Royal Jelly Exerts a Potent Anti-Obesity Effect in Rats by Activating Lipolysis and Suppressing Adipogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Subjects
2.2. Dietary Interventions
2.3. Royal Jelly Preparation
2.4. Experimental Protocol
2.5. Nutritional and Anthropometric Measurements
2.6. Blood and Adipose Tissue Collection and Adiposity Index Calculation
2.7. Serum Lipid and Adipokine Analysis
2.8. Adipokine and Free Glycerol Quantification
2.9. Cytokine Release from Adipose Tissue
2.10. Protein Extraction from Adipose Tissue
2.11. Biochemical Analysis of Adipose Tissue Proteins
2.12. Biochemical Analysis of Nuclear Adipose Tissue Fractions
2.13. Real-Time PCR Analysis
2.14. Statistical Evaluation
3. Results
3.1. RJ Attenuates the Gain in Body Weight and Decreases Adiposity Markers in HFD-Fed Obese Rats without Altering Food and Energy Intake
3.2. RJ Attenuates the Impairment in Glucose/Lipid Metabolism and Adipokines’ Release and Increases Markers of Lipolysis in the Serum of HFD Obese Rats
3.3. RJ Suppresses Levels of Inflammatory Cytokine Synthesis and Release from Adipose Tissue
3.4. RJ Activates AMPK and Suppresses ACC in the Adipose Tissue of HFD-Fed Obese Rats
3.5. RJ Attenuates the Increment in Transcription Factors of Adipogenesis
3.6. RJ Stimulates Adipose Tissue Lipolysis by Activating HSL and TGL and Upregulating PGC-1α
3.7. RJ Reduces the Size of the Adipocytes in the HFD-Fed Rats
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Atlas 2023; World Obesity Federation: London, UK, 2023. [Google Scholar]
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: Is the Sharp Rise of the Evil Empire Truly Levelling Off? Current Obesity Reports; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Wang, Q.; Sun, J.; Liu, M.; Zhou, Y.; Zhang, L.; Li, Y. The new role of AMP-activated protein kinase in regulating fat metabolism and energy expenditure in adipose tissue. Biomolecules 2021, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.-K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular mechanisms of adipogenesis: The anti-adipogenic role of AMP-activated protein kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Gaidhu, M.P.; Anthony, N.M.; Patel, P.; Hawke, T.J.; Ceddia, R.B. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: Role of ATGL, HSL, and AMPK. Am. J. Physiol.-Cell. Physiol. 2010, 298, C961–C971. [Google Scholar] [CrossRef] [PubMed]
- Morak, M.; Schmidinger, H.; Riesenhuber, G.; Rechberger, G.N.; Kollroser, M.; Haemmerle, G.; Zechner, R.; Kronenberg, F.; Hermetter, A. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) deficiencies affect expression of lipolytic activities in mouse adipose tissues. Mol. Cell. Proteom. 2012, 11, 1777–1789. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Roh, E.; Yoo, H.J. The Role of Adipose Tissue Lipolysis in Diet-Induced Obesity: Focus on Vimentin. Diabetes Metab. J. 2021, 45, 43–45. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, A.; Qi, W. The Potential to fight obesity with adipogenesis modulating compounds. Int. J. Mol. Sci. 2022, 23, 2299. [Google Scholar] [CrossRef]
