Capsaicin Improves Systemic Inflammation, Atherosclerosis, and Macrophage-Derived Foam Cells by Stimulating PPAR Gamma and TRPV1 Receptors
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vivo Study
2.1.1. Carotid Obstruction Surgery
2.1.2. Biochemical Determinations
2.1.3. Quantification of the Atherosclerosis Area
2.1.4. Immunofluorescence
2.1.5. Determination of NAG and MPO Activities
2.1.6. Cytokine Determination
2.1.7. Intravital Microscopy of Mesenteric Plexus
2.2. In Vitro Studies
2.2.1. Preparation of Dil-Oxldl
2.2.2. Cell Cultures
2.2.3. Solutions for Cell Cultures
2.3. Statistical Analysis
3. Results
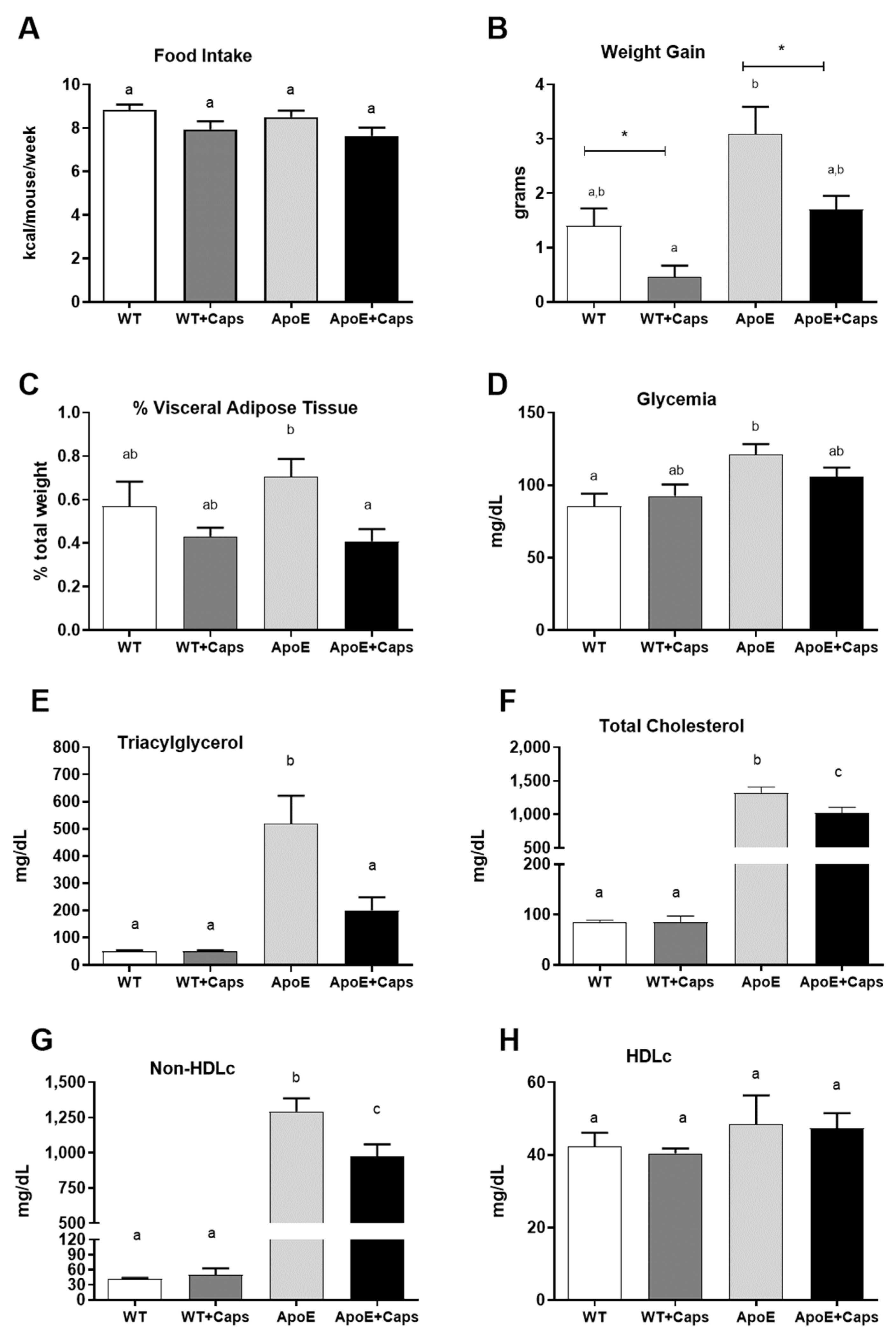
3.1. Capsaicin Improves Weight, Reduces Visceral Fat, and Enhances Glycemia and Lipid Profile in ApoE KO Mice
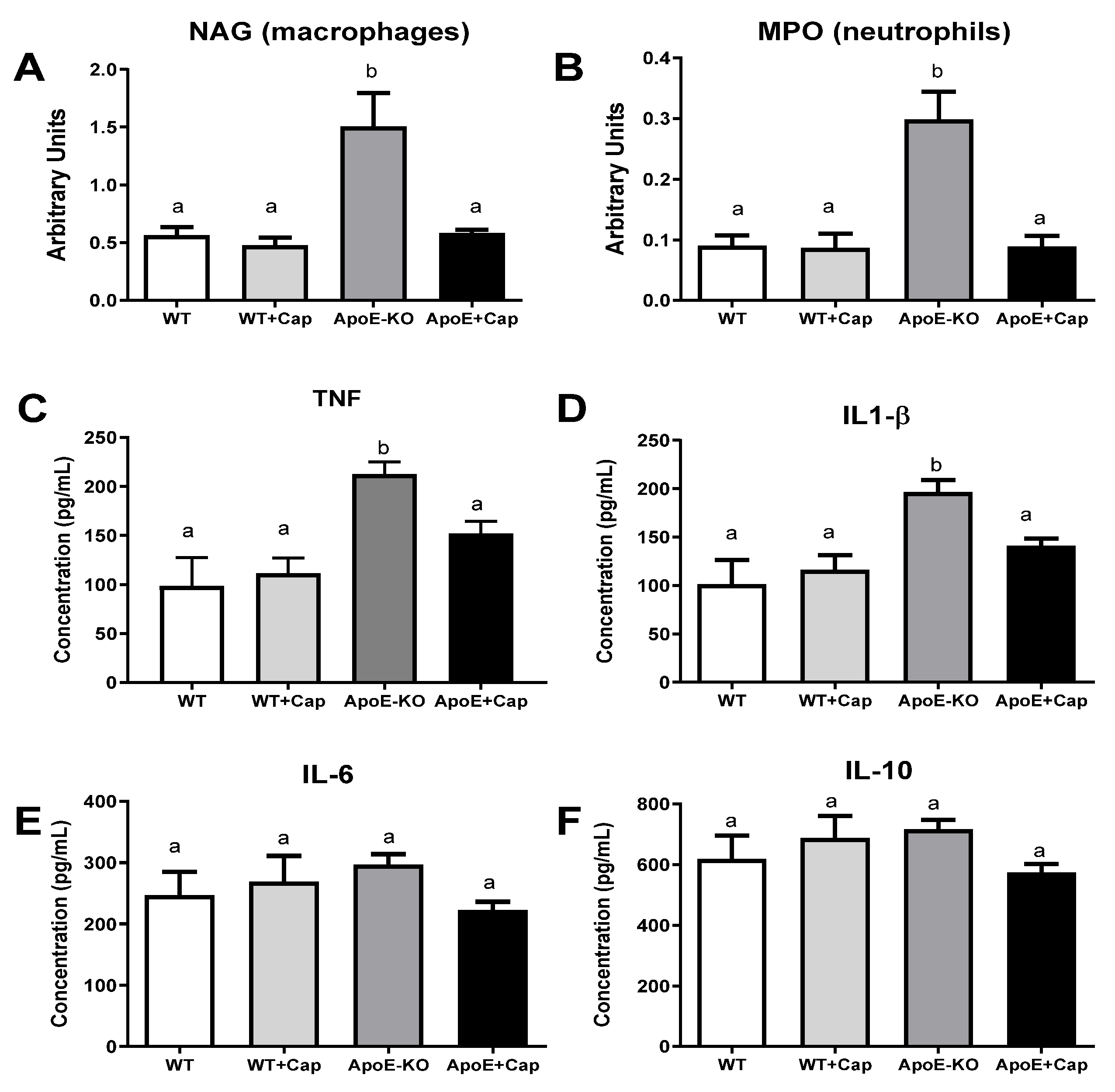
3.2. Capsaicin Reduces Inflammatory Markers in the Liver of ApoE KO Mice
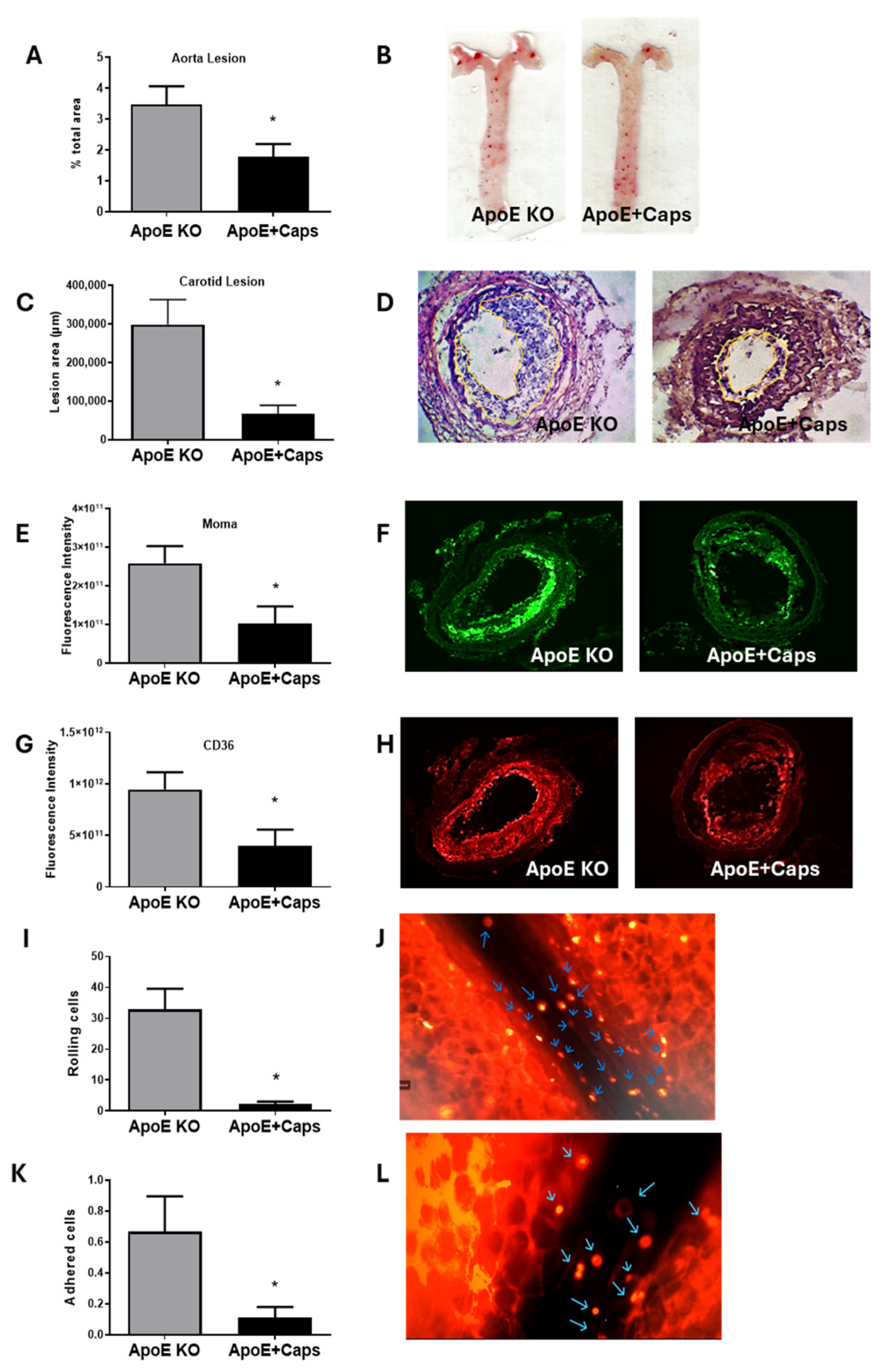
3.3. Capsaicin Treatment Reduces Atherosclerosis and Inflammation in ApoE KO Mice
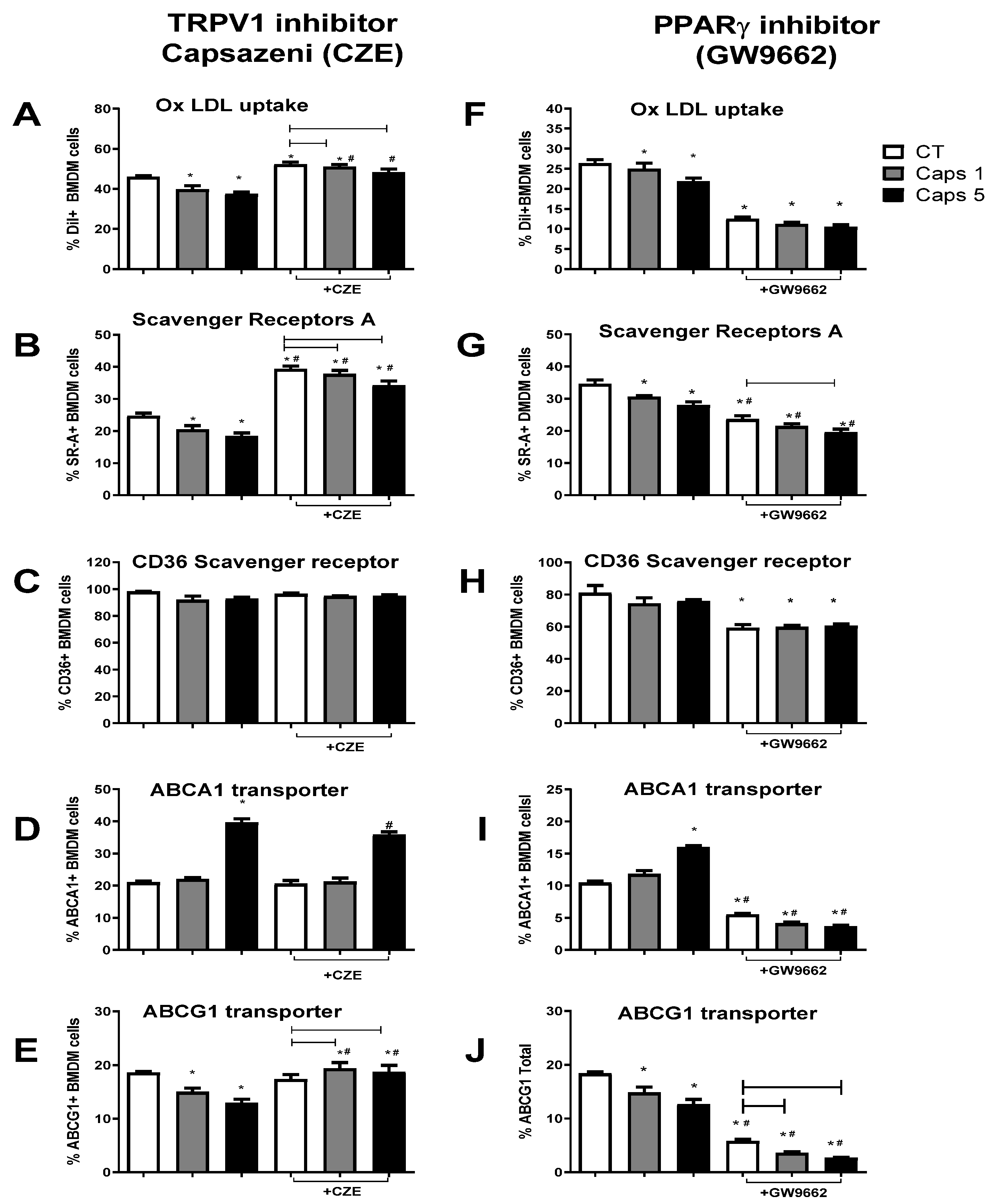
3.4. Capsaicin Reduces Foam Cell Formation by Modulating Macrophage Cholesterol Influx and Efflux Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chanda, S.; Bashir, M.; Babbar, S.; Koganti, A.; Bley, K. In vitro hepatic and skin metabolism of capsaicin. Drug Metab. Dispos. 2008, 36, 670–675. (In English) [Google Scholar] [CrossRef] [PubMed]
- TMaximiano, T.K.E.; Carneiro, J.A.; Fattori, V.; Verri, W.A. TRPV1: Receptor structure, activation, modulation and role in neuro-immune interactions and pain. Cell Calcium 2024, 119, 102870. (In English) [Google Scholar] [CrossRef] [PubMed]
