Influence of Body Composition Assessed by Computed Tomography on Mortality Risk in Young Women with Breast Cancer
Abstract
1. Introduction
2. Methods
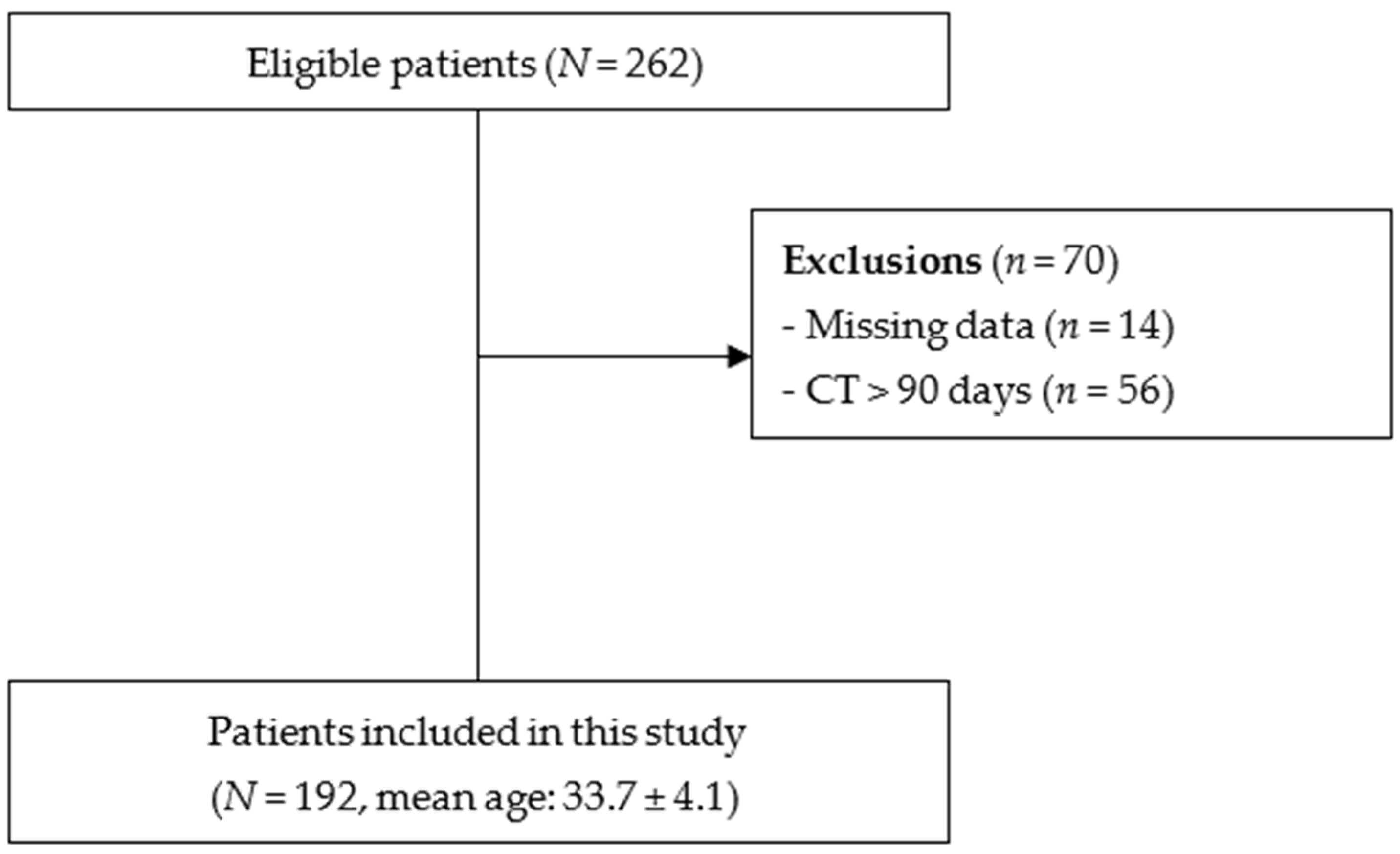
2.1. Study Design and Subjects
2.2. Outcomes and Covariates
2.3. Body Composition from Computed Tomography Scans
2.4. Statistical Analyses
3. Results
| Variables | Total | Survivors | Non-Survivors | p |
|---|---|---|---|---|
| N, % | 192 | 169 (88.0) | 23 (12.0) | |
| Ethnicity, n (%) | 0.09 | |||
| Caucasian | 28 (14.6) | 22 (78.6) | 6 (21.4) | |
| Non-Caucasian | 164 (85.4) | 147 (89.6) | 17 (10.4) | |
| Educational level, n (%) | 0.84 | |||
| Elementary | 66 (35.5) | 57 (86.4) | 9 (13.6) | |
| Secondary | 85 (45.7) | 76 (89.4) | 9 (10.6) | |
| Post-secondary | 35 (18.8) | 31 (88.6) | 4 (11.4) | |
| Marital status, n (%) | 0.51 | |||
| With partner | 117 (61.9) | 102 (87.2) | 15 (12.8) | |
| No partner | 72 (38.1) | 65 (90.3) | 7 (9.7) | |
| Age at diagnosis, y (median IQR) | 35 (31–37) | 35 (31–37) | 34 (31–37) | 0.63 |
| Menarche, y (median IQR) | 13 (12–14) | 13 (12–14) | 13 (11.5–14.5) | 0.89 |
| Presence of comorbidities, n (%) | ||||
| Type 2 diabetes mellitus | 3 (1.6) | 3 (100.0) | 0 (0.00) | 0.52 |
| Hypertension | 21 (89.1) | 18 (85.7) | 3 (14.3) | 0.73 |
| Dyslipidemia | 3 (1.6) | 3 (100.0) | 0 (0.00) | 0.52 |
| TNM stage, n (%) | 0.014 | |||
| I and II | 58 (30.5) | 56 (96.6) | 2 (3.4) | |
| III and IV | 132 (69.5) | 112 (84.8) | 20 (15.2) | |
| Lymph node status at diagnosis, n (%) | 0.046 | |||
| Positive | 153 (79.9) | 129 (85.4) | 22 (14.6) | |
| Negative | 36 (19.0) | 36 (100) | 0 (0.0) | |
| Unknown | 2 (1.1) | 1 (50.0) | 1 (50.0) | |
| Estrogen receptor status, n (%) | 0.30 | |||
| Positive | 112 (60.5) | 102 (91.1) | 10 (8.9) | |
| Negative | 73 (39.5) | 63 (86.3) | 10 (13.7) | |
| Progesterone receptor status, n (%) | 0.20 | |||
| Positive | 107 (58.2) | 98 (91.6) | 9 (8.4) | |
| Negative | 77 (41.8) | 66 (85.7) | 11 (14.3) | |
| HER2 status, n (%) | 0.64 | |||
| Positive | 61 (33.0) | 55 (90.2) | 6 (9.8) | |
| Negative | 124 (67.0) | 109 (87.9) | 15 (12.1) | |
| Immunophenotype, n (%) | 0.77 | |||
| Luminal A | 14 (8.9) | 13 (12.9) | 1 (7.1) | |
| Luminal B | 54 (34.2) | 48 (88.9) | 6 (11.1) | |
| Overexpression of ER2-HER2+ | 15 (9.5) | 42 (89.4) | 5 (10.6) | |
| Triple-negative | 47 (29.7) | 14 (93.3) | 1 (6.7) | |
| Unknown | 28 (17.7) | 23 (82.1) | 5 (17.9) | |
| Ki67 status, n (%) | 0.75 | |||
| ≤20 | 45 (28.5) | 39 (86.7) | 6 (13.3) | |
| >20 | 113 (71.5) | 100 (88.5) | 13 (11.5) |