- Jung, Y.-C.; Kim, H.W.; Min, B.K.; Cho, J.Y.; Son, H.J.; Lee, J.Y.; Kim, J.-Y.; Kwon, S.-B.; Li, Q.; Lee, H.-W. Inhibitory effect of olive leaf extract on obesity in high-fat diet-induced mice. Vivo 2019, 33, 707–715. [Google Scholar] [CrossRef]
- Dong, S.; Qi, M.; Wang, Y.; Chen, L.; Weaver, J.C.; Krilis, S.A.; Giannakopoulos, B. β2GPI exerts an anti-obesity effect in female mice by inhibiting lipogenesis and promoting lipolysis. Oncotarget 2017, 8, 92652. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.H.; Zuo, G.; Lim, S.S. Anti-obesity effect of melandrium firmum rohrbach extract in 3T3-L1 cells and high-fat diet-induced obese C57BL/6N mice. Food Sci. Nutr. 2020, 8, 2251–2261. [Google Scholar] [CrossRef]
- Yang, X.-D.; Ge, X.-C.; Jiang, S.-Y.; Yang, Y.-Y. Potential lipolytic regulators derived from natural products as effective approaches to treat obesity. Front. Endocrinol. 2022, 13, 1000739. [Google Scholar] [CrossRef]
- Choi, M.J.; Yu, H.; Kim, J.I.; Seo, H.; Kim, J.G.; Kim, S.-K.; Lee, H.S.; Cheon, H.G. Anti-obesity effects of Lactiplantibacillus plantarum SKO-001 in high-fat diet-induced obese mice. Eur. J. Nutr. 2023, 62, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, R.; Teimouri, M.; Safaei, M.; Arab Sadeghabadi, Z. AMP-activated protein kinase is a key regulator of obesity-associated factors. Cell. Biochem. Funct. 2023, 41, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.M.; Staels, B. Peroxisome proliferator-activated receptor γ and adipose tissue—Understanding obesity-related changes in regulation of lipid and glucose metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 386–395. [Google Scholar] [CrossRef]
- Villena, J.A.; Viollet, B.; Andreelli, F.; Kahn, A.; Vaulont, S.; Sul, H.S. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-α2 subunit. Diabetes 2004, 53, 2242–2249. [Google Scholar] [CrossRef]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Lopez-Mejia, I.C.; Lagarrigue, S.; Giralt, A.; Martinez-Carreres, L.; Zanou, N.; Denechaud, P.-D.; Castillo-Armengol, J.; Chavey, C.; Orpinell, M.; Delacuisine, B. CDK4 phosphorylates AMPKα2 to inhibit its activity and repress fatty acid oxidation. Mol. Cell. 2017, 68, 336–349.E6. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Gauthier, M.-S.; Hess, D.T.; Apovian, C.M.; Cacicedo, J.M.; Gokce, N.; Farb, M.; Valentine, R.J.; Ruderman, N.B. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J. Lipid Res. 2012, 53, 792–801. [Google Scholar] [CrossRef]
- Heo, M.-G.; Choung, S.-Y. Anti-obesity effects of Spirulina maxima in high fat diet induced obese rats via the activation of AMPK pathway and SIRT1. Food Funct. 2018, 9, 4906–4915. [Google Scholar] [CrossRef]
- Samuels, J.S.; Shashidharamurthy, R.; Rayalam, S. Novel anti-obesity effects of beer hops compound xanthohumol: Role of AMPK signaling pathway. Nutr. Metab. 2018, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Zhao, T.; Wang, Y.; Sun, C. Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS ONE 2013, 8, e70135. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, A.D.; Kooijman, S.; Schilperoort, M.; Rensen, P.C.; Boon, M.R. Regulation of brown fat by AMP-activated protein kinase. Trends Mol. Med. 2015, 21, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.W.; Ahmad, F.; Tang, Y.; Hockman, S.C.; Kee, H.J.; Berger, K.; Guirguis, E.; Choi, Y.H.; Schimel, D.M.; Aponte, A.M. White to beige conversion in PDE3B KO adipose tissue through activation of AMPK signaling and mitochondrial function. Sci. Rep. 2017, 7, 40445. [Google Scholar] [CrossRef]
