Global Leadership Initiative on Malnutrition Criteria and Immunonutritional Status Predict Chemoadherence and Survival in Stage II/III Gastric Cancer Treated with XELOX Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Chemotherapy Adherence
2.3. Body Composition Assessment
2.4. GLIM Criteria
Other Immunonutritional Indices
2.5. Statistical Analysis
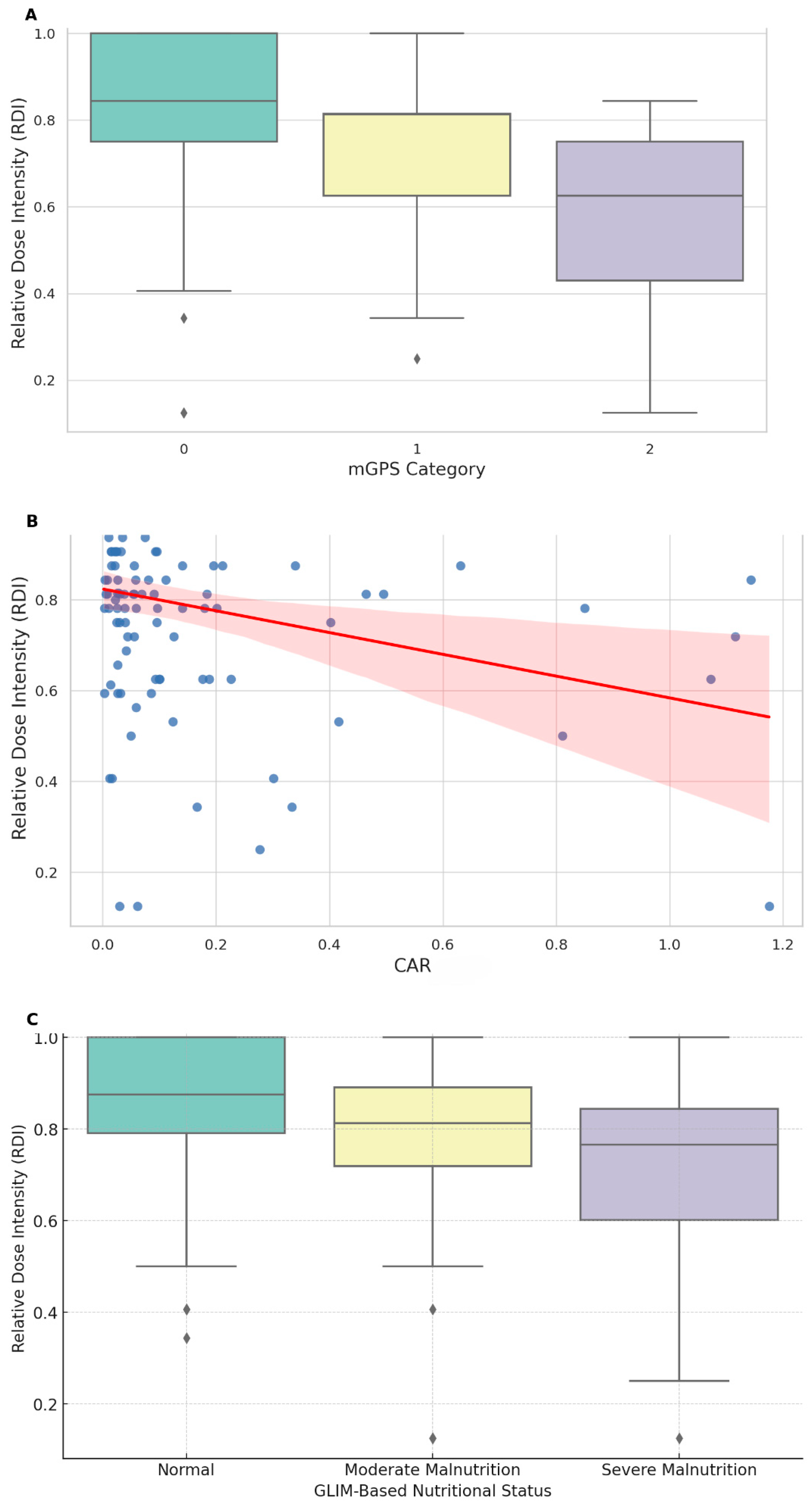
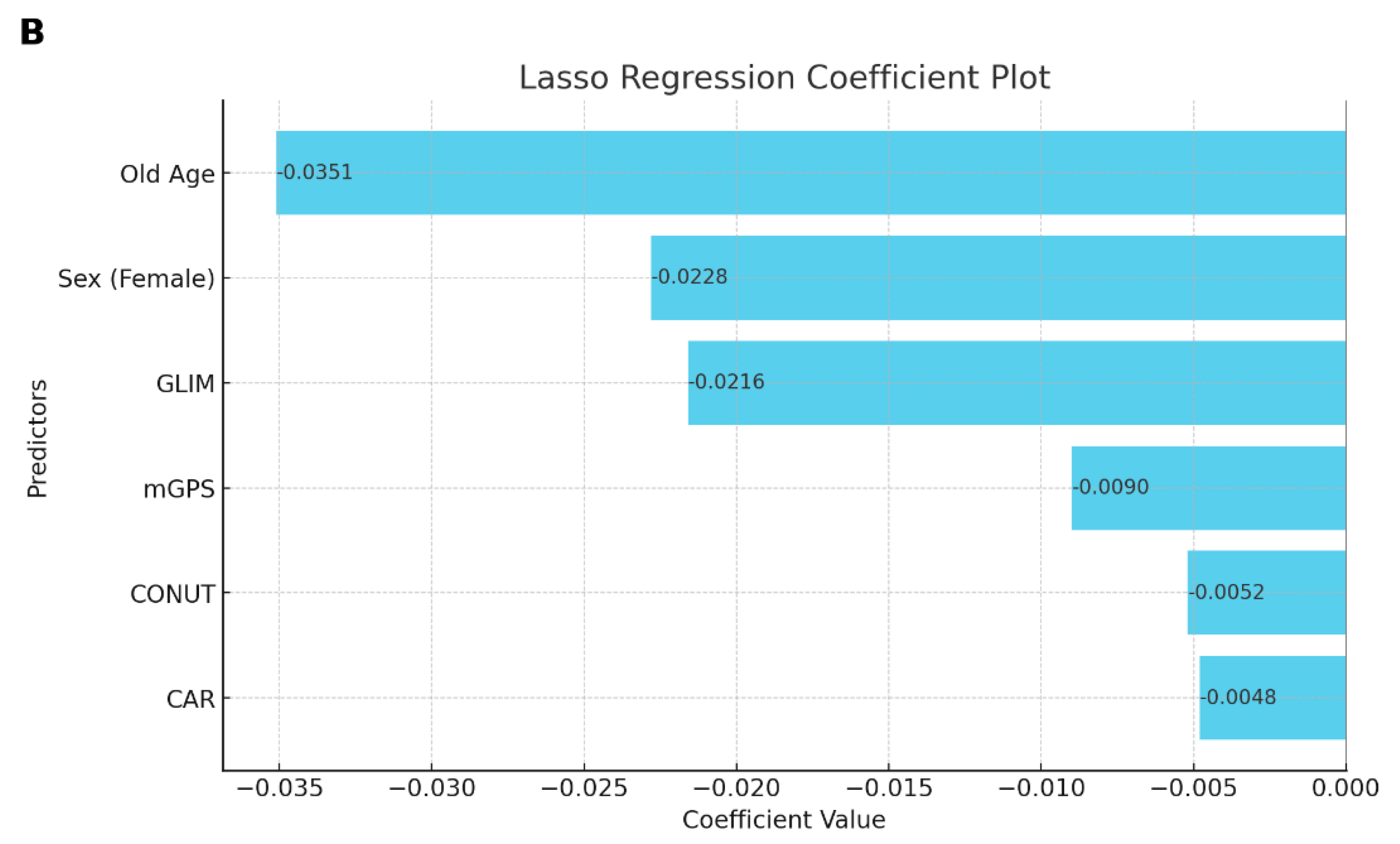
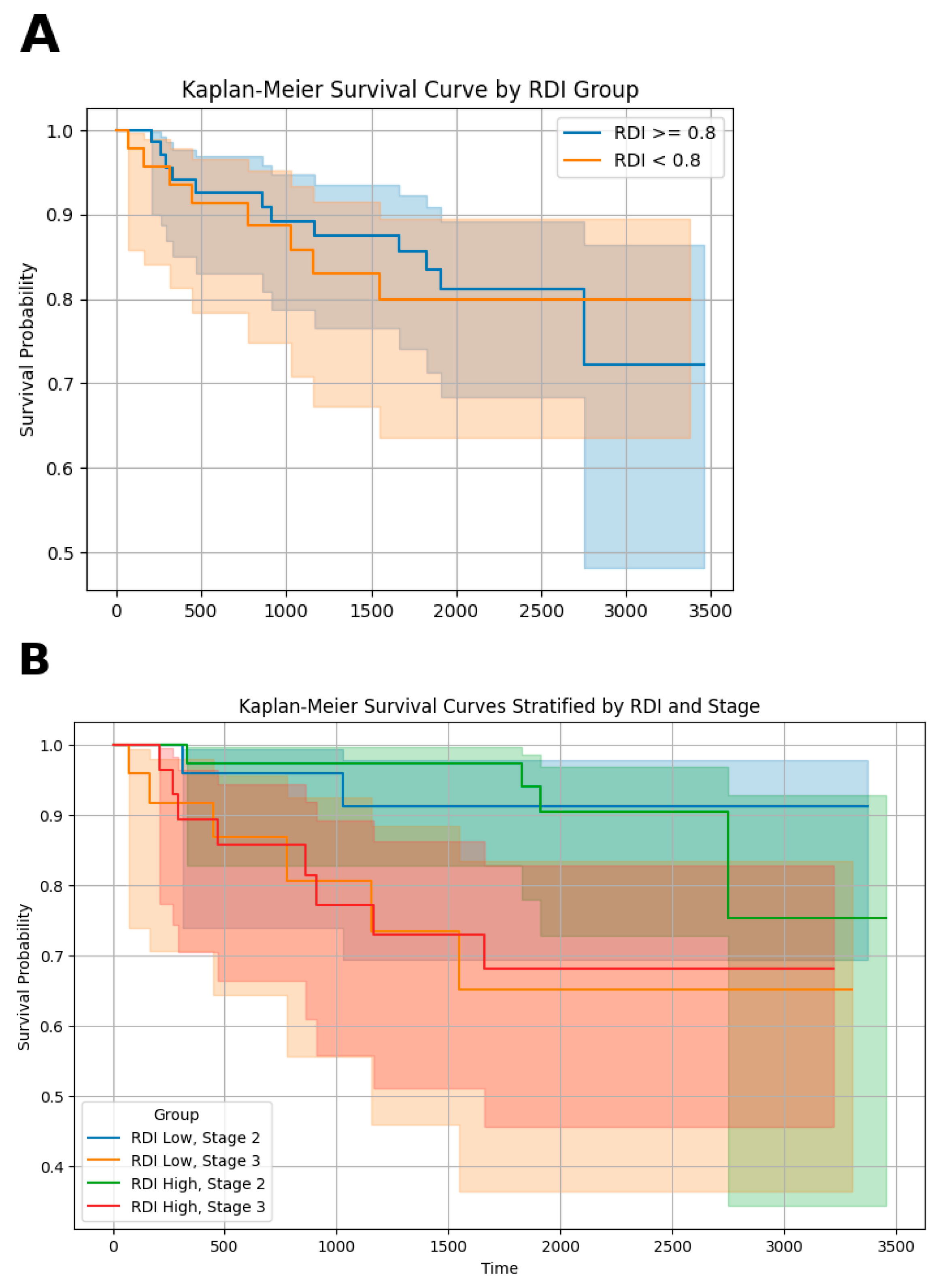
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, I.H.; Kang, S.J.; Choi, M.; Kim, B.H.; Eom, B.W.; Kim, B.J.; Min, B.H.; Choi, C.I.; Shin, C.M.; et al. Korean Practice Guidelines for Gastric Cancer 2022: An Evidence-based, Multidisciplinary Approach. J. Gastric Cancer 2023, 23, 3–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zheng, Z.F.; Wang, W.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Cao, L.L.; Lin, M.; Tu, R.H.; et al. A novel TNM staging system for gastric cancer based on the metro-ticket paradigm: A comparative study with the AJCC-TNM staging system. Gastric Cancer 2019, 22, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Longo, D.L.; Duffey, P.L.; DeVita, V.T., Jr.; Wesley, M.N.; Hubbard, S.M.; Young, R.C. The calculation of actual or received dose intensity: A comparison of published methods. J. Clin. Oncol. 1991, 9, 2042–2051. [Google Scholar] [CrossRef]
- Lalami, Y.; Klastersky, J. Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: An overview about well-established and recently emerging clinical data. Crit. Rev. Oncol. Hematol. 2017, 120, 163–179. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Chen, W.Y.; Lee, V.; Albers, K.B.; Prado, C.M.; Alexeeff, S.; Xiao, J.; Shachar, S.S.; Caan, B.J. Body Composition, Adherence to Anthracycline and Taxane-Based Chemotherapy, and Survival after Nonmetastatic Breast Cancer. JAMA Oncol. 2020, 6, 264–270. [Google Scholar] [CrossRef]
