Comparison of Dietary Supplementation with Krill Oil, Fish Oil, and Astaxanthin on an Experimental Ethanol-Induced Gastric Ulcer Model: A Biochemical and Histological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Drugs
2.2. Experimental Design
2.3. Macroscopic Analysis of Gastric Tissue
2.4. Histopathological Examination of Gastric and Hepatic Tissues
2.5. Measurement of Gastric and Hepatic Malondialdehyde and Glutathione Levels
2.6. Measurement of Gastric and Hepatic Myeloperoxidase Activity
2.7. Measurement of Gastric and Hepatic Chemiluminescence Assays
2.8. Measurement of Serum Lipids and Liver Function Tests in Serum Samples
2.9. Statistical Analysis
3. Results
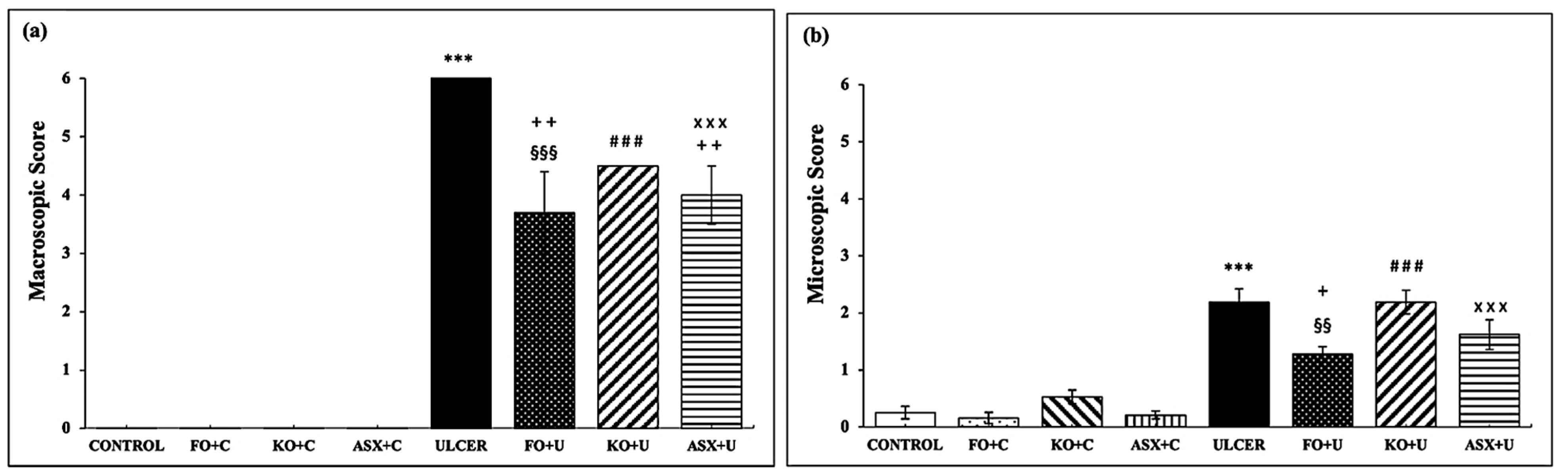
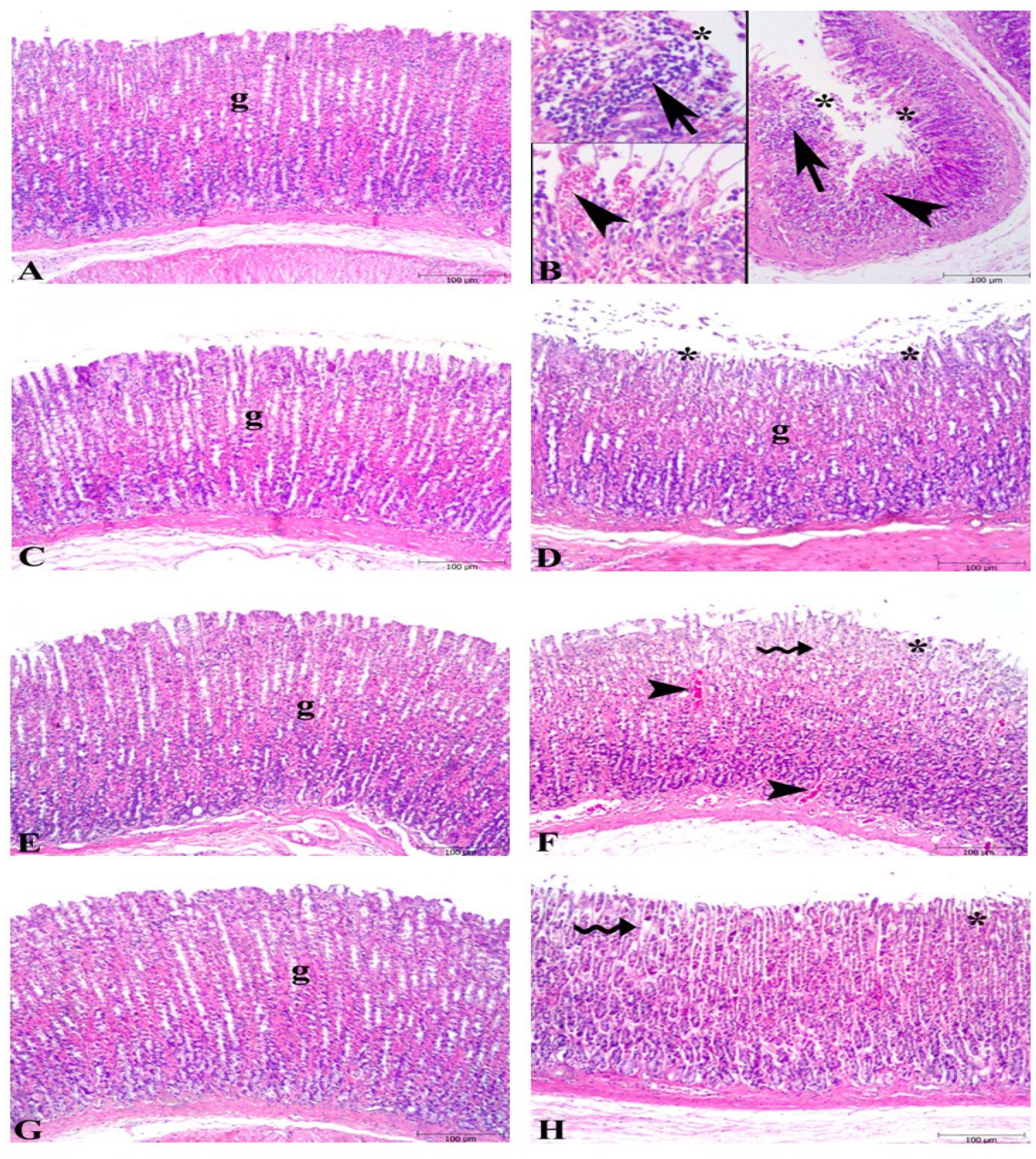
3.1. Macroscopic and Microscopic Evaluations of Gastric Tissues
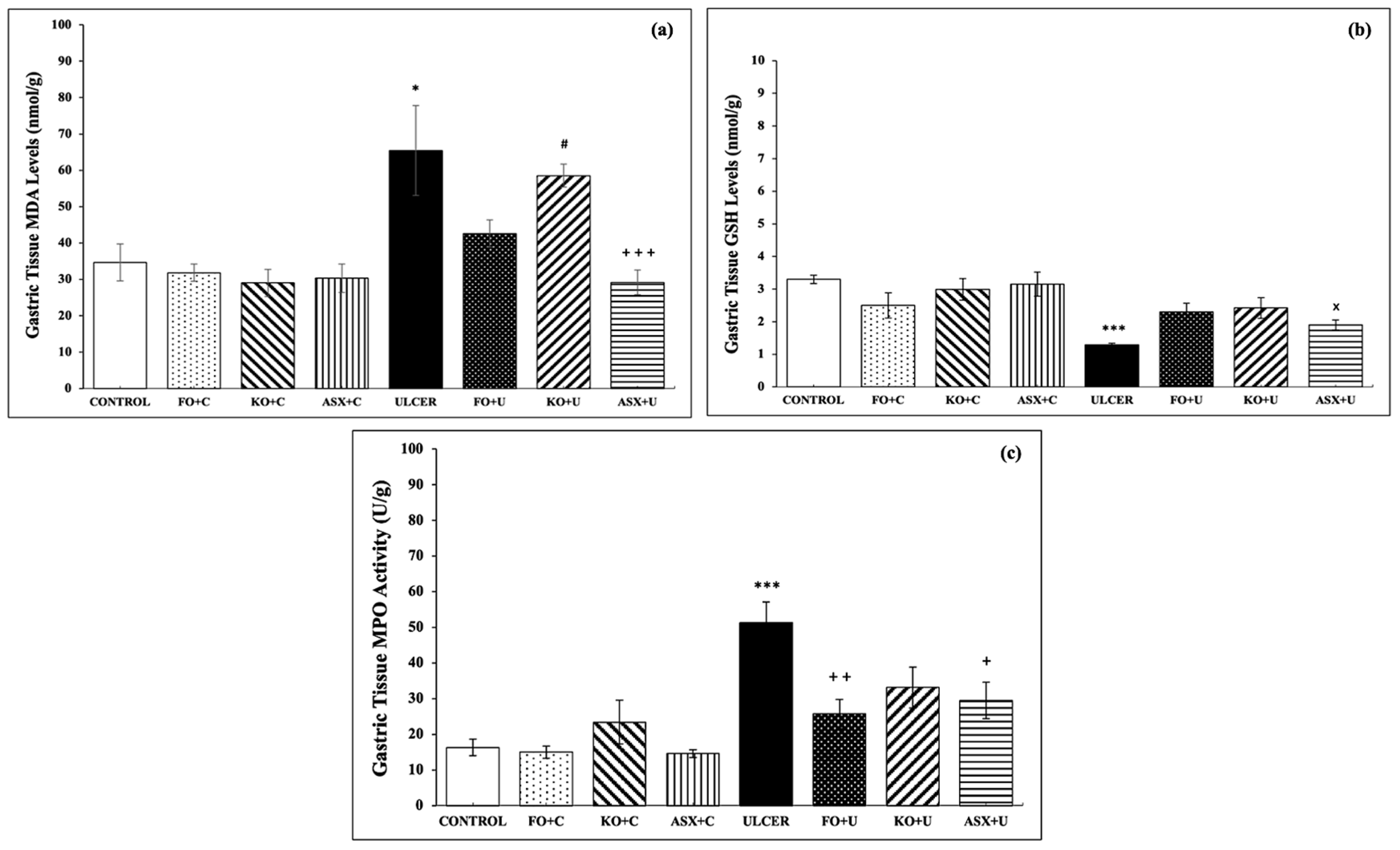
3.2. MDA–GSH Levels and MPO Activities in Gastric Tissues
3.3. Chemiluminescence Levels (CL) in Gastric Tissues
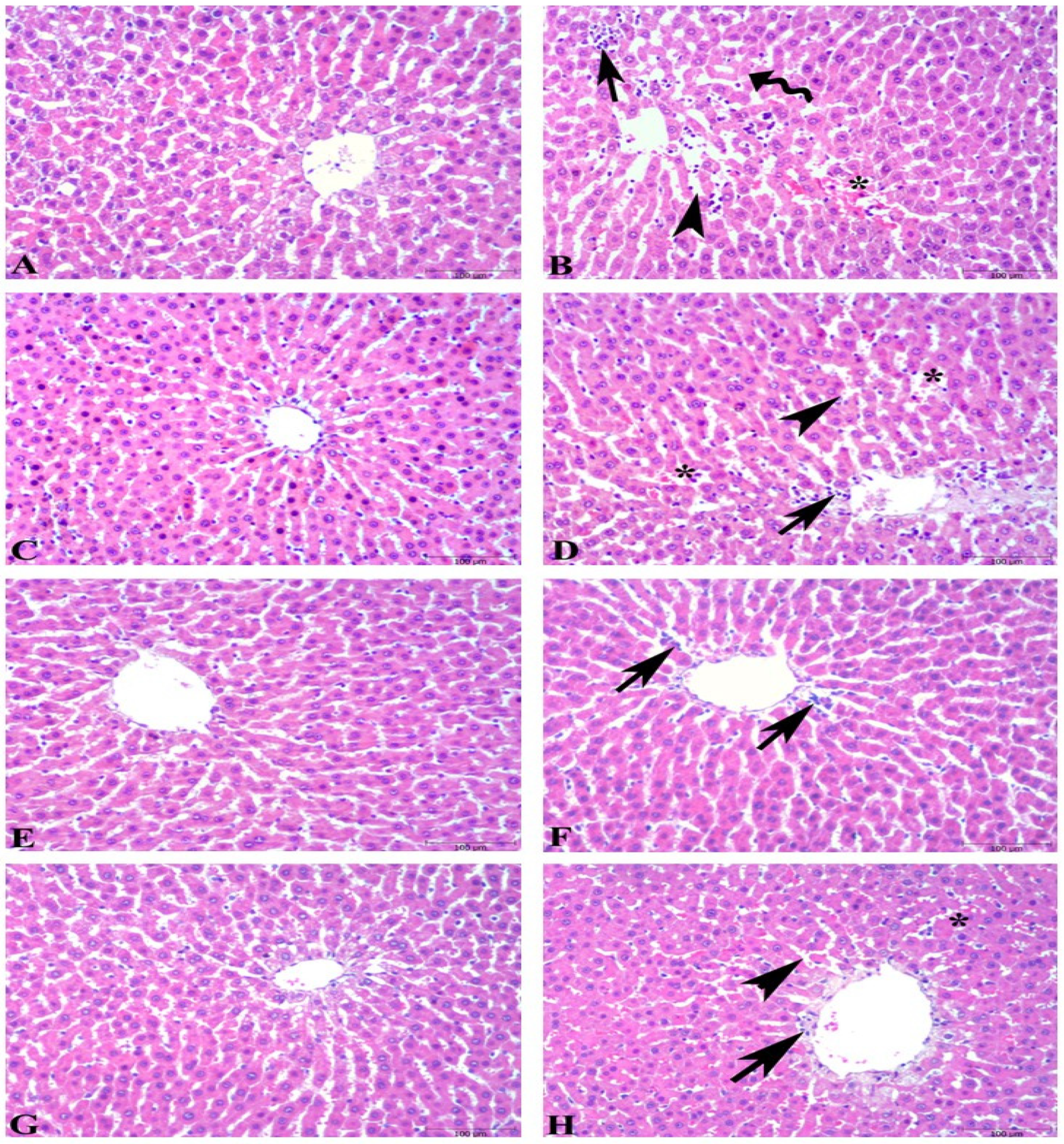
3.4. Microscopic Evaluations of Hepatic Tissues
3.5. MDA–GSH Levels and MPO Activities in Hepatic Tissues
3.6. Chemiluminescence Levels in Hepatic Tissues
3.7. Serum Lipids and Liver Function Tests in Serum Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Lian, Y.; Li, Q.; Sun, L.; Chen, R.; Lai, X.; Lai, Z.; Yuan, E.; Sun, S. Preventative and Therapeutic Potential of Flavonoids in Peptic Ulcers. Molecules 2020, 25, 4626. [Google Scholar] [CrossRef] [PubMed]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Di Mario, F.; De’angelis, G.L. Helicobacter Pylori, Transmission Routes and Recurrence of Infection: State of the Art. Acta Bio Med. Atenei Parm. 2018, 89, 72. [Google Scholar] [CrossRef]
- Woolf, A.; Rose, R. Gastric Ulcer; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Tarnawski, A.; Hollander, D.; Stachura, J.; Krause, W.J.; Gergely, H. Prostaglandin Protection of the Gastric Mucosa against Alcohol Injury—A Dynamic Time-Related Process. Role of the Mucosal Proliferative Zone. Gastroenterology 1985, 88, 334–352. [Google Scholar] [CrossRef] [PubMed]
- Musumba, C.; Pritchard, D.M.; Pirmohamed, M. Review Article: Cellular and Molecular Mechanisms of NSAID-Induced Peptic Ulcers. Aliment. Pharmacol. Ther. 2009, 30, 517–531. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Zhang, H.; He, Z.; Zhi, W.; Liu, F.; Wang, Y.; Niu, X. Anti-Ulcerogenic Effect of Cavidine against Ethanol-Induced Acute Gastric Ulcer in Mice and Possible Underlying Mechanism. Int. Immunopharmacol. 2016, 38, 450–459. [Google Scholar] [CrossRef]
- AbdelAziz, E.Y.; Tadros, M.G.; Menze, E.T. The Effect of Metformin on Indomethacin-Induced Gastric Ulcer: Involvement of Nitric Oxide/Rho Kinase Pathway. Eur. J. Pharmacol. 2021, 892, 173812. [Google Scholar] [CrossRef]
