An Oral Botanical Supplement Improves Small Intestinal Bacterial Overgrowth (SIBO) and Facial Redness: Results of an Open-Label Clinical Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Supplemental Regimen
2.3. Study Design and Recruitment
2.4. Subject Inclusion and Exclusion Criteria
2.5. Lactulose Breath Testing
2.6. Facial Imaging and Image-Based Analysis
2.7. Gut Microbiome Sequencing and Analysis
2.8. Plasma Collection and Intestinal Barrier Assessment
2.9. Statistical Analysis
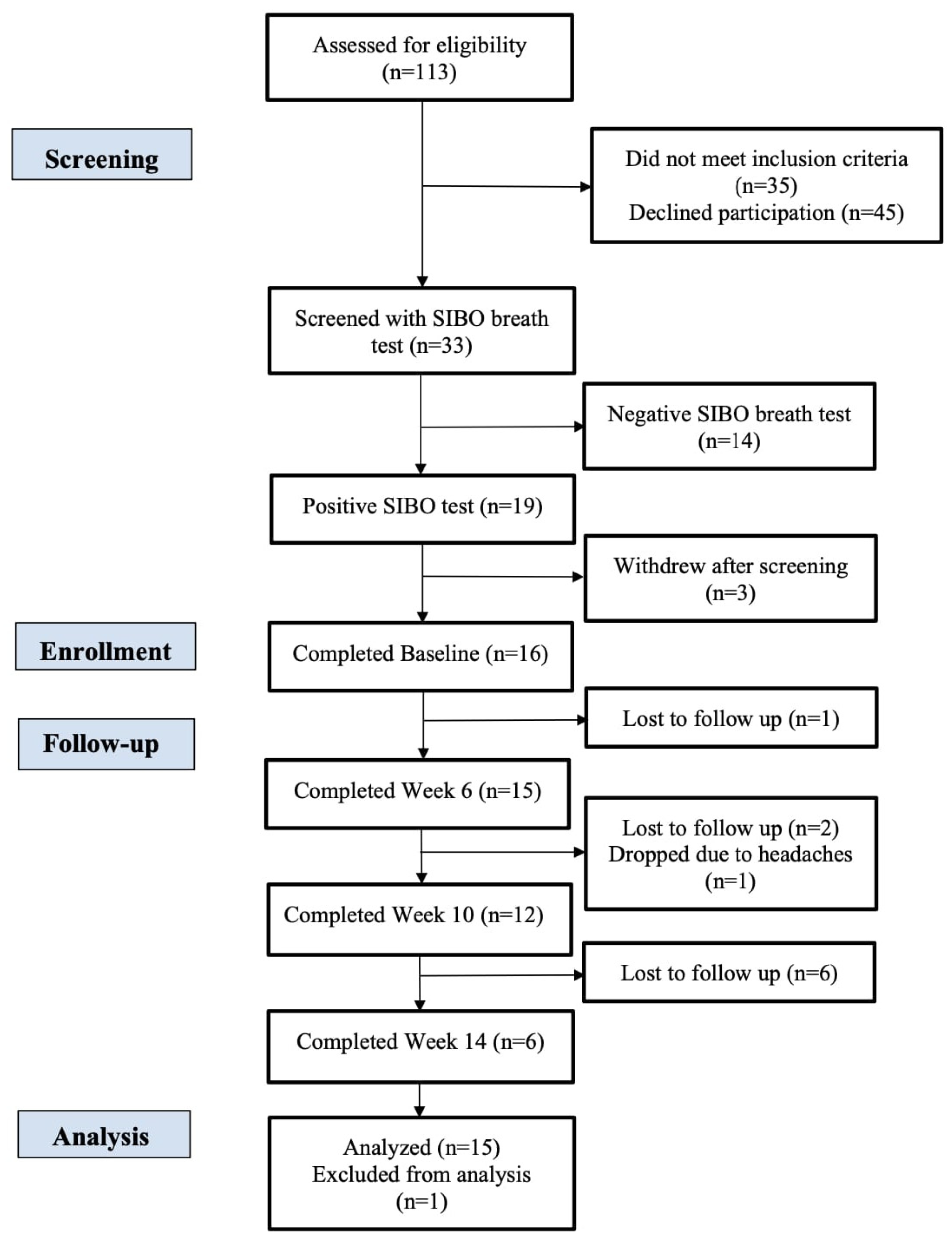
3. Results
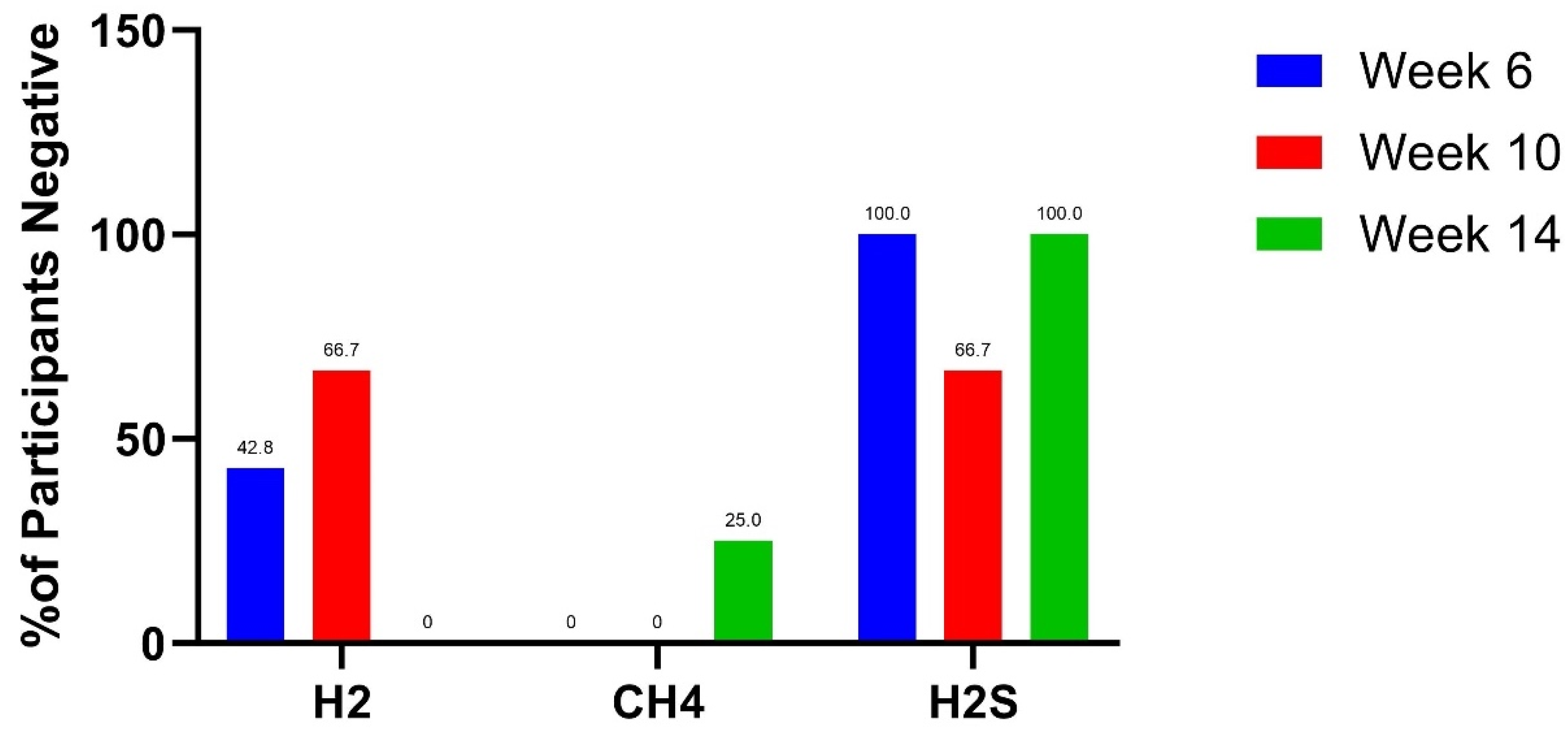
3.1. Lactulose Breath Test Results
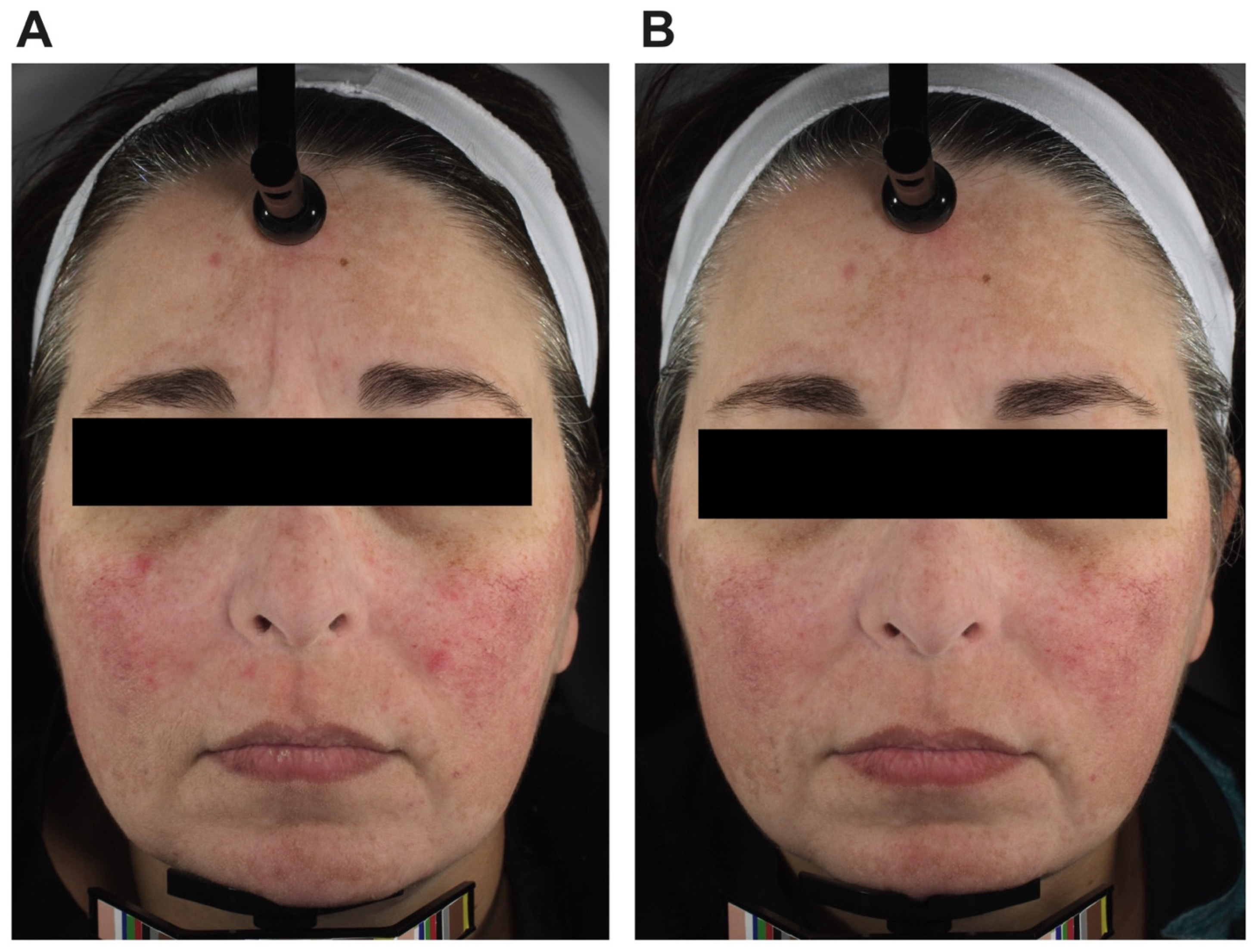
3.2. Facial Erythema
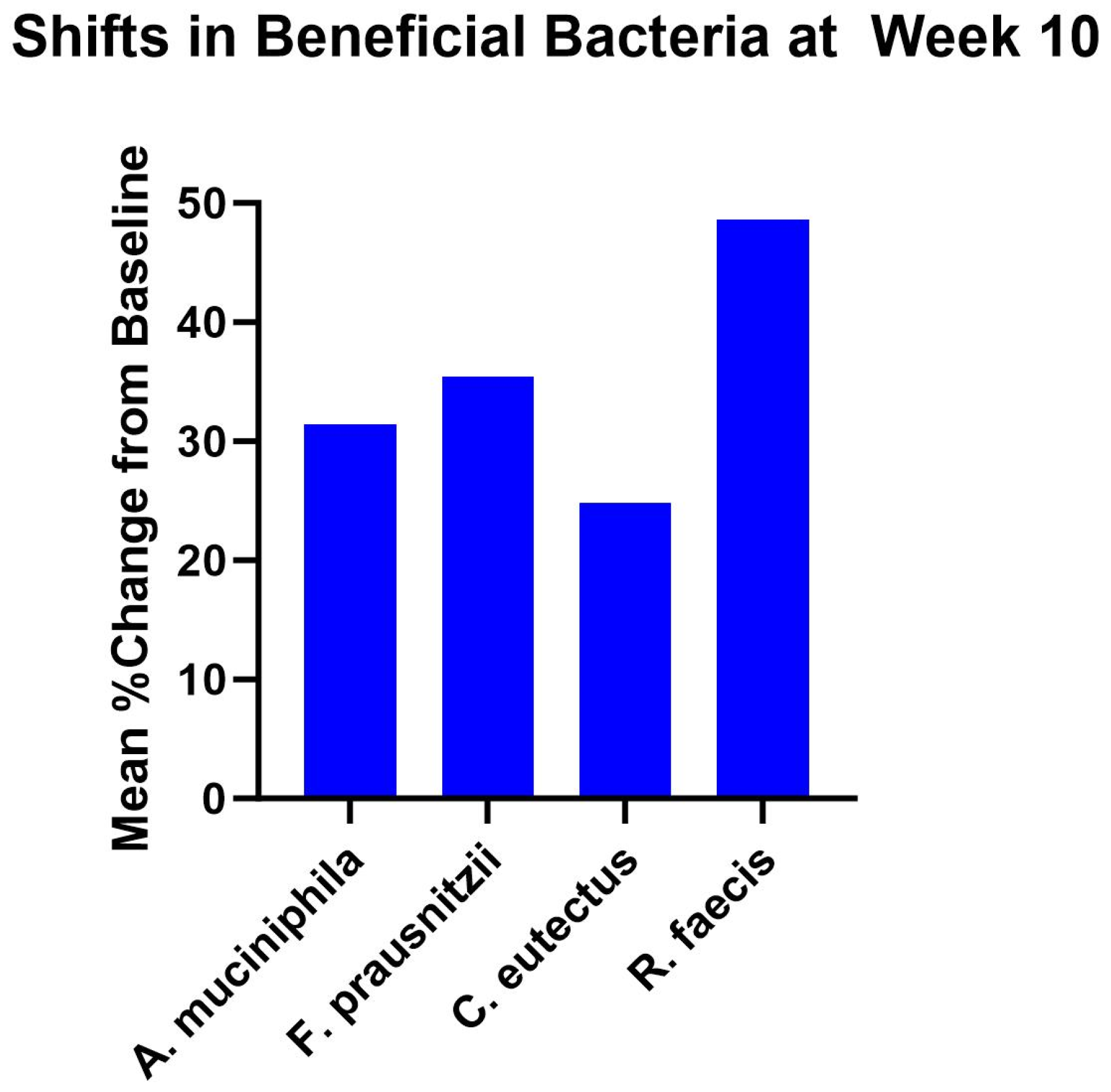
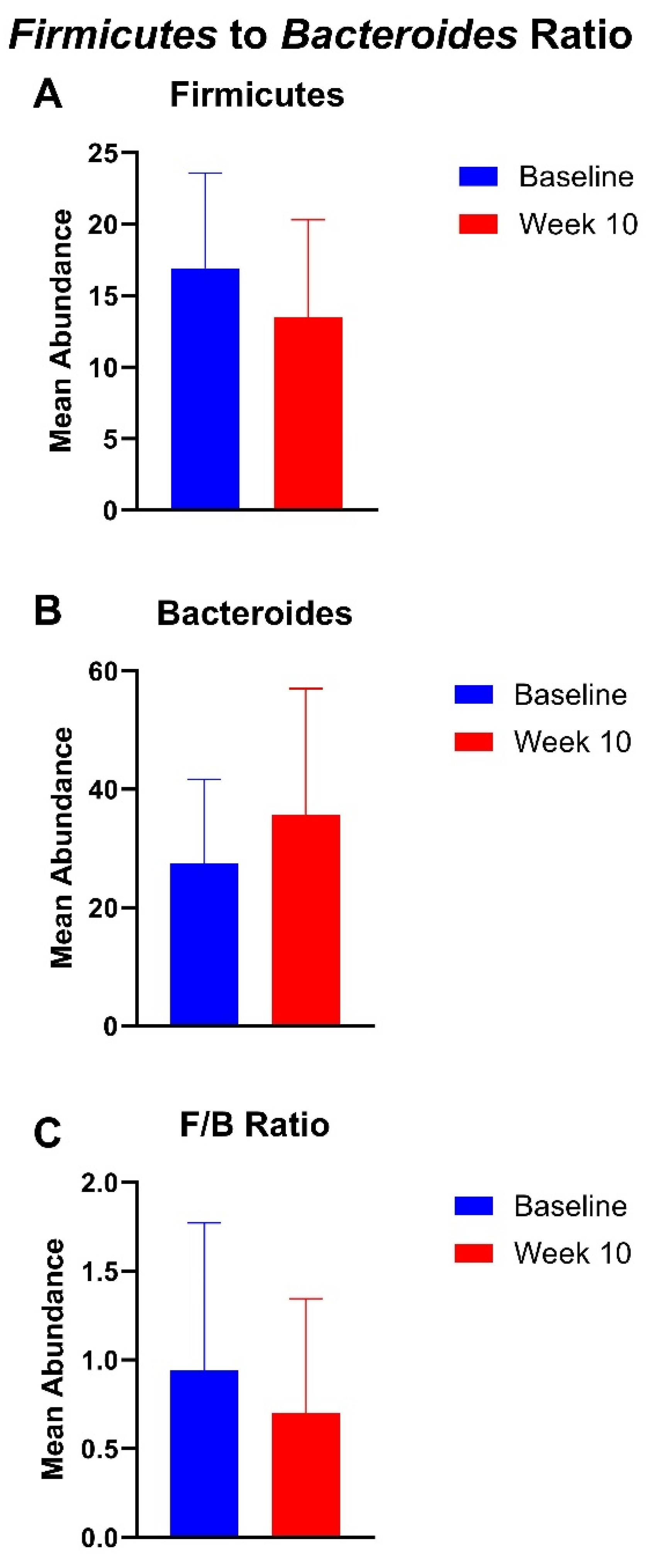
3.3. Gut Microbiome Findings
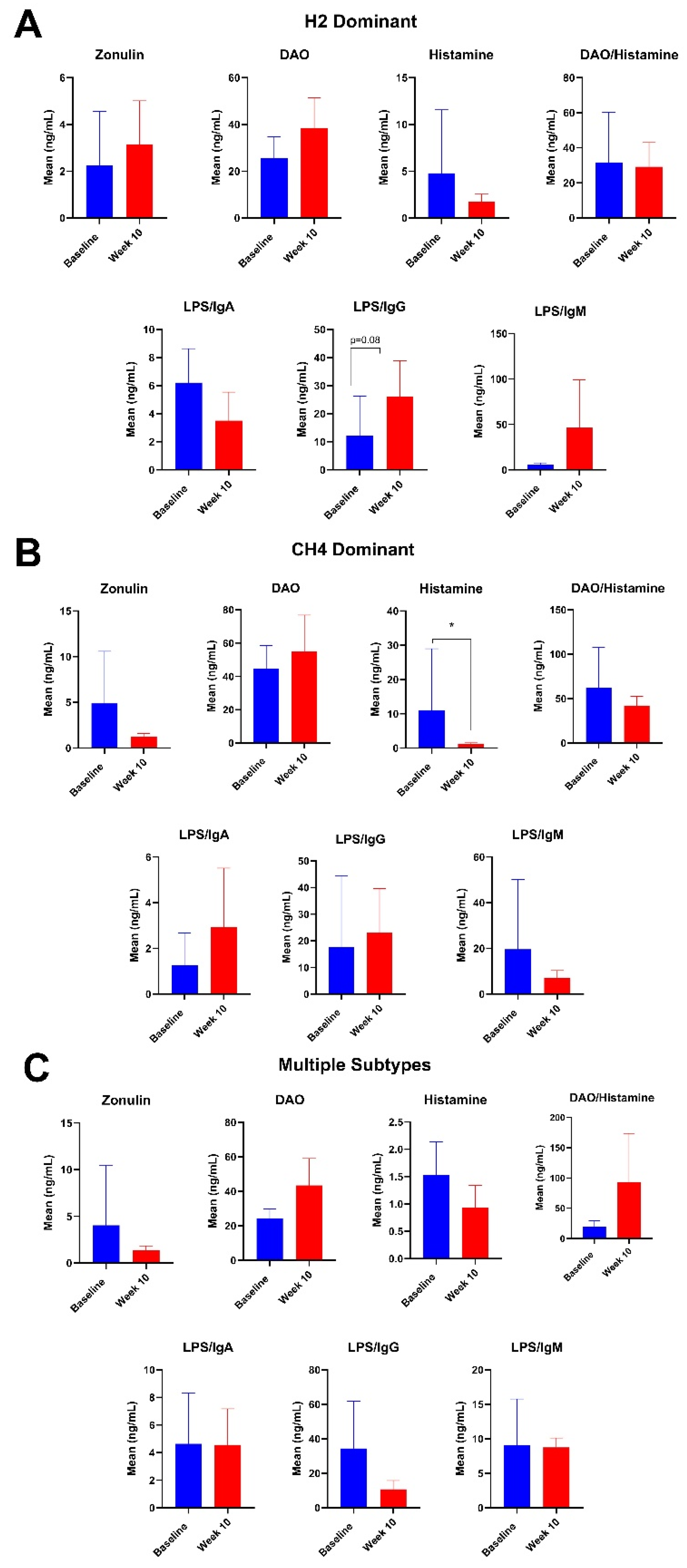
3.4. Plasma Intestinal Barrier Function Markers
3.5. Adverse Events
4. Discussion
4.1. Lactulose Breath Test Findings
4.2. Changes in Facial Erythema
4.3. Gut Microbiota
4.4. Changes Plasma Intestinal Barrier Function Markers
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Achufusi, T.G.O.; Sharma, A.; Zamora, E.A.; Manocha, D. Small Intestinal Bacterial Overgrowth: Comprehensive Review of Diagnosis, Prevention, and Treatment Methods. Cureus 2020, 12, e8860. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. The Spectrum of Small Intestinal Bacterial Overgrowth (SIBO). Curr. Gastroenterol. Rep. 2019, 21, 3. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Gorna, I.; Wozniak, D.; Przyslawski, J.; Drzymala-Czyz, S. Association between Gut Dysbiosis and the Occurrence of SIBO, LIBO, SIFO and IMO. Microorganisms 2023, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.H.; Pimentel, M. Gastrointestinal bacterial overgrowth: Pathogenesis and clinical significance. Ther. Adv. Chronic Dis. 2013, 4, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Cyrany, J.; Kohoutova, D.; Forstl, M.; Rejchrt, S.; Kvetina, J.; Vorisek, V.; Kopacova, M. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 2010, 16, 2978–2990. [Google Scholar] [CrossRef]
