Anorexia-Induced Hypoleptinemia Drives Adaptations in the JAK2/STAT3 Pathway in the Ventral and Dorsal Hippocampus of Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
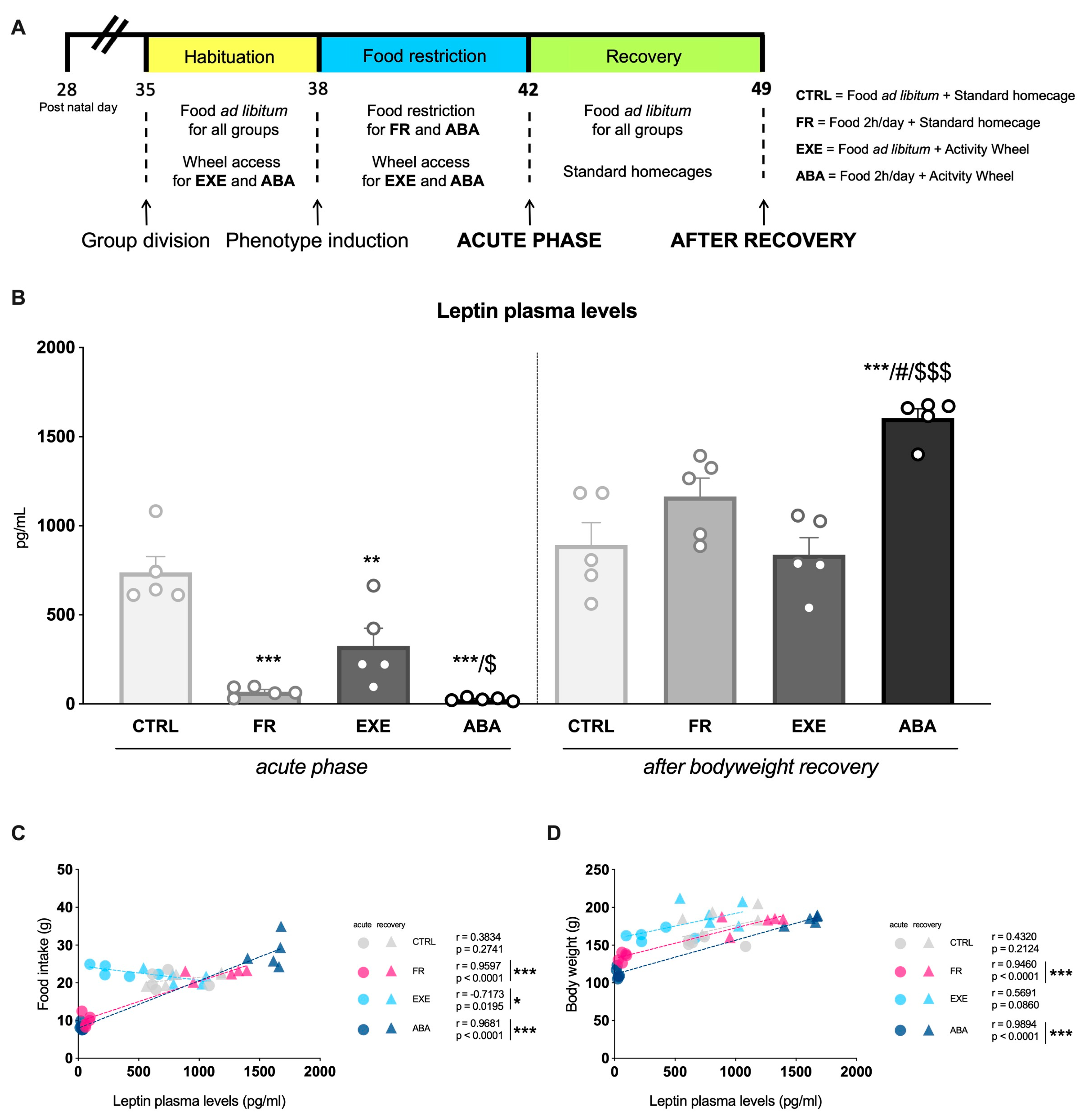
2.2. Experimental Design
2.3. Plasma Collection and ELISA Assay
2.4. Post-Synaptic Density Isolation and Western Blot Analyses
2.5. mRNA Isolation and Real-Time PCR Analysis
- Dnmt1: forward primer 5′- GCGCTCATTGGCTTTTCTAC-3′, reverse primer 5′- CTCGACCACAATCTTGCTGA-3′, probe 5′- AGCCCAGAGTATGCACCAAT-3′;
- 36B4: forward primer 5′-TCAGTGCCTCACTCCATCAT-3′, reverse primer 5′-AGGAAGGCCTTGACCTTTTC-3′, probe 5′-TGGATACAAAAGGGTCCTGG-3′.