- Sheen, E.; Triadafilopoulos, G. Adverse Effects of Long-Term Proton Pump Inhibitor Therapy. Dig. Dis. Sci. 2011, 56, 931–950. [Google Scholar] [CrossRef] [PubMed]
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, A.; Şener, G.; Çetinel, Ş.; Yüksel, M.; Yeǧen, C.; Yeǧen, B.Ç. Protective and Therapeutic Effects of Resveratrol on Acetic Acid-Induced Gastric Ulcer. Free Radic. Res. 2009, 43, 594–603. [Google Scholar] [CrossRef]
- Karakoyun, B.; Yüksel, M.; Ercan, F.; Erzik, C.; Yeǧen, B.Ç. Alpha-Lipoic Acid Improves Acetic Acid-Induced Gastric Ulcer Healing in Rats. Inflammation 2009, 32, 37–46. [Google Scholar] [CrossRef]
- Kulshreshtha, M.; Srivastava, G.; Singh, M. Pathophysiological Status and Nutritional Therapy of Peptic Ulcer: An Update. Environ. Dis. 2017, 2, 76. [Google Scholar] [CrossRef]
- Ortiz Sánchez, C.A.; Zavaleta, E.B.; García, G.R.U.; Solano, G.L.; Díaz, M.P.R. Krill Oil Microencapsulation: Antioxidant Activity, Astaxanthin Retention, Encapsulation Efficiency, Fatty Acids Profile, in Vitro Bioaccessibility and Storage Stability. LWT 2021, 147, 111476. [Google Scholar] [CrossRef]
- Krill Oil Monograph. Altern. Med. Rev. A J. Clin. Ther. 2010, 15, 84–86.
- Colletti, A.; Cravotto, G.; Citi, V.; Martelli, A.; Testai, L.; Cicero, A.F.G. Advances in Technologies for Highly Active Omega-3 Fatty Acids from Krill Oil: Clinical Applications. Mar. Drugs 2021, 19, 306. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Driscoll, D.; Schwarzbach, H.; Kinscherf, R. Krill Oil-In-Water Emulsion Protects against Lipopolysaccharide-Induced Proinflammatory Activation of Macrophages In Vitro. Mar. Drugs 2017, 15, 74. [Google Scholar] [CrossRef]
- Costanzo, M.; Cesi, V.; Prete, E.; Negroni, A.; Palone, F.; Cucchiara, S.; Oliva, S.; Leter, B.; Stronati, L. Krill Oil Reduces Intestinal Inflammation by Improving Epithelial Integrity and Impairing Adherent-Invasive Escherichia Coli Pathogenicity. Dig. Liver Dis. 2016, 48, 34–42. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef]
- Ulven, S.M.; Holven, K.B. Comparison of Bioavailability of Krill Oil versus Fish Oil and Health Effect. Vasc. Health Risk Manag. 2015, 11, 511–524. [Google Scholar] [CrossRef]
- Miki, W. Biological Functions and Activities of Animal Carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, B.; Lee, J.-Y. Astaxanthin Structure, Metabolism, and Health Benefits. J. Hum. Nutr. Food Sci. 2013, 1, 1003. [Google Scholar]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Pourbagher-Shahri, A.M.; Samarghandian, S. Anti-Inflammatory Action of Astaxanthin and Its Use in the Treatment of Various Diseases. Biomed. Pharmacother. 2022, 145, 112179. [Google Scholar] [CrossRef]
