The Impact of Complementary Feeding on Fecal Microbiota in Exclusively Breast-Fed Infants with Cystic Fibrosis (A Descriptive Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Fecal Samples Collection and Analysis
2.3. Anthropometric Measurements
2.4. Analysis of Alpha and Beta Diversity of Fecal Microbiota
3. Results and Discussion
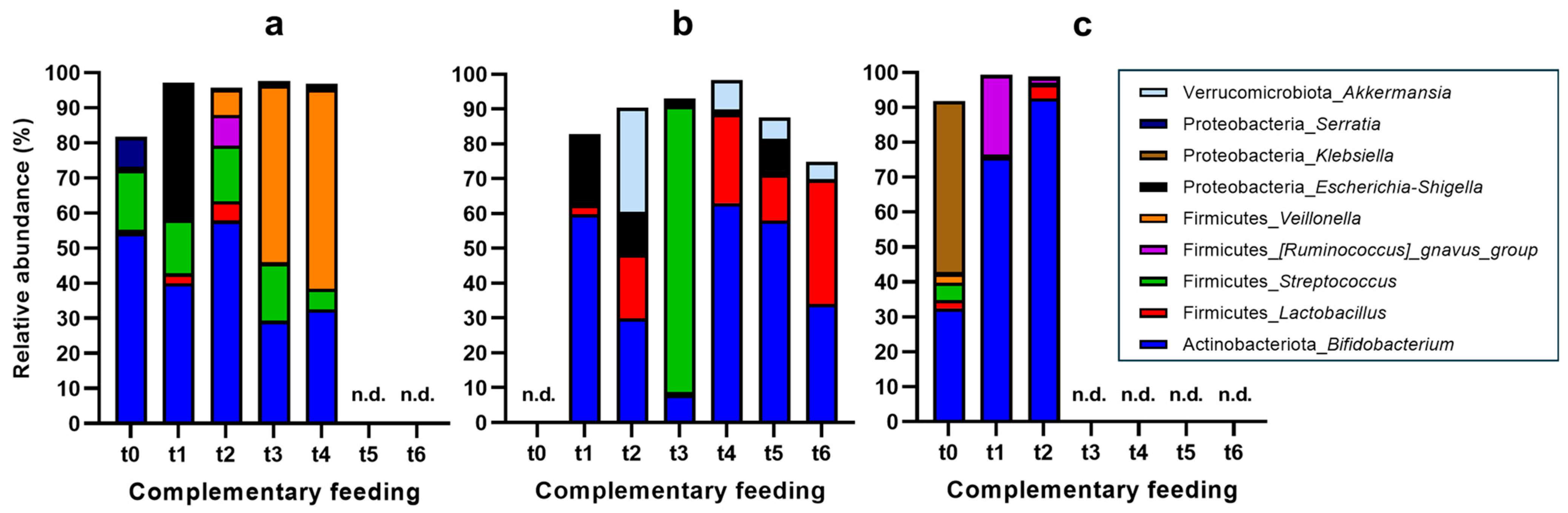
3.1. Subject and Sample Characteristics
3.2. Basal Fecal Microbiota During the Exclusive Breastfeeding Period
3.3. Changes in Fecal Microbiota Composition upon Introduction of Complementary Feeding
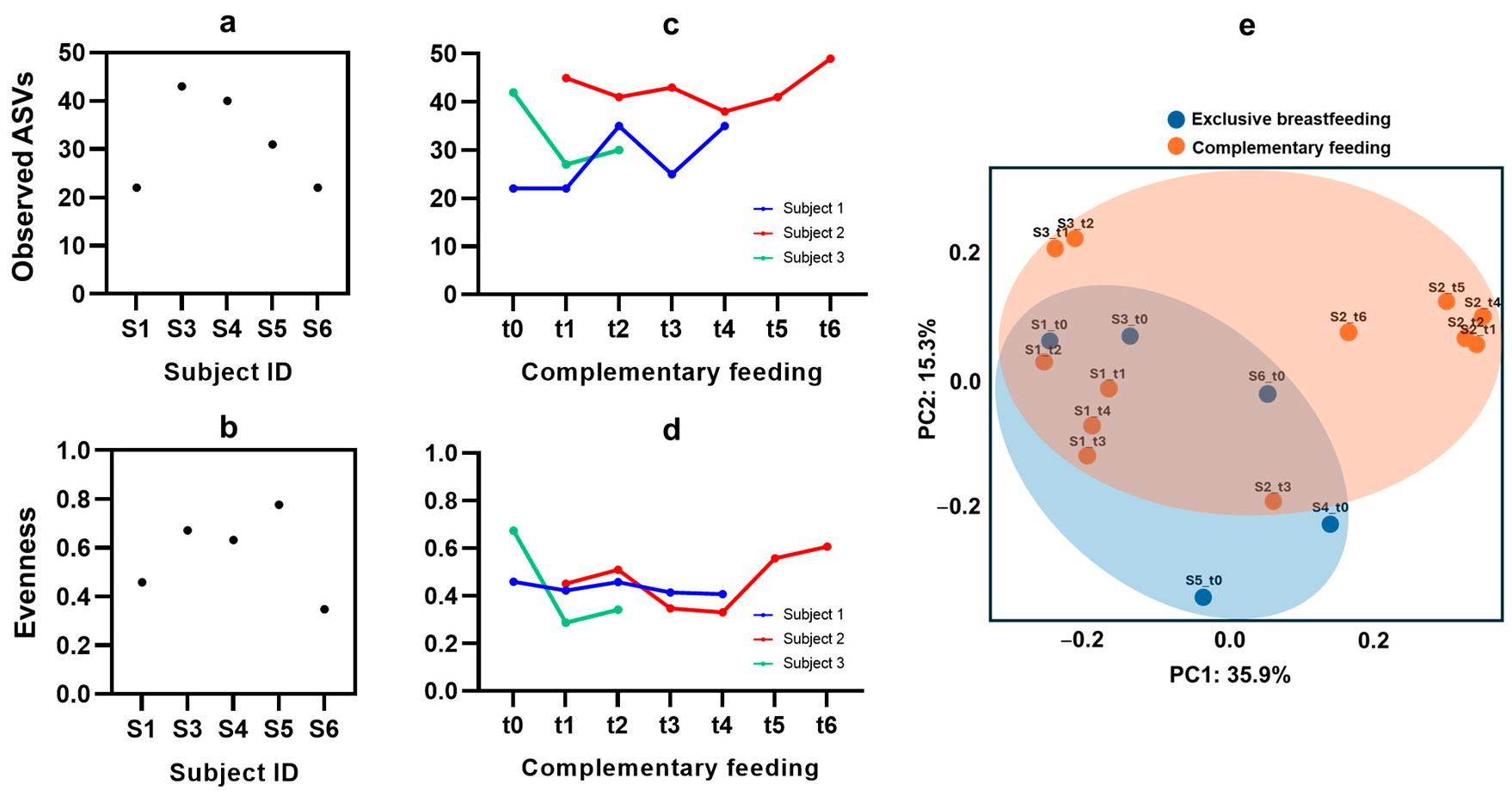
3.4. Microbiota Diversity from Exclusive Breastfeeding to Complementary Feeding
3.5. General Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Ulmer, J.; Kechaou, N.; Langella, P.; Bermúdez-Humarán, L.G. Role of commensal and probiotic bacteria in human health: A focus on inflammatory bowel disease. Microb. Cell Fact. 2016, 15, 71. [Google Scholar] [CrossRef]
- Nielsen, S.; Needham, B.; Leach, S.T.; Day, A.S.; Jaffe, A.; Thomas, T.; Ooi, C.Y. The gut microbiome of healthy and cystic fibrosis children. Microbiome 2016, 4, 7. [Google Scholar]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef]
- Caley, L.R.; White, H.; de Goffau, M.C.; Floto, R.A.; Parkhill, J.; Marsland, B.; Peckham, D.G. Cystic fibrosis-related gut dysbiosis: A systematic review. Dig. Dis. Sci. 2023, 68, 1797–1814. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guideline for Complementary Feeding of Infants and Young Children 6–23 Months of Age; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Shi, Y.; Yin, R.; Pang, J.; Chen, Y.; Li, Z.; Su, S.; Wen, Y. Impact of complementary feeding on infant gut microbiome, metabolites and early development. Food Funct. 2024, 15, 10663–10678. [Google Scholar] [CrossRef] [PubMed]
- Gelfond, D.; Borowitz, D. Gastrointestinal complications of cystic fibrosis. Clin. Gastroenterol. Hepatol. 2013, 11, 333–342. [Google Scholar] [CrossRef] [PubMed]
- De Weerth, C. Do bacteria shape our development? The gut microbiome and its role in health and disease during infancy. Front. Pediatr. 2017, 5, 178. [Google Scholar]
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.M.; Norin, E.; Young, D.; et al. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 2011, 157, 1385–1392. [Google Scholar] [CrossRef]
