Preconceptional and Periconceptional Folic Acid Supplementation in the Visegrad Group Countries for the Prevention of Neural Tube Defects
Abstract
1. Introduction
2. Historical Overview of FA Supplementation in the Visegrad Group Countries
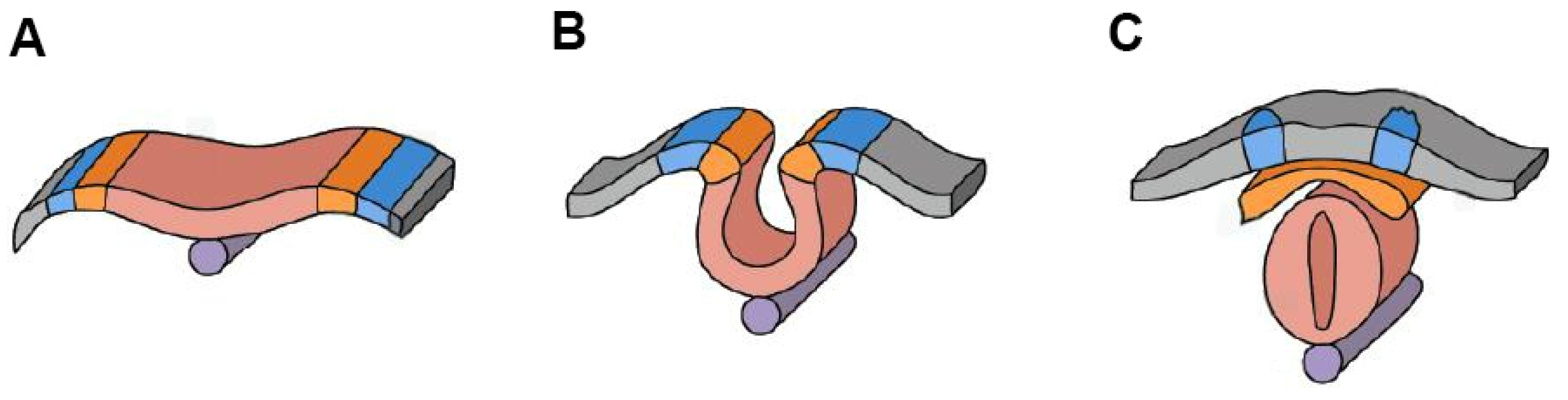
3. Neurulation and Genetic Predisposition to NTDs
| Neural Tube Defect | Characteristics | Causes | Consequences |
|---|---|---|---|
| Spina bifida | Incomplete closure of the spinal canal. Severe cases lead to paralysis of lower limbs and bladder/bowel dysfunction. | Genetic factors (e.g., MTHFR, FOLR1 SNPs), folate deficiency, infections during pregnancy, maternal diabetes, obesity. | Movement impairment, loss of sensation, incontinence, hydrocephalus, cognitive impairments [34]. |
| Anencephaly | Absence of the brain and most of the skull. | Genetic factors, teratogenic substances (e.g., alcohol, drugs), maternal folate deficiency (increased risk with certain MTHFR SNPs), environmental toxins. | Often results in fetal death or death shortly after birth [35]. |
| Encephalocele | External sac containing brain tissue on the head or neck. | Genetic factors (e.g., defects in early embryonic development), maternal folate deficiency, environmental factors. | Developmental delays, neurological abnormalities depending on sac size and location [36]. |
