Influence of Alkaline Reduced Water Supplementation on Glucose and Lipid Metabolism in Non-Diabetic and Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Alkaline Reduced Water (ARW), Chemicals, and Reagents
2.2. Animal Experiment
2.3. Determination of Plasma Biochemistry, Lipid Profile, TBARS Levels, and Antioxidant Enzyme Activities
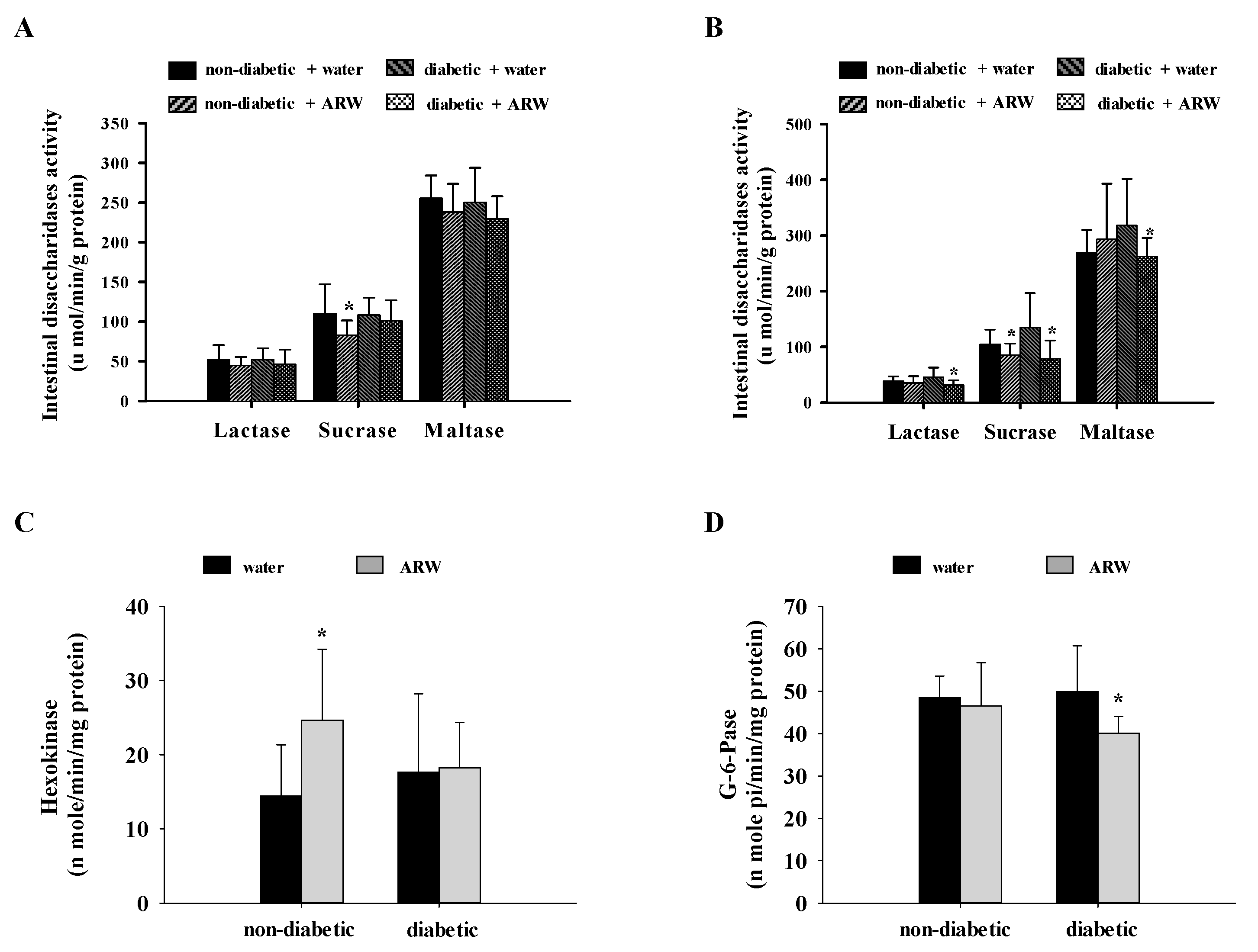
2.4. Measurement of the Activities of G-6-Pase, Hexokinase, G-6-P DeHase, and Intestinal Disaccharidase
2.5. Statistical Analysis
3. Results
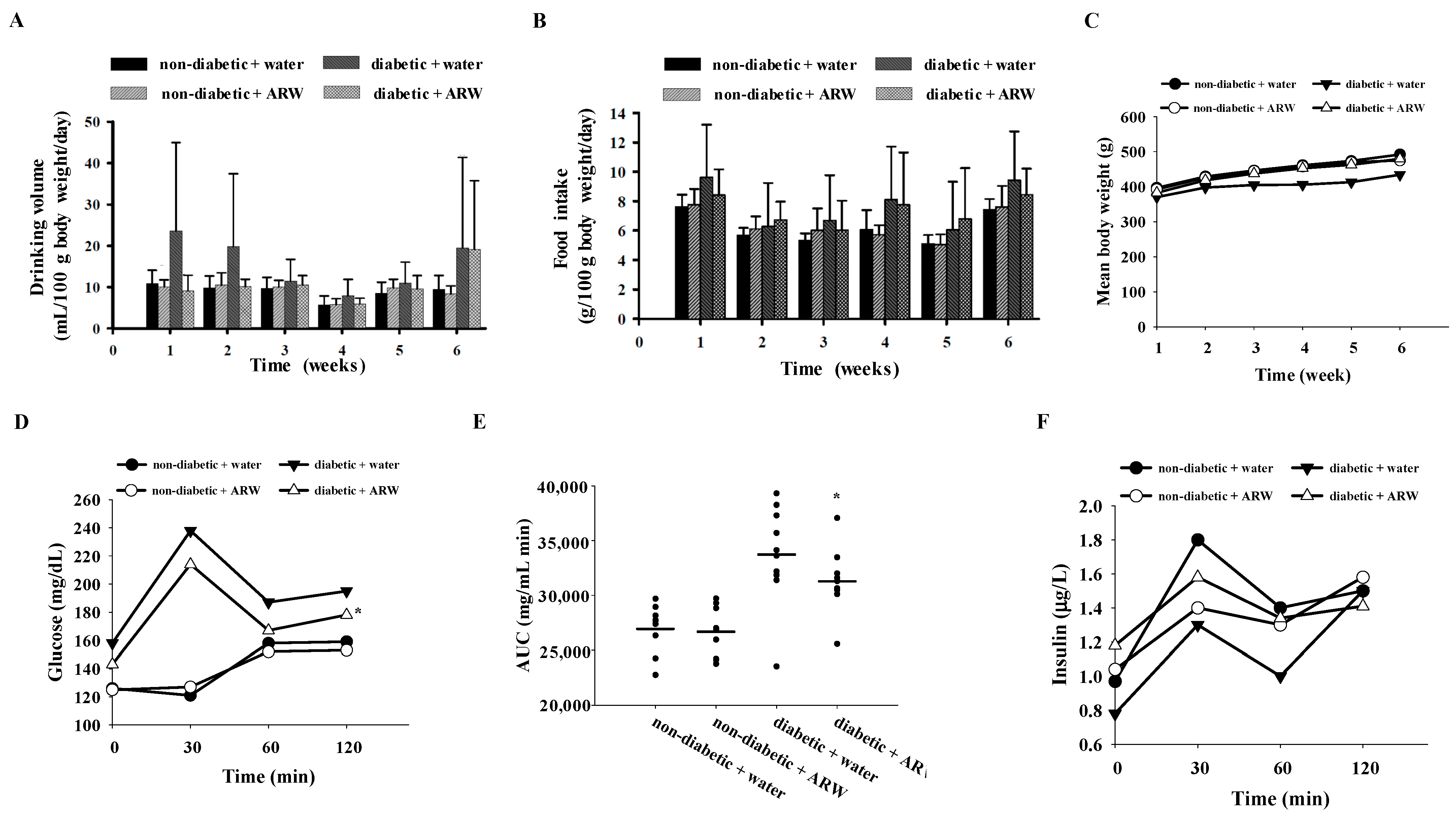
3.1. Influence of ARW Supplementation on Modulating Glucose Metabolism
3.2. Effect of ARW Supplementation on Improving Lipid Metabolism
3.3. Antioxidative Potential of ARW Supplementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Association, A.D. Standards of medical care in diabetes—2022 abridged for primary care providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.; Makaroff, L. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Riera, K.P.; Pérez-Severiano, F.; López-Meraz, M.L. Oxidative stress: A common imbalance in diabetes and epilepsy. Metab. Brain Dis. 2023, 38, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef]
- Tahara, A.; Matsuyama-Yokono, A.; Shibasaki, M. Effects of antidiabetic drugs in high-fat diet and streptozotocin–nicotinamide-induced type 2 diabetic mice. Eur. J. Pharmacol. 2011, 655, 108–116. [Google Scholar] [CrossRef]
- Hanaoka, K.; Sun, D.; Lawrence, R.; Kamitani, Y.; Fernandes, G. The mechanism of the enhanced antioxidant effects against superoxide anion radicals of reduced water produced by electrolysis. Biophys. Chem. 2004, 107, 71–82. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Joo, K.-B.; Lee, K.-J. Clinical effect and mechanism of alkaline reduced water. J. Food Drug Anal. 2012, 20, 33. [Google Scholar] [CrossRef]