- Grimstad, T.; Bjørndal, B.; Cacabelos, D.; Aasprong, O.G.; Janssen, E.A.M.; Omdal, R.; Svardal, A.; Hausken, T.; Bohov, P.; Portero-Otin, M.; et al. Dietary Supplementation of Krill Oil Attenuates Inflammation and Oxidative Stress in Experimental Ulcerative Colitis in Rats. Scand. J. Gastroenterol. 2012, 47, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia Superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Wibrand, K.; Berge, K.; Messaoudi, M.; Duffaud, A.; Panja, D.; Bramham, C.R.; Burri, L. Enhanced Cognitive Function and Antidepressant-like Effects after Krill Oil Supplementation in Rats. Lipids Health Dis. 2013, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Avramovic, N.; Dragutinovic, V.; Krstic, D.; Colovic, M.B.; Trbovic, A.; de Luka, S.; Milovanovic, I.; Popovic, T. The Effects of Omega 3 Fatty Acid Supplementation on Brain Tissue Oxidative Status in Aged Wistar Rats. Hippokratia 2012, 16, 241. [Google Scholar] [PubMed]
- Jho, D.H.; Cole, S.M.; Lee, E.M.; Espat, N.J. Role of Omega-3 Fatty Acid Supplementation in Inflammation and Malignancy. Integr. Cancer Ther. 2004, 3, 98–111. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.S.; Song, G.G.; Park, J.J.; Chang, H.I. Protective Effect of Astaxanthin on Naproxen-Induced Gastric Antral Ulceration in Rats. Eur. J. Pharmacol. 2005, 514, 53–59. [Google Scholar] [CrossRef]
- Cil, M.A.; Ghareaghaji, A.G.; Bayir, Y.; Buyuktuncer, Z.; Besler, H.T. Efficacy of Krill Oil versus Fish Oil on Obesity-Related Parameters and Lipid Gene Expression in Rats: Randomized Controlled Study. PeerJ 2021, 9, e12009. [Google Scholar] [CrossRef]
- Ferramosca, A.; Conte, L.; Zara, V. A Krill Oil Supplemented Diet Reduces the Activities of the Mitochondrial Tricarboxylate Carrier and of the Cytosolic Lipogenic Enzymes in Rats. J. Anim. Physiol. Anim. Nutr. 2012, 96, 295–306. [Google Scholar] [CrossRef]
- Ferramosca, A.; Conte, A.; Burri, L.; Berge, K.; de Nuccio, F.; Giudetti, A.M.; Zara, V. A Krill Oil Supplemented Diet Suppresses Hepatic Steatosis in High-Fat Fed Rats. PLoS ONE 2012, 7, 38797. [Google Scholar] [CrossRef] [PubMed]
- İpek, B.E.; Yüksel, M.; Cumbul, A.; Ercan, F.; Cabadak, H.; Aydın, B.; Alican, İ. The Effect of Metformin on Ethanol- and IndomethacinInduced Gastric Ulcers in Rats. Turk. J. Gastroenterol. 2022, 33, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Kolgazi, M.; Güleken, Z.; Kolbaşı, B.; Başıbüyük, C.S.; Ercan, F.; Yeğen, B. Nikotinin Sıçan Pankreatit Modelindeki Hafifletici Etkisinde Vagusun Rolünün Araştırılması. Acıbadem Üniversitesi Sağlık Bilim. Derg. 2021, 12, 166–175. [Google Scholar] [CrossRef]