| Myelomeningocele | Protrusion of meninges and part of the spinal cord from the back. | Incomplete closure of the neural tube during development, genetic factors (MTHFR polymorphisms), maternal folate deficiency, infections. | Severe paralysis, incontinence, pelvic organ dysfunction, cognitive and motor impairments [37]. |
| Closed neural tube defects (lipomyelocele, lipomyelomeningocele, meningocele, myelocystocele) | Defects involving the accumulation of fatty tissue or abnormal spinal structures. | Less well-defined causes compared to open defects but may involve genetic predispositions (MTHFR and other folate-related SNPs), maternal folate deficiency, and early developmental issues. | Less severe but may lead to spinal deformities, mild neurological symptoms and developmental delays [31]. |
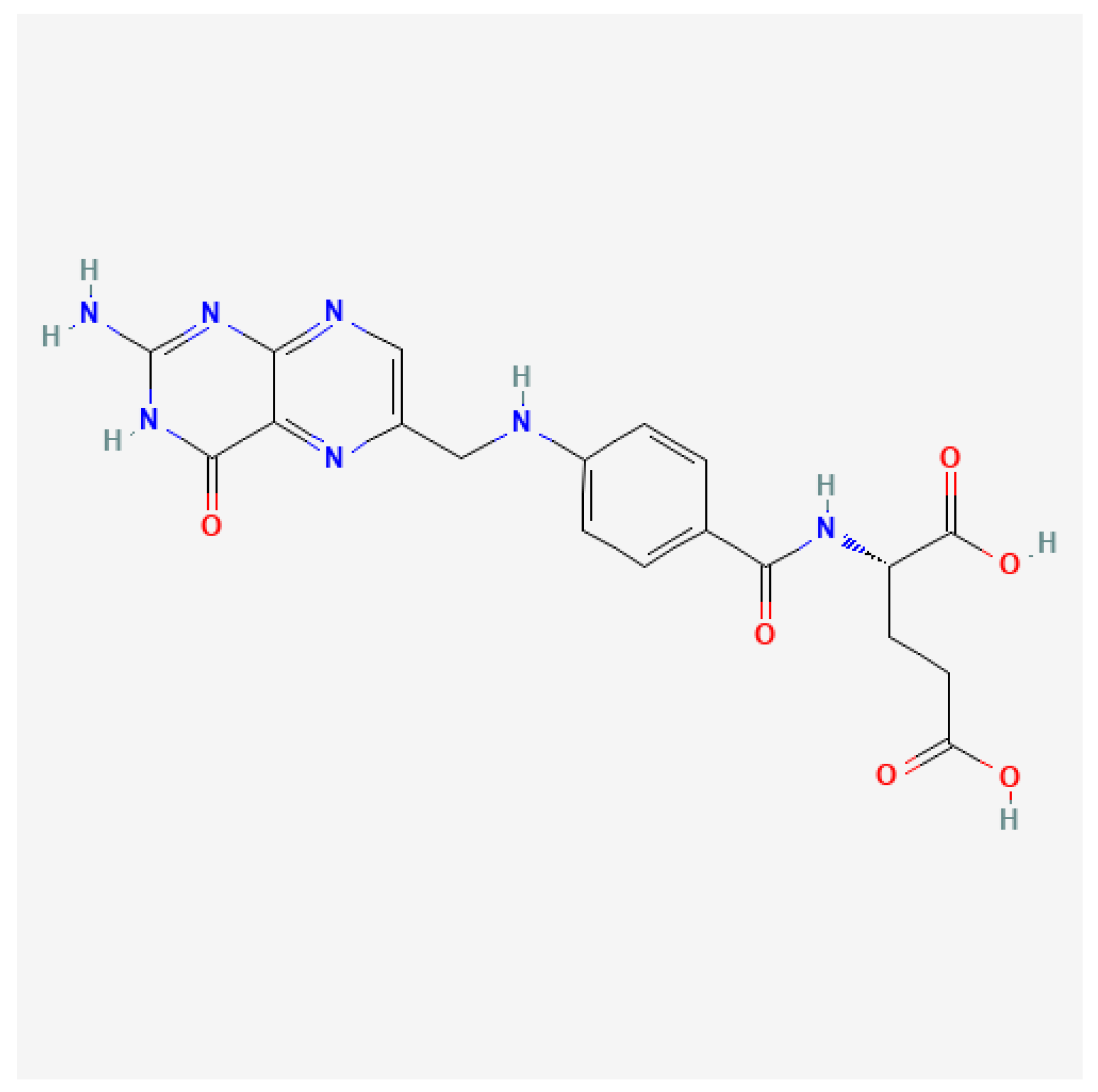
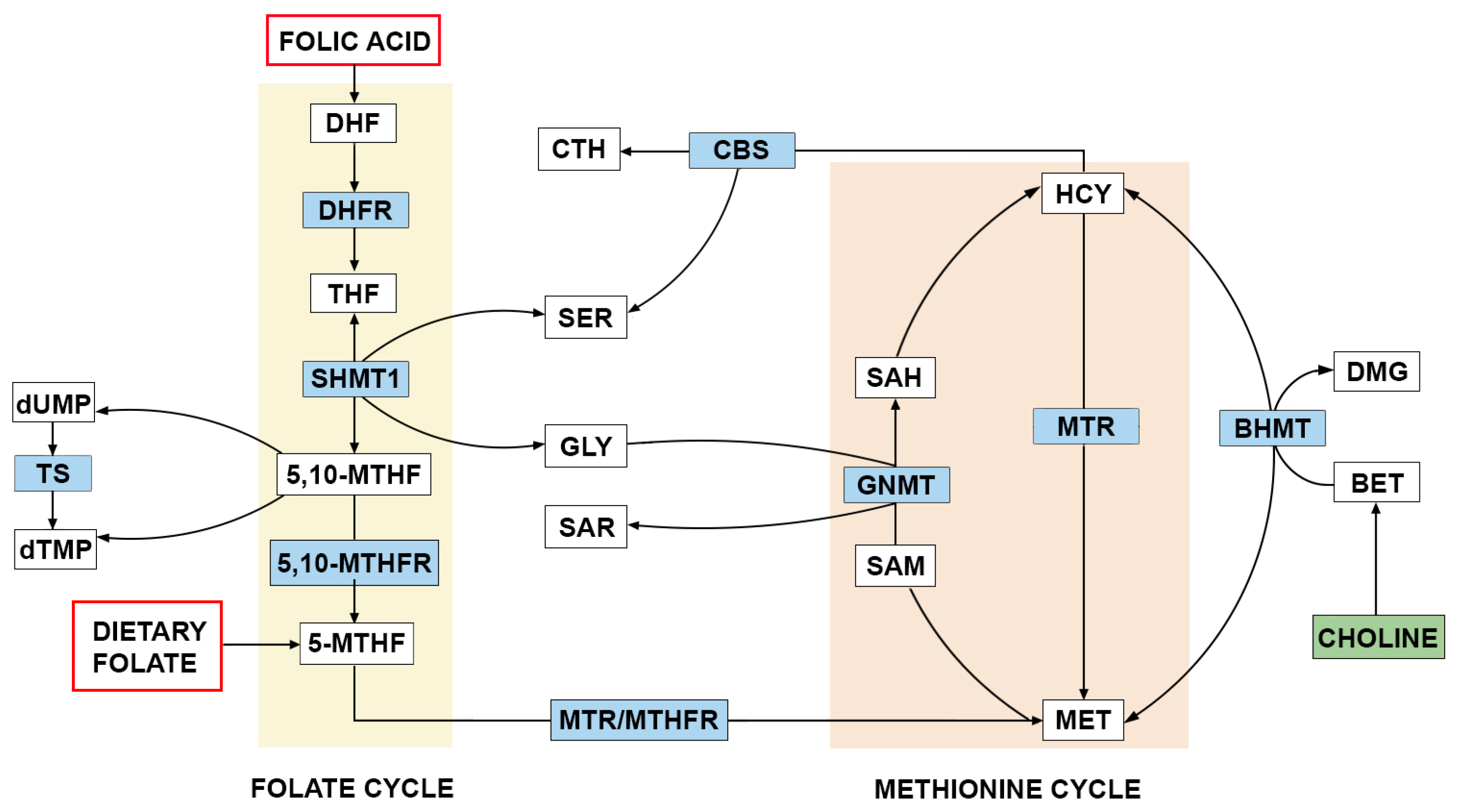
4. The Role of FA and One-Carbon Metabolism in the Etiology of NTDs
5. Preconceptional and Periconceptional FA Supplementation in Visegrad Group Countries
6. Comprehensive Strategies for NTDs Prevention
6.1. Recommended Daily Intake
6.2. Timing of Supplementation
6.3. Preconception Counselling
6.4. National Level Strategic Plan
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greene, N.D.E.; Copp, A.J. Neural Tube Defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Massa, V.; George, T.M.; Qureshy, S.; Bulfamante, G.; Finnell, R.H. Overview on Neural Tube