- Silva, J.L.; Santos, E.A.; Alvarez-Leite, J.I. Are We Ready to Recommend Capsaicin for Disorders Other Than Neuropathic Pain? Nutrients 2023, 15, 4469. (In English) [Google Scholar] [CrossRef]
- Nawaka, N.; Wanmasae, S.; Makarasen, A.; Dechtrirat, D.; Techasakul, S.; Jeenduang, N. Allicin and Capsaicin Ameliorated Hypercholesterolemia by Upregulating LDLR and Downregulating PCSK9 Expression in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 14299. (In English) [Google Scholar] [CrossRef]
- Huang, C.-J.; Pu, C.-M.; Su, S.-Y.; Lo, S.-L.; Lee, C.H.; Yen, Y.-H. Improvement of wound healing by capsaicin through suppression of the inflammatory response and amelioration of the repair process. Mol. Med. Rep. 2023, 28, 155. (In English) [Google Scholar] [CrossRef]
- Irving, G.A.; Backonja, M.; Rauck, R.; Webster, L.R.; Tobias, J.K.; Vanhove, G.F. NGX-4010, a capsaicin 8% dermal patch, administered alone or in combination with systemic neuropathic pain medications, reduces pain in patients with postherpetic neuralgia. Clin. J. Pain 2012, 28, 101–107. (In English) [Google Scholar] [CrossRef] [PubMed]
- Thongin, S.; Den-Udom, T.; Uppakara, K.; Sriwantana, T.; Sibmooh, N.; Laolob, T.; Boonthip, C.; Wichai, U.; Muta, K.; Ketsawatsomkron, P. Beneficial effects of capsaicin and dihydrocapsaicin on endothelial inflammation, nitric oxide production and antioxidant activity. Biomed. Pharmacother. 2022, 154, 113521. (In English) [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Fan, J.; Feng, Z.; Song, X. Pharmacological activity of capsaicin: Mechanisms and controversies (Review). Mol. Med. Rep. 2024, 29, 38. (In English) [Google Scholar] [CrossRef]