- Skrzydlo-Radomanska, B.; Cukrowska, B. How to Recognize and Treat Small Intestinal Bacterial Overgrowth? J. Clin. Med. 2022, 11, 6017. [Google Scholar] [CrossRef]
- Villanueva-Millan, M.J.; Leite, G.; Wang, J.; Morales, W.; Parodi, G.; Pimentel, M.L.; Barlow, G.M.; Mathur, R.; Rezaie, A.; Sanchez, M.; et al. Methanogens and Hydrogen Sulfide Producing Bacteria Guide Distinct Gut Microbe Profiles and Irritable Bowel Syndrome Subtypes. Am. J. Gastroenterol. 2022, 117, 2055–2066. [Google Scholar] [CrossRef]
- Wielgosz-Grochowska, J.P.; Domanski, N.; Drywien, M.E. Influence of Body Composition and Specific Anthropometric Parameters on SIBO Type. Nutrients 2023, 15, 4035. [Google Scholar] [CrossRef]
- Chedid, V.; Dhalla, S.; Clarke, J.O.; Roland, B.C.; Dunbar, K.B.; Koh, J.; Justino, E.; Tomakin, E.; Mullin, G.E. Herbal therapy is equivalent to rifaximin for the treatment of small intestinal bacterial overgrowth. Glob. Adv. Health Med. 2014, 3, 16–24. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.R.; Samir, R.; Jamil, M.A.-H.L.; Ramadan, M.A. Olive Leaf Extract Modulates Quorum Sensing Genes and Biofilm Formation in Multi-Drug Resistant Pseudomonas aeruginosa. Antibiotics 2020, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Wegener, T.; Wagner, H. The active components and the pharmacological multi-target principle of STW 5 (Iberogast). Phytomedicine 2006, 13 (Suppl. 5), 20–35. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Meah, D.; Ahmed, N.; Conniff-Jenkins, R.; Chileshe, E.; Phillips, C.O.; Claypole, T.C.; Forman, D.W.; Row, P.E. Comparison of the antibacterial activity of essential oils and extracts of medicinal and culinary herbs to investigate potential new treatments for irritable bowel syndrome. BMC Complement. Altern. Med. 2013, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Foolad, N.; Prakash, N.; Shi, V.Y.; Kamangar, F.; Wang, Q.; Li, C.S.; Sivamani, R.K. The use of facial modeling and analysis to objectively quantify facial redness. J. Cosmet. Dermatol. 2016, 15, 43–48. [Google Scholar] [CrossRef]
- Ma, T.; Chen, D.; Tu, Y.; Zhang, N.; Si, B.; Deng, K.; Diao, Q. Effect of supplementation of allicin on methanogenesis and ruminal microbial flora in Dorper crossbred ewes. J. Anim. Sci. Biotechnol. 2016, 7, 1. [Google Scholar] [CrossRef]
- Becker, P.M.; Wikselaar, P.G.; Ilgenfritz, J.; Beekwilder, J.; De Vos, R.; Franz, C.; Zitterl-Eglseer, K. Methane reduction by plant pigments and antioxidants in rumen fluid involves modifications, e.g. hydrogenation, or degradation of the active compounds. Wien. Tierarztl. Monatsschrift 2013, 100, 295–305. [Google Scholar]
- Ciezkowska, M.; Bajda, T.; Decewicz, P.; Dziewit, L.; Drewniak, L. Effect of Clinoptilolite and Halloysite Addition on Biogas Production and Microbial Community Structure during Anaerobic Digestion. Materials 2020, 13, 4127. [Google Scholar] [CrossRef]
- Wu, F.; Xie, J.; Xin, X.; He, J. Effect of activated carbon/graphite on enhancing anaerobic digestion of waste activated sludge. Front. Microbiol. 2022, 13, 999647. [Google Scholar] [CrossRef]
- Jia, L.; Wu, J.; Lei, Y.; Kong, F.; Zhang, R.; Sun, J.; Wang, L.; Li, Z.; Shi, J.; Wang, Y.; et al. Oregano Essential Oils Mediated Intestinal Microbiota and Metabolites and Improved Growth Performance and Intestinal Barrier Function in Sheep. Front. Immunol. 2022, 13, 908015. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Lang, X.; Liu, L.; Casper, D.P.; Wang, C.; Zhang, L.; Wei, S. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J. Dairy. Sci. 2020, 103, 2303–2314. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, J.; Kubota, T.; Takada, E.; Takai, K.; Fujiwara, N.; Arimitsu, N.; Murayama, M.A.; Ueda, Y.; Wakisaka, S.; Suzuki, T.; et al. Propionate-producing bacteria in the intestine may associate with skewed responses of IL10-producing regulatory T cells in patients with relapsing polychondritis. PLoS ONE 2018, 13, e0203657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Zhang, L.; Wu, J.; Zhao, S.; Jiao, T. Effect of Oregano Oil and Cobalt Lactate on Sheep In Vitro Digestibility, Fermentation Characteristics and Rumen Microbial Community. Animals 2022, 12, 118. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Hellwing, A.L.F.; Grevsen, K.; Haveman, L.S.; Chowdhury, M.R.; Lovendahl, P.; Weisbjerg, M.R.; Noel, S.J.; Hojberg, O.; Wiking, L.; et al. Effect of dried oregano (Origanum vulgare L.) plant material in feed on methane production, rumen fermentation, nutrient digestibility, and milk fatty acid composition in dairy cows. J. Dairy. Sci. 2019, 102, 9902–9918. [Google Scholar] [CrossRef]
- Fang, X.; Wu, H.; Wang, X.; Lian, F.; Li, M.; Miao, R.; Wei, J.; Tian, J. Modulation of Gut Microbiota and Metabolites by Berberine in Treating Mice With Disturbances in Glucose and Lipid Metabolism. Front. Pharmacol. 2022, 13, 870407. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Di, Z.; Zhang, Z.; Fu, B.; Wang, Y.; Huang, C.; Du, Y. Chinese herbal medicine for the treatment of small intestinal bacterial overgrowth (SIBO): A protocol for systematic review and meta-analysis. Medicine 2020, 99, e23737. [Google Scholar] [CrossRef]