Defects: From Development to Physical Characteristics. Birth Defects Res. 2019, 111, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.D.E.; Stanier, P.; Copp, A.J. Genetics of Human Neural Tube Defects. Hum. Mol. Genet. 2009, 18, R113–R129. [Google Scholar] [CrossRef]
- Greene, N.D.E.; Stanier, P.; Moore, G.E. The Emerging Role of Epigenetic Mechanisms in the Etiology of Neural Tube Defects. Epigenetics 2011, 6, 875–883. [Google Scholar] [CrossRef]
- Padmanabhan, R. Etiology, Pathogenesis and Prevention of Neural Tube Defects. Congenit. Anom. 2006, 46, 55–67. [Google Scholar] [CrossRef]
- Cabrera, R.M.; Hill, D.S.; Etheredge, A.J.; Finnell, R.H. Investigations into the Etiology of Neural Tube Defects. Birth Defects Res. Part C Embryo Today Rev. 2004, 72, 330–344. [Google Scholar] [CrossRef]
- Isaković, J.; Šimunić, I.; Jagečić, D.; Hribljan, V.; Mitrečić, D. Overview of Neural Tube Defects: Gene-Environment Interactions, Preventative Approaches and Future Perspectives. Biomedicines 2022, 10, 965. [Google Scholar] [CrossRef]
- Finnell, R.H.; Caiaffa, C.D.; Kim, S.-E.; Lei, Y.; Steele, J.; Cao, X.; Tukeman, G.; Lin, Y.L.; Cabrera, R.M.; Wlodarczyk, B.J. Gene Environment Interactions in the Etiology of Neural Tube Defects. Front. Genet. 2021, 12, 659612. [Google Scholar] [CrossRef]
- Wolujewicz, P.; Ross, M.E. The Search for Genetic Determinants of Human Neural Tube Defects. Curr. Opin. Pediatr. 2019, 31, 739–746. [Google Scholar] [CrossRef]