- Bozzetti, F.; Mariani, L.; Lo Vullo, S.; Group, S.W.; Amerio, M.L.; Biffi, R.; Caccialanza, G.; Capuano, G.; Correja, I.; Cozzaglio, L.; et al. The nutritional risk in oncology: A study of 1,453 cancer outpatients. Support. Care Cancer 2012, 20, 1919–1928. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hutterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Aspinall, S.L.; Good, C.B.; Zhao, X.; Cunningham, F.E.; Heron, B.B.; Geraci, M.; Passero, V.; Stone, R.A.; Smith, K.J.; Rogers, R.; et al. Adjuvant chemotherapy for stage III colon cancer: Relative dose intensity and survival among veterans. BMC Cancer 2015, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Camblor-Alvarez, M.; Ocon-Breton, M.J.; Luengo-Perez, L.M.; Viruzuela, J.A.; Sendros-Marono, M.J.; Cervera-Peris, M.; Grande, E.; Alvarez-Hernandez, J.; Jimenez-Fonseca, P. Nutritional support and parenteral nutrition in the oncological patient: An expert group consensus report. Nutr. Hosp. 2018, 35, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, C.; Martin, L.; Gramlich, L.; Scrimger, R.; Jha, N.; Debenham, B.; Chua, N.; Walker, J.; Baracos, V.E. Prevalence and prognostic significance of malnutrition in patients with cancers of the head and neck. Clin. Nutr. 2020, 39, 901–909. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Y.; Fang, Y. Nutritional status and related factors of patients with advanced gastrointestinal cancer. Br. J. Nutr. 2014, 111, 1239–1244. [Google Scholar] [CrossRef]
- Lee, H.H.; Park, J.M.; Song, K.Y.; Choi, M.G.; Park, C.H. Survival impact of postoperative body mass index in gastric cancer patients undergoing gastrectomy. Eur. J. Cancer 2016, 52, 129–137. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Liu, J.; Kong, P.; Chen, S.; Zhan, Y.; Xu, D. Preoperative C-Reactive Protein/Albumin Ratio Predicts Prognosis of Patients after Curative Resection for Gastric Cancer. Transl. Oncol. 2015, 8, 339–345. [Google Scholar] [CrossRef]
- Iwai, N.; Okuda, T.; Sakagami, J.; Harada, T.; Ohara, T.; Taniguchi, M.; Sakai, H.; Oka, K.; Hara, T.; Tsuji, T.; et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci. Rep. 2020, 10, 18758. [Google Scholar] [CrossRef]