- Kim, H.J. Capsaicin supplementation prevents western diet-induced hyperleptinemia by reducing endoplasmic reticulum stress in apolipoprotein E-deficient mice. Food Nutr. Res. 2023, 67. (In English) [Google Scholar] [CrossRef]
- Kang, J.-H.; Tsuyoshi, G.; Le Ngoc, H.; Kim, H.-M.; Tu, T.H.; Noh, H.-J.; Kim, C.-S.; Choe, S.-Y.; Kawada, T.; Yoo, H.; et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J. Med. Food 2011, 14, 310–315. (In English) [Google Scholar] [CrossRef]
- Nam, D.; Ni, C.-W.; Rezvan, A.; Suo, J.; Budzyn, K.; Llanos, A.; Harrison, D.G.; Giddens, D.P.; Jo, H. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of RNA isolation from carotid endothelium. J. Vis. Exp. 2010, 40, e1861. (In English) [Google Scholar] [CrossRef]
- Aguilar, E.; Leonel, A.; Teixeira, L.; Silva, A.; Silva, J.; Pelaez, J.; Capettini, L.; Lemos, V.; Santos, R.; Alvarez-Leite, J. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. (In English) [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.L.; Leonel, A.J.; Sad, A.P.; Beltrão, N.R.; Costa, T.F.; Ferreira, T.M.; Gomes-Santos, A.C.; Faria, A.M.; Peluzio, M.C.; Cara, D.C.; et al. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J. Nutr. Biochem. 2012, 23, 430–436. (In English) [Google Scholar] [CrossRef]
- Teixeira, L.G.; Leonel, A.J.; Aguilar, E.C.; Batista, N.V.; Alves, A.C.; Coimbra, C.C.; Ferreira, A.V.; De Faria, A.M.C.; Cara, D.C.; Alvarez Leite, J.I. The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis. 2011, 10, 204. (In English) [Google Scholar] [CrossRef] [PubMed]
- Chung, B.H.; Segrest, J.P.; Ray, M.J.; Brunzell, J.D.; Hokanson, J.E.; Krauss, R.M.; Beaudrie, K.; Cone, J.T. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 1986, 128, 181–209. (In English) [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. (In English) [Google Scholar] [CrossRef]