- Jin, D.; Ryu, S.H.; Kim, H.W.; Yang, E.J.; Lim, S.J.; Ryang, Y.S.; Chung, C.H.; Park, S.K.; Lee, K.J. Anti-diabetic effect of alkaline-reduced water on OLETF rats. Biosci. Biotechnol. Biochem. 2006, 70, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Siswantoro, E.; Purwanto, N.H. Effectiveness of alkali water consumption to reduce blood sugar levels in diabetes mellitus type 2. J. Diabetes Mellit. 2017, 7, 249–264. [Google Scholar] [CrossRef]
- Ramadhan, A.; Wicaksono, S.A.; Nugroho, T.E.; Utami, S.B. The effects of alkaline ionized water administration to the total cholesterol levels in patients with type 2 diabetes mellitus accompanied by dyslipidemia. Pak. J. Med. Health Sci. 2021, 15, 1449–1455. [Google Scholar] [CrossRef]
- Dan, J.; Yang-Suk, Y.; Young-Kun, D. Effect of mineral-induced alkaline reduced water on Sprague-Dawley rats fed on high-fat diet. Biomed. Sci. Lett. 2006, 12, 1–7. [Google Scholar]
- Delos Reyes, F.S.L.G.; Mamaril, A.C.C.; Matias, T.J.P.; Tronco, M.K.V.; Samson, G.R.; Javier, N.D.; Fadriquela, A.; Antonio, J.M.; Sajo, M.E.J.V. The search for the elixir of life: On the therapeutic potential of alkaline reduced water in metabolic syndromes. Processes 2021, 9, 1876. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chen, J.-Y.; Chiang, M.-T. Effects of Mung Bean Water Supplementation on Modulating Lipid and Glucose Metabolism in a Diabetic Rat Model. Nutrients 2024, 16, 2684. [Google Scholar] [CrossRef]
- Allison, D.B.; Paultre, F.; Maggio, C.; Mezzitis, N.; Pi-Sunyer, F.X. The use of areas under curves in diabetes research. Diabetes Care 1995, 18, 245–250. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Takehisa, F.; Suzuki, Y. Effect of guar gum and cholestyramine on plasma lipoprotein cholesterol in rats. J. Jpn. Soc. Nutr. Food Sci. 1990, 43, 269–274. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Carlberg, I.; Mannervik, B. [59] Glutathione reductase. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1985; Volume 113, pp. 484–490. [Google Scholar]