2.6. Statistical Analysis
3. Results
3.1. Effects of the Anorexic Phenotype Induction on Circulating Leptin Levels
3.2. Activity-Based Anorexia Alters Intracellular Leptin Receptor Signaling in the Ventral Hippocampus
3.3. Activity-Based Anorexia Alters Intracellular Leptin Receptor Signaling in the Dorsal Hippocampus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrios-Correa, A.A.; Estrada, J.A.; Contreras, I. Leptin Signaling in the Control of Metabolism and Appetite: Lessons from Animal Models. J. Mol. Neurosci. 2018, 66, 390–402. [Google Scholar] [CrossRef]
- Chan, J.L.; Blüher, S.; Yiannakouris, N.; Suchard, M.A.; Kratzsch, J.; Mantzoros, C.S. Regulation of Circulating Soluble Leptin Receptor Levels By Gender, Adiposity, Sex Steroids, and Leptin. Diabetes 2002, 51, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiao, T.; Liu, H. Leptin Signaling and Its Central Role in Energy Homeostasis. Front. Neurosci. 2023, 17, 1238528. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, X.; Jiang, K.; Shi, B.; Liu, L.; Hou, R.; Chen, G.; Farag, M.A.; Yan, N.; Liu, L. Dietary Polyphenols Regulate Appetite Mechanism via Gut-Brain Axis and Gut Homeostasis. Food Chem. 2024, 446, 138739. [Google Scholar] [CrossRef] [PubMed]
- Bjørbaek, C.; Uotani, S.; da Silva, B.; Flier, J.S. Divergent Signaling Capacities of the Long and Short Isoforms of the Leptin Receptor. J. Biol. Chem. 1997, 272, 32686–32695. [Google Scholar] [CrossRef] [PubMed]
- Banks, A.S.; Davis, S.M.; Bates, S.H.; Myers, M.G. Activation of Downstream Signals by the Long Form of the Leptin Receptor. J. Biol. Chem. 2000, 275, 14563–14572. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.H.; Stearns, W.H.; Dundon, T.A.; Schubert, M.; Tso, A.W.K.; Wang, Y.; Banks, A.S.; Lavery, H.J.; Haq, A.K.; Maratos-Flier, E.; et al. STAT3 Signalling Is Required for Leptin Regulation of Energy Balance but Not Reproduction. Nature 2003, 421, 856–859. [Google Scholar] [CrossRef]
- Patterson, C.M.; Leshan, R.L.; Jones, J.C.; Myers, M.G. Molecular Mapping of Mouse Brain Regions Innervated by Leptin Receptor-Expressing Cells. Brain Res. 2011, 1378, 18–28. [Google Scholar] [CrossRef]
- Harvey, J. Leptin Regulation of Neuronal Excitability and Cognitive Function. Curr. Opin. Pharmacol. 2007, 7, 643–647. [Google Scholar] [CrossRef][Green Version]
- Farr, S.A.; Banks, W.A.; Morley, J.E. Effects of Leptin on Memory Processing. Peptides 2006, 27, 1420–1425. [Google Scholar] [CrossRef]
- Guo, M.; Lu, Y.; Garza, J.C.; Li, Y.; Chua, S.C.; Zhang, W.; Lu, B.; Lu, X.-Y. Forebrain Glutamatergic Neurons Mediate Leptin Action on Depression-like Behaviors and Synaptic Depression. Transl. Psychiatry 2012, 2, e83. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Miller, K.K.; Blum, J.I.; Meenaghan, E.; Misra, M.; Eddy, K.T.; Herzog, D.B.; Klibanski, A. Leptin Levels Are Associated With Decreased Depressive Symptoms in Women Across the Weight Spectrum, Independent of Body Fat. Clin. Endocrinol. 2012, 76, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Hildebrandt, T.; Schlögl, H.; Seitz, J.; Denecke, S.; Vieira, D.; Gradl-Dietsch, G.; Peters, T.; Antel, J.; Lau, D.; et al. The Role of Hypoleptinemia in the Psychological and Behavioral Adaptation to Starvation: Implications for Anorexia Nervosa. Neurosci. Biobehav. Rev. 2022, 141, 104807. [Google Scholar] [CrossRef]
- Scharf, I. The Multifaceted Effects of Starvation on Arthropod Behaviour. Anim. Behav. 2016, 119, 37–48. [Google Scholar] [CrossRef]