| Variables | Total | Survivors | Non-Survivors | p |
|---|---|---|---|---|
| BMI (kg/m2), (median IQR) | 26.2 (23.5–29.8) | 26.2 (23.5–29.7) | 27.0 (21.7–30.1) | 0.80 a |
| Underweight, n (%) | 3 (1.6) | 3 (1.8) | 0 (0.0) | 0.77 b |
| Normal range, n (%) | 71 (37.0) | 61 (36.1) | 10 (43.5) | |
| Overweight, n (%) | 72 (37.5) | 65 (38.5) | 7 (30.4) | |
| Obesity, n (%) | 46 (24.0) | 40 (23.7) | 6 (26.1) | |
| CT-derived body composition, (median IQR) | ||||
| SAT (cm2) | 190.3 (142.7–259.3) | 189.0 (142.5–254.6) | 235.9 (149.3–283.8) | 0.22 a |
| VAT (cm2) | 67.5 (42.4–107.5) | 67.6 (39.3–106.7) | 67.5 (43.6–116.9) | 0.90 a |
| TAT (cm2) | 276.3 (197.7–367.5) | 272.9 (198.3–367.4) | 319.0 (183.8–384.0) | 0.36 a |
| IMAT (cm2) | 7.25 (4.57–10.01) | 7.24 (4.57–9.99) | 7.36 (4.44–11.05) | 0.87 a |
| SMD (HU) | 38.2 (35.3–43.1) | 38.4 (35.4–43.1) | 37.8 (34.6–41.2) | 0.25 a |
| SM (cm2) | 114.3 ± 18.8 | 115.6 ± 18.1 | 104.8 ± 21.1 | 0.029 c |
| SMI (cm2/m2) | 46.5 ± 7.6 | 47.1 ± 7.4 | 42.5 ± 8.0 | 0.015 c |

4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F.; Bsc, M.F.B.; Me, J.F.; Soerjomataram, M.I.; et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Câncer (Brasil). Estimativa 2023: Incidência de Câncer no Brasil; Instituto Nacional de Câncer, INCA: Rio de Janeiro, Brazil, 2022; 160p. [Google Scholar]
- Migowski, A.; Silva, G.A.E.; Dias, M.B.K.; Diz, M.D.P.E.; Sant’Ana, D.R.; Nadanovsky, P. Guidelines for early detection of breast cancer in Brazil. II—New national recommendations, main evidence, and controversies. Cad. Saúde Pública 2018, 34, e00074817. [Google Scholar] [PubMed]
- Lauby-Secretan, B.; Sccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, S.A.; Sousa, I.M.; Silva, F.; Lyra, C.D.; Fayh, A.P. Nutritional and environmental risk factors for breast cancer: A case-control study. Soc. Sci. Med. 2018, 28, 28723. [Google Scholar] [CrossRef]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, U.M.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Pang, Y.; Wei, Y.; Kartsonaki, C. Associations of adiposity and weight change with recurrence and survival in breast cancer patients: A systematic review and meta-analysis. Breast Cancer 2022, 29, 575–588. [Google Scholar] [CrossRef]
- Kimmick, G.G.; Balducci, L. Breast cancer and aging. Clinical interactions. Hematol. Oncol. Clin. N. Am. 2000, 14, 213–234. [Google Scholar] [CrossRef]
- Anastasiadi, Z.; Lianos, G.D.; Ignatiadou, E.; Harissis, H.V.; Mitsis, M. Breast cancer in young women: An overview. Updates Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef]
