F.; Wang, S.J. Fluoride in drinking water and the bone mineral density of women in Taiwan. Int. J. Epidemiol. 1995, 24, 1182–1187. [Google Scholar] [CrossRef]
- CDC. Fluoride—Plasma Laboratory Procedure Manual. 2017. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/FLDEP_I_MET.pdf (accessed on 1 June 2024).
- CDC. Fluoride—Water Laboratory Procedure Manual. 2017. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/FLDEW_I_MET.pdf (accessed on 1 June 2024).
- CDC. Fluoride—Urine Laboratory Procedure Manual. 2021. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/UFLDE-I-MET-508.pdf (accessed on 1 June 2024).
- CDC. Body Composition Procedures Manual. 2016. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_Body_Composition_Procedures_Manual.pdf (accessed on 1 June 2024).
- Liu, J.; Wang, L.; Sun, J.; Liu, G.; Yan, W.; Xi, B.; Xiong, F.; Ding, W.; Huang, G.; Heymsfield, S.; et al. Bone mineral density reference standards for Chinese children aged 3-18: Cross-sectional results of the 2013-2015 China Child and Adolescent Cardiovascular Health (CCACH) Study. BMJ Open 2017, 7, e014542. [Google Scholar] [CrossRef]
- CDC. Physical Activity and Physical Fitness Questionnaire Instruments. 2013. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/questionnaires/PAQ_I.pdf (accessed on 1 June 2024).
- CDC. Diet Behavior and Nutrition Questionnaire Instruments. 2015. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/questionnaires/DBQ_I.pdf (accessed on 1 June 2024).
- Sun, R.; Zhou, G.; Liu, L.; Ren, L.; Xi, Y.; Zhu, J.; Huang, H.; Li, Z.; Li, Y.; Cheng, X.; et al. Fluoride exposure and CALCA methylation is associated with the bone mineral density of Chinese women. Chemosphere 2020, 253, 126616. [Google Scholar] [CrossRef]
- Qin, M.; Gao, Y.; Zhang, M.; Wu, J.; Liu, Y.; Jiang, Y.; Zhang, X.; Wang, X.; Yang, Y.; Gao, Y. Association between ADAMTS14_rs4747096 gene polymorphism and bone mineral density of Chinese Han population residing in fluorine exposed areas in ShanXi Province, China. Environ. Sci. Pollut. Res. Int. 2023, 30, 106059–106067. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, B.H.; Zhao, J.; Ommati, M.M.; Wang, S.; Wang, H.W. Fluoride-induced osteoporosis via interfering with the RANKL/RANK/OPG pathway in ovariectomized rats: Oophorectomy shifted skeletal fluorosis from osteosclerosis to osteoporosis. Environ. Pollut. 2023, 336, 122407. [Google Scholar] [CrossRef]
- Ouyang, T.; Qin, Y.; Luo, K.; Han, X.; Yu, C.; Zhang, A.; Pan, X. miR-486-3p regulates CyclinD1 and promotes fluoride-induced osteoblast proliferation and activation. Environ. Toxicol. 2021, 36, 1817–1828. [Google Scholar] [CrossRef]
- Li, C.; Qin, Y.; Ouyang, T.; Yao, M.; Zhang, A.; Luo, P.; Pan, X. miR-122-5p Mediates Fluoride-Induced Osteoblast Activation by Targeting CDK4. Biol. Trace Elem. Res. 2021, 199, 1215–1227. [Google Scholar] [CrossRef]
- Guo, N.; Yu, Y.; Chu, Y.; Lou, Q.; Huang, W.; Wu, L.; Fan, C.; Su, M.; Zhang, M.; Yin, F.; et al. miR-21-5p and canonical Wnt signaling pathway promote osteoblast function through a feed-forward loop induced by fluoride. Toxicology 2022, 466, 153079. [Google Scholar] [CrossRef]