- Cilingir Kaya, O.T.; Bihter Gurler, E. Therapeutic Potential of Essential Oil of Melaleuca Quinquenervia (Myrtaceae) in a Rat Model of Ethanol- Induced Peptic Ulcer. Trop. J. Pharm. Res. 2021, 20, 981. [Google Scholar] [CrossRef]
- Kolgazi, M.; Cantali-Ozturk, C.; Deniz, R.; Ozdemir-Kumral, Z.N.; Yuksel, M.; Sirvanci, S.; Yeğen, B.C. Nesfatin-1 Alleviates Gastric Damage via Direct Antioxidant Mechanisms. J. Surg. Res. 2015, 193, 111–118. [Google Scholar] [CrossRef]
- Hillegass, L.M.; Griswold, D.E.; Brickson, B.; Albrightson-Winslow, C. Assessment of Myeloperoxidase Activity in Whole Rat Kidney. J. Pharmacol. Methods 1990, 24, 285–295. [Google Scholar] [CrossRef]
- Kikuchi, K.; Nagano, T.; Hayakawa, H.; Hirata, Y.; Hirobe, M. Detection of Nitric Oxide Production from a Perfused Organ by a Luminol-H2O2 System. Anal. Chem. 1993, 65, 1794–1799. [Google Scholar] [CrossRef]
- Haklar, G.; Sayin-Özveri, E.; Yüksel, M.; Aktan, A.Ö.; Yalçin, A.S. Different Kinds of Reactive Oxygen and Nitrogen Species Were Detected in Colon and Breast Tumors. Cancer Lett. 2001, 165, 219–224. [Google Scholar] [CrossRef]
- Haklar, U.; Yüksel, M.; Velioglu, A.; Turkmen, M.; Haklar, G.; Yalçin, A.S. Oxygen Radicals and Nitric Oxide Levels in Chondral or Meniscal Lesions or Both. Clin. Orthop. Relat. Res. 2002, 403, 135–142. [Google Scholar] [CrossRef]
- Jaccob, A.A. Effect of Pharmacological Doses of Garlic and Omega 3 on Gastric Lesions Induced by Ethanol in Mice. Asian J. Pharm. Clin. Res. 2016, 9, 153–157. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ghosal, S.; Bhattacharya, S.K. Effect of Fish Oil on Offensive and Defensive Factors in Gastric Ulceration in Rats. Prostaglandins Leukot. Essent. Fat. Acids 2006, 74, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Faust, T.W.; Redfern, J.S.; Podolsky, I.; Lee, E.; Grundy, S.M.; Feldman, M. Effects of Aspirin on Gastric Mucosal Prostaglandin E2 and F2α Content and on Gastric Mucosal Injury in Humans Receiving Fish Oil or Olive Oil. Gastroenterology 1990, 98, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xiang, X.; Zhou, Y.; Zhou, T.; Deng, S.; Zheng, B.; Zheng, P. Protective Effects of Antarctic Krill Oil in Dextran Sulfate Sodium-Induced Ulcerative Colitis Mice. J. Funct. Foods 2021, 79, 104394. [Google Scholar] [CrossRef]
- Kamath, B.S.; Srikanta, B.M.; Dharmesh, S.M.; Sarada, R.; Ravishankar, G.A. Ulcer Preventive and Antioxidative Properties of Astaxanthin from Haematococcus Pluvialis. Eur. J. Pharmacol. 2008, 590, 387–395. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; Zhu, X.; Ruan, H.; Fu, Y. Therapeutic Effect of Astaxanthin on Acetic Acid-Induced Gastric Ulcer in Rats. Yao Xue Xue Bao 2009, 44, 558–560. [Google Scholar] [PubMed]