- Rogers, L.M.; Cordero, A.M.; Pfeiffer, C.M.; Hausman, D.B.; Tsang, B.L.; De-Regil, L.M.; Rosenthal, J.; Razzaghi, H.; Wong, E.C.; Weakland, A.P.; et al. Global Folate Status in Women of Reproductive Age: A Systematic Review with Emphasis on Methodological Issues. Ann. N. Y. Acad. Sci. 2018, 1431, 35–57. [Google Scholar] [CrossRef]
- Wilcken, B.; Bamforth, F.; Li, Z.; Zhu, H.; Ritvanen, A.; Renlund, M.; Stoll, C.; Alembik, Y.; Dott, B.; Czeizel, A.E.; et al. Geographical and Ethnic Variation of the 677C>T Allele of 5,10 Methylenetetrahydrofolate Reductase (MTHFR): Findings from over 7000 Newborns from 16 Areas World Wide. J. Med. Genet. 2003, 40, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Burda, P.; Schäfer, A.; Suormala, T.; Rummel, T.; Bürer, C.; Heuberger, D.; Frapolli, M.; Giunta, C.; Sokolová, J.; Vlášková, H.; et al. Insights into Severe 5,10-Methylenetetrahydrofolate Reductase Deficiency: Molecular Genetic and Enzymatic Characterization of 76 Patients. Hum. Mutat. 2015, 36, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Lachenauer, E.R. Folate One-Carbon Metabolism in Mouse Models of Neural Tube Defects. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2019. [Google Scholar]
- PubChem Folic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/135398658 (accessed on 27 November 2024).
