Adiponectin Signaling Modulates Fat Taste Responsiveness in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Genotype and Phenotype of the Adipor1-Deficient Mice
2.3. Chemicals and Solutions
2.4. Taste Cell Isolation
2.5. Calcium Imaging
2.6. Brief-Access Taste Testing
2.7. Statistical Analysis
3. Results
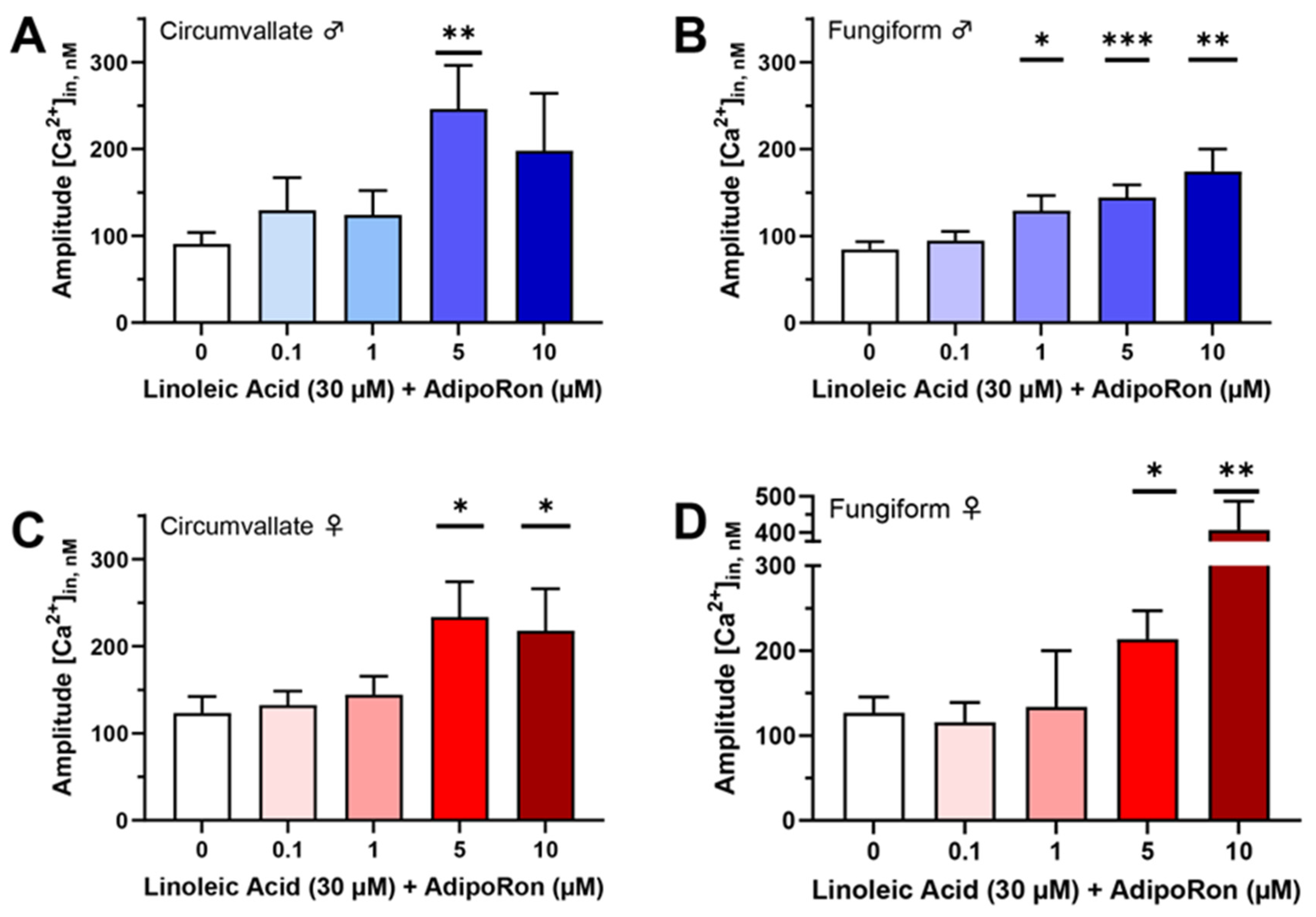
3.1. AdipoRon Enhances Cellular Responses to Fatty Acids in Mouse Taste Cells
3.2. The Effect of AdipoRon on LA-Induced Responses Is Mediated by AMPK Activation in Mouse Taste Cells
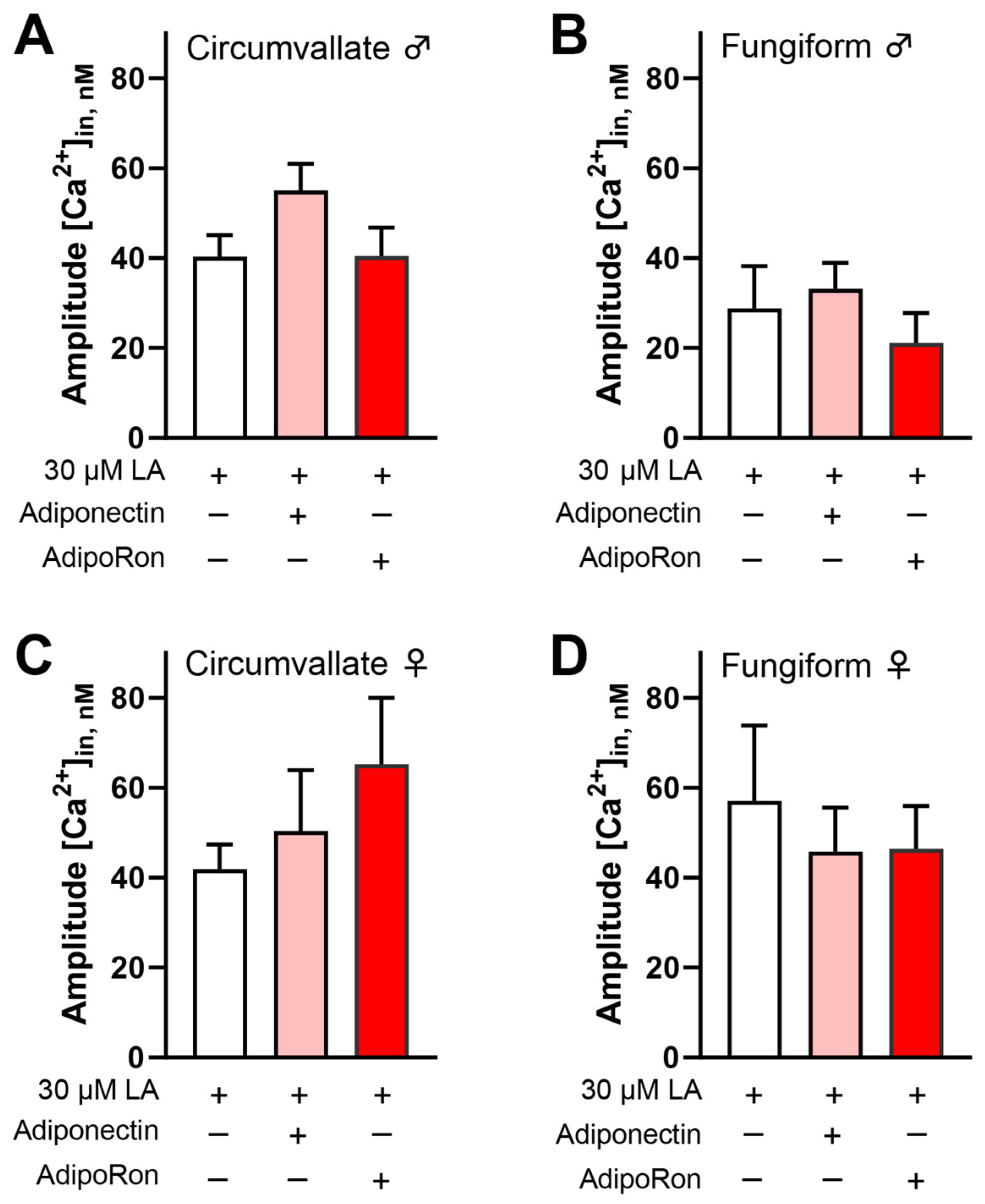
3.3. Adiponectin/AdipoRon Acts on CD36 to Increase LA-Induced Responses in Mouse Taste Cells
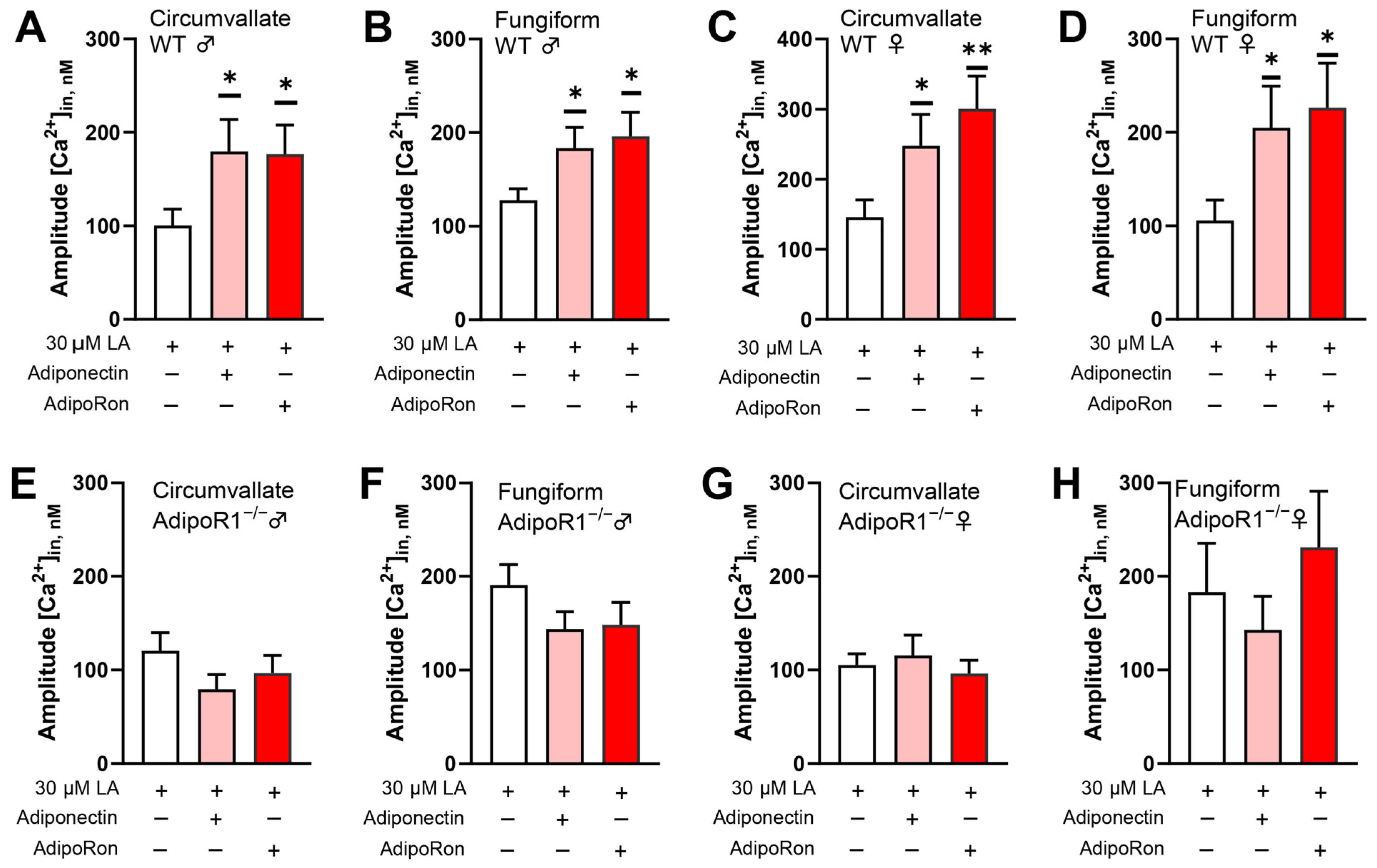
3.4. Adiponectin/AdipoRon Enhancement of LA-Induced Responses Is Dependent upon AdipoR1
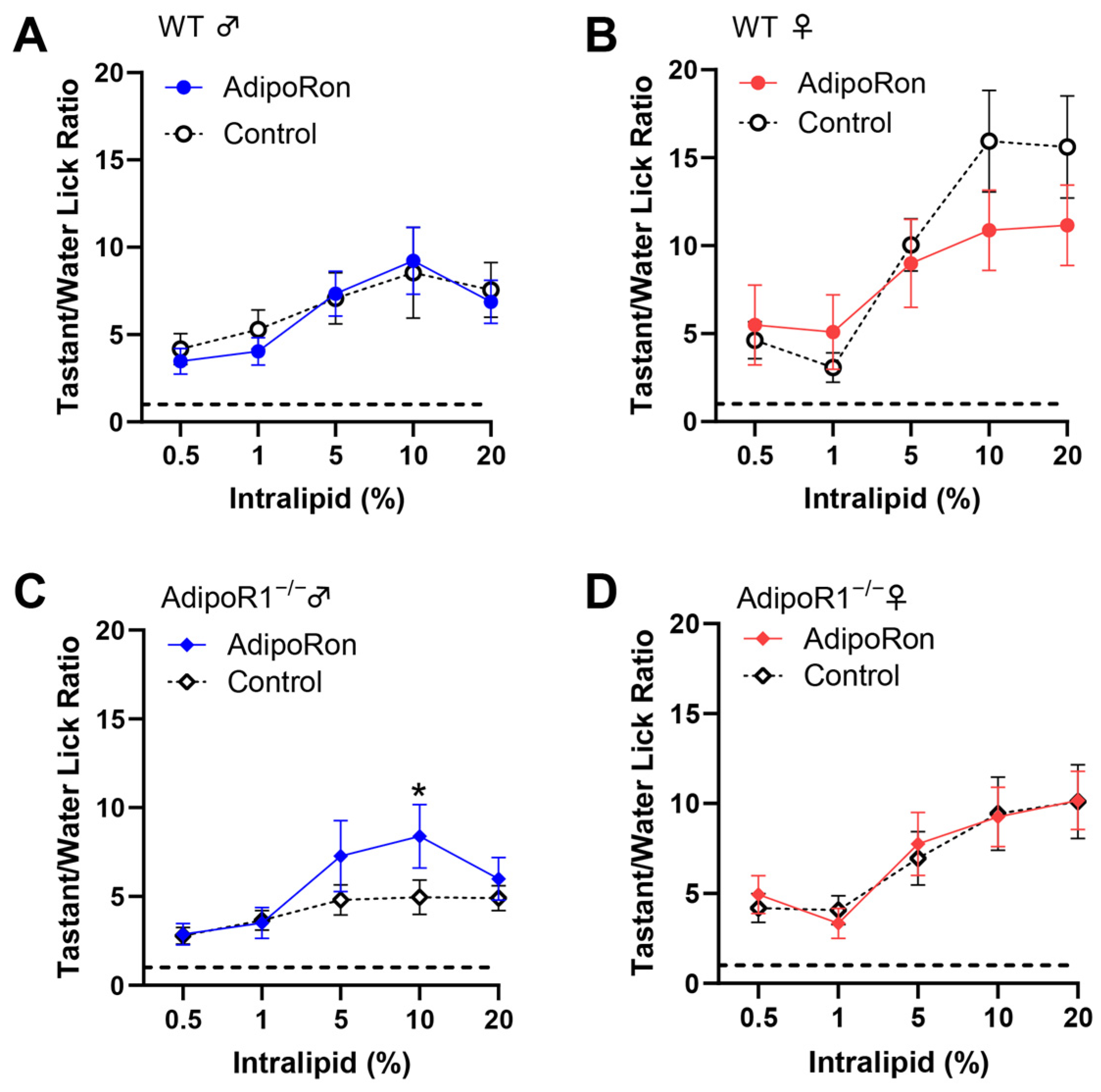
3.5. No Significant Effect of AdipoRon on Fat Taste Behavior in Mice
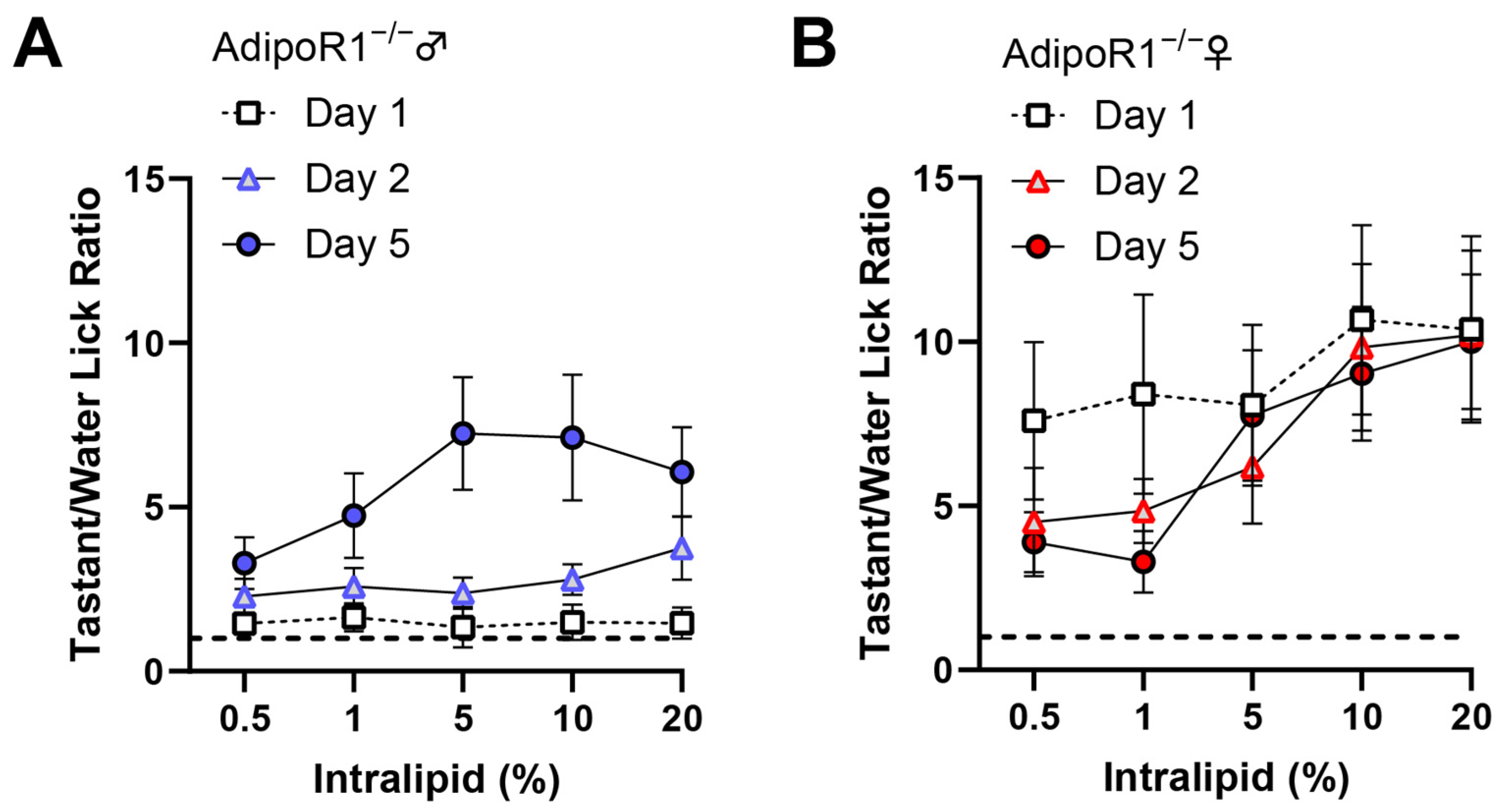
3.6. Reduction of Taste Behavioral Responsiveness to Intralipid in Adipor1−/− Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, Y.; Han, L. Adiponectin improves endothelial dysfunction caused by elevated FFAs levels, partially through cAMP-dependent pathway. Diabetes Res. Clin. Pract. 2012, 97, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, W.-Q.; Zhang, H.; Yang, X.; Fan, Q.; Christopher, T.A.; Lopez, B.L.; Tao, L.; Goldstein, B.J.; Gao, F.; et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E1703–E1708. [Google Scholar] [CrossRef]
- Xu, X.; Huang, X.; Zhang, L.; Huang, X.; Qin, Z.; Hua, F. Adiponectin protects obesity-related glomerulopathy by inhibiting ROS/NF-κB/NLRP3 inflammation pathway. BMC Nephrol. 2021, 22, 218. [Google Scholar] [CrossRef]