- Desjardins, E.M.; Steinberg, G.R. Emerging role of AMPK in brown and beige adipose tissue (BAT): Implications for obesity, insulin resistance, and type 2 diabetes. Curr. Diab. Rep. 2018, 18, 80. [Google Scholar] [CrossRef]
- Mancini, S.J.; White, A.D.; Bijland, S.; Rutherford, C.; Graham, D.; Richter, E.A.; Viollet, B.; Touyz, R.M.; Palmer, T.M.; Salt, I.P. Activation of AMP-activated protein kinase rapidly suppresses multiple pro-inflammatory pathways in adipocytes including IL-1 receptor-associated kinase-4 phosphorylation. Mol. Cell. Endocrinol. 2017, 440, 44–56. [Google Scholar] [CrossRef]
- Pollard, A.E.; Martins, L.; Muckett, P.J.; Khadayate, S.; Bornot, A.; Clausen, M.; Admyre, T.; Bjursell, M.; Fiadeiro, R.; Wilson, L. AMPK activation protects against diet-induced obesity through Ucp1-independent thermogenesis in subcutaneous white adipose tissue. Nat. Metab. 2019, 1, 340–349. [Google Scholar] [CrossRef]
- Cheng, A.-W.; Tan, X.; Sun, J.-Y.; Gu, C.-M.; Liu, C.; Guo, X. Catechin attenuates TNF-α induced inflammatory response via AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS ONE 2019, 14, e0217090. [Google Scholar] [CrossRef] [PubMed]
- Habinowski, S.A.; Witters, L.A. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2001, 286, 852–856. [Google Scholar] [CrossRef]
- Daval, M.; Foufelle, F.; Ferré, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Ono, M.; Fujimori, K. Antiadipogenic effect of dietary apigenin through activation of AMPK in 3T3-L1 cells. J. Agric. Food Chem. 2011, 59, 13346–13352. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.J.; Rodríguez-Hermosa, J.-I.; Sabater, M.; Pardo, G.; Ricart, W.; Fernández-Real, J.M. OCT1 expression in adipocytes could contribute to increased metformin action in obese subjects. Diabetes 2011, 60, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Vingtdeux, V.; Chandakkar, P.; Zhao, H.; Davies, P.; Marambaud, P. Small-molecule activators of AMP-activated protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis. Mol. Med. 2011, 17, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choe, S.S.; Jang, H.; Kim, J.; Jeong, H.W.; Jo, H.; Jeong, K.-H.; Tadi, S.; Park, M.G.; Kwak, T.H. AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J. Lipid Res. 2012, 53, 1277–1286. [Google Scholar] [CrossRef]
- Kang, M.-C.; Ding, Y.; Kim, H.-S.; Jeon, Y.-J.; Lee, S.-H. Inhibition of adipogenesis by diphlorethohydroxycarmalol (DPHC) through AMPK activation in adipocytes. Mar. Drugs 2019, 17, 44. [Google Scholar] [CrossRef]
- Lingesh, A.; Paul, D.; Naidu, V.; Satheeshkumar, N. AMPK activating and anti adipogenic potential of Hibiscus rosa sinensis flower in 3T3-L1 cells. J. Ethnopharmacol. 2019, 233, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-T.; Huang, Y.-W.; Hou, C.-Y.; Wang, J.-J.; Wu, C.-C.; Hsieh, S.-L. D-Limonene Promotes Anti-Obesity in 3T3-L1 Adipocytes and High-Calorie Diet-Induced Obese Rats by Activating the AMPK Signaling Pathway. Nutrients 2023, 15, 267. [Google Scholar] [CrossRef]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Balkanska, R.; Karadjova, I.; Ignatova, M. Comparative analyses of chemical composition of royal jelly and drone brood. Bulg. Chem. Commun 2014, 46, 412–416. [Google Scholar]