- Daou, H.; Paradiso, M.; Hennessy, K.; Seminario-Vidal, L. Rosacea and the Microbiome: A Systematic Review. Dermatol. Ther. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Parodi, A.; Paolino, S.; Greco, A.; Drago, F.; Mansi, C.; Rebora, A.; Parodi, A.; Savarino, V. Small intestinal bacterial overgrowth in rosacea: Clinical effectiveness of its eradication. Clin. Gastroenterol. Hepatol. 2008, 6, 759–764. [Google Scholar] [CrossRef]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar] [CrossRef]
- Rosso, J.Q.; Draelos, Z.D.; Effron, C.; Kircik, L.H. Oral Sarecycline for Treatment of Papulopustular Rosacea: Results of a Pilot Study of Effectiveness and Safety. J. Drugs Dermatol. 2021, 20, 426–431. [Google Scholar] [CrossRef]
- Maier, L.; Goemans, C.V.; Wirbel, J.; Kuhn, M.; Eberl, C.; Pruteanu, M.; Muller, P.; Garcia-Santamarina, S.; Cacace, E.; Zhang, B.; et al. Unravelling the collateral damage of antibiotics on gut bacteria. Nature 2021, 599, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Noh, C.K.; Lee, K.J. Fecal Microbiota Alterations and Small Intestinal Bacterial Overgrowth in Functional Abdominal Bloating/Distention. J. Neurogastroenterol. Motil. 2020, 26, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Gasaly, N.; Hermoso, M.A.; Gotteland, M. Butyrate and the Fine-Tuning of Colonic Homeostasis: Implication for Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 3061. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Palmnas-Bedard, M.S.A.; Costabile, G.; Vetrani, C.; Aberg, S.; Hjalmarsson, Y.; Dicksved, J.; Riccardi, G.; Landberg, R. The human gut microbiota and glucose metabolism: A scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022, 116, 862–874. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Phan, J.; Nair, D.; Jain, S.; Montagne, T.; Flores, D.V.; Nguyen, A.; Dietsche, S.; Gombar, S.; Cotter, P. Alterations in Gut Microbiome Composition and Function in Irritable Bowel Syndrome and Increased Probiotic Abundance with Daily Supplementation. mSystems 2021, 6, e0121521. [Google Scholar] [CrossRef]