- Rossi, L.; Mazzara, C.; Pagani, O. Diagnosis and Treatment of Breast Cancer in Young Women. Curr. Treat. Options Oncol. 2019, 20, 86. [Google Scholar] [CrossRef]
- Alvarez-Bañuelos, M.T.; Segura-Jaramillo, K.A.; Gómez-Rivera, E.D.C.; Alarcón-Rojas, C.A.; Morales-Romero, J.; Sampieri, C.L.; Guzmán-García, R.E. Age Under 30 Years As a Predictor of Poor Survival in a Cohort of Mexican Women with Breast Cancer. Cancer Control 2021, 28, 10732748211047408. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Giovannucci, E.L. The Obesity Paradox in Cancer: Insights and Epidemiological Perspectives. Curr. Nutr. Rep. 2019, 8, 175–181. [Google Scholar] [CrossRef]
- Modi, N.D.; Tan, J.Q.E.; Rowland, A.; Koczwara, B.; Abuhelwa, A.Y.; Kichenadasse, G.; McKinnon, R.A.; Wiese, M.D.; Sorich, M.J.; Hopkins, A.M. The obesity paradox in early and advanced HER2 positive breast cancer: Pooled analysis of clinical trial data. NPJ Breast Cancer 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, G.G.; Guillet, C.; Poggiogalle, E.; Pomar, M.D.B.; Batsis, J.A.; Boirie, Y.; Breton, I.; Frara, S.; Genton, L.; Gepner, Y.; et al. Sarcopenic obesity research perspectives outlined by the sarcopenic obesity global leadership initiative (SOGLI)—Proceedings from the SOGLI consortium meeting in Rome November 2022. Clin. Nutr. 2023, 42, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R. The Obesity Paradox in the Cancer Movement Beyond the Response to BMI. Cancer Epidemiol. Biomark. Prev. 2017, 26, 981. [Google Scholar] [CrossRef]
- Caan, B.J.; Feliciano, E.M.C.; Kroenke, C.H. The importance of body composition in explaining the paradox of excess weight in the counterpoint of cancer. Cancer Res. 2018, 78, 1906–1912. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Gonzalez, M.C.; Prado, C.M. Sarcopenia ≠ low muscle mass. Eur. Geriatr. Med. 2023, 14, 225–228. [Google Scholar] [CrossRef]
- Bezerra, M.R.O.; Sousa, I.M.; Miranda, A.L.; Ferreira, G.M.C.; Chaves, G.V.; Verde, S.M.M.L.; Maurício, S.F.; Pereira, J.P.d.C.; Gonzalez, M.C.; Prado, C.M.; et al. Age-adjusted Charlson comorbidity index and its association with body composition and overall survival in patients with colorectal cancer. Support. Care Cancer 2024, 16, 517. [Google Scholar] [CrossRef]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Rev. Ann. Oncol. 2018, 29, ii1–ii9. [Google Scholar] [CrossRef]
- Prado, C.M.; Gonzalez, M.C.; Heymsfield, S.B. Body composition phenotypes and obesity paradox. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 535–551. [Google Scholar] [CrossRef]