- Joksimovič, A.; Stanković, D.; Joksimović, I.; Molnar, S.; Joksimović, S. Royal jelly as a supplement for young football players. Sport Sci. 2009, 2, 62–67. [Google Scholar]
- Pourmoradian, S.; Mahdavi, R.; Mobasseri, M.; Faramarzi, E.; Mobasseri, M. Effects of royal jelly supplementation on body weight and dietary intake in type 2 diabetic females. Health Promot. Perspect. 2012, 2, 231. [Google Scholar]
- Ibrahim, S.E.M.; Kosba, A.A. Royal jelly supplementation reduces skeletal muscle lipotoxicity and insulin resistance in aged obese rats. Pathophysiology 2018, 25, 307–315. [Google Scholar] [CrossRef]
- Mesri Alamdari, N.; Irandoost, P.; Roshanravan, N.; Vafa, M.; Asghari Jafarabadi, M.; Alipour, S.; Roshangar, L.; Alivand, M.; Farsi, F.; Shidfar, F. Effects of Royal Jelly and Tocotrienol Rich Fraction in obesity treatment of calorie-restricted obese rats: A focus on white fat browning properties and thermogenic capacity. Nutr. Metab. 2020, 17, 42. [Google Scholar] [CrossRef]
- Vajdi, M.; Musazadeh, V.; Khajeh, M.; Safaei, E.; Darzi, M.; Noshadi, N.; Bazyar, H.; Askari, G. The effects of royal jelly supplementation on anthropometric indices: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1196258. [Google Scholar] [CrossRef]
- Yoshida, M.; Hayashi, K.; Watadani, R.; Okano, Y.; Tanimura, K.; Kotoh, J.; Sasaki, D.; Matsumoto, K.; Maeda, A. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Sci. 2017, 79, 299–307. [Google Scholar] [CrossRef]
- Felemban, A.H.; Alshammari, G.M.; Yagoub, A.E.A.; Al-Harbi, L.N.; Alhussain, M.H.; Yahya, M.A. Activation of AMPK entails the protective effect of royal jelly against high-fat-diet-induced hyperglycemia, hyperlipidemia, and non-alcoholic fatty liver disease in rats. Nutrients 2023, 15, 1471. [Google Scholar] [CrossRef]
- Farley, C.; Cook, J.A.; Spar, B.D.; Austin, T.M.; Kowalski, T.J. Meal pattern analysis of diet-induced obesity in susceptible and resistant rats. Obes. Res. 2003, 11, 845–851. [Google Scholar] [CrossRef]
- Hildebrandt, A.L.; Kelly-Sullivan, D.M.; Black, S.C. Antiobesity effects of chronic cannabinoid CB1 receptor antagonist treatment in diet-induced obese mice. Eur. J. Pharmacol. 2003, 462, 125–132. [Google Scholar] [CrossRef]
- Vuković, R.; Blažetić, S.; Oršolić, I.; Heffer, M.; G Vari, S.; Gajdoš, M.; Krivošíková, Z.; Kramarova, P.; Kebis, A.; Has-Schoen, E. Impact of ovariectomy, high fat diet, and lifestyle modifications on oxidative/antioxidative status in the rat liver. Croat. Med. J. 2014, 55, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Gulnaz, A.; Nadeem, J.; Han, J.-H.; Lew, L.-C.; Son, J.-D.; Park, Y.-H.; Rather, I.A.; Hor, Y.-Y. Lactobacillus SPS in reducing the risk of diabetes in high-fat diet-induced diabetic mice by modulating the gut microbiome and inhibiting key digestive enzymes associated with diabetes. Biology 2021, 10, 348. [Google Scholar] [CrossRef]