- van Eeden, A.E.; van Hoeken, D.; Hoek, H.W. Incidence, Prevalence and Mortality of Anorexia Nervosa and Bulimia Nervosa. Curr. Opin. Psychiatry 2021, 34, 515–524. [Google Scholar] [CrossRef]
- American Psychiatric Association. DSM-V Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia Nervosa: Aetiology, Assessment, and Treatment. Lancet Psychiatry 2015, 2, 1099–1111. [Google Scholar] [CrossRef]
- Hebebrand, J.; Blum, W.F.; Barth, N.; Coners, H.; Englaro, P.; Juul, A.; Ziegler, A.; Warnke, A.; Rascher, W.; Remschmidt, H. Leptin Levels in Patients with Anorexia Nervosa Are Reduced in the Acute Stage and Elevated upon Short-Term Weight Restoration. Mol. Psychiatry 1997, 2, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, K.; Hebebrand, J.; Mika, C.; Grzella, I.; Heer, M.; Heussen, N.; Herpertz-Dahlmann, B. The Effect of Therapeutically Induced Weight Gain on Plasma Leptin Levels in Patients with Anorexia Nervosa. J. Psychiatr. Res. 2003, 37, 165–169. [Google Scholar] [CrossRef]
- Mantzoros, C.; Flier, J.S.; Lesem, M.D.; Brewerton, T.D.; Jimerson, D.C. Cerebrospinal Fluid Leptin in Anorexia Nervosa: Correlation with Nutritional Status and Potential Role in Resistance to Weight Gain1. J. Clin. Endocrinol. Metab. 1997, 82, 1845–1851. [Google Scholar] [CrossRef][Green Version]
- Monteleone, P.; Di Lieto, A.; Tortorella, A.; Longobardi, N.; Maj, M. Circulating Leptin in Patients with Anorexia Nervosa, Bulimia Nervosa or Binge-Eating Disorder: Relationship to Body Weight, Eating Patterns, Psychopathology and Endocrine Changes. Psychiatry Res. 2000, 94, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Maj, M. Dysfunctions of Leptin, Ghrelin, BDNF and Endocannabinoids in Eating Disorders: Beyond the Homeostatic Control of Food Intake. Psychoneuroendocrinology 2013, 38, 312–330. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Bulik, C.M. Critical Appraisal of the Provisional DSM-5 Criteria for Anorexia Nervosa and an Alternative Proposal. Int. J. Eat. Disord. 2011, 44, 665–678. [Google Scholar] [CrossRef]
- Lob, S.; Pickel, J.; Bidlingmaier, M.; Schaaf, L.; Backmund, H.; Gerlinghoff, M.; Stalla, G. Serum Leptin Monitoring in Anorectic Patients During Refeeding Therapy. Exp. Clin. Endocrinol. Diabetes 2003, 111, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.F.; Choi, D.L.; Benoit, S.C. Insulin, Leptin and Reward. Trends Endocrinol. Metab. 2010, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Irving, A.J.; Harvey, J. Leptin Regulation of Hippocampal Synaptic Function in Health and Disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130155. [Google Scholar] [CrossRef]
- Holtkamp, K.; Herpertz-Dahlmann, B.; Hebebrand, K.; Mika, C.; Kratzsch, J.; Hebebrand, J. Physical Activity and Restlessness Correlate with Leptin Levels in Patients with Adolescent Anorexia Nervosa. Biol. Psychiatry 2006, 60, 311–313. [Google Scholar] [CrossRef]
- Hebebrand, J.; Milos, G.; Wabitsch, M.; Teufel, M.; Führer, D.; Bühlmeier, J.; Libuda, L.; Ludwig, C.; Antel, J. Clinical Trials Required to Assess Potential Benefits and Side Effects of Treatment of Patients With Anorexia Nervosa With Recombinant Human Leptin. Front. Psychol. 2019, 10, 769. [Google Scholar] [CrossRef]
- Milos, G.; Antel, J.; Kaufmann, L.-K.; Barth, N.; Koller, A.; Tan, S.; Wiesing, U.; Hinney, A.; Libuda, L.; Wabitsch, M.; et al. Short-Term Metreleptin Treatment of Patients with Anorexia Nervosa: Rapid on-Set of Beneficial Cognitive, Emotional, and Behavioral Effects. Transl. Psychiatry 2020, 10, 303. [Google Scholar] [CrossRef]
