Maternal Gut Microbiome-Mediated Epigenetic Modifications in Cognitive Development and Impairments: A New Frontier for Therapeutic Innovation
Abstract
:1. Introduction
2. Methods
3. Different Types of Factors Affecting Cognitive Performance by Alterations in Gut Microbiome
4. Links Between Gut Microbial Pathology, Gut Metabolites Involved in Epigenetic Regulation, and Cognitive Impairment
5. Relationship Between Maternal Prenatal Gut Microbiota and Offspring’s Cognitive Functions
6. Microbial and Dietary Interventions Contributing to Epigenetic Regulation for Cognitive Improvement
7. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Lehtisalo, J.; Palmer, K.; Mangialasche, F.; Solomon, A.; Kivipelto, M.; Ngandu, T. Changes in lifestyle, behaviors, and risk factors for cognitive impairment in older persons during the first wave of the coronavirus disease 2019 pandemic in Finland: Results from the FINGER study. Front. Psychiatry 2021, 12, 624125. [Google Scholar] [CrossRef] [PubMed]
- Katayama, O.; Lee, S.; Bae, S.; Makino, K.; Shinkai, Y.; Chiba, I.; Harada, K.; Shimada, H. Lifestyle activity patterns related to physical frailty and cognitive impairment in urban community-dwelling older adults in Japan. J. Am. Med. Dir. Assoc. 2021, 22, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Ruano, L.; Carvalho, O.P.; Barros, H. Global cognitive impairment prevalence and incidence in community dwelling older adults—A systematic review. Geriatrics 2020, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Emerson, E.; Llewellyn, G. The prevalence of significant cognitive delay among 3-to 4-year-old children growing up in low-and middle-income countries: Results from 126 nationally representative surveys undertaken in 73 countries. J. Intellect. Disabil. Res. 2023, 67, 1200–1215. [Google Scholar] [CrossRef]
- Bai, W.; Chen, P.; Cai, H.; Zhang, Q.; Su, Z.; Cheung, T.; Jackson, T.; Sha, S.; Xiang, Y.-T. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: A meta-analysis and systematic review of epidemiology studies. Age Ageing 2022, 51, afac173. [Google Scholar]
- Hale, J.M.; Schneider, D.C.; Mehta, N.K.; Myrskylä, M. Cognitive impairment in the US: Lifetime risk, age at onset, and years impaired. SSM-Popul. Health 2020, 11, 100577. [Google Scholar] [CrossRef]
- Day, J.J.; Sweatt, J.D. Epigenetic treatments for cognitive impairments. Neuropsychopharmacology 2012, 37, 247–260. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Zhou, J.-R. Underlying mechanisms of brain aging and neurodegenerative diseases as potential targets for preventive or therapeutic strategies using phytochemicals. Nutrients 2023, 15, 3456. [Google Scholar] [CrossRef]
- Nohesara, S.; Abdolmaleky, H.M.; Thiagalingam, S.; Zhou, J.-R. Gut microbiota defined epigenomes of Alzheimer’s and Parkinson’s diseases reveal novel targets for therapy. Epigenomics 2024, 16, 57–77. [Google Scholar] [CrossRef]
- Sun, Y.; Baptista, L.C.; Roberts, L.M.; Jumbo-Lucioni, P.; McMahon, L.L.; Buford, T.W.; Carter, C.S. The gut microbiome as a therapeutic target for cognitive impairment. J. Gerontol. Ser. A 2020, 75, 1242–1250. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: The second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- González Olmo, B.M.; Butler, M.J.; Barrientos, R.M. Evolution of the human diet and its impact on gut microbiota, immune responses, and brain health. Nutrients 2021, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Kim, M.; Bakshi, U.; Cunningham, K.Y.; Davis, J.M., III; Lazaridis, K.N.; Nelson, H.; Chia, N.; Sung, J. A predictive index for health status using species-level gut microbiome profiling. Nat. Commun. 2020, 11, 4635. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Petersen, F.C.; Shekhar, S. Commensal bacteria: An emerging player in defense against respiratory pathogens. Front. Immunol. 2019, 10, 1203. [Google Scholar] [CrossRef]