- Andersen, L.P.; Holck, S.; Kupcinskas, L.; Kiudelis, G.; Jonaitis, L.; Janciauskas, D.; Permin, H.; Wadström, T. Gastric Inflammatory Markers and Interleukins in Patients with Functional Dyspepsia Treated with Astaxanthin. FEMS Immunol. Med. Microbiol. 2007, 50, 244–248. [Google Scholar] [CrossRef]
- Şehirli, Ö.; Tatlidede, E.; Yüksel, M.; Erzik, C.; Çetinel, S.; Yeǧen, B.Ç.; Şener, G. Antioxidant Effect of Alpha-Lipoic Acid against Ethanol-Induced Gastric Mucosal Erosion in Rats. Pharmacology 2008, 81, 173–180. [Google Scholar] [CrossRef]
- Shaaban, A.A.; Shaker, M.E.; Zalata, K.R.; El-Kashef, H.A.; Ibrahim, T.M. Modulation of Carbon Tetrachloride-Induced Hepatic Oxidative Stress, Injury and Fibrosis by Olmesartan and Omega-3. Chem. Biol. Interact. 2014, 207, 81–91. [Google Scholar] [CrossRef]
- Yorulmaz, E.; Yorulmaz, H.; Gökmen, E.S.; Altınay, S.; Küçük, S.H.; Zengi, O.; Çelik, D.S.; Şit, D. Therapeutic Effectiveness of Rectally Administered Fish Oil and Mesalazine in Trinitrobenzenesulfonic Acid-Induced Colitis. Biomed. Pharmacother. 2019, 118, 109247. [Google Scholar] [CrossRef] [PubMed]
- Lima Rocha, J.É.; Mendes Furtado, M.; Mello Neto, R.S.; da Silva Mendes, A.V.; Brito, A.K.d.S.; Sena de Almeida, J.O.C.; Rodrigues Queiroz, E.I.; de Sousa França, J.V.; Silva Primo, M.G.; Cunha Sales, A.L.d.C.; et al. Effects of Fish Oil Supplementation on Oxidative Stress Biomarkers and Liver Damage in Hypercholesterolemic Rats. Nutrients 2022, 14, 426. [Google Scholar] [CrossRef]
- Şahin, Y.; Alçiğir, M.E.; Şenol, A.; Özden, H.; Ekici, H.; Yildirim, E.; Çinar, M. Protective Effect of Krill Oil against Gentamicin-Induced Oxidative Stress-Mediated Nephrotoxicity in Rats. Kocatepe Vet. J. 2022, 15, 38–46. [Google Scholar] [CrossRef]
- Kölükçü, E.; Uluocak, N.; Unsal, V. Protective Effects of Krill Oil on Ischemic Reperfusion Injury in Experimental Model of Priapism. J. Surg. Med. 2019, 3, 371–376. [Google Scholar] [CrossRef]
- Burri, L.; Johnsen, L. Krill Products: An Overview of Animal Studies. Nutrients 2015, 7, 3300–3321. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Sun, T.; Li, Y.; Zhang, D.; Zhou, J.; Su, X. Modulation of the Gut Microbiota by Krill Oil in Mice Fed a High-Sugar High-Fat Diet. Front. Microbiol. 2017, 8, 905. [Google Scholar] [CrossRef] [PubMed]
- Da Boit, M.; Mastalurova, I.; Brazaite, G.; McGovern, N.; Thompson, K.; Gray, S.R. The Effect of Krill Oil Supplementation on Exercise Performance and Markers of Immune Function. PLoS ONE 2015, 10, e0139174. [Google Scholar] [CrossRef] [PubMed]
- de Arruda, L.L.M.; Ames, F.Q.; de Morais, D.R.; Grespan, R.; Gil, A.P.M.; Silva, M.A.R.C.P.; Visentainer, J.V.; Cuman, R.K.N.; Bersani-Amado, C.A. A Single Administration of Fish Oil Inhibits the Acute Inflammatory Response in Rats. Asian Pac. J. Trop. Med. 2017, 10, 765–772. [Google Scholar] [CrossRef]