- Stephan, Z.F.; Yurachek, E.C. Rapid fluorometric assay of LDL receptor activity by DiI-labeled LDL. J. Lipid Res. 1993, 34, 325–330. (In English) [Google Scholar] [CrossRef]
- Fernandes-Braga, W.; Aguilar, E.C.; Navia-Pelaez, J.M.; Ávila, D.L.; Rezende, L.; Andrade, L.d.O.; Miranda, S.E.M.; de Barros, A.L.B.; Capettini, L.d.S.A.; Alvarez-Leite, J.I. The atheroprotective role of fucoidan involves the reduction of foam cell formation by altering cholesterol flux-associated factors in macrophages. Biochem. Biophys. Res. Commun. 2003, 650, 21–29. (In English) [Google Scholar] [CrossRef]
- Chen, C.; Lee, S.T.; Wu, W.T.; Fu, W.; Ho, F.; Lin, W.W. Signal transduction for inhibition of inducible nitric oxide synthase and cyclooxygenase-2 induction by capsaicin and related analogs in macrophages. Br. J. Pharmacol. 2003, 140, 1077–1087. (In English) [Google Scholar] [CrossRef]
- Zhao, J.-F.; Ching, L.-C.; Kou, Y.R.; Lin, S.-J.; Wei, J.; Shyue, S.-K.; Lee, T.-S. Activation of TRPV1 prevents OxLDL-induced lipid accumulation and TNF-α-induced inflammation in macrophages: Role of liver X receptor α. Mediat. Inflamm. 2013, 2013, 925171. (In English) [Google Scholar] [CrossRef]
- Min, K.-W.; Zhang, X.; Imchen, T.; Baek, S.J. A peroxisome proliferator-activated receptor ligand MCC-555 imparts anti-proliferative response in pancreatic cancer cells by PPARgamma-independent up-regulation of KLF4. Toxicol. Appl. Pharmacol. 2012, 263, 225–232. (In English) [Google Scholar] [CrossRef] [PubMed]