- Griffith, O.W.; Meister, A. Origin and turnover of mitochondrial glutathione. Proc. Natl. Acad. Sci. USA 1985, 82, 4668–4672. [Google Scholar] [CrossRef] [PubMed]
- Guthenberg, C.; Ålin, P.; Mannervik, B. [64] Glutathione transferase from rat testis. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1985; Volume 113, pp. 507–510. [Google Scholar]
- Erickson, R.H.; Zakim, D.; Vessey, D.A. Preparation and properties of a phospholipid-free form of microsomal UDP-glucuronyltransferase. Biochemistry 1978, 17, 3706–3711. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem 1951, 193, 265. [Google Scholar] [CrossRef]
- Ikeda, I.M.T. Effects of diabetes and hyperglycemia on disaccharidase activities in the rat. Scand. J. Gastroenterol. 1998, 33, 1069–1073. [Google Scholar] [CrossRef]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, H.K. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006, 79, 2288–2292. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Kang, T.-Y.; Kim, C.-S.; Kim, S.-K.; Yang, Y.-C.; Sohn, J.-H.; Lee, K.-J. Anti-obesity effect of alkaline reduced water in high fat-fed obese mice. Biol. Pharm. Bull. 2013, 36, 1052–1059. [Google Scholar] [CrossRef]
- Wicaksono, S.; Nabyla, D.H.; Utami, S.B. The effects of alkaline reduced water administration to the fasting blood glucose levels in patients with type 2 diabetes mellitus. Methods 2018, 14, 1260–1266. [Google Scholar]
- Chan, Y.M.; Shariff, Z.M.; Chin, Y.S.; Ghazali, S.S.; Lee, P.Y.; Chan, K.S. Associations of alkaline water with metabolic risks, sleep quality, muscle strength: A cross-sectional study among postmenopausal women. PLoS ONE 2022, 17, e0275640. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Fadriquela, A.; Antonio, J.M.; Kim, C.-S.; Cho, I.-Y.; Kim, K.-E.; An, W.-S.; Jang, H.-Y.; Bajgai, J.; Lee, K.-J. Effects of alkaline-reduced water on exercise-induced oxidative stress and fatigue in young male healthy adults. Processes 2022, 10, 1543. [Google Scholar] [CrossRef]
| Ingredient | HFD (Weight, %) |
|---|---|