- Takamizawa, Y.; Shida, D.; Boku, N.; Nakamura, Y.; Ahiko, Y.; Yoshida, T.; Tanabe, T.; Takashima, A.; Kanemitsu, Y. Nutritional and inflammatory measures predict survival of patients with stage IV colorectal cancer. BMC Cancer 2020, 20, 1092. [Google Scholar] [CrossRef]
- Nogueiro, J.; Santos-Sousa, H.; Pereira, A.; Devezas, V.; Fernandes, C.; Sousa, F.; Fonseca, T.; Barbosa, E.; Barbosa, J.A. The impact of the prognostic nutritional index (PNI) in gastric cancer. Langenbecks Arch. Surg. 2022, 407, 2703–2714. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Kurokawa, Y.; Yamamoto, K.; Hirota, M.; Kawabata, R.; Mikami, J.; Masuzawa, T.; Takiguchi, S.; Mori, M.; Doki, Y. Risk Factors for Poor Compliance with Adjuvant S-1 Chemotherapy for Gastric Cancer: A Multicenter Retrospective Study. Ann. Surg. Oncol. 2017, 24, 2639–2645. [Google Scholar] [CrossRef] [PubMed]
- Breadner, D.; Loree, J.M.; Cheung, W.Y.; Gipson, M.; Lakkunarajah, S.; Mulder, K.E.; Spartlin, J.L.; Kong, S.; Ding, P.Q.; Gill, S.; et al. The influence of adjuvant chemotherapy dose intensity on overall survival in resected colon cancer: A multicentered retrospective analysis. BMC Cancer 2022, 22, 1119. [Google Scholar] [CrossRef]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef]
- Touko, A.; Mboua, C.P.; Tohmuntain, P.M.; Perrot, A.B. Sexual vulnerability and HIV seroprevalence among the deaf and hearing impaired in Cameroon. J. Int. AIDS Soc. 2010, 13, 5. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, K.W.; Shin, Y.; Lee, J.; Ko, Y.; Kim, Y.J.; Lee, M.J.; Bae, S.J.; Park, S.W.; Choe, J.; et al. Reference Data and T-Scores of Lumbar Skeletal Muscle Area and Its Skeletal Muscle Indices Measured by CT Scan in a Healthy Korean Population. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 265–271. [Google Scholar] [CrossRef]
- Sakurai, K.; Ohira, M.; Tamura, T.; Toyokawa, T.; Amano, R.; Kubo, N.; Tanaka, H.; Muguruma, K.; Yashiro, M.; Maeda, K.; et al. Predictive Potential of Preoperative Nutritional Status in Long-Term Outcome Projections for Patients with Gastric Cancer. Ann. Surg. Oncol. 2016, 23, 525–533. [Google Scholar] [CrossRef]