- Estevez-Ordonez, D.; Davis, M.C.; Hopson, B.; Arynchyna, A.; Rocque, B.G.; Fieggen, G.; Rosseau, G.; Oakley, G.; Blount, J.P. Reducing Inequities in Preventable Neural Tube Defects: The Critical and Underutilized Role of Neurosurgical Advocacy for Folate Fortification. Neurosurg. Focus 2018, 45, E20. [Google Scholar] [CrossRef] [PubMed]
- Hudec, M. Development of the Visegrad Group in the Context of Efforts to Accelerate the Convergence Processes by Joining the European Union. Stud. Commer. Bratisl. 2016, 9, 26–35. [Google Scholar] [CrossRef][Green Version]
- Smithells, R.W.; Nevin, N.C.; Seller, M.J.; Sheppard, S.; Harris, R.; Read, A.P.; Fielding, D.W.; Walker, S.; Schorah, C.J.; Wild, J. Further Experience of Vitamin Supplementation for Prevention of Neural Tube Defect Recurrences. Lancet 1983, 1, 1027–1031. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I. Prevention of the First Occurrence of Neural-Tube Defects by Periconceptional Vitamin Supplementation. N. Engl. J. Med. 1992, 327, 1832–1835. [Google Scholar] [CrossRef]
- Czeizel, A.E. Reduction of Urinary Tract and Cardiovascular Defects by Periconceptional Multivitamin Supplementation. Am. J. Med. Genet. 1996, 62, 179–183. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dobó, M.; Vargha, P. Hungarian Cohort-Controlled Trial of Periconceptional Multivitamin Supplementation Shows a Reduction in Certain Congenital Abnormalities. Birt. Defects Res. A Clin. Mol. Teratol. 2004, 70, 853–861. [Google Scholar] [CrossRef]
- Tolarová, M. Orofacial Clefts in Czechoslovakia. Incidence, Genetics and Prevention of Cleft Lip and Palate over a 19-Year Period. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1987, 21, 19–25. [Google Scholar] [CrossRef]
- Brzeziński, Z. Primary prevention program for neural tube defects in Poland. Med. Wieku Rozwoj. 1999, 3, 503–508. [Google Scholar]
- Czochańska, J.; Lech, M. Prevention of neural tube defects. An important health and social problem. Przegl. Lek. 1998, 55, 174–178. [Google Scholar] [PubMed]
- Mierzejewska, E. Methylene tetrahydrofolate reductase mutations as genetic risk factors for neural tube defects (NTF). Med. Wieku Rozwoj. 1999, 3, 521–527. [Google Scholar] [PubMed]
- Wiśniewska, K.; Wysocki, J. The Importance of Folic Acid in the Primary Prevention of Congenital Malformations. Arch. Perinat. Med. 2008, 14, 32–40. [Google Scholar]
- Hobbs, C.A.; Cleves, M.A.; Karim, M.A.; Zhao, W.; MacLeod, S.L. Maternal Folate-Related Gene Environment Interactions and Congenital Heart Defects. Obstet. Gynecol. 2010, 116, 316–322. [Google Scholar] [CrossRef]
- van Rooij, I.A.L.M.; Ocké, M.C.; Straatman, H.; Zielhuis, G.A.; Merkus, H.M.W.M.; Steegers-Theunissen, R.P.M. Periconceptional Folate Intake by Supplement and Food Reduces the Risk of Nonsyndromic Cleft Lip with or without Cleft Palate. Prev. Med. 2004, 39, 689–694. [Google Scholar] [CrossRef]
- Liu, J.; Li, Z.; Ye, R.; Ren, A.; Liu, J. Folic Acid Supplementation and Risk for Congenital Limb Reduction Defects in China. Int. J. Epidemiol. 2019, 48, 2010–2017. [Google Scholar] [CrossRef]
- Schoenwolf, G.C.; Bleyl, S.B.; Brauer, P.R.; Francis-West, P.H. Larsen’s Human Embryology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; ISBN 978-1-4557-2791-9. [Google Scholar]
- Wang, X.; Yu, J.; Wang, J. Neural Tube Defects and Folate Deficiency: Is DNA Repair Defective? Int. J. Mol. Sci. 2023, 24, 2220. [Google Scholar] [CrossRef]