- Yi, W.; OuYang, Q. Adiponectin improves diabetic nephropathy by inhibiting necrotic apoptosis. Arch. Med. Sci. 2019, 15, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Yano, W.; Kubota, T.; Yamauchi, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Okamoto, S.; Shiuchi, T.; et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007, 6, 55–68. [Google Scholar] [CrossRef]
- Katsiougiannis, S.; Kapsogeorgou, E.K.; Manoussakis, M.N.; Skopouli, F.N. Salivary gland epithelial cells: A new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2006, 54, 2295–2299. [Google Scholar] [CrossRef]
- Toda, M.; Tsukinoki, R.; Morimoto, K. Measurement of salivary adiponectin levels. Acta Diabetol. 2007, 44, 20–22. [Google Scholar] [CrossRef]
- Akuailou, E.-N.; Vijayagopal, P.; Imrhan, V.; Prasad, C. Measurement and validation of the nature of salivary adiponectin. Acta Diabetol. 2013, 50, 727–730. [Google Scholar] [CrossRef]
- Nigro, E.; Piombino, P.; Scudiero, O.; Monaco, M.; Schettino, P.; Chambery, A.; Daniele, A. Evaluation of salivary adiponectin profile in obese patients. Peptides 2015, 63, 150–155. [Google Scholar] [CrossRef]
- Crosson, S.M.; Marques, A.; Dib, P.; Dotson, C.D.; Munger, S.D.; Zolotukhin, S. Taste receptor cells in mice express receptors for the hormone adiponectin. Chem. Senses 2019, 44, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Liu, Y.; Rudeski-Rohr, T.; Dahir, N.; Calder, A.; Gilbertson, T.A. Adiponectin enhances fatty acid signaling in human taste cells by increasing surface expression of CD36. Int. J. Mol. Sci. 2023, 24, 5801. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, J.; Shingai, T.; Kajii, Y.; Takahashi, Y.; Taguchi, Y.; Matsumoto, S. Leptin modulates the response to oleic acid in the pharynx. Neurosci. Lett. 2007, 423, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Khan, A.S.; Murtaza, B.; Hichami, A.; Khan, N.A. Tongue leptin decreases oro-sensory perception of dietary fatty acids. Nutrients 2021, 14, 197. [Google Scholar] [CrossRef] [PubMed]