- Hebebrand, J.; Hinney, A.; Antel, J. Could Leptin Substitution Therapy Potentially Terminate Entrapment in Anorexia Nervosa? Nat. Rev. Endocrinol. 2023, 19, 435–436. [Google Scholar] [CrossRef]
- Exner, C.; Hebebrand, J.; Remschmidt, H.; Wewetzer, C.; Ziegler, A.; Herpertz, S.; Schweiger, U.; Blum, W.F.; Preibisch, G.; Heldmaier, G.; et al. Leptin Suppresses Semi-Starvation Induced Hyperactivity in Rats: Implications for Anorexia Nervosa. Mol. Psychiatry 2000, 5, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, J.J.G.; Koeners, M.P.; de Rijke, C.E.; Kas, M.J.H.; Adan, R.A.H. Leptin Treatment in Activity-Based Anorexia. Biol. Psychiatry 2005, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, L.A.W.; Luijendijk, M.C.M.; Adan, R.A.H. Leptin Reduces Hyperactivity in an Animal Model for Anorexia Nervosa via the Ventral Tegmental Area. Eur. Neuropsychopharmacol. 2011, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Foldi, C.J. Taking Better Advantage of the Activity-Based Anorexia Model. Trends Mol. Med. 2023, 30, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Ahima, R.S. Leptin Signaling. F1000Prime Rep. 2014, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Day, J.J.; Sweatt, J.D. DNA Methylation and Memory Formation. Nat. Neurosci. 2010, 13, 1319–1323. [Google Scholar] [CrossRef]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in Human Disease and Prospects for Epigenetic Therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Mottarlini, F.; Rizzi, B.; Targa, G.; Fumagalli, F.; Caffino, L. Long-Lasting BDNF Signaling Alterations in the Amygdala of Adolescent Female Rats Exposed to the Activity-Based Anorexia Model. Front. Behav. Neurosci. 2022, 16, 1087075. [Google Scholar] [CrossRef]
- Gutierrez, E. A Rat in the Labyrinth of Anorexia Nervosa: Contributions of the Activity-Based Anorexia Rodent Model to the Understanding of Anorexia Nervosa. Int. J. Eat. Disord. 2013, 46, 289–301. [Google Scholar] [CrossRef]
- Chapman, R.H.; Stern, J.M. Maternal Stress and Pituitary-Adrenal Manipulations during Pregnancy in Rats: Effects on Morphology and Sexual Behavior of Male Offspring. J. Comp. Physiol. Psychol. 1978, 92, 1074–1083. [Google Scholar] [CrossRef]
- Boakes, R.A.; Dwyer, D.M. Weight Loss in Rats Produced by Running: Effects of Prior Experience and Individual Housing. Q. J. Exp. Psychol. B 1997, 50, 129–148. [Google Scholar] [PubMed]
- Tezenas Du Montcel, C.; Cao, J.; Mattioni, J.; Hamelin, H.; Lebrun, N.; Ramoz, N.; Gorwood, P.; Tolle, V.; Viltart, O. Chronic Food Restriction in Mice and Increased Systemic Ghrelin Induce Preference for Running Wheel Activity. Psychoneuroendocrinology 2023, 155, 106311. [Google Scholar] [CrossRef] [PubMed]
- Carrera, O.; Fraga, Á.; Pellón, R.; Gutiérrez, E. Rodent Model of Activity-Based Anorexia. Curr. Protoc. Neurosci. 2014, 67, 9.47.1–9.47.11. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Targa, G.; Mottarlini, F.; Rizzi, B.; Leo, D.; Caffino, L.; Fumagalli, F. Dysregulation of AMPA Receptor Trafficking and Intracellular Vesicular Sorting in the Prefrontal Cortex of Dopamine Transporter Knock-Out Rats. Biomolecules 2023, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Caffino, L.; Verheij, M.M.M.; Roversi, K.; Targa, G.; Mottarlini, F.; Popik, P.; Nikiforuk, A.; Golebiowska, J.; Fumagalli, F.; Homberg, J.R. Hypersensitivity to Amphetamine’s Psychomotor and Reinforcing Effects in Serotonin Transporter Knockout Rats: Glutamate in the Nucleus Accumbens. Br. J. Pharmacol. 2020, 177, 4532–4547. [Google Scholar] [CrossRef] [PubMed]