- Jia, W.; Whitehead, R.N.; Griffiths, L.; Dawson, C.; Waring, R.H.; Ramsden, D.B.; Hunter, J.O.; Cole, J.A. Is the abundance of Faecalibacterium prausnitzii relevant to Crohn’s disease? FEMS Microbiol. Lett. 2010, 310, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.I.; Kim, N.; Nam, R.H.; Jang, J.Y.; Kim, E.H.; Ha, S.; Kang, K.; Lee, W.; Choi, H.; Kim, Y.R.; et al. The Protective Effect of Roseburia faecis Against Repeated Water Avoidance Stress-induced Irritable Bowel Syndrome in a Wister Rat Model. J. Cancer Prev. 2023, 28, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shan, S.; Shi, J.; Li, H.; An, N.; Li, S.; Cui, K.; Guo, H.; Li, Z. Coprococcus eutactus, a Potent Probiotic, Alleviates Colitis via Acetate-Mediated IgA Response and Microbiota Restoration. J. Agric. Food Chem. 2023, 71, 3273–3284. [Google Scholar] [CrossRef] [PubMed]
- Spychala, M.S.; Venna, V.R.; Jandzinski, M.; Doran, S.J.; Durgan, D.J.; Ganesh, B.P.; Ajami, N.J.; Putluri, N.; Graf, J.; Bryan, R.M.; et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 2018, 84, 23–36. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Strukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Sabater, M.; Ortega, F.; Ricart, W.; Fernandez-Real, J.M. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE 2012, 7, e37160. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Fasano, A. All disease begins in the (leaky) gut: Role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res 2020, 9, F1000. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Schenk, M.; Lackner, S.; Enko, D.; Mangge, H.; Forster, F. Diamine oxidase supplementation improves symptoms in patients with histamine intolerance. Food Sci. Biotechnol. 2019, 28, 1779–1784. [Google Scholar] [CrossRef]