- Abt, M.C.; Pamer, E.G. Commensal bacteria mediated defenses against pathogens. Curr. Opin. Immunol. 2014, 29, 16–22. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Antibiotic-therapy-induced gut dysbiosis affecting gut microbiota—Brain axis and cognition: Restoration by intake of probiotics and synbiotics. Int. J. Mol. Sci. 2023, 24, 3074. [Google Scholar] [CrossRef]
- Alsegiani, A.S.; Shah, Z.A. The influence of gut microbiota alteration on age-related neuroinflammation and cognitive decline. Neural Regen. Res. 2022, 17, 2407–2412. [Google Scholar]
- Mayer, E.A.; Nance, K.; Chen, S. The gut–brain axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.-J.; Xie, J.; Chen, J.-J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiatry 2020, 10, 350. [Google Scholar] [CrossRef]
- Gao, W.; Baumgartel, K.L.; Alexander, S.A. The gut microbiome as a component of the gut–brain axis in cognitive health. Biol. Res. Nurs. 2020, 22, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Ni Lochlainn, M.; Bowyer, R.C.; Moll, J.M.; García, M.P.; Wadge, S.; Baleanu, A.-F.; Nessa, A.; Sheedy, A.; Akdag, G.; Hart, D. Effect of gut microbiome modulation on muscle function and cognition: The PROMOTe randomised controlled trial. Nat. Commun. 2024, 15, 1859. [Google Scholar] [CrossRef] [PubMed]
- Kolobaric, A.; Andreescu, C.; Jašarević, E.; Hong, C.H.; Roh, H.W.; Cheong, J.; Kim, Y.; Shin, T.; Kang, C.; Kwon, C. Gut microbiome predicts cognitive function and depressive symptoms in late life. Mol. Psychiatry 2024, 29, 3064–3075. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.-M.; Serino, M.; Blasco, G.; Puig, J.; Daunis-i-Estadella, J.; Ricart, W.; Burcelin, R.; Fernández-Aranda, F.; Portero-Otin, M. Gut microbiota interacts with brain microstructure and function. J. Clin. Endocrinol. Metab. 2015, 100, 4505–4513. [Google Scholar] [CrossRef]
- Liang, X.; Fu, Y.; Cao, W.-T.; Wang, Z.; Zhang, K.; Jiang, Z.; Jia, X.; Liu, C.-Y.; Lin, H.-R.; Zhong, H. Gut microbiome, cognitive function and brain structure: A multi-omics integration analysis. Transl. Neurodegener. 2022, 11, 49. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, Y.; Singh, S.; Singh, R.B. Gut microbiome-mediated epigenetic regulation of brain disorder and application of machine learning for multi-omics data analysis. Genome 2021, 64, 355–371. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- Mehta, R.S.; Lochhead, P.; Wang, Y.; Ma, W.; Nguyen, L.H.; Kochar, B.; Huttenhower, C.; Grodstein, F.; Chan, A.T. Association of midlife antibiotic use with subsequent cognitive function in women. PLoS ONE 2022, 17, e0264649. [Google Scholar] [CrossRef]
- Mosaferi, B.; Jand, Y.; Salari, A.-A. Antibiotic-induced gut microbiota depletion from early adolescence exacerbates spatial but not recognition memory impairment in adult male C57BL/6 mice with Alzheimer-like disease. Brain Res. Bull. 2021, 176, 8–17. [Google Scholar] [CrossRef]
- Hickey, M.K.; Miller, N.C.; Haapala, J.; Demerath, E.W.; Pfister, K.M.; Georgieff, M.K.; Gale, C.A. Infants exposed to antibiotics after birth have altered recognition memory responses at one month of age. Pediatr. Res. 2021, 89, 1500–1507. [Google Scholar] [CrossRef]
- Li, J.; Pu, F.; Peng, C.; Wang, Y.; Zhang, Y.; Wu, S.; Wang, S.; Shen, X.; Li, Y.; Cheng, R. Antibiotic cocktail-induced gut microbiota depletion in different stages could cause host cognitive impairment and emotional disorders in adulthood in different manners. Neurobiol. Dis. 2022, 170, 105757. [Google Scholar] [CrossRef] [PubMed]