| Casein | 20.0 |
| Lard | 10.0 |
| AIN-76 Vitamin mixture | 1.0 |
| AIN-76 Mineral mixture | 4.0 |
| Cholesterol | 0.5 |
| Cholic acid | 0.1 |
| Choline chloride | 0.2 |
| Cellulose | 5.0 |
| Corn starch | 59.2 |
| Total | 100 |
| Non-Diabetic | Diabetic | Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Water | ARW | Water | ARW | DM | ARW | DM × ARW | |
| Glucose (mg/dL) | 177.2 ± 10.0 | 166.2 ± 11.8 * | 206.1 ± 36.2 | 175.5 ± 14.4 * | 0.0090 ** | 0.0050 ** | NS |
| Insulin (μg/L) | 3.00 ± 0.11 | 1.91 ± 0.68 * | 1.38 ± 0.76 | 1.09 ± 0.43 | 0.0001 ** | 0.0030 ** | NS |
| Leptin (ng/mL) | 4.96 ± 0.55 | 4.32 ± 0.86 * | 3.24 ± 1.32 | 3.66 ± 1.60 | 0.0040 ** | NS | NS |
| Fructosamine (μmol/L) | 175.2 ± 62.9 | 144.8 ± 66.1 | 199.6 ± 63.8 | 169.6 ± 64.8 | NS | NS | NS |
| Non-Diabetic | Diabetic | Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Water | ARW | Water | ARW | DM | ARW | DM × ARW | |
| Total cholesterol (TC) (mg/dL) | 113.7 ± 24.9 | 92.3 ± 17.8 * | 125.9 ± 41.4 | 131.1 ± 37.2 | NS | NS | 0.024 |
| HDL-C (mg/dL) | 22.7 ± 5.5 | 24.1 ± 5.5 | 34.6 ± 11.2 | 27.2 ± 7.8 * | 0.024 | NS | 0.017 |
| LDL + VLDL-C (mg/dL) | 91.0 ± 25.0 | 68.2 ± 19.1 * | 93.6 ± 36.0 | 116.7 ± 46.5 | 0.030 | NS | 0.050 |
| TC/HDL-C | 5.25 ± 1.63 | 4.03 ± 1.26 * | 4.00 ± 1.18 | 5.34 ± 1.68 | NS | NS | 0.015 |
| (LDL + VLDL-C)/HDL-C | 4.25 ± 1.63 | 3.03 ± 1.26 * | 3.00 ± 1.18 | 4.34 ± 1.68 | NS | NS | 0.015 |
| Triglyceride (TG) (mg/dL) | 101.8 ± 22.8 | 77.8 ± 17.8 * | 90.1 ± 25.7 | 106.5 ± 30.9 | NS | NS | 0.020 |
| HDL-TG (mg/dL) | 16.5 ± 3.3 | 13.9 ± 1.0 * | 14.8 ± 0.9 | 16.9 ± 3.4 | NS | NS | 0.010 ** |
| LDL + VLDL-TG (mg/dL) | 85.3 ± 22.3 | 62.5 ± 19.9 * | 63.5 ± 15.8 | 85.3 ± 30.7 * | NS | NS | 0.008 ** |
| HDL-TG/(LDL + VLDL-TG) | 0.21 ± 0.09 | 0.27 ± 0.05 | 0.27 ± 0.08 | 0.21 ± 0.20 * | NS | NS | NS |
| Non-Diabetic | Diabetic | Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Water | ARW | Water | ARW | DM | ARW | DM × ARW | |
