Preparation of Rutin–Whey Protein Pickering Emulsion and Its Effect on Zebrafish Skeletal Muscle Movement Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Rutin–Whey Protein Pickering Emulsion (RWP)
2.3. Characterization of RWP
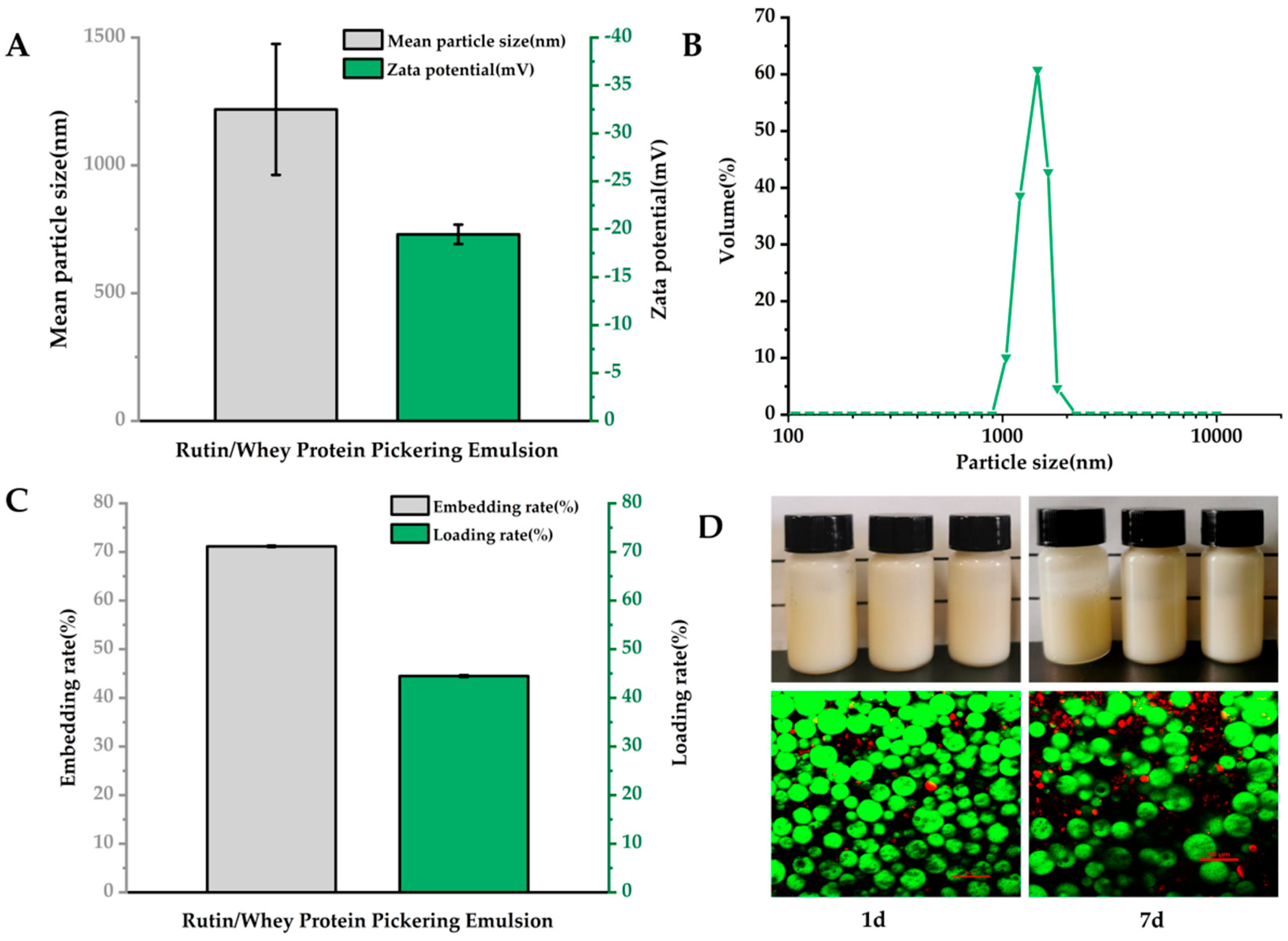
2.3.1. Particle Size and Zeta (ζ) Potential
2.3.2. Confocal Laser Scanning Microscopy (CLSM) Observation
2.3.3. Encapsulation Efficiency and Loading Capacity
2.4. Storage Stability
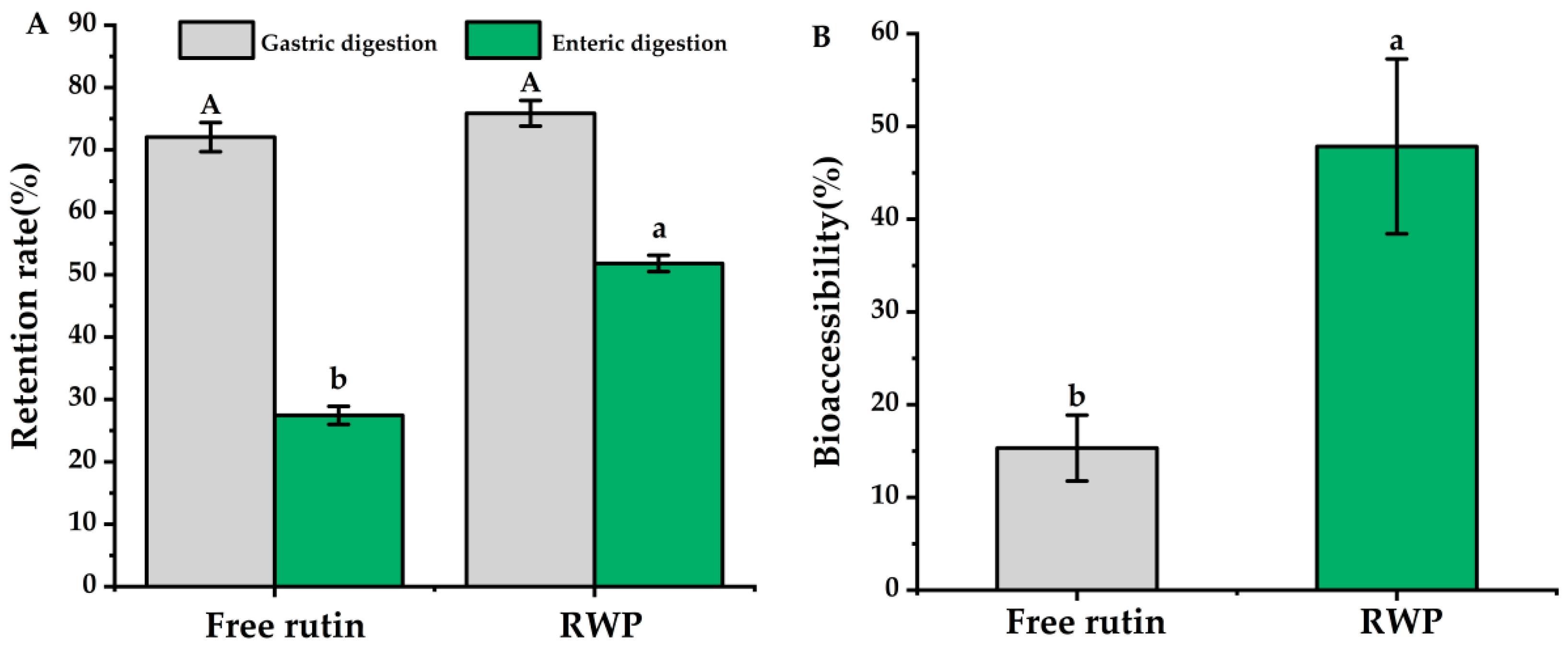
2.5. In Vitro Digestion of Pickering Emulsion
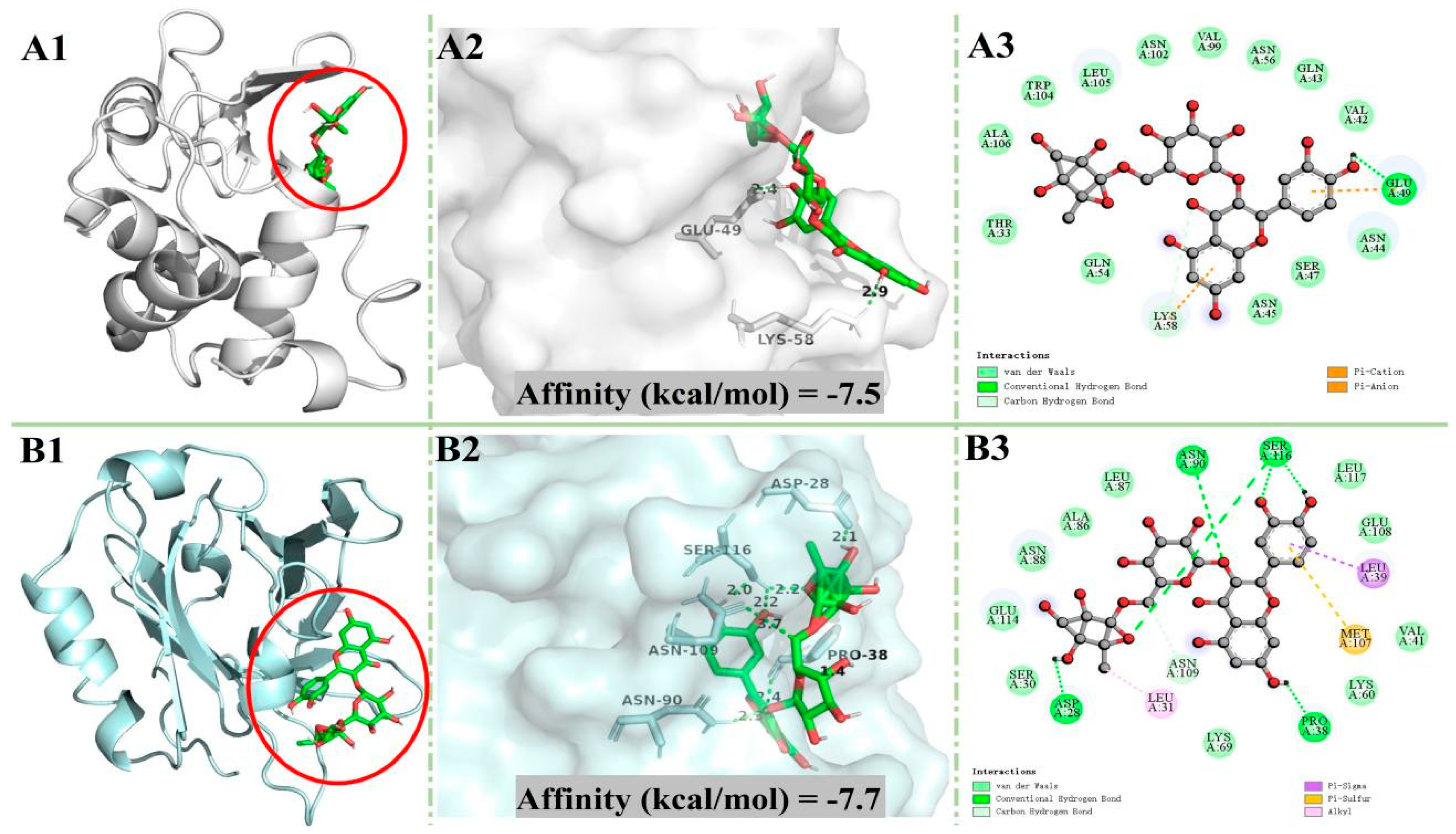
2.6. Molecular Docking
2.7. Zebrafish Experiment
2.7.1. Experimental Animals
2.7.2. Maximum Tolerated Dose Assay
2.7.3. Mobility Ability Evaluation
2.7.4. ATP Level Test
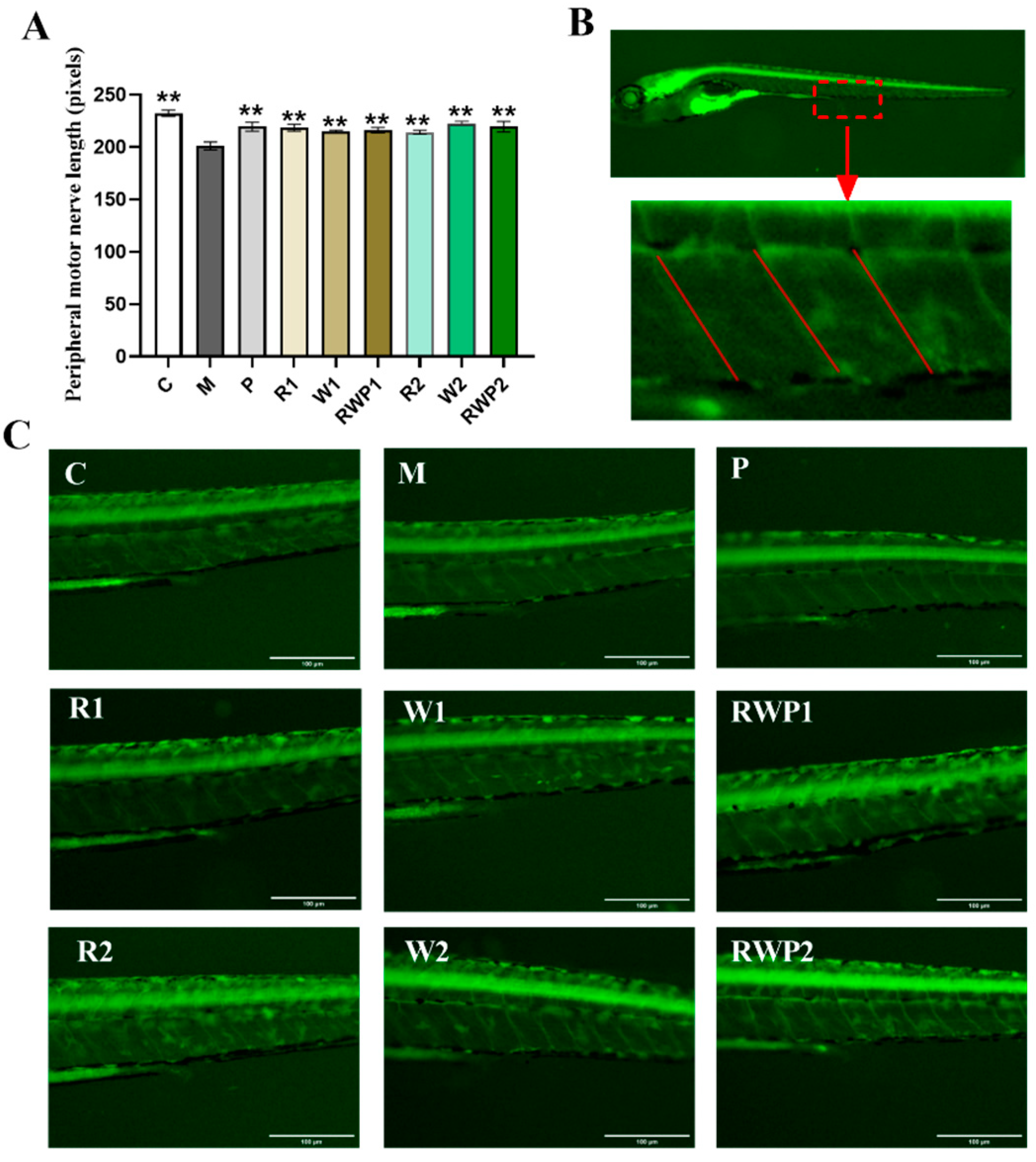
2.7.5. Peripheral Motor Nerve Length Measurement
2.7.6. Histopathological Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Structural Characteristics and Loading Efficiency of Emulsions
3.2. Storage Stability
3.3. In Vitro Simulation of Digestion and Bioaccessibility
3.4. Molecular Docking
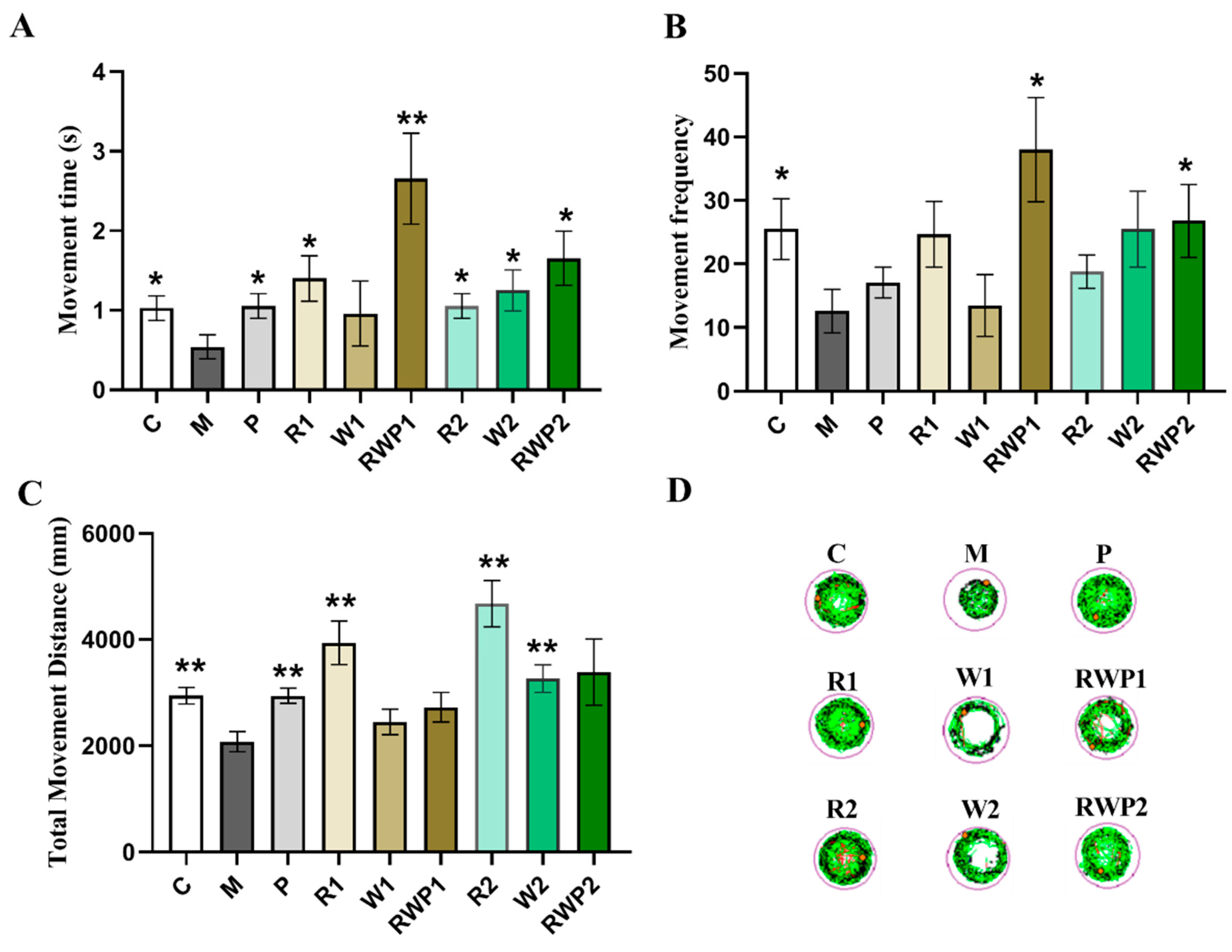
3.5. Effects of the RWP on Zebrafish Muscle Motor Function
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, L.; Zong, Y.; Zhao, J.; Yang, Y.; Li, L.; Li, N.; Gao, Y.; Xie, X.; Bao, Q.; Jiang, L.; et al. Characterizing the skeletal muscle immune microenvironment for sarcopenia: Insights from transcriptome analysis and histological validation. Front. Immunol. 2024, 15, 1414387. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metab. Clin. Exp. 2023, 144, 155533. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Y.; Han, F. Prevalence of sarcopenia in the community-dwelling, elderly Chinese population: A systematic review and meta-analysis. Lancet 2017, 390, S35. [Google Scholar] [CrossRef]
- Sallfeldt, E.S.; Mallmin, H.; Karlsson, M.K.; Mellström, D.; Hailer, N.P.; Ribom, E.L. Sarcopenia prevalence and incidence in older men—A MrOs Sweden study. Geriatr. Nurs. 2023, 50, 102–108. [Google Scholar] [CrossRef]