- Garcia-Malpartida, K.; Aragon-Valera, C.; Botella-Romero, F.; Ocon-Breton, M.J.; Lopez-Gomez, J.J. Effects of Immunonutrition on Cancer Patients Undergoing Surgery: A Scoping Review. Nutrients 2023, 15, 1776. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Lee, S.; Kang, D.H.; Ahn, T.S.; Kim, S.S.; Yun, J.H.; Kim, H.J.; Seo, S.H.; Kim, T.W.; Kong, H.J.; Baek, M.J. The Impact of Pre-Chemotherapy Body Composition and Immunonutritional Markers on Chemotherapy Adherence in Stage III Colorectal Cancer Patients. J. Clin. Med. 2023, 12, 1423. [Google Scholar] [CrossRef]
- Fonseca-Nunes, A.; Agudo, A.; Aranda, N.; Arija, V.; Cross, A.J.; Molina, E.; Sanchez, M.J.; Bueno-de-Mesquita, H.B.; Siersema, P.; Weiderpass, E.; et al. Body iron status and gastric cancer risk in the EURGAST study. Int. J. Cancer 2015, 137, 2904–2914. [Google Scholar] [CrossRef] [PubMed]
- Engle, J.A.; Traynor, A.M.; Campbell, T.C.; Wisinski, K.B.; LoConte, N.; Liu, G.; Wilding, G.; Kolesar, J.M. Assessment of adherence and relative dose intensity with oral chemotherapy in oncology clinical trials at an academic medical center. J. Oncol. Pharm. Pract. 2018, 24, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Ohashi, M.; Hiki, N.; Takahari, D.; Chin, K.; Yamaguchi, K.; Ida, S.; Kumagai, K.; Sano, T.; Nunobe, S. Facilitated completion of 1-year adjuvant S-1 monotherapy for pathological stage II or III gastric cancer by medical oncologists. Surg. Today 2020, 50, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Prado, C.M.; Sullivan, E.S.; Power, D.G.; Daly, L.E. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition 2019, 67–68, 110539. [Google Scholar] [CrossRef]
- Ramsden, K.; Laskin, J.; Ho, C. Adjuvant Chemotherapy in Resected Stage II Non-small Cell Lung Cancer: Evaluating the Impact of Dose Intensity and Time to Treatment. Clin. Oncol. (R. Coll. Radiol.) 2015, 27, 394–400. [Google Scholar] [CrossRef]
- Nielson, C.M.; Bylsma, L.C.; Fryzek, J.P.; Saad, H.A.; Crawford, J. Relative Dose Intensity of Chemotherapy and Survival in Patients with Advanced Stage Solid Tumor Cancer: A Systematic Review and Meta-Analysis. Oncologist 2021, 26, e1609–e1618. [Google Scholar] [CrossRef]