| Hepatic total cholesterol (mg/g) | 84.9 ± 16.7 | 109.8 ± 22.2 * | 127.6 ± 28.3 | 126.3 ± 20.6 | 0.0001 ** | NS | NS |
| Hepatic total cholesterol (g/liver) | 1.7 ± 0.7 | 2.2 ± 0.6 | 2.6 ± 1.0 | 3.0 ± 0.6 | 0.0020 ** | NS | NS |
| Hepatic triglyceride (mg/g) | 109.7 ± 30.7 | 124.8 ± 32.0 | 126.2 ± 30.4 | 122.7 ± 21.0 | NS | NS | NS |
| Hepatic triglyceride (g/liver) | 2.2 ± 0.9 | 2.5 ± 0.7 | 2.5 ± 0.6 | 2.9 ± 0.5 | NS | NS | NS |
| Fecal total cholesterol (mg/g) | 24.7 ± 3.2 | 20.3 ± 3.9 * | 15.4 ± 5.5 | 15.3 ± 4.0 | 0.0001 ** | NS | NS |
| Fecal total cholesterol (mg/day) | 45.5 ± 8.4 | 35.7 ± 9.6 * | 33.5 ± 13.0 | 33.6 ± 9.0 | NS | NS | NS |
| Fecal triglyceride (mg/g) | 2.1 ± 0.5 | 2.3 ± 0.4 | 2.8 ± 0.9 | 6.7 ± 2.5 * | 0.0001 ** | 0.0001 ** | 0.0001 ** |
| Fecal triglyceride (mg/day) | 3.9 ± 0.9 | 4.1 ± 1.3 | 6.7 ± 4.5 | 11.4 ± 3.4 * | 0.0001 ** | 0.0220 | 0.0350 ** |
| Fecal bile acid (μmol/g) | 43.8 ± 4.0 | 44.3 ± 4.8 | 38.3 ± 6.7 | 40.1 ± 7.2 | 0.0190 ** | NS | NS |
| Fecal bile acid (μmol/day) | 79.7 ± 9.7 | 82.6 ± 8.0 | 67.9 ±17.6 | 83.6 ± 14.3 * | NS | 0.0470 | NS |
| Non-Diabetic | Diabetic | Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Water | ARW | Water | ARW | DM | ARW | DM × ARW | |
| Hepatic TBARS (n mole/g) | 24.9 ± 3.4 | 24.9 ± 5.2 | 23.0 ± 3.2 | 25.4 ± 4.9 | NS | NS | NS |
| Nephric TBARS (nmole/g) | 26.6 ± 10.2 | 30.3 ± 11.2 | 22.3 ± 7.7 | 20.9 ± 8.6 | 0.034 | NS | NS |
| Hepatic GSH (nmol/g liver) | 1.7 ± 0.8 | 2.3 ± 0.4 * | 2.5 ± 0.5 | 1.8 ± 0.7 * | NS | NS | 0.007 ** |
| Hepatic GSSG (nmol/g liver) | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.001 ** | NS | NS |
| Hepatic GSH/GSSG ratio | 3.6 ± 3.5 | 4.0 ± 1.4 | 7.8 ± 6.5 | 6.5 ± 3.6 | 0.038 | NS | NS |
| Hepatic GSH Px (nmol NADPH oxidized/min/mg protein) | 66.8 ± 9.9 | 82.5 ± 10.8 * | 77.5 ± 12.8 | 75.7 ± 10.2 | NS | 0.048 | 0.029 |
| Hepatic GSH Rd (nmol NADPH oxidized/min/mg protein) | 13.1 ± 2.1 | 16.3 ± 3.5 * | 13.8 ± 2.9 | 15.7 ± 4.0 | NS | 0.025 | NS |