- Fayh, A.P.T.; Sousa, I.M.; Gonzalez, M.C. New insights on how and where to measure muscle mass. Curr. Opin. Support. Palliat. Care 2020, 14, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Ford, K.L.; Gonzalez, M.C.; Murnane, L.C.; Gillis, C.; Wischmeyer, P.E.; Morrison, C.A.; Lobo, D.N. Nascent to novel methods to evaluate malnutrition and frailty in the surgical patient. J. Parenter. Enter. Nutr. 2023, 47, S54–S68. [Google Scholar] [CrossRef]
- Liguori, A.A.L.; Fayh, A.P.T. Computed tomography: An efficient, opportunistic method for assessing body composition and predicting adverse outcomes in cancer patients. Radiol. Bras. 2023, 56, VIII–IX. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Feliciano, E.M.C.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography with Survival in Patients with Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, E.C.; Chen, W.Y. Clinical implications of low skeletal muscle mass in early-stage breast and colorectal cancer. Proc. Nutr. Soc. 2018, 77, 382–387. [Google Scholar] [CrossRef]
- WHO. Physical Status: The Use and Interpretation of Anthropometry; Report of a WHO Expert Committee; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1995; Volume 854, pp. 1–452. [Google Scholar]
- Shachar, S.S.; Williams, G.R. The Obesity Paradox in Cancer-Moving Beyond BMI. Cancer Epidemiol. Biomark. Prev. 2017, 26, 13–16. [Google Scholar] [CrossRef]
- Villaseñor, A.; Ballard-Barbash, R.; Baumgartner, K.; Baumgartner, R.; Bernstein, L.; McTiernan, A.; Neuhouser, M.L. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: The HEAL Study. J. Cancer Surviv. 2012, 6, 398–406. [Google Scholar] [CrossRef]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef]
- Del Fabbro, E.; Parsons, H.; Warneke, C.L.; Pulivarthi, K.; Litton, J.K.; Dev, R.; Palla, S.L.; Brewster, A.; Bruera, E. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 2012, 17, 1240–1245. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Rier, H.N.; Jager, A.; Sleijfer, S.; Rosmalen, J.V.; Kock, M.C.J.M.; Levin, M.D. Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast 2017, 31, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, K. Immunological capabilities of skeletal muscle cells. Acta Physiol. Scand. 2001, 171, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Giudice, J.; Taylor, J.M. Muscle as a paracrine and endocrine organ. Curr. Opin. Pharmacol. 2017, 34, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.C.; Segal, R.J.; McKenzie, D.C.; Vallerand, J.R.; Morielli, A.R.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Reid, R.D.; Courneya, K.S. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy; a multicenter randomized controlled trial. Breast Cancer Res. Treat. 2016, 158, 497–507. [Google Scholar] [CrossRef]