- Wolvekamp, M.C.; de Bruin, R.W. Diamine oxidase: An overview of historical, biochemical and functional aspects. Dig. Dis. 1994, 12, 2–14. [Google Scholar] [CrossRef]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, S.; Jutel, M.; Crameri, R.; O’Mahony, L. Histamine and gut mucosal immune regulation. Allergy 2014, 69, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: Data from the randomized controlled LIBRE trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Liu, H.; Sui, X.; Liu, Y.; Wei, X.; Liu, C.; Cheng, Y.; Ye, W.; Gao, B.; et al. The Anti-Inflammatory Effect and Mucosal Barrier Protection of Clostridium butyricum RH2 in Ceftriaxone-Induced Intestinal Dysbacteriosis. Front. Cell Infect. Microbiol. 2021, 11, 647048. [Google Scholar] [CrossRef]


| Baseline to Week 10 | Until Week 14 | |
|---|---|---|
| Biocidin liquid tincture | 15 drops orally, morning and evening | 15 drops orally, morning and evening |
| GI Detox+ | 2 capsules every evening, 1 h away from food | 2 capsules every evening, 1 h away from food |
| Olivirex | - | 2 capsules orally, morning and evening, with liquid tincture |
| Subject | Subtype |
|---|---|
| S01 | H2 |
| S02 | H2 |
| S03 | H2 |
| S04 | H2 |
| S05 | CH4 |
| S06 | CH4 |
| S07 | H2, H2S |
| S10 | CH4, H2S |
| S11 | H2, CH4 |
| S13 | CH4 |
| S14 | H2, H2S |
| S15 | H2 |
| S16 | H2 |
| S17 | CH4 |
| S18 | H2, H2S |
| S19 | CH4 |
| Hydrogen SIBO | ||||
|---|---|---|---|---|
| Subject | Baseline | Week 6 | Week 10 | Week 14 |
| S01 | + | + | - | N/A |
| S02 | + | - | + | LTFU |
| S03 | + | + | LTFU | |
| S04 | + | - | - | LTFU |
| S07 * | + | + | - | + |
| S08 | + | Dropped after baseline | ||
| S09 | + | Dropped after baseline | ||
| S11 * | + | - | LTFU | |
| S12 | + | Dropped after baseline | ||
| S14 * | + | Dropped after baseline | ||
| S15 | + | + | - | N/A |
| S16 | + | pd | LTFU | |
| S18 * | + | + | + | + |
| Methane SIBO | ||||
| Subject | Baseline | Week 6 | Week 10 | Week 14 |
| S05 | + | + | + | - |