- Saiyasit, N.; Chunchai, T.; Prus, D.; Suparan, K.; Pittayapong, P.; Apaijai, N.; Pratchayasakul, W.; Sripetchwandee, J.; Chattipakorn, N.; Chattipakorn, S.C. Gut dysbiosis develops before metabolic disturbance and cognitive decline in high-fat diet–induced obese condition. Nutrition 2020, 69, 110576. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Dong, W.; Zhou, W.; Yan, Y.; Lu, L.; Mi, J.; Cao, Y.; Sun, Y.; Zeng, X. The polysaccharides from the fruits of Lycium barbarum ameliorate high-fat and high-fructose diet-induced cognitive impairment via regulating blood glucose and mediating gut microbiota. Int. J. Biol. Macromol. 2024, 258, 129036. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhu, S.; Peng, X.; Li, K.; Peng, W.; Zhong, Y.; Kang, C.; Cao, X.; Liu, Z.; Zhao, B. High salt elicits brain inflammation and cognitive dysfunction, accompanied by alternations in the gut microbiota and decreased SCFA production. J. Alzheimer’s Dis. 2020, 77, 629–640. [Google Scholar] [CrossRef]
- Olson, C.A.; Iñiguez, A.J.; Yang, G.E.; Fang, P.; Pronovost, G.N.; Jameson, K.G.; Rendon, T.K.; Paramo, J.; Barlow, J.T.; Ismagilov, R.F. Alterations in the gut microbiota contribute to cognitive impairment induced by the ketogenic diet and hypoxia. Cell Host Microbe 2021, 29, 1378–1392.e6. [Google Scholar] [CrossRef]
- Li, B.; Ma, Y.; Wang, X.; Zhao, D.; Wang, Z.; Wang, G.; Li, C.; Yang, L.; Ji, H.; Liu, K. Ketogenic Diets Alter the Gut Microbiome, Resulting in Decreased Susceptibility to and Cognitive Impairment in Rats with Pilocarpine-Induced Status Epilepticus. Neurochem. Res. 2024, 49, 2726–2742. [Google Scholar] [CrossRef]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, D.; Zhang, Y.; Wang, J.; Dong, L.; Hu, Y.; Wang, S. Long-term exposure to advanced lipid peroxidation end products impairs cognitive function through microbiota-gut-brain axis. Food Chem. 2024, 461, 140864. [Google Scholar] [CrossRef]
- Brunt, V.E.; LaRocca, T.J.; Bazzoni, A.E.; Sapinsley, Z.J.; Miyamoto-Ditmon, J.; Gioscia-Ryan, R.A.; Neilson, A.P.; Link, C.D.; Seals, D.R. The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 2021, 43, 377–394. [Google Scholar] [CrossRef]
- Komanduri, M.; Savage, K.; Lea, A.; McPhee, G.; Nolidin, K.; Deleuil, S.; Stough, C.; Gondalia, S. The relationship between gut microbiome and cognition in older Australians. Nutrients 2021, 14, 64. [Google Scholar] [CrossRef]
- Teng, Y.; Mu, J.; Xu, F.; Zhang, X.; Sriwastva, M.K.; Liu, Q.M.; Li, X.; Lei, C.; Sundaram, K.; Hu, X. Gut bacterial isoamylamine promotes age-related cognitive dysfunction by promoting microglial cell death. Cell Host Microbe 2022, 30, 944–960.e8. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, X.; Yan, X.; Wang, J.; Yu, G.; Ma, W.; Xiao, B.; Quinones, S.; Tian, X.; Ren, X. Gut microbiota perturbations and neurodevelopmental impacts in offspring rats concurrently exposure to inorganic arsenic and fluoride. Environ. Int. 2020, 140, 105763. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.; Liu, Y.; Xie, Y.; Hu, J.; Hou, Y.; Tan, X.; Ning, X.; Li, G.; Sang, N. Tebuconazole mediates cognitive impairment via the microbe-gut-brain axis (MGBA) in mice. Environ. Int. 2023, 173, 107821. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, D.S.; Jain, S.; Yata, V.K.; Mishra, S.P.; Kumar, A.; Fraser, A.; Kociolek, J.; Dangiolo, M.; Smith, A.; Golden, A. Unique trans-kingdom microbiome structural and functional signatures predict cognitive decline in older adults. Geroscience 2023, 45, 2819–2834. [Google Scholar] [CrossRef]
- Mishra, S.P.; Jain, S.; Wang, B.; Wang, S.; Miller, B.C.; Lee, J.Y.; Borlongan, C.V.; Jiang, L.; Pollak, J.; Taraphder, S. Abnormalities in microbiota/butyrate/FFAR3 signaling in aging gut impair brain function. JCI Insight 2024, 9, e168443. [Google Scholar] [CrossRef]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity-and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Coradduzza, D.; Sedda, S.; Cruciani, S.; De Miglio, M.R.; Ventura, C.; Nivoli, A.; Maioli, M. Age-related cognitive decline, focus on microbiome: A systematic review and meta-analysis. Int. J. Mol. Sci. 2023, 24, 13680. [Google Scholar] [CrossRef]