- Pratesi, A.; Tarantini, F.; Bari, M.D. Skeletal muscle: An endocrine organ. Clin. Cases Miner. Bone Metab. 2013, 10, 11–14. [Google Scholar] [CrossRef]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: Results from the C SCANS study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Malietzis, G.; Johns, N.; Al-Hassi, H.O.; Cavaleiro, S.C.; Kennedy, R.H.; Fearon, K.C.H.; Aziz, O.P.; Jenkins, J.T.M. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann. Surg. 2016, 263, 320–325. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; López-Soriano, F.J. Cachexia and sarcopenia: Mechanisms and potential targets for intervention. Curr. Opin. Pharmacol. 2015, 22, 100–106. [Google Scholar] [CrossRef]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.M.; Gonzalez, M.C.; Sousa, I.M.; das Virgens, I.P.A.; Medeiros, G.O.C.; Oliveira, M.N.; Dantas, J.C.A.D.S.; Trussardi Fayh, A.P. Low skeletal muscle radiodensity is the best predictor for short-term major surgical complications in gastrointestinal surgical cancer: A cohort study. PLoS ONE 2021, 16, e0247322. [Google Scholar] [CrossRef] [PubMed]
- Rocha, I.M.G.; Marcadenti, A.; Medeiros, G.O.C.; Bezerra, R.A.; Rêgo, J.F.M.; González, M.C.; Fayh, A.P.T. Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J. Cachexia Sarcopenia Muscle 2019, 10, 445–454. [Google Scholar] [CrossRef]
- Veja, M.C.M.D.; Laviano, A.; Pimentel, G.D. Sarcopenia and chemotherapy-mediated toxicity. Einstein 2016, 14, 580–584. [Google Scholar]
- Ryan, A.M.; Prado, C.M.; Sullivan, E.S.; Power, D.G.; Daly, L.E. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition 2019, 67–68, 110539. [Google Scholar] [CrossRef]
- Souza, A.P.S.; Silva, L.C.; Fayh, A.P.T. Nutritional Intervention Contributes to the Improvement of Symptoms Related to Quality of Life in Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy: A Randomized Clinical Trial. Nutrients 2021, 13, 589. [Google Scholar] [CrossRef]
- Bandera, E.V.; Maskarinec, G.; Romieu, I.; John, E.M. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: A global perspective. Adv. Nutr. 2015, 6, 803–819. [Google Scholar] [CrossRef]
- Poggio, F.; Blondeaux, E.; Tagliamento, M.; Perachino, M.; Nardin, S.; Conte, B.; Giuliano, M.; Arpino, G.; De Laurentiis, M.; Gravina, A.; et al. Efficacy of adjuvant chemotherapy schedules for breast cancer according to body mass index: Results from the phase III GIM2 trial. ESMO Open 2024, 9, 103650. [Google Scholar] [CrossRef]