- Damanti, S.; Azzolino, D.; Roncaglione, C.; Arosio, B.; Rossi, P.; Cesari, M. Efficacy of Nutritional Interventions as Stand-Alone or Synergistic Treatments with Exercise for the Management of Sarcopenia. Nutrients 2019, 11, 1991. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef]
- Alaseem, A.M. Continued elevation of creatinine and uric acid in a male athlete: A case report. SAGE Open Med. Case Rep. 2024, 12, 2050313x241260229. [Google Scholar] [CrossRef]
- Singh, R.G.; Guérin-Deremaux, L.; Lefranc-Millot, C.; Perreau, C.; Crowley, D.C.; Lewis, E.D.; Evans, M.; Moulin, M. Ef-ficacy of Pea Protein Supplementation in Combination with a Resistance Training Program on Muscle Performance in a Sedentary Adult Population: A Randomized, Comparator-Controlled, Parallel Clinical Trial. Nutrients 2024, 16, 2017. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Kim, J.S.; Wilson, J.M.; Lee, S.R. Dietary implications on mechanisms of sarcopenia: Roles of protein, amino acids and antioxidants. J. Nutr. Biochem. 2010, 21, 1–13. [Google Scholar] [CrossRef]
- Park, C.Y.; Shin, S. Low dietary vitamin C intake is associated with low muscle strength among elderly Korean women. Nutr. Res. 2024, 127, 75–83. [Google Scholar] [CrossRef]
- Zhao, X.; Hua, L.; Jin, K.; Sun, Q.; Wang, R. Association between oxidative balance score and skeletal muscle mass and strength: NHANES from 2011 to 2018. Front. Nutr. 2024, 11, 1414161. [Google Scholar] [CrossRef]
- Hosoda, R.; Nakashima, R.; Yano, M.; Iwahara, N.; Asakura, S.; Nojima, I.; Saga, Y.; Kunimoto, R.; Horio, Y.; Kuno, A. Resveratrol, a SIRT1 activator, attenuates aging-associated alterations in skeletal muscle and heart in mice. J. Pharmacol. Sci. 2023, 152, 112–122. [Google Scholar] [CrossRef]
- Hedya, S.; Hawila, N.; Abdin, A.; Maaly, A.E. Luteolin Attenuates Dexamethasone-Induced Skeletal Muscle Atrophy in Male Albino Rats. Med. J. Cairo Univ. 2019, 87, 3365–3374. [Google Scholar] [CrossRef]
- Guo, R.; Wei, P. Studies on the antioxidant effect of rutin in the microenvironment of cationic micelles. Microchim. Acta 2008, 161, 233–239. [Google Scholar] [CrossRef]
- Lee, C.C.; Shen, S.R.; Lai, Y.J.; Wu, S.C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food Funct. 2013, 4, 794–802. [Google Scholar] [CrossRef]