- Caffino, L.; Mottarlini, F.; Mingardi, J.; Zita, G.; Barbon, A.; Fumagalli, F. Anhedonic-like Behavior and BDNF Dysregulation Following a Single Injection of Cocaine during Adolescence. Neuropharmacology 2020, 175, 108161. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J. Food for Thought: Leptin and Hippocampal Synaptic Function. Front. Pharmacol. 2022, 13, 882158. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Solovyova, N.; Irving, A. Leptin and Its Role in Hippocampal Synaptic Plasticity. Prog. Lipid Res. 2006, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, T.; Thom, E.; Watson, A.; Roth, J.; Klaus, S.; Meyerhof, W. Leptin-Induced Nuclear Translocation of STAT3 Immunoreactivity in Hypothalamic Nuclei Involved in Body Weight Regulation. J. Neurosci. 2001, 21, 2413–2424. [Google Scholar] [CrossRef]
- Endo, T.A.; Masuhara, M.; Yokouchi, M.; Suzuki, R.; Sakamoto, H.; Mitsui, K.; Matsumoto, A.; Tanimura, S.; Ohtsubo, M.; Misawa, H.; et al. A New Protein Containing an SH2 Domain That Inhibits JAK Kinases. Nature 1997, 387, 921–924. [Google Scholar] [CrossRef]
- Halder, R.; Hennion, M.; Vidal, R.O.; Shomroni, O.; Rahman, R.-U.; Rajput, A.; Centeno, T.P.; van Bebber, F.; Capece, V.; Garcia Vizcaino, J.C.; et al. DNA Methylation Changes in Plasticity Genes Accompany the Formation and Maintenance of Memory. Nat. Neurosci. 2016, 19, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.G.; Fenton, A.A.; Aoki, C. Effects of Adolescent Experience of Food Restriction and Exercise on Spatial Learning and Open Field Exploration of Female Rats. Hippocampus 2021, 31, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Bahnsen, K.; Wronski, M.-L.; Keeler, J.L.; King, J.A.; Preusker, Q.; Kolb, T.; Weidner, K.; Roessner, V.; Bernardoni, F.; Ehrlich, S. Differential Longitudinal Changes of Hippocampal Subfields in Patients with Anorexia Nervosa. Psychiatry Clin. Neurosci. 2023, 78, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Collantoni, E.; Tenconi, E.; Solmi, M.; Meneguzzo, P.; Marzola, E.; D’Agata, F.; Gotti, S.; Daga, G.A.; Manara, R.; Favaro, A. Hippocampal Volumes in Anorexia Nervosa at Different Stages of the Disorder. Eur. Eat. Disord. Rev. 2021, 29, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Keeler, J.; Patsalos, O.; Thuret, S.; Ehrlich, S.; Tchanturia, K.; Himmerich, H.; Treasure, J. Hippocampal Volume, Function, and Related Molecular Activity in Anorexia Nervosa: A Scoping Review. Expert. Rev. Clin. Pharmacol. 2020, 13, 1367–1387. [Google Scholar] [CrossRef] [PubMed]
- Alzaid, H.; Simon, J.J.; Brugnara, G.; Vollmuth, P.; Bendszus, M.; Friederich, H.-C. Hypothalamic Subregion Alterations in Anorexia Nervosa and Obesity: Association with Appetite-Regulating Hormone Levels. Int. J. Eat. Disord. 2024, 57, 581–592. [Google Scholar] [CrossRef]
- Arner, E.; Westermark, P.O.; Spalding, K.L.; Britton, T.; Rydén, M.; Frisén, J.; Bernard, S.; Arner, P. Adipocyte Turnover: Relevance to Human Adipose Tissue Morphology. Diabetes 2010, 59, 105–109. [Google Scholar] [CrossRef]