| Hepatic GSH-S-T (nmol CDNB-GSH conjugate formed oxidized/min/mg protein) | 300.9 ± 63.5 | 306.8 ± 37.0 | 268.1 ± 49.0 | 267.0 ± 50.9 | 0.044 | NS | NS |
| Hepatic Catalase (K−2/mg protein) | 38.1 ± 14.3 | 43.0 ± 19.8 | 38.6 ± 12.5 | 28.2 ± 8.5 | NS | NS | NS |
| Hepatic SOD (U/mg protein) | 74.1 ± 5.9 | 72.1 ± 7.7 | 63.0 ± 16.6 | 70.4 ± 6.5 | NS | NS | NS |
| Hepatic G-6-P DeHase (nmole/min/mg protein) | 12.9 ± 6.2 | 19.5 ± 7.4 * | 15.4 ± 8.4 | 18.3 ± 4.9 | NS | 0.045 | NS |
| Non-Diabetic | Diabetic | Two-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Water | ARW | Water | ARW | DM | ARW | DM × ARW | |
| GOT (U/L) | 44.39 ± 13.29 | 60.64 ± 23.63 * | 58.98 ± 24.74 | 50.61 ± 9.68 | NS | NS | NS |
| GPT (U/L) | 20.03 ± 6.85 | 27.32 ± 12.77 | 28.64 ± 11.53 | 24.20 ± 3.95 | NS | NS | NS |
| Uric acid (mg/dL) | 1.28 ± 0.27 | 1.25 ± 1.89 | 1.42 ± 0.47 | 1.31 ± 0.39 | NS | NS | NS |
| Creatinine (mg/dL) | 0.62 ± 0.04 | 0.53 ± 0.05 * | 0.55 ± 0.07 | 0.65 ± 0.10 * | NS | NS | 0.0001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Chao, Y.-C.; Chiang, M.-T. Influence of Alkaline Reduced Water Supplementation on Glucose and Lipid Metabolism in Non-Diabetic and Diabetic Rats. Nutrients 2024, 16, 4082. https://doi.org/10.3390/nu16234082
Huang C-H, Chao Y-C, Chiang M-T. Influence of Alkaline Reduced Water Supplementation on Glucose and Lipid Metabolism in Non-Diabetic and Diabetic Rats. Nutrients. 2024; 16(23):4082. https://doi.org/10.3390/nu16234082
Chicago/Turabian StyleHuang, Chung-Hsiung, Ying-Chiun Chao, and Meng-Tsan Chiang. 2024. "Influence of Alkaline Reduced Water Supplementation on Glucose and Lipid Metabolism in Non-Diabetic and Diabetic Rats" Nutrients 16, no. 23: 4082. https://doi.org/10.3390/nu16234082
APA StyleHuang, C.-H., Chao, Y.-C., & Chiang, M.-T. (2024). Influence of Alkaline Reduced Water Supplementation on Glucose and Lipid Metabolism in Non-Diabetic and Diabetic Rats. Nutrients, 16(23), 4082. https://doi.org/10.3390/nu16234082