| S06 | + | + | + | LTFU |
| S10 * | + | + | + | + |
| S11 * | + | + | LTFU | |
| S13 | + | + | + | + |
| S17 | + | pd | + | LTFU |
| S19 | + | + | + | + |
| Hydrogen Sulfide SIBO | ||||
| Subject | Baseline | Week 6 | Week 10 | Week 14 |
| S03 | + | - | LTFU | LTFU |
| S07 * | + | - | + | - |
| S10 * | + | - | - | - |
| S14 * | + | Dropped after baseline | ||
| S18 * | + | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, M.; Nadora, D.; Chakkalakal, M.; Afzal, N.; Subramanyam, C.; Gahoonia, N.; Pan, A.; Thacker, S.; Nong, Y.; Chambers, C.J.; et al. An Oral Botanical Supplement Improves Small Intestinal Bacterial Overgrowth (SIBO) and Facial Redness: Results of an Open-Label Clinical Study. Nutrients 2024, 16, 3149. https://doi.org/10.3390/nu16183149
Min M, Nadora D, Chakkalakal M, Afzal N, Subramanyam C, Gahoonia N, Pan A, Thacker S, Nong Y, Chambers CJ, et al. An Oral Botanical Supplement Improves Small Intestinal Bacterial Overgrowth (SIBO) and Facial Redness: Results of an Open-Label Clinical Study. Nutrients. 2024; 16(18):3149. https://doi.org/10.3390/nu16183149
Chicago/Turabian StyleMin, Mildred, Dawnica Nadora, Mincy Chakkalakal, Nasima Afzal, Chaitra Subramanyam, Nimrit Gahoonia, Adrianne Pan, Shivani Thacker, Yvonne Nong, Cindy J. Chambers, and et al. 2024. "An Oral Botanical Supplement Improves Small Intestinal Bacterial Overgrowth (SIBO) and Facial Redness: Results of an Open-Label Clinical Study" Nutrients 16, no. 18: 3149. https://doi.org/10.3390/nu16183149
APA StyleMin, M., Nadora, D., Chakkalakal, M., Afzal, N., Subramanyam, C., Gahoonia, N., Pan, A., Thacker, S., Nong, Y., Chambers, C. J., & Sivamani, R. K. (2024). An Oral Botanical Supplement Improves Small Intestinal Bacterial Overgrowth (SIBO) and Facial Redness: Results of an Open-Label Clinical Study. Nutrients, 16(18), 3149. https://doi.org/10.3390/nu16183149