- Föcker, M.; Timmesfeld, N.; Scherag, S.; Bühren, K.; Langkamp, M.; Dempfle, A.; Sheridan, E.M.; de Zwaan, M.; Fleischhaker, C.; Herzog, W.; et al. Screening for Anorexia Nervosa via Measurement of Serum Leptin Levels. J. Neural Transm. 2011, 118, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Dardennes, R.; Tolle, V.; Lavoisy, G.; Grouselle, D.; Alanbar, N.; Duriez, P.; Gorwood, P.; Ramoz, N.; Epelbaum, J. Lower Leptin Level at Discharge in Acute Anorexia Nervosa Is Associated with Early Weight-Loss. Eur. Eat. Disord. Rev. 2021, 29, 634–644. [Google Scholar] [CrossRef]
- Scherma, M.; Satta, V.; Collu, R.; Boi, M.F.; Usai, P.; Fratta, W.; Fadda, P. Cannabinoid CB1 /CB2 Receptor Agonists Attenuate Hyperactivity and Body Weight Loss in a Rat Model of Activity-Based Anorexia. Br. J. Pharmacol. 2017, 174, 2682–2695. [Google Scholar] [CrossRef]
- Gilman, T.L.; Owens, W.A.; George, C.M.; Metzel, L.; Vitela, M.; Ferreira, L.; Bowman, M.A.; Gould, G.G.; Toney, G.M.; Daws, L.C. Age- and Sex-Specific Plasticity in Dopamine Transporter Function Revealed by Food Restriction and Exercise in a Rat Activity-Based Anorexia Paradigm. J. Pharmacol. Exp. Ther. 2019, 371, 268–277. [Google Scholar] [CrossRef] [PubMed]
- de Assis, G.G.; Murawska-Ciałowicz, E. Exercise and Weight Management: The Role of Leptin-A Systematic Review and Update of Clinical Data from 2000-2022. J Clin Med 2023, 12, 4490. [Google Scholar] [CrossRef] [PubMed]
- Kaye, W.H.; Gwirtsman, H.E.; Obarzanek, E.; George, T.; Jimerson, D.C.; Ebert, M.H. Caloric Intake Necessary for Weight Maintenance in Anorexia Nervosa: Nonbulimics Require Greater Caloric Intake than Bulimics. Am. J. Clin. Nutr. 1986, 44, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Weltzin, T.E.; Fernstrom, M.H.; Hansen, D.; McConaha, C.; Kaye, W.H. Abnormal Caloric Requirements for Weight Maintenance in Patients with Anorexia and Bulimia Nervosa. Am. J. Psychiatry 1991, 148, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, K.; Hebebrand, J.; Mika, C.; Heer, M.; Heussen, N.; Herpertz-Dahlmann, B. High Serum Leptin Levels Subsequent to Weight Gain Predict Renewed Weight Loss in Patients with Anorexia Nervosa. Psychoneuroendocrinology 2004, 29, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Eijkenboom, M.; Van Der Staay, F.J. Spatial Learning Deficits in Rats after Injection of Vincristine into the Dorsal Hippocampus. Neuroscience 1999, 91, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Dong, H.-W. Are The Dorsal and Ventral Hippocampus Functionally Distinct Structures? Neuron 2010, 65, 7. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, D.M.; Nordquist, R.E.; Vanderschuren, L.J.M.J.; van der Staay, F.-J. Spatial Memory Deficits after Vincristine-Induced Lesions to the Dorsal Hippocampus. PLoS ONE 2020, 15, e0231941. [Google Scholar] [CrossRef]
- Ragu-Varman, D.; Macedo-Mendoza, M.; Labrada-Moncada, F.E.; Reyes-Ortega, P.; Morales, T.; Martínez-Torres, A.; Reyes-Haro, D. Anorexia Increases Microglial Density and Cytokine Expression in the Hippocampus of Young Female Rats. Behav. Brain Res. 2019, 363, 118–125. [Google Scholar] [CrossRef]
- Webster, M.; Ungerleider, L.; Bachevalier, J. Connections of Inferior Temporal Areas TE and TEO with Medial Temporal- Lobe Structures in Infant and Adult Monkeys. J. Neurosci. 1991, 11, 1095–1116. [Google Scholar] [CrossRef]
- Guo, M.; Huang, T.-Y.; Garza, J.C.; Chua, S.C.; Lu, X.-Y. Selective Deletion of Leptin Receptors in Adult Hippocampus Induces Depression-Related Behaviors. Int. J. Neuropsychopharmacol. 2013, 16, 857–867. [Google Scholar] [CrossRef]
- Wable, G.S.; Min, J.-Y.; Chen, Y.-W.; Aoki, C. Anxiety Is Correlated with Running in Adolescent Female Mice Undergoing Activity-Based Anorexia. Behav. Neurosci. 2015, 129, 170–182. [Google Scholar] [CrossRef]