- Fujita, S.; Sakuramoto, S.; Matsui, K.; Ebara, G.; Nishibeppu, K.; Oya, S.; Fujihata, S.; Lee, S.; Miyawaki, Y.; Sugita, H.; et al. Relative dose intensity and 1-year psoas muscle index reduction rate as prognostic factors in gastric cancer patients with postoperative adjuvant chemotherapy. Int. J. Clin. Oncol. 2023, 28, 110–120. [Google Scholar] [CrossRef]
- Yu, Q.; Li, K.Z.; Fu, Y.J.; Tang, Y.; Liang, X.Q.; Liang, Z.Q.; Bai, J.H. Clinical significance and prognostic value of C-reactive protein/albumin ratio in gastric cancer. Ann. Surg. Treat. Res. 2021, 100, 338–346. [Google Scholar] [CrossRef]
- Aoyama, T.; Komori, K.; Nakazano, M.; Hara, K.; Tamagawa, H.; Kazama, K.; Hashimoto, I.; Yamada, T.; Maezawa, Y.; Segami, K.; et al. The Clinical Influence of the CONUT Score on Survival of Patients with Gastric Cancer Receiving Curative Treatment. In Vivo 2022, 36, 942–948. [Google Scholar] [CrossRef]
- Hacker, U.T.; Hasenclever, D.; Baber, R.; Linder, N.; Busse, H.; Obermannova, R.; Zdrazilova-Dubska, L.; Valik, D.; Lordick, F. Modified Glasgow prognostic score (mGPS) is correlated with sarcopenia and dominates the prognostic role of baseline body composition parameters in advanced gastric and esophagogastric junction cancer patients undergoing first-line treatment from the phase III EXPAND trial. Ann. Oncol. 2022, 33, 685–692. [Google Scholar] [CrossRef]
- Zheng, H.L.; Lin, J.; Shen, L.L.; Yang, H.B.; Xu, B.B.; Xue, Z.; Wu, D.; Huang, J.B.; Lin, G.S.; Zheng, C.H.; et al. The GLIM criteria as an effective tool for survival prediction in gastric cancer patients. Eur. J. Surg. Oncol. 2023, 49, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Song, G.J.; Ahn, H.; Son, M.W.; Yun, J.H.; Lee, M.S.; Lee, S.M. Adipose Tissue Quantification Improves the Prognostic Value of GLIM Criteria in Advanced Gastric Cancer Patients. Nutrients 2024, 16, 728. [Google Scholar] [CrossRef] [PubMed]
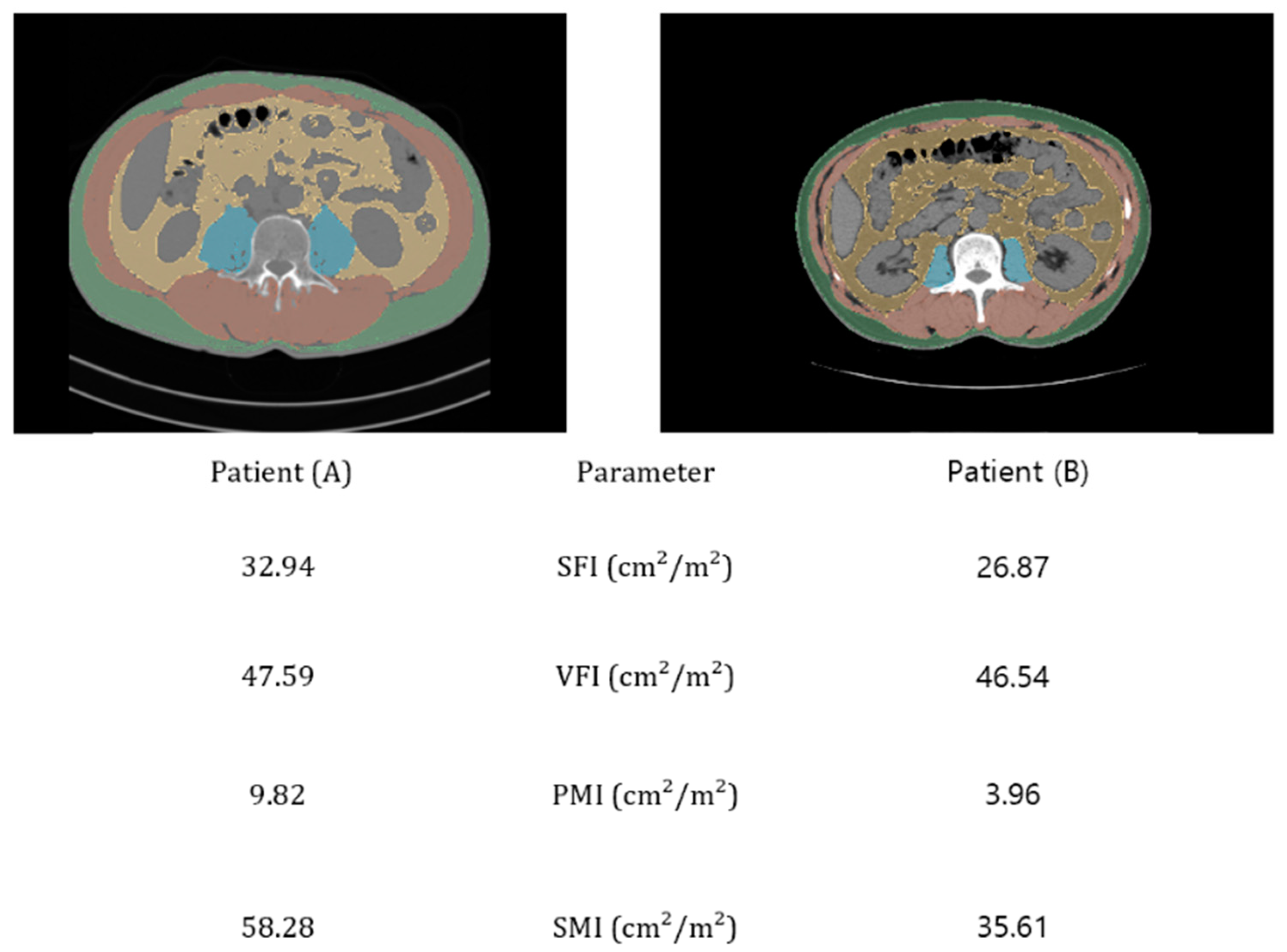


| Variable | No Malnutrition | Moderate | Severe | Total |
|---|---|---|---|---|
| (N = 51) | (N = 43) | (N = 22) | (N = 116) | |