- Seo, S.; Lee, M.S.; Chang, E.; Shin, Y.; Oh, S.; Kim, I.H.; Kim, Y. Rutin Increases Muscle Mitochondrial Biogenesis with AMPK Activation in High-Fat Diet-Induced Obese Rats. Nutrients 2015, 7, 8152–8169. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Xin, T.; Dong, L.; Deng, M.; Han, L.; Huang, F.; Su, D. Effects of polysaccharides on colonic targeting and colonic fermentation of ovalbumin-ferulic acid based emulsion. Food Chem. 2024, 453, 139630. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of chrysin and rutin using self-assembled nanoparticles of debranched quinoa, maize, and waxy maize starches. Carbohydr. Polym. 2024, 337, 122118. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein nanoparticles for Pickering emulsions: A comprehensive review on their shapes, preparation methods, and modification methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Gantumur, M.A.; Guo, L.; Lian, L.; Wang, B.; Yu, C.; Jiang, Z. Insight into oil-water interfacial adsorption of protein particles towards regulating Pickering emulsions: A review. Int. J. Biol. Macromol. 2024, 272, 2937. [Google Scholar] [CrossRef]
- Jia, N.; Lin, S.; Yu, Y.; Zhang, G.; Li, L.; Zheng, D.; Liu, D. The Effects of Ethanol and Rutin on the Structure and Gel Properties of Whey Protein Isolate and Related Mechanisms. Foods 2022, 11, 3480. [Google Scholar] [CrossRef]
- Sánchez-García, Y.I.; Gutiérrez-Méndez, N.; Landeros-Martínez, L.L.; Ramos-Sánchez, V.H.; Orozco-Mena, R.; Salmerón, I.; Leal-Ramos, M.Y.; Sepúlveda, D.R. Crystallization of Lactose-Protein Solutions in the Presence of Flavonoids. J. Agric. Food Chem. 2022, 70, 2684–2694. [Google Scholar] [CrossRef]
- Gagliardi, A.; Paolino, D.; Costa, N.; Fresta, M.; Cosco, D. Zein- vs PLGA-based nanoparticles containing rutin: A comparative investigation. Mater. Sci. Eng. C 2021, 118, 111538. [Google Scholar] [CrossRef]