- Kundu, P.; Torres, E.R.S.; Stagaman, K.; Kasschau, K.; Okhovat, M.; Holden, S.; Ward, S.; Nevonen, K.A.; Davis, B.A.; Saito, T. Integrated analysis of behavioral, epigenetic, and gut microbiome analyses in App NL-GF, App NL-F, and wild type mice. Sci. Rep. 2021, 11, 4678. [Google Scholar] [CrossRef]
- Fan, K.-C.; Lin, C.-C.; Liu, Y.-C.; Chao, Y.-P.; Lai, Y.-J.; Chiu, Y.-L.; Chuang, Y.-F. Altered gut microbiota in older adults with mild cognitive impairment: A case-control study. Front. Aging Neurosci. 2023, 15, 1162057. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Wang, L.; Hao, L.; Ju, M.; Xiao, R. Diet quality, gut microbiota, and microRNAs associated with mild cognitive impairment in middle-aged and elderly Chinese population. Am. J. Clin. Nutr. 2021, 114, 429–440. [Google Scholar] [CrossRef]
- McLeod, A.; Penalver Bernabe, B.; Xia, Y.; Sanchez-Flack, J.; Lamar, M.; Schiffer, L.; Castellanos, K.; Fantuzzi, G.; Maki, P.; Fitzgibbon, M. Comparing the gut microbiome of obese, African American, older adults with and without mild cognitive impairment. PLoS ONE 2023, 18, e0280211. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Li, J.; Liang, J.; Yang, M.; Xia, G.; Ren, Y.; Zhou, H.; Wu, Q.; He, Y. Gut microbiota is causally associated with poststroke cognitive impairment through lipopolysaccharide and butyrate. J. Neuroinflammation 2022, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Li, X.; An, Y.; Song, Y.; Lu, Y. Association of gut microbiota with sort-chain fatty acids and inflammatory cytokines in diabetic patients with cognitive impairment: A cross-sectional, non-controlled study. Front. Nutr. 2022, 9, 930626. [Google Scholar] [CrossRef]
- Kirschner, S.K.; Deutz, N.E.; Rijnaarts, I.; Smit, T.J.; Larsen, D.J.; Engelen, M.P. Impaired intestinal function is associated with lower muscle and cognitive health and well-being in patients with congestive heart failure. J. Parenter. Enter. Nutr. 2022, 46, 660–670. [Google Scholar] [CrossRef]
- Zheng, M.; Ye, H.; Yang, X.; Shen, L.; Dang, X.; Liu, X.; Gong, Y.; Wu, Q.; Wang, L.; Ge, X. Probiotic Clostridium butyricum ameliorates cognitive impairment in obesity via the microbiota-gut-brain axis. Brain Behav. Immun. 2024, 115, 565–587. [Google Scholar] [CrossRef]
- Ma, C.; Li, Y.; Mei, Z.; Yuan, C.; Kang, J.H.; Grodstein, F.; Ascherio, A.; Willett, W.C.; Chan, A.T.; Huttenhower, C. Association between bowel movement pattern and cognitive function: Prospective cohort study and a metagenomic analysis of the gut microbiome. Neurology 2023, 101, e2014–e2025. [Google Scholar] [CrossRef]
- Yamashiro, K.; Takabayashi, K.; Kamagata, K.; Nishimoto, Y.; Togashi, Y.; Yamauchi, Y.; Ogaki, K.; Li, Y.; Hatano, T.; Motoi, Y. Free water in gray matter linked to gut microbiota changes with decreased butyrate producers in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Dis. 2024, 193, 106464. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome 2023, 11, 17. [Google Scholar] [CrossRef]
- Zheng, H.; Xu, P.; Jiang, Q.; Xu, Q.; Zheng, Y.; Yan, J.; Ji, H.; Ning, J.; Zhang, X.; Li, C. Depletion of acetate-producing bacteria from the gut microbiota facilitates cognitive impairment through the gut-brain neural mechanism in diabetic mice. Microbiome 2021, 9, 145. [Google Scholar]
- Kong, Y.; Jiang, B.; Luo, X. Gut microbiota influences Alzheimer’s disease pathogenesis by regulating acetate in Dro-sophila model. Future Microbiol. 2018, 13, 1117–1128. [Google Scholar] [CrossRef]