- La Sala, M.S.; Hurtado, M.D.; Brown, A.R.; Bohórquez, D.V.; Liddle, R.A.; Herzog, H.; Zolotukhin, S.; Dotson, C.D. Modulation of taste responsiveness by the satiation hormone peptide YY. FASEB J. 2013, 27, 5022–5033. [Google Scholar] [CrossRef]
- Treesukosol, Y.; Moran, T.H. Administration of Exendin-4 but not CCK alters lick responses and trial initiation to sucrose and intralipid during brief-access tests. Chem. Senses 2022, 47, bjac004. [Google Scholar] [CrossRef]
- Martin, C.; Passilly-Degrace, P.; Chevrot, M.; Ancel, D.; Sparks, S.M.; Drucker, D.J.; Besnard, P. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: Putative involvement of GPR120 and impact on taste sensitivity. J. Lipid Res. 2012, 53, 2256–2265. [Google Scholar] [CrossRef]
- Cai, H.; Cong, W.-N.; Daimon, C.M.; Wang, R.; Tschöp, M.H.; Sévigny, J.; Martin, B.; Maudsley, S. Altered lipid and salt taste responsivity in ghrelin and GOAT null mice. PLoS ONE 2013, 8, e76553. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.N.; Yu, T.; Dahir, N.S.; Sun, Y.; Gilbertson, T.A. Ghrelin receptors enhance fat taste responsiveness in female mice. Nutrients 2021, 13, 1045. [Google Scholar] [CrossRef]
- Brissard, L.; Leemput, J.; Hichami, A.; Passilly-Degrace, P.; Maquart, G.; Demizieux, L.; Degrace, P.; Khan, N.A. Orosensory detection of dietary fatty acids is altered in CB1R−/− mice. Nutrients 2018, 10, 1347. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Bjursell, M.; Ahnmark, A.; Bohlooly-Y, M.; William-Olsson, L.; Rhedin, M.; Peng, X.-R.; Ploj, K.; Gerdin, A.-K.; Arnerup, G.; Elmgren, A.; et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 2007, 56, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Nio, Y.; Maki, T.; Kobayashi, M.; Takazawa, T.; Iwabu, M.; Okada-Iwabu, M.; Kawamoto, S.; Kubota, N.; Kubota, T.; et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007, 13, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Dahir, N.S.; Calder, A.N.; McKinley, B.J.; Liu, Y.; Gilbertson, T.A. Sex differences in fat taste responsiveness are modulated by estradiol. Am. J. Physiol.