- Pereira, J.P.C.; Gonzalez, M.C.; Prado, C.M.; Cabral, P.C.; Nascimento, T.G.; do Nascimento, M.K.; da Silva Diniz, A.; Ramiro, C.P.S.P.; Fayh, A.P.T. Body mass index-adjusted calf circumference is associated with mortality in hospitalized older patients with excess weight. Nutrition 2024, 125, 112505. [Google Scholar] [CrossRef]
| Variables | HRcrude (95% CI) | p | HRadjusted (95% CI) | p | Cutoffs |
|---|---|---|---|---|---|
| SM (cm2) | 0.97 (0.95;0.99) | 0.026 | 0.97 (0.94;0.99) | 0.014 | <94.2 |
| SMI (cm2/m2) | 0.93 (0.88;0.99) | 0.018 | 0.91 (0.85;0.97) | 0.004 | <39 |
| SMD (HU) | 0.93 (0.88;0.99) | 0.029 | 0.94 (0.88;1.01) | 0.07 | <42.5 |
| IMAT (cm2) | 1.03 (0.96;1.11) | 0.46 | 1.01 (0.92;1.10) | 0.90 | |
| SAT (cm2) | 1.00 (0.99;1.01) | 0.29 | 1.01 (1.00;1.02) | 0.030 | >216 |
| VAT (cm2) | 1.00 (0.99;1.01) | 0.37 | 1.00 (0.99;1.02) | 0.26 | |
| TAT (cm2) | 1.00 (0.99;1.00) | 0.27 | 1.01 (1.00;1.01) | 0.021 | >273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima Bezerra, A.D.; da Costa Pereira, J.P.; de Macedo Soares, I.F.; Ferreira, G.M.C.; Miranda, A.L.; de Medeiros, G.O.C.; Verde, S.M.M.L.; Fayh, A.P.T. Influence of Body Composition Assessed by Computed Tomography on Mortality Risk in Young Women with Breast Cancer. Nutrients 2024, 16, 3175. https://doi.org/10.3390/nu16183175
de Lima Bezerra AD, da Costa Pereira JP, de Macedo Soares IF, Ferreira GMC, Miranda AL, de Medeiros GOC, Verde SMML, Fayh APT. Influence of Body Composition Assessed by Computed Tomography on Mortality Risk in Young Women with Breast Cancer. Nutrients. 2024; 16(18):3175. https://doi.org/10.3390/nu16183175
Chicago/Turabian Stylede Lima Bezerra, Agnes Denise, Jarson Pedro da Costa Pereira, Ingryd Fernandes de Macedo Soares, Glaucia Mardrini Cassiano Ferreira, Ana Lúcia Miranda, Galtieri Otávio Cunha de Medeiros, Sara Maria Moreira Lima Verde, and Ana Paula Trussardi Fayh. 2024. "Influence of Body Composition Assessed by Computed Tomography on Mortality Risk in Young Women with Breast Cancer" Nutrients 16, no. 18: 3175. https://doi.org/10.3390/nu16183175
APA Stylede Lima Bezerra, A. D., da Costa Pereira, J. P., de Macedo Soares, I. F., Ferreira, G. M. C., Miranda, A. L., de Medeiros, G. O. C., Verde, S. M. M. L., & Fayh, A. P. T. (2024). Influence of Body Composition Assessed by Computed Tomography on Mortality Risk in Young Women with Breast Cancer. Nutrients, 16(18), 3175. https://doi.org/10.3390/nu16183175