- Liao, H.; Li, H.; Bao, H.; Jiang, L.; Du, J.; Guo, Y.; Si, Y. Short chain fatty acids protect the cognitive function of sepsis associated encephalopathy mice via GPR43. Front. Neurol. 2022, 13, 909436. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.L.; Xia, K.; Azcarate-Peril, M.A.; Goldman, B.D.; Ahn, M.; Styner, M.A.; Thompson, A.L.; Geng, X.; Gilmore, J.H.; Knickmeyer, R.C. Infant gut microbiome associated with cognitive development. Biol. Psychiatry 2018, 83, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef]
- Dawson, S.L.; O’Hely, M.; Jacka, F.N.; Ponsonby, A.-L.; Symeonides, C.; Loughman, A.; Collier, F.; Moreno-Betancur, M.; Sly, P.; Burgner, D. Maternal prenatal gut microbiota composition predicts child behaviour. EBioMedicine 2021, 68, 103400. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Ederveen, T.H.; Rizzo, F.; Weisz, A.; Collado, M.C.; Muratori, F.; Gross, G.; Alkema, W.; Iozzo, P. Maternal pre-pregnancy overweight and neonatal gut bacterial colonization are associated with cognitive development and gut microbiota composition in pre-school-age offspring. Brain Behav. Immun. 2022, 100, 311–320. [Google Scholar] [CrossRef]
- Contu, L.; Hawkes, C.A. A review of the impact of maternal obesity on the cognitive function and mental health of the offspring. Int. J. Mol. Sci. 2017, 18, 1093. [Google Scholar] [CrossRef]
- Monthé-Drèze, C.; Rifas-Shiman, S.L.; Gold, D.R.; Oken, E.; Sen, S. Maternal obesity and offspring cognition: The role of inflammation. Pediatr. Res. 2019, 85, 799–806. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zou, Q.; Zeng, Z.; Zhao, W.; Yan, S.; Li, L.; Yuan, S. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021, 33, 923–938.e6. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, L.; Schröder, J.; Schuster, I.S.; Nakai, M.; Sun, G.; Sun, Y.B.; Mariño, E.; Degli-Esposti, M.A.; Marques, F.Z. Dietary fiber and microbiota metabolite receptors enhance cognition and alleviate disease in the 5xFAD mouse model of Alzheimer’s disease. J. Neurosci. 2023, 43, 6460–6475. [Google Scholar] [CrossRef]
- Yu, L.; Zhong, X.; He, Y.; Shi, Y. Butyrate, but not propionate, reverses maternal diet-induced neurocognitive deficits in offspring. Pharmacol. Res. 2020, 160, 105082. [Google Scholar] [CrossRef]
- Leyrolle, Q.; Decoeur, F.; Briere, G.; Amadieu, C.; Quadros, A.R.A.d.A.; Voytyuk, I.; Lacabanne, C.; Benmamar-Badel, A.; Bourel, J.; Aubert, A. Maternal dietary omega-3 deficiency worsens the deleterious effects of prenatal inflammation on the gut-brain axis in the offspring across lifetime. Neuropsychopharmacology 2021, 46, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-Y.; Chen, Y.-C.; Hsu, M.-H.; Yu, H.-R.; Su, C.-H.; Tain, Y.-L.; Huang, L.-T.; Sheen, J.-M. Maternal iron deficiency programs offspring cognition and its relationship with gastrointestinal microbiota and metabolites. Int. J. Environ. Res. Public Health 2020, 17, 6070. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, L.; Kell, D.B.; Pretorius, E. Iron dysregulation and dormant microbes as causative agents for impaired blood rheology and pathological clotting in Alzheimer’s type dementia. Front. Neurosci. 2018, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.L.H.; Liu, W.-C.; Wu, C.-W.; Fu, M.-H.; Huang, H.-M.; Tain, Y.-L.; Liang, C.-K.; Hung, C.-Y.; Chen, I.-C.; Hung, P.-L. Butyrate reduction and HDAC4 increase underlie maternal high fructose-induced metabolic dysfunction in hippocampal astrocytes in female rats. J. Nutr. Biochem. 2024, 126, 109571. [Google Scholar] [CrossRef]
- Silva Valvassori, S.; Bitencourt Varela, R.; Orlandi Arent, C.; Colombo Dal-Pont, G.; Sarate Bobsin, T.; Budni, J.; Zilli Reus, G.; Quevedo, J. Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr. Neurovascular Res. 2014, 11, 359–366. [Google Scholar]