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Østbye, T.; Mueller, N.T. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Eng, A.; Hayden, H.S.; Pope, C.E.; Brittnacher, M.J.; Vo, A.T.; Weiss, E.J.; Hager, K.R.; Leung, D.H.; Heltshe, S.L.; Raftery, D. Infants with cystic fibrosis have altered fecal functional capacities with potential clinical and metabolic consequences. BMC Microbiol. 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Thavamani, A.; Salem, I.; Sferra, T.J.; Sankararaman, S. Impact of altered gut microbiota and its metabolites in cystic fibrosis. Metabolites 2021, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Grau, A.; Heredia, A.; García-Hernández, J.; Cabrera-Rubio, R.; Masip, E.; Ribes-Koninckx, C.; Collado, M.C.; Andrés, A.; Calvo-Lerma, J. Effect of beta-glucan supplementation on cystic fibrosis colonic microbiota: An in vitro study. Pediatr. Res. 2024, 95, 1519–1527. [Google Scholar] [CrossRef]
- Asensio-Grau, A.; Calvo-Lerma, J.; Ribes-Koninckx, C.; Heredia, A.; Andrés, A. Complementary feeding in infants with cystic fibrosis: In vitro nutrient digestibility and impact on colonic microbiota. Food Biosci. 2024, 59, 104249. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. Dada2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Nutritional Application of the Spanish Society of Pediatric Gastroenterology, Hepatology and Nutrition (SEGHNP). Available online: https://www.seghnp.org/nutricional/ (accessed on 11 September 2024).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Daftary, A.; Acton, J.; Heubi, J.; Amin, R. Fecal elastase-1: Utility in pancreatic function in cystic fibrosis. J. Cyst. Fibros. 2006, 5, 71–76. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Kuang, Y.S.; Li, S.H.; Guo, Y.; Lu, J.H.; He, J.R.; Luo, B.J.; Qiu, X. Composition of gut microbiota in infants in China and global comparison. Sci. Rep. 2016, 6, 36666. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, Z.; Liu, L.; Zhang, R.; Zhang, X.; Peng, C.; Hou, X. Characteristics of gut microbiota of term small gestational age infants within 1 week and their relationship with neurodevelopment at 6 months. Front. Microbiol. 2022, 13, 912968. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.-L.; Jang, M.-H. Development of gut microbiota in infants and its impact on early life health. Nutrients 2019, 11, 1388. [Google Scholar]