- Dammak, I.; do Amaral Sobral, P.J. Investigation into the physicochemical stability and rheological properties of rutin emulsions stabilized by chitosan and lecithin. J. Food Eng. 2018, 229, 12–20. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Ma, Y.; Liu, Y.; Liu, G.; Wang, Y.; Tan, B. Dietary fatty acid-mediated protein encapsulation simultaneously improving the water-solubility, storage stability, and oral absorption of astaxanthin. Food Hydrocoll. 2022, 123, 7152. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Chen, M.; Liu, C.; Dai, M.; Wang, Q.; Li, C.; Hung, W. Bifidobacterium lactis BL-99 modulates intestinal inflammation and functions in zebrafish models. PLoS ONE 2022, 17, e0262942. [Google Scholar] [CrossRef]
- Zhao, B.; Jia, X.; Feng, H.; Tang, C.; Huang, Y.; Zhao, Z.; Hao, J.; Li, H.; Du, J.; Liu, Y.; et al. Nutrient combinations exhibit universal antianxiety, antioxidant, neuro-protecting, and memory-improving activities. Front. Nutr. 2023, 9, 96692. [Google Scholar] [CrossRef]
- Li, Q.; Wu, L.; Wang, G.; Zheng, F.; Sun, J.; Zhang, Y.; Li, Z.; Li, L.; Sun, B. Inhibitory Effects of Jiuzao Polysaccharides on Alcoholic Fatty Liver Formation in Zebrafish Larvae and Their Regulatory Impact on Intestinal Microbiota. Foods 2024, 13, 276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhong, W.; Lin, H.; Huang, P.; Ma, N.; Zhang, Y.; Zhou, C.; Lai, Y.; Huang, S.; Huang, S.; et al. Hesperidin Protects against Acute Alcoholic Injury through Improving Lipid Metabolism and Cell Damage in Zebrafish Larvae. Evid. Based Complement. Altern. Med. 2017, 2017, 7282653. [Google Scholar] [CrossRef]
- Fu, Y.; McClements, D.J.; Luo, S.; Ye, J.; Liu, C. Degradation kinetics of rutin encapsulated in oil-in-water emulsions: Impact of particle size. J. Sci. Food Agric. 2022, 103, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Qiu, S.; Zhang, W.; Dang, Z.; Xie, C.; Xiong, Y.L. Fabrication and characterization of glycosylated-zein as an effective delivery system for rutin: Formation mechanism, physicochemical properties, and bioaccessibility in vitro. Food Biosci. 2024, 59, 104056. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, Y.; Xia, Q.; Du, L.; He, J.; Xu, J.; Zhou, C.; Pan, D. Hydrophobic interaction at the O/W interface: Impacts on the interfacial stability, encapsulation and bioaccessibility of polyphenols. Food Hydrocoll. 2023, 140, 108622. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.-Y.; Guo, J.; Yin, S.-W.; Yang, X.-Q. Microfluidization initiated cross-linking of gliadin particles for structured algal oil emulsions. Food Hydrocoll. 2017, 73, 153–161. [Google Scholar] [CrossRef]