-Endocrinol. Metab. 2021, 320, E566–E580. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, H.; Dahir, N.S.; Calder, A.N.; Lin, F.; Gilbertson, T.A. GPR84 is essential for the taste of medium chain saturated fatty acids. J. Neurosci. 2021, 41, 5219–5228. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Iwata, S.; Inoue, M.; Ninomiya, Y. Fatty acid taste quality information via GPR120 in the anterior tongue of mice. Acta Physiol. 2019, 226, e13215. [Google Scholar] [CrossRef]
- Murtaza, B.; Hichami, A.; Khan, A.S.; Shimpukade, B.; Ulven, T.; Ozdener, M.H.; Khan, N.A. Novel GPR120 agonist TUG891 modulates fat taste perception and preference and activates tongue-brain-gut axis in mice. J. Lipid Res. 2020, 61, 133–142. [Google Scholar] [CrossRef]
- Ozdener, M.H.; Subramaniam, S.; Sundaresan, S.; Sery, O.; Hashimoto, T.; Asakawa, Y.; Besnard, P.; Abumrad, N.A.; Khan, N.A. CD36-and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 2014, 146, 995–1005.e5. [Google Scholar] [CrossRef]
- Martin, C.; Passilly-Degrace, P.; Gaillard, D.; Merlin, J.-F.; Chevrot, M.; Besnard, P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: Impact on spontaneous fat preference. PLoS ONE 2011, 6, e24014. [Google Scholar] [CrossRef]
- Laugerette, F.; Passilly-Degrace, P.; Patris, B.; Niot, I.; Febbraio, M.; Montmayeur, J.-P.; Besnard, P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Investig. 2005, 115, 3177–3184. [Google Scholar] [CrossRef]