- Koren, O.; Knights, D.; Gonzalez, A.; Waldron, L.; Segata, N.; Knight, R.; Huttenhower, C.; Ley, R. A guide to enterotypes across the human body: Meta-analysis of 16S rRNA microbial community data. Sci. Rep. 2014, 4, 5289. [Google Scholar]
- Huda, M.N.; Lewis, Z.; Kalanetra, K.M.; Rashid, M.; Ahmad, S.M.; Raqib, R.; Qadri, F.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Stool microbiota and vaccine responses in infants. J. Pediatr. 2014, 165, 1154–1160. [Google Scholar] [CrossRef]
- Moubareck, C.A. Human milk microbiota and oligosaccharides: A glimpse into benefits, diversity, and correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef] [PubMed]
- Pantoja-Feliciano, I.G.; Clemente, J.C.; Costello, E.K.; Perez, M.E.; Blaser, M.J.; Knight, R.; Dominguez-Bello, M.G. Biphasic assembly of the murine intestinal microbiota during early development. ISME J. 2013, 7, 1112–1115. [Google Scholar] [CrossRef]
- Calderon-Gonzalez, R.; Lee, A.; Lopez-Campos, G.; Hancock, S.J.; Sa-Pessoa, J.; Dumigan, A.; McMullan, R.; Campbell, E.L.; Bengoechea, J.A. Modelling the gastrointestinal carriage of Klebsiella pneumoniae infections. Mbio 2023, 14, e03121-22. [Google Scholar] [CrossRef]
- Łoniewski, I.; Skonieczna-Żydecka, K.; Stachowska, L.; Fraszczyk-Tousty, M.; Tousty, P.; Łoniewska, B. Breastfeeding affects concentration of faecal short chain fatty acids during the first year of life: Results of the systematic review and meta-analysis. Front. Nutr. 2022, 9, 939194. [Google Scholar] [CrossRef]
- Cherny, K.E.; Muscat, E.B.; Reyna, M.E.; Kociolek, L.K. Clostridium innocuum: Microbiological and Clinical Characteristics of a Potential Emerging Pathogen. Anaerobe 2021, 71, 102418. [Google Scholar] [CrossRef]
- Coffey, M.J.; Nielsen, S.; Wemheuer, B.; Kaakoush, N.O.; Garg, M.; Needham, B.; Pickford, R.; Jaffe, A.; Thomas, T.; Ooi, C.Y. Gut microbiota in children with cystic fibrosis: A taxonomic and functional dysbiosis. Sci. Rep. 2019, 9, 18593. [Google Scholar] [CrossRef] [PubMed]