- Atarés, L.; Marshall, L.J.; Akhtar, M.; Murray, B.S. Structure and oxidative stability of oil in water emulsions as affected by rutin and homogenization procedure. Food Chem. 2012, 134, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Y.; Yuan, F.; Gao, Y.; Mao, L. Emulsion gels with different proteins at the interface: Structures and delivery functionality. Food Hydrocoll. 2021, 116, 6637. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Zheng, H.; Chen, H.; Gao, Y. Characterization of β-carotene loaded emulsion gels containing denatured and native whey protein. Food Hydrocoll. 2020, 102, 105600. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of Emulsion Gels for the Delivery of Functional Food Ingredients: From Structure to Functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Vertuani, S.; Bino, A.; De Lucia, D.; Lampronti, I.; Milani, R.; Gambari, R.; Manfredini, S. Design, synthesis and biological activity of a novel Rutin analogue with improved lipid soluble properties. Bioorganic Med. Chem. 2015, 23, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, H.; Su, S.; Shi, M.; Qin, S.; Zeng, C. Enhanced bioaccessibility of interfacial delivered oleanolic acid through self-constructed Pickering emulsion: Effects of oil types. Food Res. Int. 2024, 191, 114708. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Qin, W.; Zhang, Q.; Chen, D.; Lin, D.; Liu, S.; Huang, Z.; Chen, H. Physicochemical stability and in vitro bi-oaccessibility of β-carotene emulsions stabilized with arabinoxylan hydrolysates-soy protein isolate conjugates. LWT 2022, 157, 113120. [Google Scholar] [CrossRef]
- Berthelsen, R.; Klitgaard, M.; Rades, T.; Müllertz, A. In vitro digestion models to evaluate lipid based drug delivery systems; present status and current trends. Adv. Drug Deliv. Rev. 2019, 142, 35–49. [Google Scholar] [CrossRef]