- Aoki, C.; Santiago, A.N. Pathway-Specific GABAergic Inhibition Contributes to the Gain of Resilience against Anorexia-like Behavior of Adolescent Female Mice. Front. Behav. Neurosci. 2022, 16, 990354. [Google Scholar] [CrossRef]
- Anckarsäter, H.; Hofvander, B.; Billstedt, E.; Gillberg, I.C.; Gillberg, C.; Wentz, E.; Råstam, M. The Sociocommunicative Deficit Subgroup in Anorexia Nervosa: Autism Spectrum Disorders and Neurocognition in a Community-Based, Longitudinal Study. Psychol. Med. 2012, 42, 1957–1967. [Google Scholar] [CrossRef]
- Bora, E.; Köse, S. Meta-Analysis of Theory of Mind in Anorexia Nervosa and Bulimia Nervosa: A Specific İmpairment of Cognitive Perspective Taking in Anorexia Nervosa? Int. J. Eat. Disord. 2016, 49, 739–740. [Google Scholar] [CrossRef]
- Kaye, W.H.; Bulik, C.M.; Thornton, L.; Barbarich, N.; Masters, K. Comorbidity of Anxiety Disorders with Anorexia and Bulimia Nervosa. Am. J. Psychiatry 2004, 161, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y. Role of Mammalian DNA Methyltransferases in Development. Annu. Rev. Biochem. 2020, 89, 135–158. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the Interplay of Inflammation, Metabolism and Immune System Disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Da Ré, C.; Souza, J.M.; Fróes, F.; Taday, J.; Dos Santos, J.P.; Rodrigues, L.; Sesterheim, P.; Gonçalves, C.A.; Leite, M.C. Neuroinflammation Induced by Lipopolysaccharide Leads to Memory Impairment and Alterations in Hippocampal Leptin Signaling. Behav. Brain Res. 2020, 379, 112360. [Google Scholar] [CrossRef] [PubMed]
- Dees, C.; Pötter, S.; Zhang, Y.; Bergmann, C.; Zhou, X.; Luber, M.; Wohlfahrt, T.; Karouzakis, E.; Ramming, A.; Gelse, K.; et al. TGF-β-Induced Epigenetic Deregulation of SOCS3 Facilitates STAT3 Signaling to Promote Fibrosis. J. Clin. Invest. 2020, 130, 2347–2363. [Google Scholar] [CrossRef]
- Foerde, K.; Steinglass, J.E. Decreased Feedback Learning in Anorexia Nervosa Persists after Weight Restoration. Int. J. Eat. Disord. 2017, 50, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Guardia, D.; Carey, A.; Cottencin, O.; Thomas, P.; Luyat, M. Disruption of Spatial Task Performance in Anorexia Nervosa. PLoS ONE 2013, 8, e54928. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Akad, A.; Aderogba, R.; Chowdhury, T.G.; Aoki, C. Dendrites of the Dorsal and Ventral Hippocampal CA1 Pyramidal Neurons of Singly Housed Female Rats Exhibit Lamina-Specific Growths and Retractions during Adolescence That Are Responsive to Pair Housing. Synapse 2018, 72, e22034. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.; Chowdhury, T.G.; Wable, G.S.; Chen, Y.-W. Synaptic Changes in the Hippocampus of Adolescent Female Rodents Associated with Resilience to Anxiety and Suppression of Food Restriction-Evoked Hyperactivity in an Animal Model for Anorexia Nervosa. Brain Res. 2017, 1654, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.G.; Barbarich-Marsteller, N.C.; Chan, T.E.; Aoki, C. Activity-Based Anorexia Has Differential Effects on Apical Dendritic Branching in Dorsal and Ventral Hippocampal CA1. Brain Struct. Funct. 2014, 219, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.G.; Ríos, M.B.; Chan, T.E.; Cassataro, D.S.; Barbarich-Marsteller, N.C.; Aoki, C. Activity-Based Anorexia during Adolescence Disrupts Normal Development of the CA1 Pyramidal Cells in the Ventral Hippocampus of Female Rats. Hippocampus 2014, 24, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Berner, L.A.; Brown, T.A.; Lavender, J.M.; Lopez, E.; Wierenga, C.E.; Kaye, W.H. Neuroendocrinology of Reward in Anorexia Nervosa and Bulimia Nervosa: Beyond Leptin and Ghrelin. Mol. Cell Endocrinol. 2019, 497, 110320. [Google Scholar] [CrossRef]