- Peake, J.M.; Gobe, G.C.; Fassett, R.G.; Coombes, J.S. The Effects of Dietary Fish Oil on Inflammation, Fibrosis and Oxidative Stress Associated with Obstructive Renal Injury in Rats. Mol. Nutr. Food Res. 2011, 55, 400–410. [Google Scholar] [CrossRef]
- Murata, K.; Oyagi, A.; Takahira, D.; Tsuruma, K.; Shimazawa, M.; Ishibashi, T.; Hara, H. Protective Effects of Astaxanthin from Paracoccus Carotinifaciens on Murine Gastric Ulcer Models. Phytother. Res. 2012, 26, 1126–1132. [Google Scholar] [CrossRef]
- Olaizola, M.; Huntley, M. Recent Advances in Commercial Production of Astaxanthin from Microalgae. In Recent Advances in Marine Biotechnology; Science Publishers: Enfield, NH, USA, 2003; pp. 143–164. [Google Scholar]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Potter, D.W.; Tran, T.B. Apparent Rates of Glutathione Turnover in Rat Tissues. Toxicol. Appl. Pharmacol. 1993, 120, 186–192. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of Hepatic Glutathione Synthesis: Current Concepts and Controversies. FASEB J. 1999, 13, 1169–1183. [Google Scholar] [CrossRef]
- Huang, L.; Wu, W.; Huang, L.; Zhong, J.; Chen, L.; Wang, M.; Chen, H. Antarctic Krill (Euphausia superba) Oil Modulatory Effects on Ethanol-Induced Acute Injury of the Gastric Mucosa in Rats. Front. Nutr. 2022, 9, 1003627. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, M.H.; Kim, H. Anti-Oxidant and Anti-Inflammatory Effects of Astaxanthin on Gastrointestinal Diseases. Int. J. Mol. Sci. 2022, 23, 15471. [Google Scholar] [CrossRef] [PubMed]
- Gürler, E.B.; Özbeyli, D.; Buzcu, H.; Çam, M.E.; Yüksel, M. Astaxanthin and Coenzyme Q10 Are Not Synergistic against Oxidative Damage in Cerulein-Induced Acute Pancreatitis. J. Surg. Med. 2021, 5, 307–310. [Google Scholar] [CrossRef]
- Nishida, Y.; Miki, W.; Yamashita, E. Quenching Activities of Common Hydrophilic and Lipophilic Antioxidants against Singlet Oxygen Using Chemiluminescence Detection System Astaxanthin View Project Quenching Activities of Common Hydrophilic and Lipophilic Antioxidants against Singlet Oxygen Using Chemiluminescence Detection System. Carotenoid Sci. 2007, 11, 16–20. [Google Scholar] [CrossRef]
- Ekpe, L.; Inaku, K.; Ekpe, V. Antioxidant Effects of Astaxanthin in Various Diseases—A Review. J. Mol. Pathophysiol. 2018, 7, 1. [Google Scholar] [CrossRef]
- Budriesi, R.; Micucci, M.; Daglia, M.; Corazza, I.; Biotti, G.; Mattioli, L.B. Chemical Features and Biological Effects of Astaxanthin Extracted from Haematococcus pluvialis Flotow: Focus on Gastrointestinal System. Biol. Life Sci. Forum 2022, 12, 31. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Sen, A.; Yuksel, M.; Bulut, G.; Bitis, L.; Ercan, F.; Ozyilmaz-Yay, N.; Akbulut, O.; Cobanoğlu, H.; Ozkan, S.; Sener, G. Therapeutic Potential of Myrtus communis Subsp. communis Extract Against Acetic ACID-Induced Colonic Inflammation in Rats. J. Food Biochem. 2017, 41, e12297. [Google Scholar] [CrossRef]