- Shah, S.A.; Amin, F.U.; Khan, M.; Abid, M.N.; Rehman, S.U.; Kim, T.H.; Kim, M.W.; Kim, M.O. Anthocyanins abrogate glutamate-induced AMPK activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. J. Neuroinflamm. 2016, 13, 286. [Google Scholar] [CrossRef]
- Yahya, M.A.; Alshammari, G.M.; Osman, M.A.; Al-Harbi, L.N.; Yagoub, A.E.A.; AlSedairy, S.A. Liquorice root extract and isoliquiritigenin attenuate high-fat diet-induced hepatic steatosis and damage in rats by regulating AMPK. Arch. Physiol. Biochem. 2022, 130, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Almohawes, Z.N.; El-Kott, A.; Morsy, K.; Shati, A.A.; El-Kenawy, A.E.; Khalifa, H.S.; Elsaid, F.G.; Abd-Lateif, A.-E.-K.M.; Abu-Zaiton, A.; Ebealy, E.R. Salidroside inhibits insulin resistance and hepatic steatosis by downregulating miR-21 and subsequent activation of AMPK and upregulation of PPARα in the liver and muscles of high fat diet-fed rats. Arch. Physiol. Biochem. 2022, 130, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Jafaripour, L.; Esmaeilpour, K.; Maneshian, M.; Bashiri, H.; Rajizadeh, M.A.; Ahmadvand, H.; Asadi-Shekaari, M. The effect of gallic acid on memory and anxiety-like behaviors in rats with bile duct ligation-induced hepatic encephalopathy: Role of AMPK pathway. Avicenna J. Phytomed. 2022, 12, 425. [Google Scholar]
- Lee, M.O. Determination of the surface area of the white rat with its application to the expression of metabolic results. Am. J. Physiol.-Leg. Content 1929, 89, 24–33. [Google Scholar] [CrossRef]
- Novelli, E.; Diniz, Y.; Galhardi, C.; Ebaid, G.; Rodrigues, H.; Mani, F.; Fernandes, A.A.H.; Cicogna, A.C.; Novelli Filho, J. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007, 41, 111–119. [Google Scholar] [CrossRef]
- Arika, W.M.; Kibiti, C.M.; Njagi, J.M.; Ngugi, M.P. Anti-obesity effects of dichloromethane leaf extract of Gnidia glauca in high fat diet-induced obese rats. Heliyon 2019, 5, e02800. [Google Scholar] [CrossRef]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol.-Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strains of mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- Martins, T.; Ferreira, T.; Nascimento-Gonçalves, E.; Castro-Ribeiro, C.; Lemos, S.; Rosa, E.; Antunes, L.M.; Oliveira, P.A. Obesity rodent models applied to research with food products and natural compounds. Obesities 2022, 2, 171–204. [Google Scholar] [CrossRef]
- James, J.V.; Varghese, J.; John, N.M.; Deschemin, J.-C.; Vaulont, S.; McKie, A.T.; Jacob, M. Insulin resistance and adipose tissue inflammation induced by a high-fat diet are attenuated in the absence of hepcidin. J. Nutr. Biochem. 2023, 111, 109175. [Google Scholar] [CrossRef]
- Carreres, L.; Jílková, Z.M.; Vial, G.; Marche, P.N.; Decaens, T.; Lerat, H. Modeling diet-induced NAFLD and NASH in rats: A comprehensive review. Biomedicines 2021, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Adeyanju, M.M.; Saheed, I.A.; Oyelekan, O.I.; Dele-Osibanjo, T.A.; Adelegan, A.A.; Raimi, A.J.; Olalekan, S.O.; Alabi, O.S.; Alli, K.M. Sesamum indicum diet prevents hyperlipidemia in experimental rats. Food Chem. Mol. Sci. 2022, 4, 100092. [Google Scholar] [CrossRef]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef]