- Amorim, T.; Khiyami, A.; Latif, T.; Fazeli, P.K. Neuroendocrine Adaptations to Starvation. Psychoneuroendocrinology 2023, 157, 106365. [Google Scholar] [CrossRef]
- Mottarlini, F.; Bottan, G.; Tarenzi, B.; Colciago, A.; Fumagalli, F.; Caffino, L. Activity-Based Anorexia Dynamically Dysregulates the Glutamatergic Synapse in the Nucleus Accumbens of Female Adolescent Rats. Nutrients 2020, 12, 3661. [Google Scholar] [CrossRef]
- Mottarlini, F.; Targa, G.; Bottan, G.; Tarenzi, B.; Fumagalli, F.; Caffino, L. Cortical Reorganization of the Glutamate Synapse in the Activity-Based Anorexia Rat Model: Impact on Cognition. J. Neurochem. 2022, 161, 350–365. [Google Scholar] [CrossRef]
- Hsuchou, H.; Pan, W.; Barnes, M.J.; Kastin, A.J. Leptin Receptor mRNA in Rat Brain Astrocytes. Peptides 2009, 30, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, V.; Contreras, A.; Merino, B.; Plaza, A.; Lorenzo, M.P.; García-Cáceres, C.; García, A.; Chowen, J.A.; Ruiz-Gayo, M.; Del Olmo, N.; et al. Specific Deletion of the Astrocyte Leptin Receptor Induces Changes in Hippocampus Glutamate Metabolism, Synaptic Transmission and Plasticity. Neuroscience 2020, 447, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Plieger, M.; Milos, G.; Peters, T.; Hinney, A.; Antel, J. Does Hypoleptinemia Trigger Entrapment in Anorexia Nervosa? Etiological and Clinical Considerations. Eur. Eat. Disord. Rev. 2024, 32, 557–574. [Google Scholar] [CrossRef]
- Rajcsanyi, L.S.; Zheng, Y.; Herpertz-Dahlmann, B.; Seitz, J.; de Zwaan, M.; Herzog, W.; Ehrlich, S.; Zipfel, S.; Giel, K.; Egberts, K.; et al. Unexpected Identification of Obesity-Associated Mutations in LEP and MC4R Genes in Patients with Anorexia Nervosa. Sci. Rep. 2024, 14, 7067. [Google Scholar] [CrossRef] [PubMed]
- Keeler, J.L.; Kan, C.; Treasure, J.; Himmerich, H. Novel Treatments for Anorexia Nervosa: Insights from Neuroplasticity Research. Eur. Eat. Disord. Rev. 2023. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Targa, G.; Mottarlini, F.; Rizzi, B.; Taddini, S.; Parolaro, S.; Fumagalli, F.; Caffino, L. Anorexia-Induced Hypoleptinemia Drives Adaptations in the JAK2/STAT3 Pathway in the Ventral and Dorsal Hippocampus of Female Rats. Nutrients 2024, 16, 1171. https://doi.org/10.3390/nu16081171
Targa G, Mottarlini F, Rizzi B, Taddini S, Parolaro S, Fumagalli F, Caffino L. Anorexia-Induced Hypoleptinemia Drives Adaptations in the JAK2/STAT3 Pathway in the Ventral and Dorsal Hippocampus of Female Rats. Nutrients. 2024; 16(8):1171. https://doi.org/10.3390/nu16081171
Chicago/Turabian StyleTarga, Giorgia, Francesca Mottarlini, Beatrice Rizzi, Sofia Taddini, Susanna Parolaro, Fabio Fumagalli, and Lucia Caffino. 2024. "Anorexia-Induced Hypoleptinemia Drives Adaptations in the JAK2/STAT3 Pathway in the Ventral and Dorsal Hippocampus of Female Rats" Nutrients 16, no. 8: 1171. https://doi.org/10.3390/nu16081171
APA StyleTarga, G., Mottarlini, F., Rizzi, B., Taddini, S., Parolaro, S., Fumagalli, F., & Caffino, L. (2024). Anorexia-Induced Hypoleptinemia Drives Adaptations in the JAK2/STAT3 Pathway in the Ventral and Dorsal Hippocampus of Female Rats. Nutrients, 16(8), 1171. https://doi.org/10.3390/nu16081171