| Age | 58.43 ± 8.39 | 58.98 ± 11.85 | 62.23 ± 14.17 | 59.35 ± 10.98 |
| BMI | 25.21 ± 2.96 | 23.12 ± 2.57 | 20.67 ± 2.75 | 23.57 ± 3.24 |
| Sex | ||||
| Female | 3 (5.89%) | 12 (27.91%) | 13 (59.09%) | 28 (24.14%) |
| Male | 48 (94.11%) | 31 (72.09%) | 9 (40.91%) | 88 (75.86%) |
| TTAC(days) | 37.61 ± 9.78 | 39.05 ± 6.07 | 39.05 ± 10.16 | 38.41 ± 8.63 |
| WIC(kg) | 63.90 ± 10.33 | 56.49 ± 6.84 | 49.59 ± 8.04 | 58.44 ± 10.24 |
| Number of retrived lymph nodes | 39.58 ± 13.06 | |||
| Surgical approach | ||||
| Open | 48 (90.56%) | 39 (90.69%) | 20 (90.90%) | 105 (90.52%) |
| Laparoscopy | 5 (9.54%) | 4 (9.31%) | 2 (9.10%) | 11 (9.48%) |
| Extent of resection | ||||
| Distal gastrectomy | 38 (74.51%) | 28 (65.21%) | 18 (81.82%) | 84 (72.41%) |
| Total gastrectomy | 13 (25.49%) | 13 (30.23%) | 4 (18.18%) | 30 (25.86%) |
| Proximal gastrectomy | 2(4.65%) | 2 (1.72%) | ||
| T stage | ||||
| T1 | 5 (9.8%) | 3 (6.98%) | 1 (4.55%) | 9 (7.76%) |
| T2 | 10 (19.61%) | 5 (11.63%) | 4 (18.18%) | 19 (16.38%) |
| T3 | 23 (45.1%) | 22 (51.16%) | 10 (45.45%) | 55 (47.41%) |
| T4 | 13 (25.49%) | 13 (30.24%) | 7 (31.82%) | 33 (28.45%) |
| N_stage | ||||
| N0 | 11 (21.57%) | 10 (23.26%) | 3 (13.64%) | 24 (20.69%) |
| N1 | 13 (25.49%) | 13 (30.23%) | 6 (27.27%) | 32 (27.59%) |
| N2 | 12 (23.53%) | 9 (20.93%) | 4 (18.18%) | 25 (21.55%) |
| N3 | 15 (29.41%) | 11 (25.58%) | 9 (40.90%) | 35 (30.17%) |
| AJCC Stage | ||||
| II | 34 (64.15%) | 24 (55.81%) | 8 (36.37%) | 64 (55.17%) |
| III | 19 (35.85%) | 19 (44.19%) | 14 (63.63%) | 52 (44.83%) |
| Lymphatic invasion | ||||
| Not identified | 12 (23.53%) | 16 (37.21%) | 5 (22.73%) | 33 (28.45%) |
| present | 39 (76.47%) | 27 (62.79%) | 17 (77.27%) | 83 (71.55%) |
| Venous invasion | ||||
| Not identified | 42 (82.35%) | 35 (81.40%) | 18 (81.82%) | 95 (81.90%) |
| present | 9 (17.65%) | 8 (18.60%) | 4 (18.18%) | 21 (18.10%) |
| Perineural invasion | ||||
| Not identified | 23 (45.10%) | 18 (41.86%) | 11 (50.00%) | 52 (44.83%) |
| present | 28 (54.90%) | 25 (58.14%) | 11 (50.00%) | 64 (55.17%) |
| 30-day postoperative complications | ||||