- Abdoul-Azize, S.; Selvakumar, S.; Sadou, H.; Besnard, P.; Khan, N.A. Ca2+ signaling in taste bud cells and spontaneous preference for fat: Unresolved roles of CD36 and GPR120. Biochimie 2014, 96, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, T.A.; Khan, N.A. Cell signaling mechanisms of oro-gustatory detection of dietary fat: Advances and challenges. Prog. Lipid Res. 2014, 53, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.; Longuet, C.; Maida, A.; Bahrami, J.; Xu, E.; Baker, C.L.; Brubaker, P.L.; Drucker, D.J.; Adeli, K. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology 2009, 137, 997–1005.e4. [Google Scholar] [CrossRef] [PubMed]
- Luiken, J.J.; Dyck, D.J.; Han, X.-X.; Tandon, N.N.; Arumugam, Y.; Glatz, J.F.; Bonen, A. Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am. J. Physiol.-Endocrinol. Metab. 2002, 282, E491–E495. [Google Scholar] [CrossRef]
- Fang, X.; Palanivel, R.; Cresser, J.; Schram, K.; Ganguly, R.; Thong, F.S.; Tuinei, J.; Xu, A.; Abel, E.D.; Sweeney, G. An APPL1-AMPK signaling axis mediates beneficial metabolic effects of adiponectin in the heart. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E721–E729. [Google Scholar] [CrossRef]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef]
- Tanaka, T.; Katsuma, S.; Adachi, T.; Koshimizu, T.-a.; Hirasawa, A.; Tsujimoto, G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 377, 523–527. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Li, X.-C.; Liang, X.-Y.; Zhao, Y.-Y.; Xie, R.; Zhang, L.-J.; Zhang, X.-C.; Chen, C. GPR120 regulates pancreatic polypeptide secretion from male mouse islets via PLC-mediated calcium mobilization. Endocrinology 2020, 161, bqaa157. [Google Scholar] [CrossRef]
- Fang, X.; Fetros, J.; Dadson, K.; Xu, A.; Sweeney, G. Leptin prevents the metabolic effects of adiponectin in L6 myotubes. Diabetologia 2009, 52, 2190–2200. [Google Scholar] [CrossRef]