- Radford-Smith, D.E.; Probert, F.; Burnet, P.W.; Anthony, D.C. Modifying the maternal microbiota alters the gut–brain metabolome and prevents emotional dysfunction in the adult offspring of obese dams. Proc. Natl. Acad. Sci. USA 2022, 119, e2108581119. [Google Scholar] [CrossRef]
- Luo, H.; Li, W.; Wu, L.; Zhong, S.; Du, C.; Liu, Y.; Xu, Y.; Huang, X.; Bahru, A.H.; Tang, X. Differences in cognition, short-chain fatty acids and related metabolites in pregnant versus non-pregnant women: A cross-sectional study. BMC Pregnancy Childbirth 2022, 22, 533. [Google Scholar] [CrossRef]
- Cruz-Rodríguez, J.; Díaz-López, A.; Canals-Sans, J.; Arija, V. Maternal vitamin B12 status during pregnancy and early infant neurodevelopment: The ECLIPSES study. Nutrients 2023, 15, 1529. [Google Scholar] [CrossRef]
- Oliphant, K.; Cruz Ayala, W.; Ilyumzhinova, R.; Mbayiwa, K.; Sroka, A.; Xie, B.; Andrews, B.; Keenan, K.; Claud, E.C. Microbiome function and neurodevelopment in Black infants: Vitamin B12 emerges as a key factor. Gut Microbes 2024, 16, 2298697. [Google Scholar] [CrossRef]
- Matsunaga, M.; Takeuchi, M.; Watanabe, S.; Takeda, A.K.; Kikusui, T.; Mogi, K.; Nagasawa, M.; Hagihara, K.; Myowa, M. Intestinal microbiome and maternal mental health: Preventing parental stress and enhancing resilience in mothers. Commun. Biol. 2024, 7, 235. [Google Scholar] [CrossRef]
- Veena, S.R.; Krishnaveni, G.V.; Srinivasan, K.; Wills, A.K.; Muthayya, S.; Kurpad, A.V.; Yajnik, C.S.; Fall, C.H. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9-to 10-year-old children in South India. J. Nutr. 2010, 140, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, X.; Wang, Y.; Zeng, Y.; Pei, P.; Zhan, X.; Zhang, M.; Zhang, T. Intergenerational association of gut microbiota and metabolism with perinatal folate metabolism and neural tube defects. Iscience 2023, 26, 107514. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S.; Kim, D.S. Folate and vitamin B-12 deficiencies additively impaire memory function and disturb the gut microbiota in amyloid-β infused rats. Int. J. Vitam. Nutr. Res. 2019, 92, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Lee, J.H.; Shin, J.; Kim, J.-S.; Cha, B.; Lee, S.; Kwon, K.S.; Shin, Y.W.; Choi, S.H. Cognitive function improvement after fecal microbiota transplantation in Alzheimer’s dementia patient: A case report. Curr. Med. Res. Opin. 2021, 37, 1739–1744. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Lin, Z.; Zheng, C.; Chen, S.; Zhou, H.; Liu, Z. Preliminary evidence for developing safe and efficient fecal microbiota transplantation as potential treatment for aged related cognitive impairments. Front. Cell. Infect. Microbiol. 2023, 13, 1103189. [Google Scholar] [CrossRef]
- Su, S.H.; Chen, M.; Wu, Y.F.; Lin, Q.; Wang, D.P.; Sun, J.; Hai, J. Fecal microbiota transplantation and short-chain fatty acids protected against cognitive dysfunction in a rat model of chronic cerebral hypoperfusion. CNS Neurosci. Ther. 2023, 29, 98–114. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, Q.; Sang, Y.; Ge, S.; Wang, Q.; Wang, R.; He, J. Probiotic Bifidobacterium longum BB68S improves cognitive functions in healthy older adults: A randomized, double-blind, placebo-controlled trial. Nutrients 2022, 15, 51. [Google Scholar] [CrossRef]
- Aljumaah, M.R.; Bhatia, U.; Roach, J.; Gunstad, J.; Peril, M.A.A. The gut microbiome, mild cognitive impairment, and probiotics: A randomized clinical trial in middle-aged and older adults. Clin. Nutr. 2022, 41, 2565–2576. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, J.; Wang, G.; Chen, W. Bifidobacterium breve HNXY26M4 attenuates cognitive deficits and neuroinflammation by regulating the gut–brain axis in APP/PS1 mice. J. Agric. Food Chem. 2023, 71, 4646–4655. [Google Scholar] [CrossRef]