- Catassi, G.; Aloi, M.; Giorgio, V.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. The role of diet and nutritional interventions for the infant gut microbiome. Nutrients 2024, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108, 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Rinne, M.M.; Gueimonde, M.; Kalliomäki, M.; Hoppu, U.; Salminen, S.J.; Isolauri, E. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Microbiol. Immunol. 2005, 43, 59–65. [Google Scholar] [CrossRef]
- Penders, J.; Vink, C.; Driessen, C.; London, N.; Thijs, C.; Stobberingh, E.E. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol. Lett. 2005, 243, 141–147. [Google Scholar] [CrossRef]
- Crost, E.H.; Le Gall, G.; Laverde-Gomez, J.A.; Mukhopadhya, I.; Flint, H.J.; Juge, N. Mechanistic insights into the cross-feeding of Ruminococcus gnavus and Ruminococcus bromii on host and dietary carbohydrates. Front. Microbiol. 2018, 9, 2558. [Google Scholar] [CrossRef]
- den Bogert, B.V.; Erkus, O.; Boekhorst, J.; Goffau, M.D.; Smid, E.J.; Zoetendal, E.G.; Kleerebezem, M. Diversity of human small intestinal Streptococcus and Veillonella populations. FEMS Microbiol. Ecol. 2013, 85, 376–388. [Google Scholar] [CrossRef]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2015, 307, 1915–1920. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S. Bacterial metabolism and health-related bioactive compounds. J. Appl. Microbiol. 2017, 83, 341S–349S. [Google Scholar]
- Miller, T.L.; Wolin, M.J. The role of Streptococcus in the digestion of dietary fibers. J. Appl. Microbiol. 2015, 119, 1431–1438. [Google Scholar]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2013, 29, 465–470. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K. Breastfeeding supports healthy gut bacteria in infants. Nat. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; D Lieberman, T.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef]
- Manor, O.; Levy, R.; Borenstein, E. Mapping the inner workings of the microbiome: Genomic tools for the functional characterization of the microbiota. Cell Host Microbe 2016, 18, 746–761. [Google Scholar]
- Laursen, M.F.; Andersen, L.B.; Michaelsen, K.F.; Mølgaard, C.; Trolle, E.; Bahl, M.I.; Licht, T.R. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. mSphere 2016, 1, e00069-15. [Google Scholar] [CrossRef]

| Subject | Age (Months) | Sex | Mutation 1 | Mutation 2 | Mode of Delivery | Fecal Elastase (µg/g Feces) | Weight (kg) | Weight (z-Score) | Height (cm) | Height (z-Score) |