- Habets, D.D.; Coumans, W.A.; Voshol, P.J.; den Boer, M.A.; Febbraio, M.; Bonen, A.; Glatz, J.F.; Luiken, J.J. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem. Biophys. Res. Commun. 2007, 355, 204–210. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Liu, Q.; Shan, T.; Wang, X.; Feng, J.; Wang, Y. AMPK facilitates intestinal long-chain fatty acid uptake by manipulating CD36 expression and translocation. FASEB J. 2020, 34, 4852–4869. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P.; et al. Impaired multimerization of human adiponectin mutants associated with diabetes: Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Maeda, K.; Fukuhara, A.; Shimomura, I.; Ito, T. Molecular expression of adiponectin in human saliva. Biochem. Biophys. Res. Commun. 2014, 445, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Hug, C.; Wang, J.; Ahmad, N.S.; Bogan, J.S.; Tsao, T.-S.; Lodish, H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 2004, 101, 10308–10313. [Google Scholar] [CrossRef]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, physiological functions, role in diseases, and effects of nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.V.; Nguyen, T.K.; Nguyen, B.L.; Kim, J.O.; Jeong, J.H.; Choi, I.; Park, P.H. Adiponectin restores the obesity-induced impaired immunomodulatory function of mesenchymal stromal cells via glycolytic reprogramming. Acta Pharm. Sin. B 2024, 14, 273–291. [Google Scholar] [CrossRef]
- Mohammed Saeed, W.; Nasser Binjawhar, D. Association of serum leptin and adiponectin concentrations with type 2 diabetes biomarkers and complications among Saudi women. Diabetes Metab. Syndr. Obes. 2023, 16, 2129–2140. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Bartlomiejczyk, M.A.; Sakowicz, A.; Maciejewski, M.; Banach, M. The role of adipokines in the development of arterial stiffness and hypertension. Angiology 2020, 71, 754–761. [Google Scholar] [CrossRef]