- Bagameri, L.; Botezan, S.; Bobis, O.; Bonta, V.; Dezmirean, D.S. Molecular Insights into Royal Jelly Anti-Inflammatory Properties and Related Diseases. Life 2023, 13, 1573. [Google Scholar] [CrossRef]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-orchestrated metabolic homeostasis in the adipose tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef]
- Reusch, J.E.; Colton, L.A.; Klemm, D.J. CREB activation induces adipogenesis in 3T3-L1 cells. Mol. Cell. Biol. 2000, 20, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.M.; Janssen, R.C.; Choudhury, M.; Baquero, K.C.; Aikens, R.M.; de la Houssaye, B.A.; Friedman, J.E. CCAAT/enhancer-binding protein β (C/EBPβ) expression regulates dietary-induced inflammation in macrophages and adipose tissue in mice. J. Biol. Chem. 2012, 287, 34349–34360. [Google Scholar] [CrossRef]
- Qu, Q.; Zeng, F.; Liu, X.; Wang, Q.; Deng, F. Fatty acid oxidation and carnitine palmitoyltransferase I: Emerging therapeutic targets in cancer. Cell. Death Dis. 2016, 7, e2226. [Google Scholar] [CrossRef]
- Kim, J.B.; Wright, H.M.; Wright, M.; Spiegelman, B.M. ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proc. Natl. Acad. Sci. USA 1998, 95, 4333–4337. [Google Scholar] [CrossRef]
- Pandeya, P.R.; Lamichhane, R.; Lee, K.-H.; Kim, S.-G.; Lee, D.-H.; Lee, H.-K.; Jung, H.-J. Bioassay-guided isolation of active anti-adipogenic compound from royal jelly and the study of possible mechanisms. BMC Complement. Altern. Med. 2019, 19, 33. [Google Scholar] [CrossRef]
- Kobayashi, M.; Deguchi, Y.; Nozaki, Y.; Higami, Y. Contribution of pgc-1α to obesity-and caloric restriction-related physiological changes in white adipose tissue. Int. J. Mol. Sci. 2021, 22, 6025. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-F.; Ku, H.-C.; Lin, H. PGC-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [PubMed]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Ning, C.; Zhang, M.; Fan, P.; Lei, D.; Du, J.; Gale, M.; Ma, Y.; Yang, Y. Ellagic acid promotes browning of white adipose tissues in high-fat diet-induced obesity in rats through suppressing white adipocyte maintaining genes. Endocr. J. 2019, 66, 923–936. [Google Scholar] [CrossRef]
- Li, J.; Yi, X.; Li, T.; Yao, T.; Li, D.; Hu, G.; Ma, Y.; Chang, B.; Cao, S. Effects of exercise and dietary intervention on muscle, adipose tissue, and blood IRISIN levels in obese male mice and their relationship with the beigeization of white adipose tissue. Endocr. Connect. 2022, 11, e210625. [Google Scholar] [CrossRef]
- Shen, S.-H.; Singh, S.P.; Raffaele, M.; Waldman, M.; Hochhauser, E.; Ospino, J.; Arad, M.; Peterson, S.J. Adipocyte-specific expression of PGC1α promotes adipocyte browning and alleviates obesity-induced metabolic dysfunction in an HO-1-Dependent fashion. Antioxidants 2022, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Even, P.C.; Viollet, B. AMPK activation reduces hepatic lipid content by increasing fat oxidation in vivo. Int. J. Mol. Sci. 2018, 19, 2826. [Google Scholar] [CrossRef]
- Dagon, Y.; Avraham, Y.; Berry, E.M. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2α in adipocytes. Biochem. Biophys. Res. Commun. 2006, 340, 43–47. [Google Scholar] [CrossRef]