- Shiota, A.; Shimabukuro, M.; Fukuda, D.; Soeki, T.; Sato, H.; Uematsu, E.; Hirata, Y.; Kurobe, H.; Maeda, N.; Sakaue, H.; et al. Telmisartan ameliorates insulin sensitivity by activating the AMPK/SIRT1 pathway in skeletal muscle of obese db/db mice. Cardiovasc. Diabetol. 2012, 11, 139. (In English) [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Ni, Y.; Feng, X.; Zhao, Z.; Wang, P.; Sun, J.; Yu, H.; Yan, Z.; Liu, D.; et al. TRPV1 activation prevents nonalcoholic fatty liver through UCP2 upregulation in mice. Pflug. Arch. 2012, 463, 727–732. (In English) [Google Scholar] [CrossRef]
- Ohyama, K.; Nogusa, Y.; Suzuki, K.; Shinoda, K.; Kajimura, S.; Bannai, M. A combination of exercise and capsinoid supplementation additively suppresses diet-induced obesity by increasing energy expenditure in mice. Am. J. Physiol. Endocrinol. Metab. 2013, 308, E315–E323. (In English) [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-W.; Ma, X.; Huang, J.-L.; Mao, X.-R.; Yang, J.-Y.; Zhao, J.-Y.; Li, S.-F.; Qiu, Y.-R.; Yang, J.; Zheng, L.; et al. Dihydrocapsaicin Attenuates Plaque Formation through a PPARγ/LXRα Pathway in apoE(−/−) Mice Fed a High-Fat/High-Cholesterol Diet. PLoS ONE 2013, 8, e66876. (In English) [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shao, M.; Zheng, Y.; Sun, W.; Qin, S.; Sun, Z.; Zhu, L.; Guan, Y.; Wang, Q.; Wang, Y.; et al. PPARs in atherosclerosis: The spatial and temporal features from mechanism to druggable targets. J. Adv. Res. 2024, 29. (In English) [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. (In English) [Google Scholar] [CrossRef]
- González, L.; Rivera, K.; Andia, M.E.; Rodriguez, G.M. The IL-1 Family and Its Role in Atherosclerosis. Int. J. Mol. Sci. 2022, 24, 17. (In English) [Google Scholar] [CrossRef]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. (In English) [Google Scholar] [CrossRef]