- Han, C.; Zheng, Y.; Huang, S.; Xu, L.; Zhou, C.; Sun, Y.; Wu, Z.; Wang, Z.; Pan, D.; Cao, J.; et al. Exploring the binding mechanisms of thermally and ultrasonically induced molten globule-like β-lactoglobulin with heptanal as revealed by multi-spectroscopic techniques and molecular simulation. Int. J. Biol. Macromol. 2024, 263, 130300. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, H.; Tian, J.; Shu, C.; Sun, R.; Li, B.; Meng, X. Protective effects of α-casein or β-casein on the stability and antioxidant capacity of blueberry anthocyanins and their interaction mechanism. LWT 2019, 115, 108434. [Google Scholar] [CrossRef]
- Aranda-Martínez, P.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Guerra-Librero, A.; Rodríguez-Santana, C.; Escames, G.; Acuña-Castroviejo, D. The Zebrafish, an Outstanding Model for Biomedical Research in the Field of Melatonin and Hu-man Diseases. Int. J. Mol. Sci. 2022, 23, 7438. [Google Scholar] [CrossRef]
- Christian, C.J.; Benian, G.M. Animal models of sarcopenia. Aging Cell 2020, 19, e13223. [Google Scholar] [CrossRef]
- Daya, A.; Donaka, R.; Karasik, D. Zebrafish models of sarcopenia. Dis. Models Mech. 2020, 13, dmm042689. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Martínez, P.; Sayed, R.K.; Fernández-Martínez, J.; Ramírez-Casas, Y.; Yang, Y.; Escames, G.; Acuña-Castroviejo, D. Zebrafish as a Human Muscle Model for Studying Age-Dependent Sarcopenia and Frailty. Int. J. Mol. Sci. 2024, 25, 6166. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hwang, J.K. Flavonoids: Nutraceutical potential for counteracting muscle atrophy. Food Sci. Biotechnol. 2020, 29, 1619–1640. [Google Scholar] [CrossRef]
- Yu, F.R.; Liu, Y.; Cui, Y.Z.; Chan, E.Q.; Xie, M.R.; McGuire, P.P.; Yu, F.H. Effects of a flavonoid extract from Cynomorium songaricum on the swimming endurance of rats. Am. J. Chin. Med. 2010, 38, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Supplemental protein in support of muscle mass and health: Advantage whey. J. Food Sci. 2015, 80 (Suppl. S1), A8–A15. [Google Scholar] [CrossRef]
- Chen, W.C.; Huang, W.C.; Chiu, C.C.; Chang, Y.K.; Huang, C.C. Whey protein improves exercise performance and biochemical profiles in trained mice. Med. Sci. Sports Exerc. 2014, 46, 1517–1524. [Google Scholar] [CrossRef]