- Kim, S.-J.; Tang, T.; Abbott, M.; Viscarra, J.A.; Wang, Y.; Sul, H.S. AMPK phosphorylates desnutrin/ATGL and hormone-sensitive lipase to regulate lipolysis and fatty acid oxidation within adipose tissue. Mol. Cell. Biol. 2016, 36, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-activated protein kinase (AMPK) regulates energy metabolism through modulating thermogenesis in adipose tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Malik, S.; Tariq, K.; Israr, M.; Iqbal, S. (2023). Major royal jelly proteins (MRJPs) of Honeybees, Functions, and Biological Activities. Egypt. J. Agric. Res. 2023, 101, 253–266. [Google Scholar]
- Gaullier, J.M.; Halse, J.; Høye, K.; Kristiansen, K.; Fagertun, H.; Vik, H.; Gudmundsen, O. Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. Am. J. Clin. Nutr. 2004, 79, 1118–1125. [Google Scholar] [CrossRef]
- Bo, T.; Wang, T.; Li, S. Flavonoids and their role in obesity management. Phytother. Res. 2019, 33, 1825–1834. [Google Scholar]
- Kuo, H.C.; Lu, T.H.; Kuo, W.H. Polyphenols in royal jelly and their potential effects on lipid metabolism and obesity. Nutrients 2021, 13, 1980. [Google Scholar]
- Gaullier, M.F.; Hussain, S.; Muneer, M. Impact of polyphenols on lipid metabolism and obesity: Mechanistic insights. Food Chem. 2022, 366, 130623. [Google Scholar]
- Biesalski, H.K.; Glei, M. Nutritional effects of royal jelly: A review. Nutrients 2019, 11, 1323. [Google Scholar]
- Kim, S.H.; Park, S.Y. Royal jelly and its potential anti-obesity effects. Food Function 2020, 11, 7432–7444. [Google Scholar]
- Huang, J.; Xu, X. Royal jelly as a functional food: Its role in weight management and metabolic health. Food Chem. 2022, 374, 131782. [Google Scholar]
- Shimizu, K.; Mizuno, M. Effects of royal jelly on metabolic health: A review of clinical and preclinical studies. J. Funct. Foods. 2021, 82, 104529. [Google Scholar]
- Wu, X.; Wang, H. Royal jelly supplementation and its effects on obesity: A systematic review and meta-analysis. Clin. Nutr. 2018, 37, 461–469. [Google Scholar]
- Lee, J.H.; Kim, J.H. Royal jelly supplementation reduces body fat and improves metabolic markers in overweight individuals: A randomized controlled trial. J. Funct. Foods. 2021, 85, 104631. [Google Scholar]
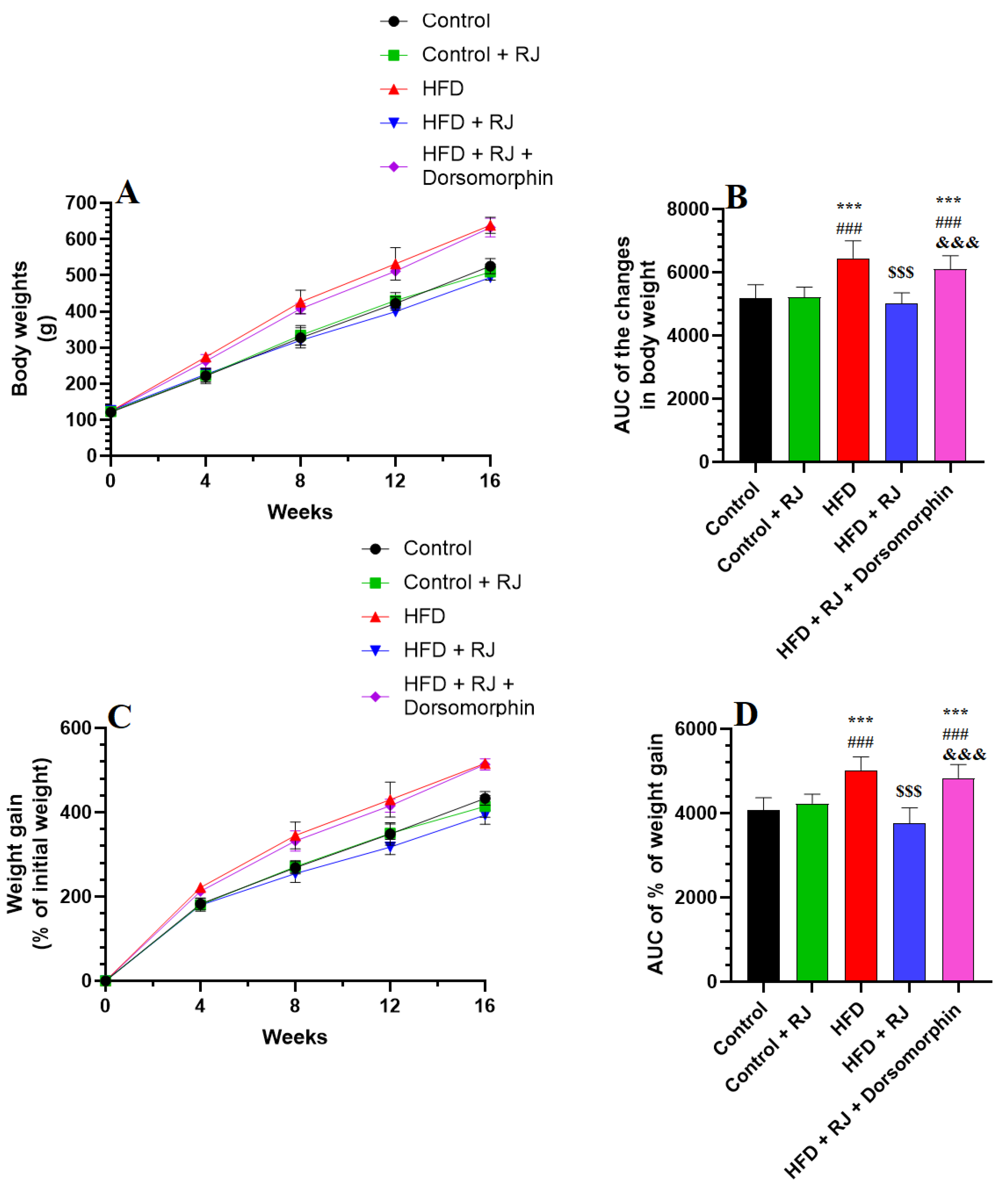

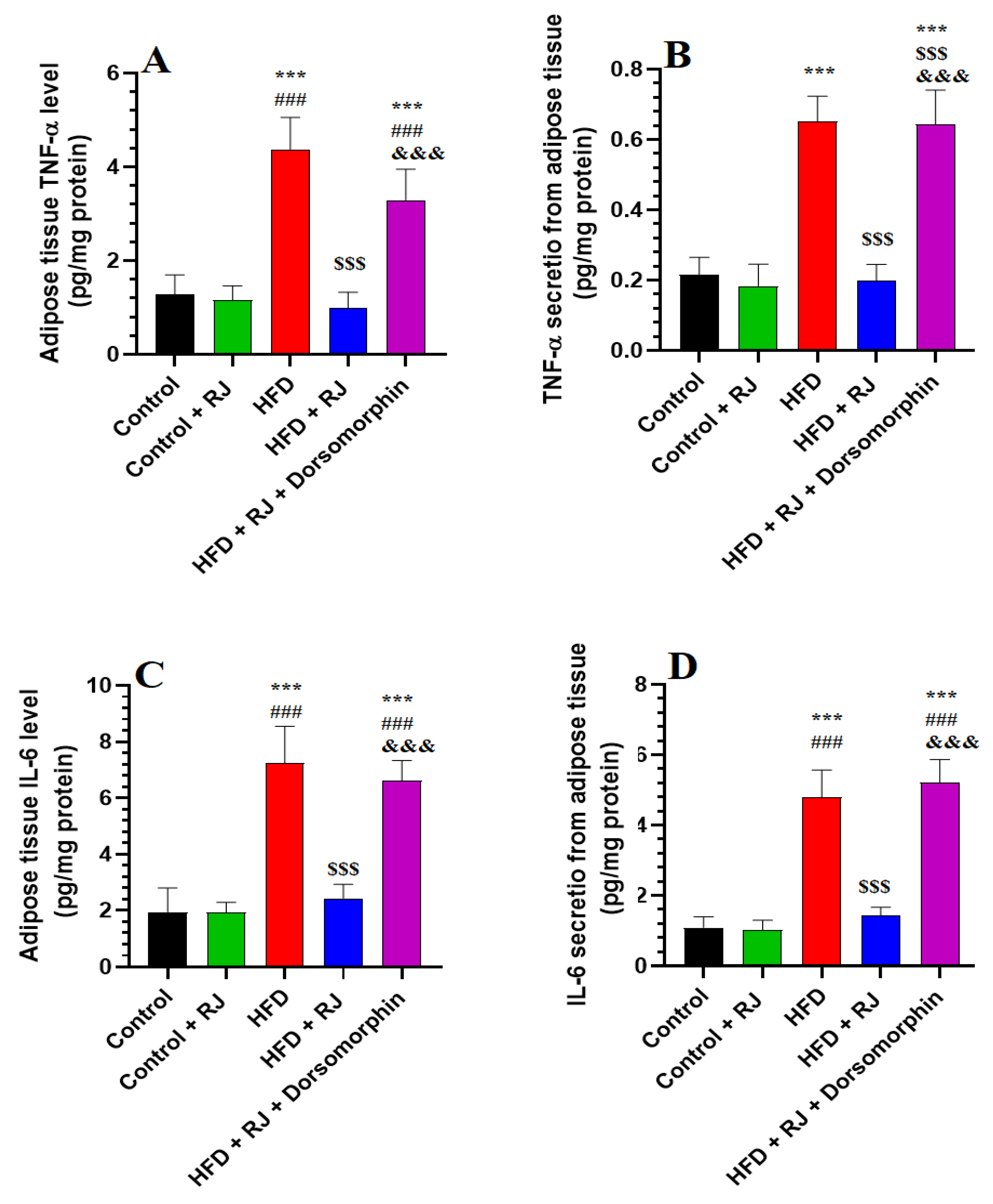
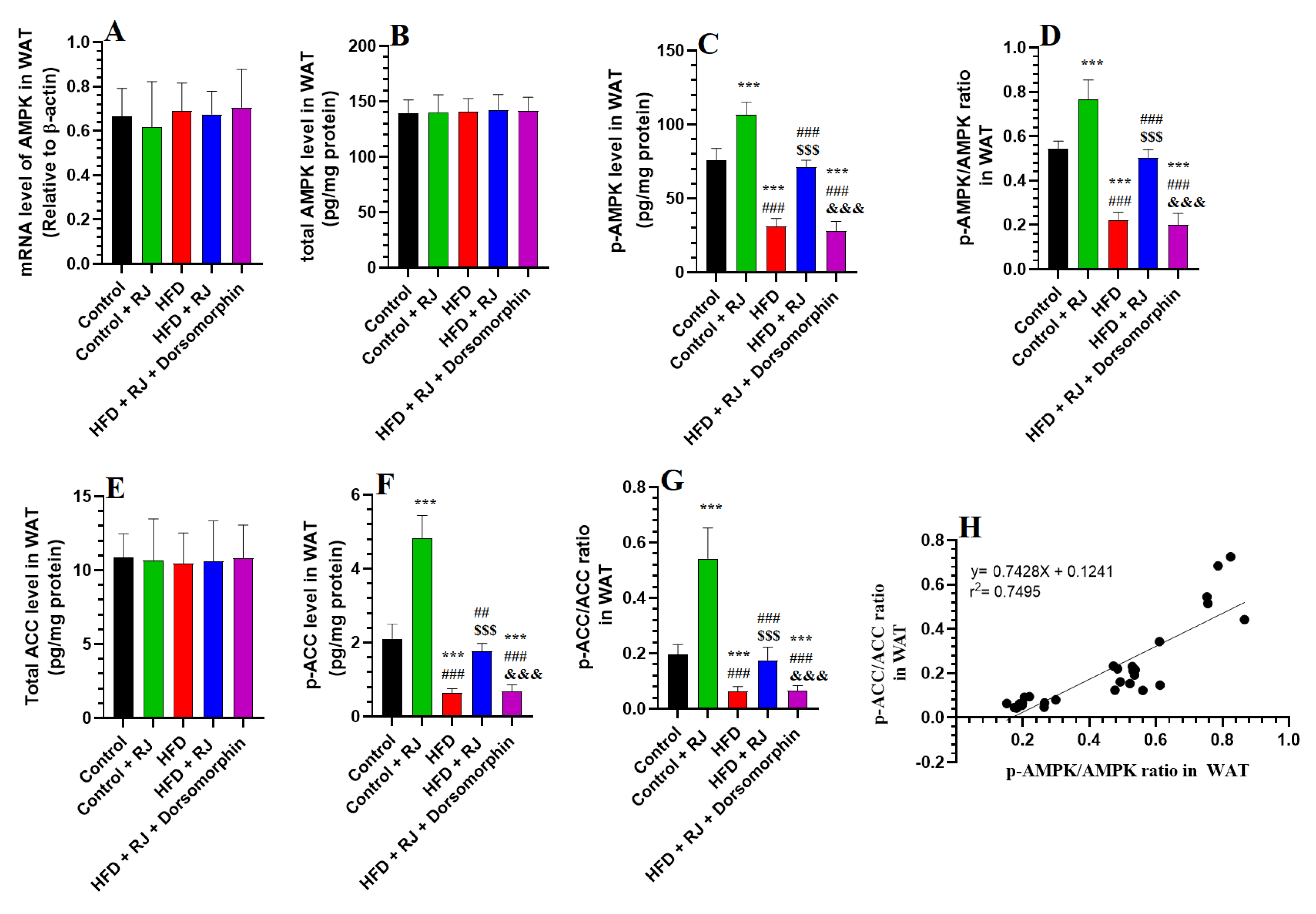
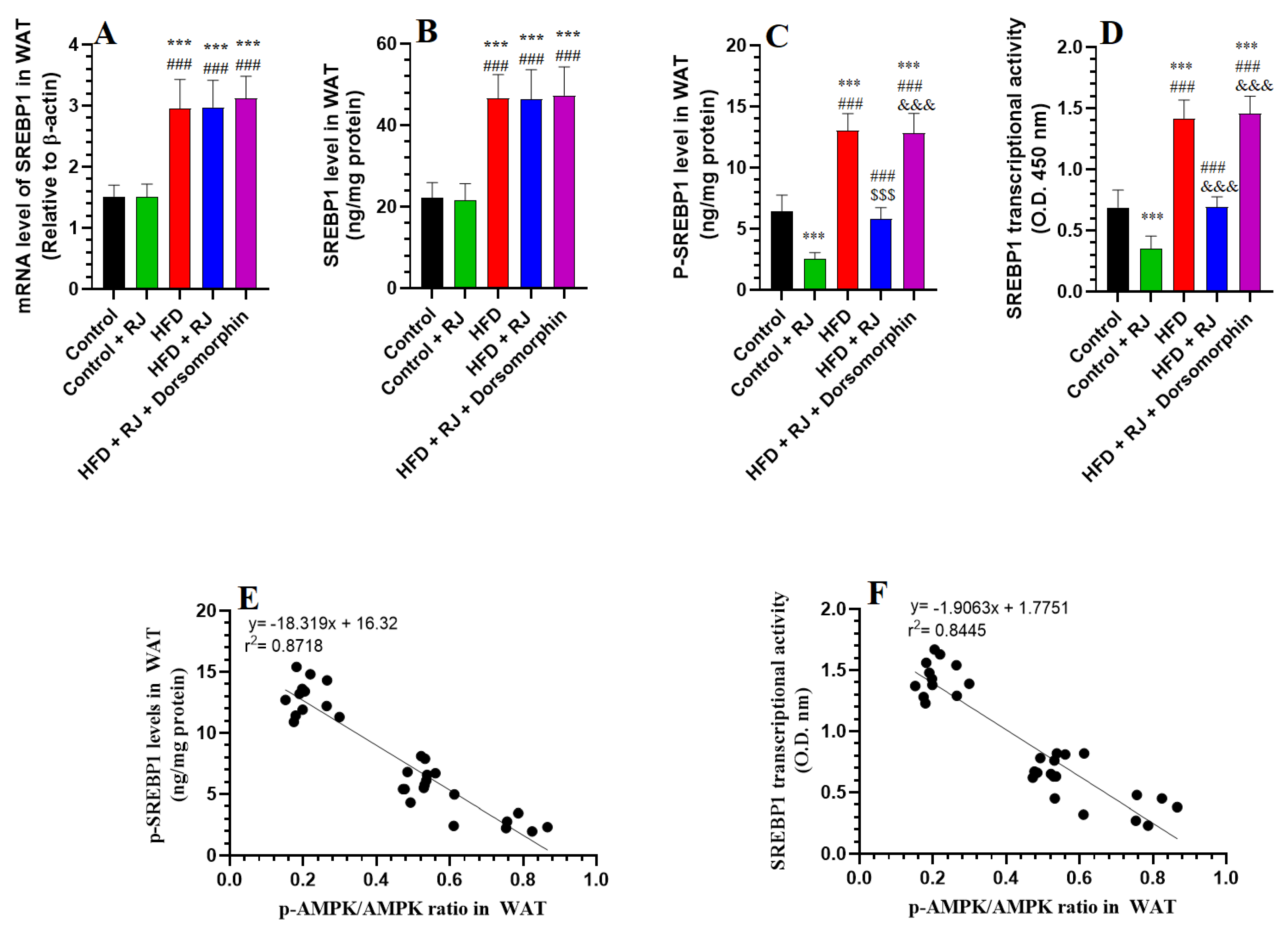


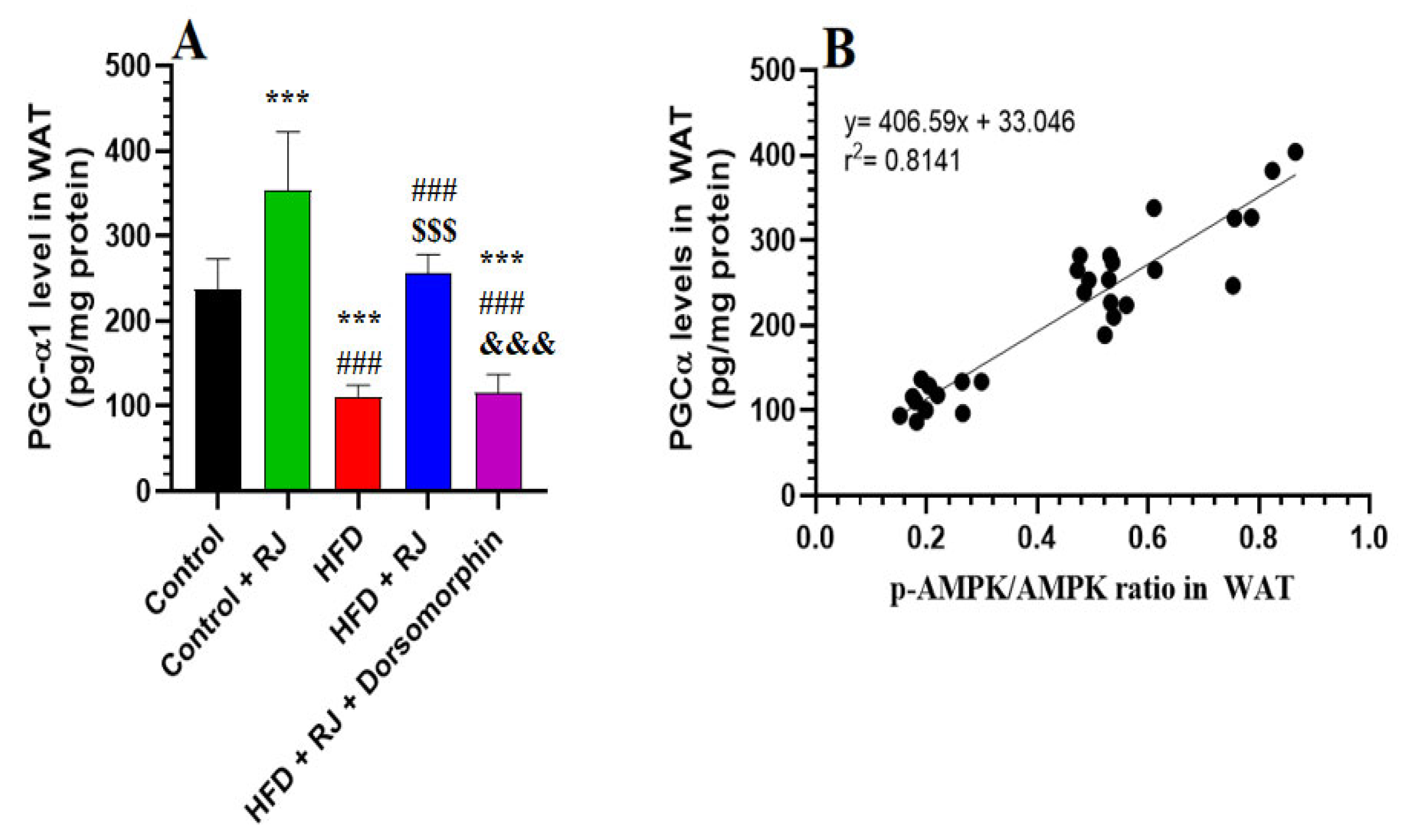
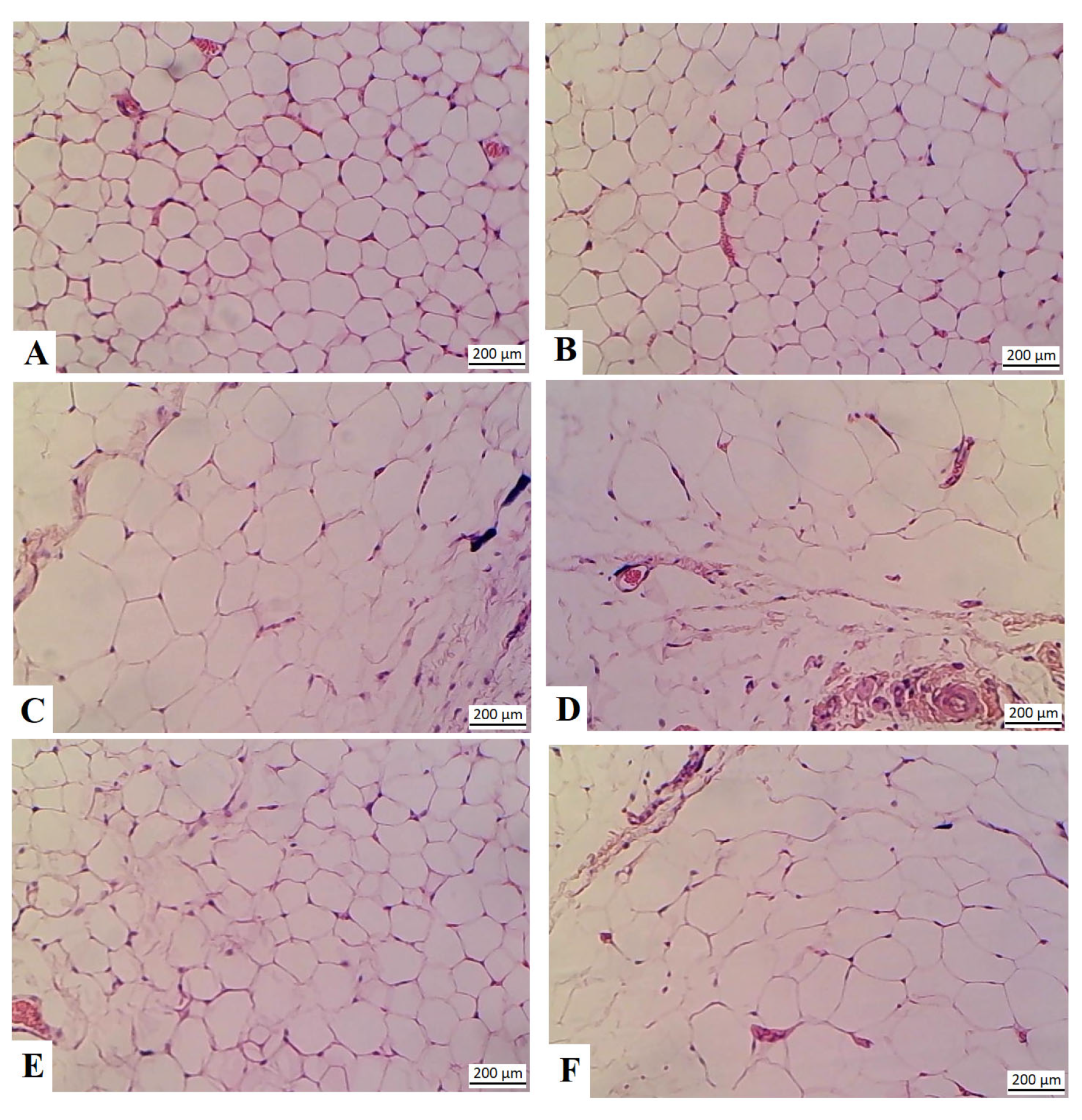
| Control | Control + RJ | HFD | HFD + RJ | HFD + RJ + Dorsomorphin | |
|---|---|---|---|---|---|
| Final body weights | 505 ± 25.4 | 494 ± 31.4 | 642 ± 47.8 *** ### | 499 ± 38.5 $$$ | 634 ± 42.3 *** ### &&& |
| Body length (cm) | 27.73 ± 0.66 | 27.03 ± 0.8 | 27.3 ± 0.5 | 26.99 ± 0.55 | 27.0 ± 0.87 *** ### &&& |
| BMI | 0.65 ± 0.04 | 0.62 ± 0.4 | 0.88 ± 0.1 *** ### | 0.67 ± 0.03 $$$ | 0.87 ± 0.08 *** ### &&& |
| AC (cm) | 14.4 ± 0.89 | 15.1 ± 1.1 | 20.8 ± 1.8 *** ### | 16.4 ± 1.3 $$$ | 21.3 ± 1.6 *** ### &&& |
| Lee index | 287.3 ± 12.2 | 293.4 ± 10.5 | 321.3 ± 13.7 *** ### | 291.6 ± 11.4 $$$ | 318.4 ± 12.8 *** ### &&& |
| Control | Control + RJ | HFD | HFD + RJ | HFD + RJ + Dorsomorphin | |
|---|---|---|---|---|---|