- Vigerust, N.F.; Bjørndal, B.; Bohov, P.; Brattelid, T.; Svardal, A.; Berge, R.K. Krill Oil versus Fish Oil in Modulation of Inflammation and Lipid Metabolism in Mice Transgenic for TNF-α. Eur. J. Nutr. 2013, 52, 1315–1325. [Google Scholar] [CrossRef]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J. Enhanced Increase of Omega-3 Index in Healthy Individuals with Response to 4-Week n-3 Fatty Acid Supplementation from Krill Oil versus Fish Oil. Lipids Health Dis. 2013, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Reeves, M.S.; Farmer, M.; Griinari, M.; Berge, K.; Vik, H.; Hubacher, R.; Rains, T.M. Krill Oil Supplementation Increases Plasma Concentrations of Eicosapentaenoic and Docosahexaenoic Acids in Overweight and Obese Men and Women. Nutr. Res. 2009, 29, 609–615. [Google Scholar] [CrossRef]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, J.I. Metabolic Effects of Krill Oil Are Essentially Similar to Those of Fish Oil but at Lower Dose of EPA and DHA, in Healthy Volunteers. Lipids 2011, 46, 37–46. [Google Scholar] [CrossRef]
- Tillander, V.; Bjørndal, B.; Burri, L.; Bohov, P.; Skorve, J.; Berge, R.K.; Alexson, S.E. Fish Oil and Krill Oil Supplementations Differentially Regulate Lipid Catabolic and Synthetic Pathways in Mice. Nutr. Metab. 2014, 11, 20. [Google Scholar] [CrossRef]
- Sistilli, G.; Kalendova, V.; Cajka, T.; Irodenko, I.; Bardova, K.; Oseeva, M.; Zacek, P.; Kroupova, P.; Horakova, O.; Lackner, K.; et al. Krill Oil Supplementation Reduces Exacerbated Hepatic Steatosis Induced by Thermoneutral Housing in Mice with Diet-Induced Obesity. Nutrients 2021, 13, 437. [Google Scholar] [CrossRef]
- Hwang, S.M.; Kim, Y.U.; Kim, J.K.; Chun, Y.S.; Kwon, Y.S.; Ku, S.K.; Song, C.H. Preventive and Therapeutic Effects of Krill Oil on Obesity and Obesity-Induced Metabolic Syndromes in High-Fat Diet-Fed Mice. Mar. Drugs 2022, 20, 483. [Google Scholar] [CrossRef] [PubMed]
| Lesion Size | Scoring |
|---|---|
| No damage | 0 |
| Presence of blood in the lumen | 1 |
| Point-type erosions; | 2 |
| 1–5 small erosions (<2 mm); | 3 |
| >5 small erosions; | 4 |
| Large erosions (>2 mm); | 5 |
| >3 large erosions | 6 |
| Gastric Tissue CL Values | C | FO + C | KO + C | ASX + C | U | FO + U | KO + U | ASX + U |
|---|---|---|---|---|---|---|---|---|
| Luminol CL (rlu/mg) | 74.1 ± 2.0 | 53.8 ± 2.4 | 64.9 ± 5.6 | 28.2 ± 1.3 | 122.3 ± 4.2 *** | 102.9 ± 2.8 ++ | 94.2 ± 3.2 +++ | 33.9 ± 3.1 +++ |
| Lucigenin CL (rlu/mg) | 54.3 ± 1.0 | 55.1 ± 6.5 | 52.3 ± 4.6 | 17.3 ± 1.3 | 140.3 ± 14.0 ** | 91.5 ± 4.7 +++ | 99.0 ± 26.9 +++ | 31.1 ± 1.0 +++ |