- McEver, R.P.; Zhu, C. Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 2010, 26, 363–396. (In English) [Google Scholar] [CrossRef]
- Schneider, L.; Hackert, T.; Heck, M.; Hartwig, W.; Fritz, S.; Strobel, O.; Gebhard, M.-M.; Werner, J. Capsaicin reduces tissue damage in experimental acute pancreatitis. Pancreas 2009, 38, 676–680. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Kawada, T.; Kim, B.-S.; Han, I.-S.; Choe, S.-Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal 2003, 15, 299–306. (In English) [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Febbraio, M.; Silverstein, R.L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Investig. 2009, 119, 136–145. (In English) [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Luo, K.; Li, Y.; Chen, Q.; Tang, D.; Wang, D.; Xiao, J. Capsaicin attenuates LPS-induced inflammatory cytokine production by upregulation of LXRalpha. Int. Immunopharmacol. 2015, 28, 264–269. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-J.; Lee, S.; Lee, K.-S.; Park, J.-H.; Jang, Y.; Lee, E.J.; Park, H.-Y. PPARgamma activation induces CD36 expression and stimulates foam cell like changes in rVSMCs. Prostaglandins Other Lipid Mediat. 2006, 80, 165–174. (In English) [Google Scholar] [CrossRef]
- Lazar, M.A. Progress in cardiovascular biology: PPAR for the course. Nat. Med. 2001, 7, 23–24. (In English) [Google Scholar] [CrossRef]