- De Carli, L.; Gambino, R.; Lubrano, C.; Rosato, R.; Bongiovanni, D.; Lanfranco, F.; Broglio, F.; Ghigo, E.; Bo, S. Impaired taste sensation in type 2 diabetic patients without chronic complications: A case–control study. J. Endocrinol. Investig. 2018, 41, 765–772. [Google Scholar] [CrossRef]
- Kaufman, A.; Kim, J.; Noel, C.; Dando, R. Taste loss with obesity in mice and men. Int. J. Obes. 2020, 44, 739–743. [Google Scholar] [CrossRef]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.-i.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Joharapurkar, A.; Das, N.; Khatoon, S.; Kushwaha, S.; Gurjar, A.A.; Singh, A.K.; Shree, S.; Ahmed, M.Z.; China, S.P.; et al. Estradiol overcomes adiponectin-resistance in diabetic mice by regulating skeletal muscle adiponectin receptor 1 expression. Mol. Cell. Endocrinol. 2022, 540, 111525. [Google Scholar] [CrossRef] [PubMed]
- Mester, P.; Räth, U.; Schmid, S.; Müller, M.; Buechler, C.; Pavel, V. Exploring the relationship between plasma adiponectin, gender, and underlying diseases in severe illness. Biomedicines 2023, 11, 3287. [Google Scholar] [CrossRef]
- Klobučar, I.; Habisch, H.; Klobučar, L.; Trbušić, M.; Pregartner, G.; Berghold, A.; Kostner, G.M.; Scharnagl, H.; Madl, T.; Frank, S.; et al. Sex-related differences in the associations between adiponectin and serum lipoproteins in healthy subjects and patients with metabolic syndrome. Biomedicines 2024, 12, 1972. [Google Scholar] [CrossRef] [PubMed]
- Pfabigan, D.M.; Vezzani, C.; Thorsby, P.M.; Sailer, U. Sex difference in human olfactory sensitivity is associated with plasma adiponectin. Horm. Behav. 2022, 145, 105235. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.-Y.; Bench, E.M.; Allerton, T.D.; Schreiber, A.L.; Arceneaux, K.P., III; Primeaux, S.D. Preference for linoleic acid in obesity-prone and obesity-resistant rats is attenuated by the reduction of CD36 on the tongue. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2013, 305, R1346–R1355. [Google Scholar] [CrossRef]