|---|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | 2.9 | Female | F508del | N1303K | C-section | <15 | 4.81 | −1.56 | 58.5 | −0.65 |
| Subject 2 | 6.1 | Male | F508del | L206W | Vaginal | 377 | 6.49 | −1.83 | 66 | −0.79 |
| Subject 3 | 5.4 | Female | F508del | 3272-26A->G | C-section | >500 | 7.53 | 0.44 | 66 | 0.42 |
| Subject 4 | 1.7 | Female | F508del | L571S | Vaginal | <15 | 4.77 | −0.18 | 55.5 | −0.33 |
| Subject 5 | 2.4 | Female | 1609delCA | 2594delGT | Vaginal | <15 | 5.21 | −0.4 | 56.5 | −0.92 |
| Subject 6 | 1.6 | Female | F508del | L206W | Vaginal | >500 | 4.01 | −1.34 | 53 | −1.42 |
| Subject | Time 0 (t0) | → | Time 1 (t1) | → | Time 2 (t2) | → | Time 3 (t3) | → | Time 4 (t4) | → | Time 5 (t5) | → | Time 6 (t6) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | Exclusive breast-feeding | 90 d | Infant formula | 16 d | Cereal | 10 d | Fruit + cereal | 27 d | Chicken + vegetables | - | - | ||
| Subject 2 | - | Fruit | 4 d | Vegetables | 4 d | Chicken | 19 d | Cereals | 13 d | Beef | 16 d | Fish | |
| Subject 3 | Exclusive breast-feeding | 18 d | Fruit | 18 d | Vegetables | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asensio-Grau, A.; Garriga, M.; Vicente, S.; Andrés, A.; Ribes-Koninckx, C.; Calvo-Lerma, J. The Impact of Complementary Feeding on Fecal Microbiota in Exclusively Breast-Fed Infants with Cystic Fibrosis (A Descriptive Study). Nutrients 2024, 16, 4071. https://doi.org/10.3390/nu16234071
Asensio-Grau A, Garriga M, Vicente S, Andrés A, Ribes-Koninckx C, Calvo-Lerma J. The Impact of Complementary Feeding on Fecal Microbiota in Exclusively Breast-Fed Infants with Cystic Fibrosis (A Descriptive Study). Nutrients. 2024; 16(23):4071. https://doi.org/10.3390/nu16234071
Chicago/Turabian StyleAsensio-Grau, Andrea, María Garriga, Saioa Vicente, Ana Andrés, Carmen Ribes-Koninckx, and Joaquim Calvo-Lerma. 2024. "The Impact of Complementary Feeding on Fecal Microbiota in Exclusively Breast-Fed Infants with Cystic Fibrosis (A Descriptive Study)" Nutrients 16, no. 23: 4071. https://doi.org/10.3390/nu16234071
APA StyleAsensio-Grau, A., Garriga, M., Vicente, S., Andrés, A., Ribes-Koninckx, C., & Calvo-Lerma, J. (2024). The Impact of Complementary Feeding on Fecal Microbiota in Exclusively Breast-Fed Infants with Cystic Fibrosis (A Descriptive Study). Nutrients, 16(23), 4071. https://doi.org/10.3390/nu16234071