- Lee, M.; Arikawa, K.; Nagahama, F. Micromolar Levels of Sodium Fluoride Promote Osteoblast Differentiation through Runx2 Signaling. Biol. Trace Elem. Res. 2017, 178, 283–291. [Google Scholar] [CrossRef]
- Yao, Y.; Ma, Y.; Zhong, N.; Pei, J. The Inverted U-Curve Association of Fluoride and Osteoclast Formation in Mice. Biol. Trace Elem. Res. 2019, 191, 419–425. [Google Scholar] [CrossRef]
- Jiao, Y.; Sun, J.; Li, Y.; Zhao, J.; Shen, J. Association between Adiposity and Bone Mineral Density in Adults: Insights from a National Survey Analysis. Nutrients 2023, 15, 3492. [Google Scholar] [CrossRef]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Lavalle-Carrasco, J.; Molina-Frechero, N.; Nevárez-Rascón, M.; Sánchez-Pérez, L.; Hamdan-Partida, A.; González-González, R.; Cassi, D.; Isiordia-Espinoza, M.A.; Bologna-Molina, R. Recent Biomarkers for Monitoring the Systemic Fluoride Levels in Exposed Populations: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 317. [Google Scholar] [CrossRef]
- Lavalle-Carrasco, J.; Vergara-Onofre, M.; González-González, R.; Bologna-Molina, R.; Isiordia-Espinoza, M.A.; Gaona, E.; Molina-Frechero, N. A Systematic Review and Meta-Analysis of the Relationship Between the Severity of Dental Fluorosis and Fluoride Biomarkers in Endemic Areas. Biol. Trace Elem. Res. 2023, 201, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Kumah, E.A.; Eskandari, F.; Azevedo, L.B.; John, S.; Zohoori, F.V. Mapping the evidence for monitoring fluoride exposure in community prevention programmes for oral health using nail clippings and spot urine samples: A scoping review. BMC Oral. Health 2022, 22, 575. [Google Scholar] [CrossRef] [PubMed]
- Aylward, L.L.; Hays, S.M.; Vezina, A.; Deveau, M.; St-Amand, A.; Nong, A. Biomonitoring Equivalents for interpretation of urinary fluoride. Regul. Toxicol. Pharmacol. 2015, 72, 158–167. [Google Scholar] [CrossRef]
- Martinez-Mier, E.A.; Soto-Rojas, A.E. Differences in exposure and biological markers of fluoride among White and African American children. J. Public. Health Dent. 2010, 70, 234–240. [Google Scholar] [CrossRef]
- Barlabà, A.; Grella, C.; Tammaro, M.; Petrone, D.; Guarino, S.; Miraglia Del Giudice, E.; Marzuillo, P.; Di Sessa, A. Kidney function evaluation in children and adolescents with obesity: A not-negligible need. Eur. J. Pediatr. 2024, 183, 3655–3664. [Google Scholar] [CrossRef]
- Jowsey, J.; Riggs, B.L. Effect of concurrent calcium ingestion on intestinal absorption of fluoride. Metabolism 1978, 27, 971–974. [Google Scholar] [CrossRef]
- Mahmood, M.; Azevedo, L.B.; Maguire, A.; Buzalaf, M.; Zohoori, F.V. Pharmacokinetics of fluoride in human adults: The effect of exercise. Chemosphere 2021, 262, 127796. [Google Scholar] [CrossRef]


| Characteristic | Overall | Male | Female | p Value |
|---|---|---|---|---|
| Number of participants, n (weighted %) | 1413 (100.0) | 728 (51.5) | 685 (48.5) | |
| Age group, n (weighted %) | 0.6 | |||
| Adolescents | 862 (68.5) | 450 (69.2) | 412 (67.7) | |
| Children | 551 (31.5) | 278 (30.8) | 273 (32.3) | |
| Race, n (weighted %) | 0.8 | |||
| Hispanic | 501 (23.7) | 240 (23.3) | 261 (24.0) | |