- Martín-Fuentes, P.; Civeira, F.; Recalde, D.; García-Otín, A.L.; Jarauta, E.; Marzo, I.; Cenarro, A. Individual variation of scavenger receptor expression in human macrophages with oxidized low-density lipoprotein is associated with a differential inflammatory response. J. Immunol. 2007, 179, 3242–3248. (In English) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila, D.L.; Fernandes-Braga, W.; Silva, J.L.; Santos, E.A.; Campos, G.; Leocádio, P.C.L.; Capettini, L.S.A.; Aguilar, E.C.; Alvarez-Leite, J.I. Capsaicin Improves Systemic Inflammation, Atherosclerosis, and Macrophage-Derived Foam Cells by Stimulating PPAR Gamma and TRPV1 Receptors. Nutrients 2024, 16, 3167. https://doi.org/10.3390/nu16183167
Ávila DL, Fernandes-Braga W, Silva JL, Santos EA, Campos G, Leocádio PCL, Capettini LSA, Aguilar EC, Alvarez-Leite JI. Capsaicin Improves Systemic Inflammation, Atherosclerosis, and Macrophage-Derived Foam Cells by Stimulating PPAR Gamma and TRPV1 Receptors. Nutrients. 2024; 16(18):3167. https://doi.org/10.3390/nu16183167
Chicago/Turabian StyleÁvila, Danielle Lima, Weslley Fernandes-Braga, Janayne Luihan Silva, Elandia Aparecida Santos, Gianne Campos, Paola Caroline Lacerda Leocádio, Luciano Santos Aggum Capettini, Edenil Costa Aguilar, and Jacqueline Isaura Alvarez-Leite. 2024. "Capsaicin Improves Systemic Inflammation, Atherosclerosis, and Macrophage-Derived Foam Cells by Stimulating PPAR Gamma and TRPV1 Receptors" Nutrients 16, no. 18: 3167. https://doi.org/10.3390/nu16183167
APA StyleÁvila, D. L., Fernandes-Braga, W., Silva, J. L., Santos, E. A., Campos, G., Leocádio, P. C. L., Capettini, L. S. A., Aguilar, E. C., & Alvarez-Leite, J. I. (2024). Capsaicin Improves Systemic Inflammation, Atherosclerosis, and Macrophage-Derived Foam Cells by Stimulating PPAR Gamma and TRPV1 Receptors. Nutrients, 16(18), 3167. https://doi.org/10.3390/nu16183167