| No | 50 | 43 | 22 | 115 (99.14%) |
| Yes | 1 | 0 | 0 | 1 (0.86%) |
| Reason for Intervention (CTCAE Version 5.0 Terminology) | No Malnutrion (N = 30) | Moderate (N = 33) | Severe (N = 21) | Total (N = 84) |
|---|---|---|---|---|
| Blood and lymphatic system disorders | 1 | 12 | 8 | 21 |
| Nausea and vomiting | 15 | 6 | 3 | 24 |
| Peripheral sensory neuropathy | 4 | 4 | 2 | 10 |
| Diarrhea | 2 | 3 | 1 | 6 |
| Palmar–plantar erythrodysesthesia syndrome | 1 | 2 | 0 | 3 |
| Hepatobiliary disorders | 0 | 0 | 1 | 1 |
| Acute kidney injury | 1 | 0 | 0 | 1 |
| General physical health deterioration | 6 | 6 | 6 | 18 |
| Variable | No Malnutrition | Moderate | Severe | Total or Mean | RDI |
|---|---|---|---|---|---|
| (N = 51) | (N = 43) | (N = 22) | (N = 116) | ||
| SFA (cm2) | 121.33 ± 59.84 | 115.57 ± 59.92 | 104.98 ± 46.81 | 116.10 ± 57.48 | |
| VFA (cm2) | 136.38 ± 79.83 | 91.44 ± 56.05 | 63.17 ± 49.66 | 105.84 ± 72.17 | |
| PMA (cm2) | 18.52 ± 4.58 | 14.14 ± 3.30 | 10.60 ± 4.66 | 15.40 ± 5.14 | |
| SMA (cm2) | 126.81 ± 19.23 | 105.01 ± 15.48 | 80.45 ± 17.99 | 125.33 ± 28.51 | |
| VFI (cm2/m2) | 49.38 ± 27.97 | 34.36 ± 21.07 | 24.94 ± 19.95 | 39.18 ± 25.86 | |
| SFI (cm2/m2) | 44.24 ± 20.50 | 44.26 ± 25.67 | 41.46 ± 19.68 | 43.72 ± 22.26 | |
| PMI (cm2/m2) | 6.79 ± 1.46 | 5.24 ± 1.06 | 4.07 ± 1.55 | 5.70 ± 1.70 | |
| SMI (cm2/m2) | 53.39 ± 6.25 | 44.23 ± 5.15 | 35.17 ± 6.17 | 46.54 ± 9.01 | |
| PNI | |||||
| ≥45 | 44 (86.27%) | 35 (81.40%) | 17 (77.27%) | 96 (82.75%) | 0.811 ± 0.182 |
| <45 | 7 (13.73%) | 35 (81.40%) | 5 (22.73%) | 20 (17.25%) | 0.687 ± 0.268 |
| NLR | 1.77 ± 0.87 | 2.05 ± 0.91 | 1.90 ± 0.77 | 1.90 ± 0.87 | |
| CAR | 0.10 ± 0.06 | 0.19 ± 0.10 | 0.14 ± 0.09 | 0.14 ± 0.24 | |
| CONUT score | |||||
| Normal (0–1) | 29 (56.86%) | 25 (58.14%) | 9 (40.91%) | 63 (54.32%) | 0.806 ± 0.201 |
| High (≥2) | 22 (43.14%) | 18 (41.86%) | 13 (59.09%) | 53 (45.68%) | 0.778 ± 0.199 |
| mGPS | |||||
| Low risk | 46 (90.2%) | 33 (76.74%) | 16 (72.73%) | 95 (81.90%) | 0.814 ± 0.188 |
| Intermediate risk | 5 (9.8%) | 7 (16.28%) | 5 (22.73%) | 17 (14.66%) | 0.710 ± 0.218 |