- Rossi, A.; Biancheri, R.; Cama, A.; Piatelli, G.; Ravegnani, M.; Tortori-Donati, P. Imaging in Spine and Spinal Cord Malformations. Eur. J. Radiol. 2004, 50, 177–200. [Google Scholar] [CrossRef]
- Wang, M.; de Marco, P.; Capra, V.; Kibar, Z. Update on the Role of the Non-Canonical Wnt/Planar Cell Polarity Pathway in Neural Tube Defects. Cells 2019, 8, 1198. [Google Scholar] [CrossRef]
- Feinberg, T.E.; Mallatt, J. The Evolutionary and Genetic Origins of Consciousness in the Cambrian Period over 500 Million Years Ago. Front. Psychol. 2013, 4, 667. [Google Scholar] [CrossRef]
- MRC Vitamin Study Research Group. Prevention of Neural Tube Defects: Results of the Medical Research Council Vitamin Study. Lancet 1991, 338, 131–137. [Google Scholar]
- Botto, L.D.; Moore, C.A.; Khoury, M.J.; Erickson, J.D. Neural-Tube Defects. N. Engl. J. Med. 1999, 341, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Veerabathini, B.C.; Manthani, K.; Hussain, A. Congenital Central Nervous System Malformations: A Rare Case of an Encephalocele and Literature Review of Its Associations, Imaging Modalities, Radiological Findings, and Treatments. Cureus 2021, 13, e15959. [Google Scholar] [CrossRef]
- Adzick, N.S.; Thom, E.A.; Spong, C.Y.; Brock, J.W.; Burrows, P.K.; Johnson, M.P.; Howell, L.J.; Farrell, J.A.; Dabrowiak, M.E.; Sutton, L.N.; et al. A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele. N. Engl. J. Med. 2011, 364, 993–1004. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Wang, B.; Ding, C.; Liu, H. Association between MTHFR C677T Polymorphism and Neural Tube Defect Risks: A Comprehensive Evaluation in Three Groups of NTD Patients, Mothers, and Fathers. Birt. Defects Res. A Clin. Mol. Teratol. 2015, 103, 488–500. [Google Scholar] [CrossRef]
- O’Byrne, M.R.; Au, K.S.; Morrison, A.C.; Lin, J.-I.; Fletcher, J.M.; Ostermaier, K.K.; Tyerman, G.H.; Doebel, S.; Northrup, H. Association of Folate Receptor (FOLR1, FOLR2, FOLR3) and Reduced Folate Carrier (SLC19A1) Genes with Meningomyelocele. Birt. Defects Res. A Clin. Mol. Teratol. 2010, 88, 689–694. [Google Scholar] [CrossRef]
- Peng, M.-Z.; Shao, Y.-X.; Li, X.-Z.; Zhang, K.-D.; Cai, Y.-N.; Lin, Y.-T.; Jiang, M.-Y.; Liu, Z.-C.; Su, X.-Y.; Zhang, W.; et al. Mitochondrial FAD Shortage in SLC25A32 Deficiency Affects Folate-Mediated One-Carbon Metabolism. Cell. Mol. Life Sci. 2022, 79, 375. [Google Scholar] [CrossRef]
- Steele, J.W.; Kim, S.-E.; Finnell, R.H. One-Carbon Metabolism and Folate Transporter Genes: Do They Factor Prominently in the Genetic Etiology of Neural Tube Defects? Biochimie 2020, 173, 27–32. [Google Scholar] [CrossRef]
- Zimmer, M.; Sieroszewski, P.; Oszukowski, P.; Huras, H.; Fuchs, T.; Pawlosek, A. Polish Society of Gynecologists and Obstetricians Recommendations on Supplementation during Pregnancy. Ginekol. Pol. 2020, 91, 644–653. [Google Scholar] [CrossRef]
- Cai, S.; Quan, S.; Yang, G.; Ye, Q.; Chen, M.; Yu, H.; Wang, G.; Wang, Y.; Zeng, X.; Qiao, S. One Carbon Metabolism and Mammalian Pregnancy Outcomes. Mol. Nutr. Food Res. 2021, 65, e2000734. [Google Scholar] [CrossRef]
- Jindasereekul, P.; Jirarattanarangsri, W.; Khemacheewakul, J.; Leksawasdi, N.; Thiennimitr, P.; Taesuwan, S. Usual Intake of One-Carbon Metabolism Nutrients in a Young Adult Population Aged 19–30 Years: A Cross-Sectional Study. J. Nutr. Sci. 2023, 12, e51. [Google Scholar] [CrossRef] [PubMed]