- Bloemendaal, M.; Szopinska-Tokov, J.; Belzer, C.; Boverhoff, D.; Papalini, S.; Michels, F.; van Hemert, S.; Arias Vasquez, A.; Aarts, E. Probiotics-induced changes in gut microbial composition and its effects on cognitive performance after stress: Exploratory analyses. Transl. Psychiatry 2021, 11, 300. [Google Scholar] [CrossRef]
- Ge, X.; Zheng, M.; Hu, M.; Fang, X.; Geng, D.; Liu, S.; Wang, L.; Zhang, J.; Guan, L.; Zheng, P. Butyrate ameliorates quinolinic acid–induced cognitive decline in obesity models. J. Clin. Investig. 2023, 133, e154612. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, D.; Weng, F.; Jin, Y.; He, L. Sodium butyrate ameliorates the cognitive impairment of Alzheimer’s disease by regulating the metabolism of astrocytes. Psychopharmacology 2022, 239, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Reolon, G.K.; Maurmann, N.; Werenicz, A.; Garcia, V.A.; Schröder, N.; Wood, M.A.; Roesler, R. Posttraining systemic administration of the histone deacetylase inhibitor sodium butyrate ameliorates aging-related memory decline in rats. Behav. Brain Res. 2011, 221, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, B.; Hui, Y.; Chu, C.; Zhao, Z.; Zhang, Y.; Zhao, B.; Shi, R.; Ren, J.; Dai, X. Methionine restriction regulates cognitive function in high-fat diet-fed mice: Roles of diurnal rhythms of SCFAs producing-and inflammation-related microbes. Mol. Nutr. Food Res. 2020, 64, 2000190. [Google Scholar] [CrossRef]
- Cuervo-Zanatta, D.; Syeda, T.; Sánchez-Valle, V.; Irene-Fierro, M.; Torres-Aguilar, P.; Torres-Ramos, M.A.; Shibayama-Salas, M.; Silva-Olivares, A.; Noriega, L.G.; Torres, N. Dietary fiber modulates the release of gut bacterial products preventing cognitive decline in an Alzheimer’s mouse model. Cell. Mol. Neurobiol. 2023, 43, 1595–1618. [Google Scholar] [CrossRef]
- Ran, Z.; Ju, B.; Cao, L.; Hou, Q.; Wen, L.; Geng, R.; Liao, Y.; Hu, J.; Yang, J. Microbiome–metabolomics analysis reveals the potential effect of verbascoside in alleviating cognitive impairment in db/db mice. Food Funct. 2023, 14, 3488–3508. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.-L.; Li, D.; Wu, J.; Guo, Y.-X.; Fan, J.; Wu, Q.; Wang, H.-P.; Wan, Z.; Xu, J.-Y. Lactoferrin alleviates Western diet-induced cognitive impairment through the microbiome-gut-brain axis. Curr. Res. Food Sci. 2023, 7, 100533. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Han, X.; Zhang, B.; Chen, J.; Lang, J.; Zhang, Q. Effect of melatonin on gut microbiome and metabolomics in diabetic cognitive impairment. Front. Pharmacol. 2024, 15, 1489834. [Google Scholar] [CrossRef]
- Gallo, A.; Martone, A.M.; Liperoti, R.; Cipriani, M.C.; Ibba, F.; Camilli, S.; Rognoni, F.M.; Landi, F.; Montalto, M. Mild cognitive impairment and microbiota: What is known and future perspectives. Front. Med. 2024, 11, 1410246. [Google Scholar] [CrossRef]
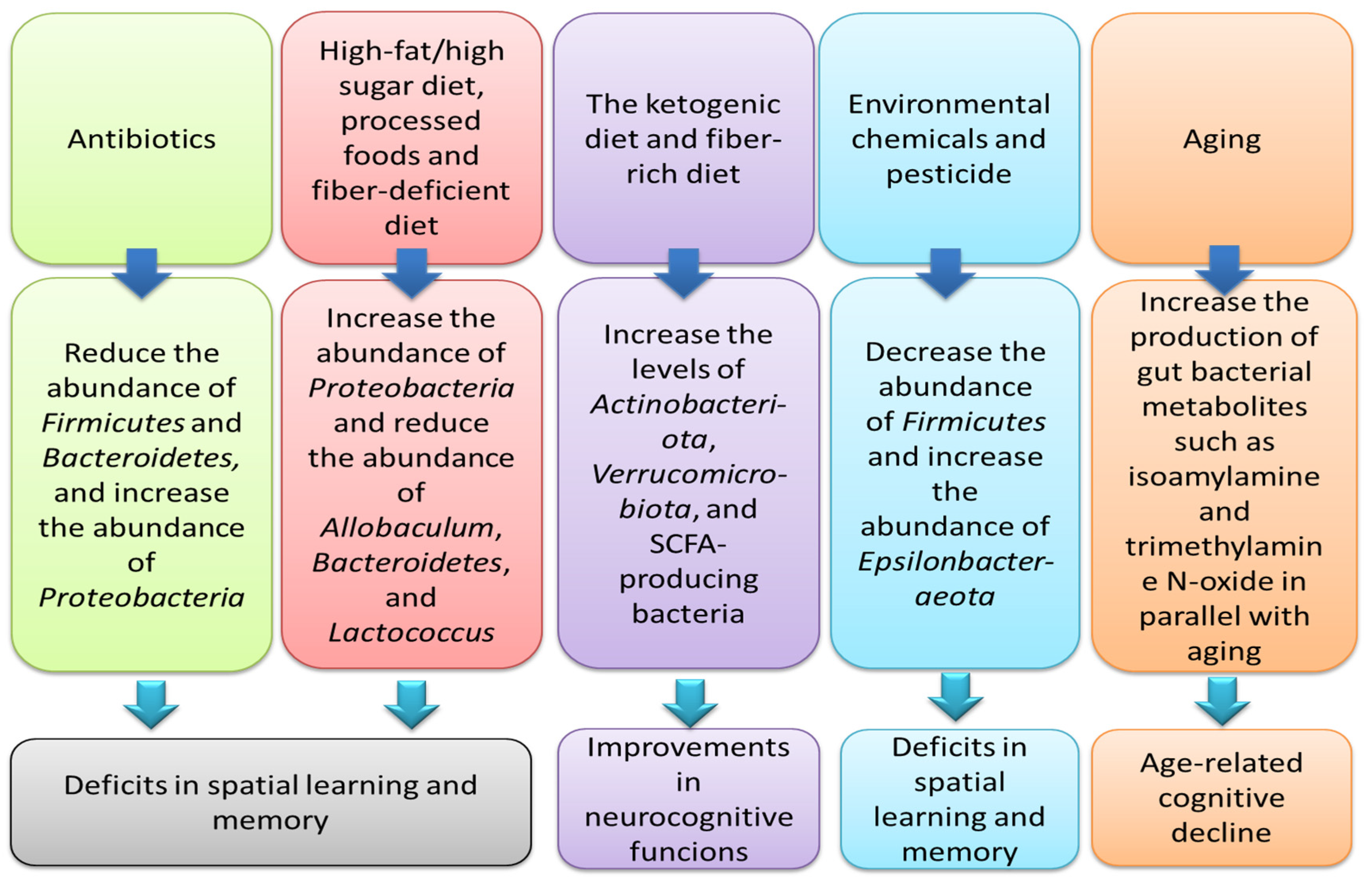

| Factor/Type of Study | Effect on Microbiome | Impact on Cognitive Performance | Reference |
|---|---|---|---|
| Antibiotic (a cocktail including vancomycin, neomycin, ampicillin, metronidazole, and amphotericin-B)/in Aβ1–42-treated mice | Antibiotic-induced gut microbiota depletion | Exacerbation of cognitive deficits after treatment with antibiotic cocktail | [29] |
| Antibiotic/in human | Derangements in the usual composition of gut microbial communities due to neonatal exposure to antibiotics | Changes in discrimination responses and auditory processing at 1 month of age in infants exposed to antibiotics | [30] |
| Antibiotic (a cocktail, including vancomycin, imipenem, bacitracin, neomycin, and amphotericin B)/in mice | Reduced abundance of Firmicutes and Bacteroidetes, but increased abundance of Proteobacteria | Association between gut microbiota depletion by antibiotics and impairments in memory and cognitive functions | [31] |