- Tian, M.; Cheng, J.; Guo, M. Stability, Digestion, and Cellular Transport of Soy Isoflavones Nanoparticles Stabilized by Polymerized Goat Milk Whey Protein. Antioxidants 2024, 13, 567. [Google Scholar] [CrossRef]
- Korin, A.; Gouda, M.M.; Youssef, M.; Elsharkawy, E.; Albahi, A.; Zhan, F.; Sobhy, R.; Li, B. Whey Protein Sodium-Caseinate as a Deliverable Vector for EGCG: In Vitro Optimization of Its Bioaccessibility, Bioavailability, and Bioactivity Mode of Actions. Molecules 2024, 29, 2588. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- John, A.A.; Rossettie, S.; Rafael, J.; Cox, C.T.; Ducic, I.; Mackay, B.J. Assessment of Motor Function in Peripheral Nerve Injury and Recovery. Orthop. Rev. 2022, 14, 37578. [Google Scholar] [CrossRef]
- Lenka, A.; Jankovic, J. Peripherally-induced Movement Disorders: An Update. Tremor Other Hyperkinetic Mov. 2023, 13, 8. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Wu, Y.-Y.; Xia, B.; Dai, M.-Z.; Huang, Y.-F.; Yang, H.; Li, C.-Q.; Li, P. Fenobucarb-induced developmental neurotoxicity and mechanisms in zebrafish. NeuroToxicology 2020, 79, 11–19. [Google Scholar] [CrossRef]
- Kedlian, V.R.; Wang, Y.; Liu, T.; Chen, X.; Bolt, L.; Tudor, C.; Shen, Z.; Fasouli, E.S.; Prigmore, E.; Kleshchevnikov, V.; et al. Human skeletal muscle aging atlas. Nat. Aging 2024, 4, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.S.; Lee, W.K.; Lee, S.J.; Lee, S.Y.; Seo, J.H.; Kim, E.J.; Choe, Y.I.; Kim, S.G.; Yoo, J.I. Rutin Prevents Dexame-thasone-Induced Muscle Loss in C2C12 Myotube and Mouse Model by Controlling FOXO3-Dependent Signaling. Antioxidants 2023, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Valle, M.S.; D’Angeli, F.; Surdo, S.; Malaguarnera, L. Resveratrol and Vitamin D: Eclectic Molecules Promoting Mitochondrial Health in Sarcopenia. Int. J. Mol. Sci. 2024, 25, 7503. [Google Scholar] [CrossRef] [PubMed]
- Kimball, S.R.; Jefferson, L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006, 136, 227s–231s. [Google Scholar] [CrossRef]
- Carbone, J.W.; Pasiakos, S.M. Dietary Protein and Muscle Mass: Translating Science to Application and Health Benefit. Nutrients 2019, 11, 1136. [Google Scholar] [CrossRef]