| High risk | 3 (6.98%) | 1 (4.55%) | 4 (3.45%) | 0.555 ± 0.314 | |
| GLIM criteria | |||||
| No malnutrition | 51 (43.97%) | 0.844 ± 0.173 | |||
| Moderate malnutrition | 43 (37.07%) | 0.771 ± 0.208 | |||
| Severe malnutrition | 22 (18.97%) | 0.702 ± 0.232 | |||
| History of weight loss | |||||
| Normal | 51 (100%) | 30 (69.76%) | 18 (81.81%) | 99 (85.34%) | |
| Moderate | 0 | 13 (30.24%) | 3 (13.63%) | 16 (13.79%) | |
| Severe | 0 | 0 | 1(4.55%) | 1 (0.86%) | |
| Low BMI | |||||
| Normal | 51 (100%) | 41 (95.34%) | 14 (63.63%) | 106 (91.38%) | |
| Moderate | 0 | 2 (4.66%) | 6 (27.27%) | 8 (6.90%) | |
| Severe | 0 | 0 | 2 (9.09%) | 2 (1.72%) | |
| Reduced muscle mass | |||||
| Normal | 51 (100%) | 8 (18.61%) | 1 (4.55%) | 60 (51.72%) | |
| Moderate | 0 | 35 (81.39%) | 0 | 35 (30.17%) | |
| Severe | 0 | 0 | 21 (95.45%) | 21 (18.10%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.H.; Song, G.J.; Son, M.W.; Lee, M.S. Global Leadership Initiative on Malnutrition Criteria and Immunonutritional Status Predict Chemoadherence and Survival in Stage II/III Gastric Cancer Treated with XELOX Chemotherapy. Nutrients 2024, 16, 3468. https://doi.org/10.3390/nu16203468
Yun JH, Song GJ, Son MW, Lee MS. Global Leadership Initiative on Malnutrition Criteria and Immunonutritional Status Predict Chemoadherence and Survival in Stage II/III Gastric Cancer Treated with XELOX Chemotherapy. Nutrients. 2024; 16(20):3468. https://doi.org/10.3390/nu16203468
Chicago/Turabian StyleYun, Jong Hyuk, Geum Jong Song, Myoung Won Son, and Moon Soo Lee. 2024. "Global Leadership Initiative on Malnutrition Criteria and Immunonutritional Status Predict Chemoadherence and Survival in Stage II/III Gastric Cancer Treated with XELOX Chemotherapy" Nutrients 16, no. 20: 3468. https://doi.org/10.3390/nu16203468
APA StyleYun, J. H., Song, G. J., Son, M. W., & Lee, M. S. (2024). Global Leadership Initiative on Malnutrition Criteria and Immunonutritional Status Predict Chemoadherence and Survival in Stage II/III Gastric Cancer Treated with XELOX Chemotherapy. Nutrients, 16(20), 3468. https://doi.org/10.3390/nu16203468