| Long-term consumption of a high-fat diet/in male Wistar rats | Gut dysbiosis and systemic inflammation | Elevated amyloid-β in the brain and cognitive decline after 40 weeks of high-fat diet utilization | [32] |
| High-fat and high-fructose diet/in mice | Increased abundance of Proteobacteria and reduced abundance of Allobaculum and Lactococcus | Cognitive impairment | [33] |
| High-salt diet/in mice | Reduced concentrations of butyrate, acetate, and propionate | Impaired learning and memory capacities after 8 weeks | [34] |
| Ketogenic diet and hypoxia/in mice | Microbiota-mediated cognitive impairment | Association between Bilophila wadsworthia and cognitive disturbances | [35] |
| Ketogenic diet/in rats with pilocarpine-induced status epilepticus | Microbiota-mediated cognitive impairment | Association between higher Actinobacteriota and Verrucomicrobiota levels after a ketogenic diet intake and improvements in learning and memory | [36] |
| A fiber-deprived diet/in mice | Reduced abundance of Bacteroidetes and elevated abundance of Proteobacteria | Impairments in the cognitive functions | [37] |
| Dietary advanced lipoxidation end-products present in ultra-processed, heat-processed, and fat-enriched foods/in mice | Increased abundance of Muribaculum and Parasutterella and decreased abundance of Faecalibaculum and an unclassified Bacteroidales | Deficits in cognition functions | [38] |
| Age/in old mice | Elevated levels of TMAO (trimethylamine N-oxide), a gut microbiome-derived metabolite, in parallel with aging | Association between TMAO and cognitive decline in aging | [39] |
| Age/in human | An association between higher abundance of the bacterial family Carnobacteriaceae and improved episodic secondary memory; a greater abundance of Clostridiaceae is linked to better continuity of attention | Reductions in cognitive performance in older Australians | [40] |
| Age/in mice | Increased amounts of isoamylamine, a gut bacterial metabolite in parallel with aging | Association between elevated levels of isoamylamine and age-related cognitive decline | [41] |
| Environmental chemicals (exposure to inorganic arsenic and fluoride)/in rats | Reduced Firmicutes, but increased abundance of Epsilonbacteraeota and Bacteroidetes in animals exposed to arsenic | Deficits in spatial learning and memory | [42] |
| Pesticide (exposure to Tebuconazole)/in mice | Disturbances in the Firmicutes/Bacteroidetes ratio, systemic immune factors, and production of neurotransmitters | Derangements in synaptic function integrity, memory, and spatial learning | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nohesara, S.; Abdolmaleky, H.M.; Dickerson, F.; Pinto-Tomás, A.A.; Jeste, D.V.; Thiagalingam, S. Maternal Gut Microbiome-Mediated Epigenetic Modifications in Cognitive Development and Impairments: A New Frontier for Therapeutic Innovation. Nutrients 2024, 16, 4355. https://doi.org/10.3390/nu16244355
Nohesara S, Abdolmaleky HM, Dickerson F, Pinto-Tomás AA, Jeste DV, Thiagalingam S. Maternal Gut Microbiome-Mediated Epigenetic Modifications in Cognitive Development and Impairments: A New Frontier for Therapeutic Innovation. Nutrients. 2024; 16(24):4355. https://doi.org/10.3390/nu16244355
Chicago/Turabian StyleNohesara, Shabnam, Hamid Mostafavi Abdolmaleky, Faith Dickerson, Adrián A. Pinto-Tomás, Dilip V. Jeste, and Sam Thiagalingam. 2024. "Maternal Gut Microbiome-Mediated Epigenetic Modifications in Cognitive Development and Impairments: A New Frontier for Therapeutic Innovation" Nutrients 16, no. 24: 4355. https://doi.org/10.3390/nu16244355
APA StyleNohesara, S., Abdolmaleky, H. M., Dickerson, F., Pinto-Tomás, A. A., Jeste, D. V., & Thiagalingam, S. (2024). Maternal Gut Microbiome-Mediated Epigenetic Modifications in Cognitive Development and Impairments: A New Frontier for Therapeutic Innovation. Nutrients, 16(24), 4355. https://doi.org/10.3390/nu16244355