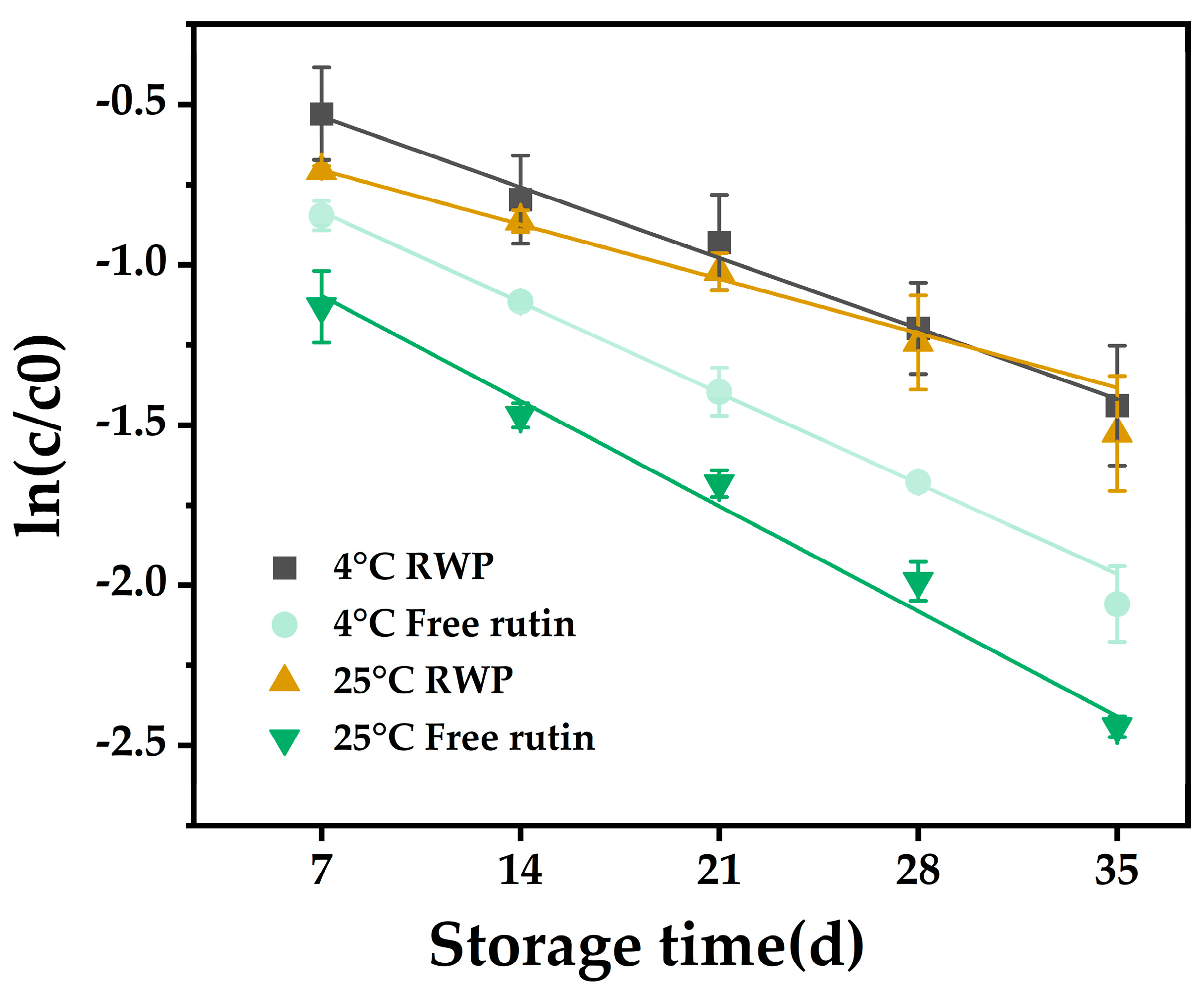


| Storage Temperatures | Specimens | K (d−1) | t1/2 (d) | R2 |
|---|---|---|---|---|
| 4 °C | RWP | 0.0411 | 16.86 | 0.9931 |
| Free rutin | 0.0588 | 11.79 | 0.9979 | |
| 25 °C | RWP | 0.0436 | 15.90 | 0.9816 |
| Free rutin | 0.0698 | 9.93 | 0.9733 |
| Complexes | Affinity (kcal/mol) | Number of van der Waals Forces | Amino Acid Residues Involved in van der Waals Forces | Number of Hydrogen Bonds | Amino Acid Residues Involved in Hydrogen Bonds |
|---|---|---|---|---|---|
| α-LA + Rutin | −7.5 | 13 | GLN (54,43), THR33, ALA106, TRP104, LEU105, ASN (102,45,56,44) VAL (99,42) SER47 | 1 | GLU49(2.4 Å) |
| β-LG + Rutin | −7.7 | 10 | SER30, GLU (114,108), ASN88, ALA86, LEU (87,117), VAL41, LYS (60,69) | 6 | SER116 (2.0 Å, 2.2 Å, 2.2 Å), ASN90 (2.3 Å), PRO38 (2.4 Å), ASP28 (2.1 Å) |
| Groups 1 | Concentrations | Number of Deaths | Mortality Rate (%) |
|---|---|---|---|
| Rutin | 6.25 ng/fish | 0 | 0 |
| 12.5 ng/fish | 0 | 0 | |
| 25.0 ng/fish | 0 | 0 | |
| 50.0 ng/fish | 0 | 0 | |
| 100.0 ng/fish | 1 | 3.33 | |
| Whey protein | 125.0 ng/fish | 0 | 0 |
| 250.0 ng/fish | 0 | 0 | |
| 500.0 ng/fish | 0 | 0 | |
| 1000.0 ng/fish | 0 | 0 | |
| 2000.0 ng/fish | 0 | 0 | |
| Pickering emulsion | 6.25% | 0 | 0 |
| 12.5% | 0 | 0 | |
| 25.0% | 0 | 0 | |
| 50% | 0 | 0 | |
| 100% | 5 | 16.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xiong, W.; Ren, Y.; Huang, J.; Wang, X.; Wang, O.; Cai, S. Preparation of Rutin–Whey Protein Pickering Emulsion and Its Effect on Zebrafish Skeletal Muscle Movement Ability. Nutrients 2024, 16, 3050. https://doi.org/10.3390/nu16183050
Zhang Y, Xiong W, Ren Y, Huang J, Wang X, Wang O, Cai S. Preparation of Rutin–Whey Protein Pickering Emulsion and Its Effect on Zebrafish Skeletal Muscle Movement Ability. Nutrients. 2024; 16(18):3050. https://doi.org/10.3390/nu16183050
Chicago/Turabian StyleZhang, Yiting, Wenyun Xiong, Yijing Ren, Jian Huang, Xiaoying Wang, Ou Wang, and Shengbao Cai. 2024. "Preparation of Rutin–Whey Protein Pickering Emulsion and Its Effect on Zebrafish Skeletal Muscle Movement Ability" Nutrients 16, no. 18: 3050. https://doi.org/10.3390/nu16183050