| Non–Hispanic White and Other | 481 (59.4) | 256 (60.1) | 225 (58.7) | |
| Non–Hispanic Black | 299 (12.4) | 160 (12.2) | 139 (12.5) | |
| Non–Hispanic Asian | 132 (4.6) | 72 (4.4) | 60 (4.8) | |
| Physical activity, n (weighted %) | 0.8 | |||
| <2 days/week | 479 (39.7) | 257 (40.8) | 222 (38.5) | |
| 2–4 days/week | 519 (35.8) | 258 (35.2) | 261 (36.5) | |
| >4 days/week | 415 (24.5) | 213 (24.0) | 202 (25.0) | |
| Milk product consumption, n (weighted %) | 0.001 | |||
| <1. times/week | 198 (14.6) | 74 (10.2) | 124 (19.5) | |
| 1–6 times/week | 367 (26.8) | 184 (25.6) | 183 (28.1) | |
| >6 times/week | 848 (58.6) | 470 (64.3) | 378 (52.4) | |
| Body Mass Index (kg/m2) | 22.64 (5.89) | 22.26 (5.91) | 23.05 (5.85) | 0.045 |
| Poverty income ratio (means ± SD) | 2.49 (1.56) | 2.58 (1.56) | 2.39 (1.56) | 0.13 |
| Bone mineral density (g/cm2, means ± SD) | 0.84 (0.15) | 0.85 (0.17) | 0.83 (0.14) | 0.058 |
| Water fluoride (mg/L, means ± SD) | 0.44 (0.35) | 0.44 (0.36) | 0.43 (0.34) | >0.9 |
| Plasma fluoride (μmol/L, means ± SD) | 0.34 (0.21) | 0.36 (0.20) | 0.32 (0.21) | <0.001 |
| Urine fluoride (mg/L, means ± SD) | 0.619 (0.510) | 0.677 (0.498) | 0.555 (0.517) | <0.001 |
| Crude β (95%CIs) | Model 1 β (95%CIs) | Model 2 β (95%CIs) | |
|---|---|---|---|
| Water fluoride (mg/L) | |||
| Q1 (≤0.16) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Q2 (0.17–0.42) | −0.03 (−0.05, −0.02) ** | −0.01 (−0.03, 0.00) | −0.02 (−0.04, 0.00) |
| Q3 (0.43–0.68) | −0.06 (−0.11, −0.02) ** | −0.04 (−0.09, 0.00) | −0.04 (−0.10, 0.02) |
| Q4 (>0.68) | −0.08 (−0.13, −0.03) ** | −0.06 (−0.10, −0.01) * | −0.06 (−0.12, 0.00) |
| p for trend | <0.001 | <0.001 | <0.001 |
| Plasma fluoride (μmmol/L) | |||
| Q1 (≤0.18) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Q2 (0.18–0.29) | −0.02 (−0.05, 0.01) | −0.01 (−0.03, 0.01) | −0.01 (−0.03, 0.01) |
| Q3 (0.30–0.40) | −0.04 (−0.06, −0.02) ** | −0.03 (−0.05, −0.01) * | −0.03 (−0.06, 0.00) * |
| Q4 (>0.40) | −0.04 (−0.08, 0.00) | −0.03 (−0.06, 0.00) * | −0.03 (−0.07, 0.00) |
| p for trend | <0.001 | 0.009 | 0.004 |
| Urine fluoride (mg/L) | |||
| Q1 (≤0.299) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Q2 (0.300–0.496) | 0.00 (−0.02, 0.02) | 0.00 (−0.02, 0.02) | 0.00 (−0.03, 0.02) |
| Q3 (0.497–0.804) | −0.01 (−0.04, 0.03) | 0.00 (−0.02, 0.03) | −0.01 (−0.04, 0.03) |
| Q4 (>0.804) | −0.02 (−0.05, 0.02) | −0.01 (−0.03, 0.01) | −0.01 (−0.04, 0.01) |
| p for trend | 0.7 | 0.8 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, H.; He, Z.; Li, H.; Xing, D.; Lin, J. The Association between Fluoride and Bone Mineral Density in US Children and Adolescents: A Pilot Study. Nutrients 2024, 16, 2948. https://doi.org/10.3390/nu16172948
Kong H, He Z, Li H, Xing D, Lin J. The Association between Fluoride and Bone Mineral Density in US Children and Adolescents: A Pilot Study. Nutrients. 2024; 16(17):2948. https://doi.org/10.3390/nu16172948
Chicago/Turabian StyleKong, Haichen, Zihao He, Hui Li, Dan Xing, and Jianhao Lin. 2024. "The Association between Fluoride and Bone Mineral Density in US Children and Adolescents: A Pilot Study" Nutrients 16, no. 17: 2948. https://doi.org/10.3390/nu16172948