| Mesenteric fat (g) | 3.2 ± 0.35 | 2.9 ± 0.39 | 6.8 ± 0.52 *** ### | 3.5 ± 0.46 # $$$ | 6.4 ± 0.71 *** ### &&& |
| Retroperitoneal fat (g) | 1.41 ± 0.12 | 1.22 ± 0.18 | 4.8 ± 0.39 *** ### | 1.87 ± 0.14 * # $$$ | 4.4 ± 0.61 *** ### &&& |
| Parametrial fat (g) | 0.38 ± 0.05 | 0.31 ± 0.3 | 0.55 ± 0.03 * # | 0.58 ± 0.05 * # | 0.51 ± 0.05 * # |
| Perirenal fat (g) | 1.94 ± 0.21 | 2.11 ± 0.26 | 5.2 ± 0.48 *** ### | 2.6 ± 0.51 ** # $$$ | 4.9 ± 0.07 *** ### &&& |
| subcutaneous fat (g) | 3.8 ± 0.05 | 3.3 ± 0.07 | 6.1 ± 0.08 *** ### | 3.5 ± 0.07 $$$ | 6.7 ± 0.08 *** ### &&& |
| Total fat weight (g) | 10.65 ± 0.83 | 9.95 ± 0.78 | 22.93 ± 1.6 *** ### | 12.82 ± 1.1 # $$$ | 23.6 ± 1.96 *** ### &&& |
| AI | 2.07 ± 0.14 | 1.93 ± 0.23 | 3.48 ± 0.26 *** ### | 2.47 ± 0.34 * # $$$ | 3.26 ± 0.23 *** ### &&& |
| Control | Control + RJ | HFD | HFD + RJ | HFD + RJ + Dorsomorphin | |
|---|---|---|---|---|---|
| Fasting glucose (mg/dL) | 101.6 ± 7.5 | 108.3 ± 12.6 | 185.4 ± 15.4 *** ### | 131.3 ± 11.8 * # $$$ | 186.3 ± 17.5 *** ### &&& |
| Fasting Insulin (ng/mL) | 3.9 ± 0.26 | 4.2 ± 0.8 | 6.7 ± 0.7 *** ### | 5.1 ± 0.36 * # $$$ | 6.8 ± 0.6 *** ### &&& |
| HOMA-IR | 0.95 ± 0.14 | 1.01 ± 0.26 | 3.09 ± 0.42 *** ### | 1.62 ± 0.0.21 * # $$$ | 3.18 ± 0.39 *** ### &&& |
| Serum-free fatty acids (μmol/L) | 334.1 ± 39 | 268.4 ± 24 ** | 1098.4 ± 107 *** ### | 383.1 ± 46 * ### $$$ | 1109.4 ± 106 *** ### &&& |
| Serum glycerol (μmol/L) | 56.5 ± 4.8 | 88.8 ± 3.4 ** | 178.8 ± 14.7 *** ### | 303.3 ± 5.8 * ### $$$ | 147.3 ± 15.9 *** ### &&& |
| Serum Leptin (ng/mL) | 30.2 ± 4.7 | 27.8 ± 3.4 | 71.5 ± 6.2 *** ### | 36.5 ± 4.8 * # $$ | 76.5 ± 8.1 *** ### &&& |
| Serum adiponectin (μg/mL) | 35.6 ± 3.2 | 47.6 ± 4.8 ** | 15.8 ± 1.7 *** ### | 25.6 ± 2.9 * # $$ | 13.9 ± 1.4 *** ### &&& |
| Serum Triglycerides (mg/dL) | 46.4 ± 4.9 | 31.6 ± 3.8 *** | 114.7 ± 19.9 *** ### | 56.4 ± 4.9 * # $$ | 105.5 ± 10.1 *** ### &&& |
| Serum Cholesterol (mg/kg) | 98.5 ± 8.6 | 80.1 ± 7.1 *** | 218.2 ± 18.9 *** ### | 94.5 ± 9.7 ## $$$ | 227.3 ± 21.8 *** ### &&& |
| Serum LDL-c (mg/dL) | 33.9 ± 2.9 | 26.2 ± 2.1 * | 113.4 ± 11.7 *** ### | 40.3 ± 3.9 * ## $$$ | 103.5 ± 9.8 *** ### &&& |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felemban, A.H.; Alshammari, G.M.; Yagoub, A.E.A.; Saleh, A.; Yahya, M.A. Royal Jelly Exerts a Potent Anti-Obesity Effect in Rats by Activating Lipolysis and Suppressing Adipogenesis. Nutrients 2024, 16, 3174. https://doi.org/10.3390/nu16183174
Felemban AH, Alshammari GM, Yagoub AEA, Saleh A, Yahya MA. Royal Jelly Exerts a Potent Anti-Obesity Effect in Rats by Activating Lipolysis and Suppressing Adipogenesis. Nutrients. 2024; 16(18):3174. https://doi.org/10.3390/nu16183174
Chicago/Turabian StyleFelemban, Alaa Hasanain, Ghedeir M. Alshammari, Abu ElGasim Ahmed Yagoub, Ali Saleh, and Mohammed Abdo Yahya. 2024. "Royal Jelly Exerts a Potent Anti-Obesity Effect in Rats by Activating Lipolysis and Suppressing Adipogenesis" Nutrients 16, no. 18: 3174. https://doi.org/10.3390/nu16183174
APA StyleFelemban, A. H., Alshammari, G. M., Yagoub, A. E. A., Saleh, A., & Yahya, M. A. (2024). Royal Jelly Exerts a Potent Anti-Obesity Effect in Rats by Activating Lipolysis and Suppressing Adipogenesis. Nutrients, 16(18), 3174. https://doi.org/10.3390/nu16183174