| NO Release (rlu/mg) | 62.4 ± 2.4 | 51.9 ± 6.3 | 49.6 ± 2.6 | 24.3 ± 2.3 | 147.5 ± 10.6 *** | 97.6 ± 3.7 +++ | 89.1 ± 5.1 +++ | 24.6 ± 3.3 +++ |
| Peroxynitrite Release (rlu/mg) | 60.1 ± 1.3 | 36.2 ± 1.2 | 57.6 ± 4.7 | 17.8 ± 0.3 | 125.4 ± 4.7 *** | 97.8 ± 1.6 +++ | 90.5 ± 4.5 +++ | 21.6 ± 1.0 +++ |
| Liver Tissue CL Values | C | FO + C | KO + C | ASX + C | U | FO + U | KO + U | ASX + U |
|---|---|---|---|---|---|---|---|---|
| Luminol CL (rlu/mg) | 20.4 ± 1.4 | 19.7 ± 1.0 | 18.0 ± 0.7 | 20.3 ± 1.0 | 23.6 ± 2.9 | 17.3 ± 1.1 | 18.6 ± 1.7 | 19.3 ± 2.5 |
| Lucigenin CL (rlu/mg) | 19.5 ± 1.6 | 20.0 ± 3.2 | 19.3 ± 1.1 | 17.9 ± 0.9 | 21.6 ± 0.9 | 17.7 ± 0.7 | 21.3 ± 1.0 | 20.3 ± 0.8 |
| NO Release (rlu/mg) | 15.4 ± 0.6 | 17.6 ± 0.6 | 17.8 ± 1.1 | 14.6 ± 0.5 | 22.1 ± 1.2 *** | 18.9 ± 0.8 | 17.5 ± 1.0 ++ | 14.6 ± 0.3 +++ |
| Peroxynitrite Release (rlu/mg) | 15.8 ± 0.5 | 13.4 ± 0.5 | 17.8 ± 0.9 | 12.8 ± 0.6 | 19.8 ± 1.0 ** | 15.3 ± 0.9 +++ | 16.1 ± 0.5 + | 14.7 ± 0.5 +++ |
| C + U | FO | KO | ASX | |
|---|---|---|---|---|
| Lipid Parameters | ||||
| Total Cholesterol (mg/dL) | 54.8 ± 4.8 | 37.7 ± 2.2 *** | 49.6 ± 2.4 | 52.3 ± 1.4 |
| Triglyceride (mg/dL) | 55.0 ± 4.4 | 40.1 ± 3.2 | 49.6 ± 5.4 | 59.9 ± 5.0 |
| LDL cholesterol (mg/dL) | 10.7 ± 1.2 | 6.2 ±0.5 *** | 9.4 ± 0.7 | 10.6 ± 0.6 |
| HDL cholesterol (mg/dL) | 42.9 ± 4.1 | 28.9 ± 1.9 ** | 37.6 ± 1.9 | 40.3 ± 1.5 |
| Liver Function Tests | ||||
| ALT (U/L) | 40.2 ± 3.0 | 44.5 ± 3.3 | 41.8 ± 3.5 | 38.8 ± 2.3 |
| AST (U/L) | 88.2 ± 9.5 | 82.5 ± 3.3 | 119.2 ± 7.0 * | 94.8 ± 8.9 |
| ALP (U/L) | 89.0 ± 5.0 | 90.9 ± 3.9 | 104.6 ± 6.7 | 62.7 ± 2.0 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarıyer, E.T.; Baş, M.; Çolak, H.; Özkan Yenal, N.; Unay Demirel, Ö.; Yüksel, M. Comparison of Dietary Supplementation with Krill Oil, Fish Oil, and Astaxanthin on an Experimental Ethanol-Induced Gastric Ulcer Model: A Biochemical and Histological Study. Nutrients 2024, 16, 3426. https://doi.org/10.3390/nu16203426
Sarıyer ET, Baş M, Çolak H, Özkan Yenal N, Unay Demirel Ö, Yüksel M. Comparison of Dietary Supplementation with Krill Oil, Fish Oil, and Astaxanthin on an Experimental Ethanol-Induced Gastric Ulcer Model: A Biochemical and Histological Study. Nutrients. 2024; 16(20):3426. https://doi.org/10.3390/nu16203426
Chicago/Turabian StyleSarıyer, Esra Tansu, Murat Baş, Hatice Çolak, Naziye Özkan Yenal, Özlem Unay Demirel, and Meral Yüksel. 2024. "Comparison of Dietary Supplementation with Krill Oil, Fish Oil, and Astaxanthin on an Experimental Ethanol-Induced Gastric Ulcer Model: A Biochemical and Histological Study" Nutrients 16, no. 20: 3426. https://doi.org/10.3390/nu16203426