- Lewandowski, D.; Gao, F.; Imanishi, S.; Tworak, A.; Bassetto, M.; Dong, Z.; Pinto, A.F.; Tabaka, M.; Kiser, P.D.; Imanishi, Y.; et al. Restoring retinal polyunsaturated fatty acid balance and retina function by targeting ceramide in AdipoR1 deficient mice. J. Biol. Chem. 2024, 300, 107291. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K.; Abumrad, N.A. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 293, R1823–R1832. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Ackroff, K. Maltodextrin and fat preference deficits in “taste-blind” P2 × 2/P2 × 3 knockout mice. Chem. Senses 2014, 39, 507–514. [Google Scholar] [CrossRef][Green Version]
- Sclafani, A.; Ackroff, K. Greater reductions in fat preferences in CALHM1 than CD36 knockout mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 315, R576–R585. [Google Scholar] [CrossRef]
- Sclafani, A.; Ackroff, K. Fat preference deficits and experience-induced recovery in global taste-deficient Trpm5 and Calhm1 knockout mice. Physiol. Behav. 2022, 246, 113695. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.; Masterson, E.; Gilbertson, T.A. Adiponectin Signaling Modulates Fat Taste Responsiveness in Mice. Nutrients 2024, 16, 3704. https://doi.org/10.3390/nu16213704
Lin F, Masterson E, Gilbertson TA. Adiponectin Signaling Modulates Fat Taste Responsiveness in Mice. Nutrients. 2024; 16(21):3704. https://doi.org/10.3390/nu16213704
Chicago/Turabian StyleLin, Fangjun, Emeline Masterson, and Timothy A. Gilbertson. 2024. "Adiponectin Signaling Modulates Fat Taste Responsiveness in Mice" Nutrients 16, no. 21: 3704. https://doi.org/10.3390/nu16213704
APA StyleLin, F., Masterson, E., & Gilbertson, T. A. (2024). Adiponectin Signaling Modulates Fat Taste Responsiveness in Mice. Nutrients, 16(21), 3704. https://doi.org/10.3390/nu16213704