- Martiniova, L.; Field, M.S.; Finkelstein, J.L.; Perry, C.A.; Stover, P.J. Maternal Dietary Uridine Causes, and Deoxyuridine Prevents, Neural Tube Closure Defects in a Mouse Model of Folate-Responsive Neural Tube Defects. Am. J. Clin. Nutr. 2015, 101, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Findley, T.; Tenpenny, J.C.; O’Byrne, M.R.; Morrison, A.C.; Hixson, J.E.; Northrup, H.; Au, K.S. Mutations in Folate Transporter Genes and Risk for Human Myelomeningocele. Am. J. Med. Genet. A 2017, 173, 2973–2984. [Google Scholar] [CrossRef]
- Cai, C.-Q.; Fang, Y.-L.; Shu, J.-B.; Zhao, L.-S.; Zhang, R.-P.; Cao, L.-R.; Wang, Y.-Z.; Zhi, X.-F.; Cui, H.-L.; Shi, O.-Y.; et al. Association of Neural Tube Defects with Maternal Alterations and Genetic Polymorphisms in One-Carbon Metabolic Pathway. Ital. J. Pediatr. 2019, 45, 37. [Google Scholar] [CrossRef]
- Zarembska, E.; Ślusarczyk, K.; Wrzosek, M. The Implication of a Polymorphism in the Methylenetetrahydrofolate Reductase Gene in Homocysteine Metabolism and Related Civilisation Diseases. Int. J. Mol. Sci. 2024, 25, 193. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, L.; Long, Y.; Zou, P.; Ji, G.; Gu, A.; Zhao, P. Association of the Maternal MTHFR C677T Polymorphism with Susceptibility to Neural Tube Defects in Offsprings: Evidence from 25 Case-Control Studies. PLoS ONE 2012, 7, e41689. [Google Scholar] [CrossRef]
- Behunova, J.; Klimcakova, L.; Zavadilikova, E.; Potocekova, D.; Sykora, P.; Podracka, L. Methylenetetrahydrofolate Reductase Gene Polymorphisms and Neural Tube Defects Epidemiology in the Slovak Population. Birt. Defects Res. A Clin. Mol. Teratol. 2010, 88, 695–700. [Google Scholar] [CrossRef]
- Doležálková, E.; Unzeitig, V. Folic acid and prevention of the neural tube defects. Ceska Gynekol. 2014, 79, 134–139. [Google Scholar]
- de la Fournière, B.; Dhombres, F.; Maurice, P.; de Foucaud, S.; Lallemant, P.; Zérah, M.; Guilbaud, L.; Jouannic, J.-M. Prevention of Neural Tube Defects by Folic Acid Supplementation: A National Population-Based Study. Nutrients 2020, 12, 3170. [Google Scholar] [CrossRef]
- Bar-Oz, B.; Koren, G.; Nguyen, P.; Kapur, B.M. Folate Fortification and Supplementation—Are We There Yet? Reprod. Toxicol. 2008, 25, 408–412. [Google Scholar] [CrossRef]
- Skrypnik, D.; Moszak, M.; Wender-Ozegowska, E.; Bogdanski, P. Comparison of Polish and International Guidelines on Diet Supplements in Pregnancy—Review. Ginekol. Pol. 2021, 92, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Eljazzar, S.; Ganji, V. Intended and Unintended Benefits of Folic Acid Fortification-A Narrative Review. Foods 2023, 12, 1612. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, J. The Evolution of Folate Supplementation—From One Size for All to Personalized, Precision, Poly-Paths. J. Transl. Intern. Med. 2023, 11, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Bobrowski-Khoury, N.; Sequeira, J.M.; Arning, E.; Bottiglieri, T.; Quadros, E.V. Absorption and Tissue Distribution of Folate Forms in Rats: Indications for Specific Folate Form Supplementation during Pregnancy. Nutrients 2022, 14, 2397. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic Acid versus 5- Methyl Tetrahydrofolate Supplementation in Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef] [PubMed]
- USDA FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 2 December 2024).
- USDA Database for the Choline Content of Common Foods, Release 2 (2008) 2015. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=221425 (accessed on 19 December 2024).
- Assefa, N.; Abdullahi, Y.Y.; Abraham, A.; Hemler, E.C.; Madzorera, I.; Dessie, Y.; Roba, K.T.; Fawzi, W.W. Consumption of Dietary Folate Estimates and Its Implication for Reproductive Outcome among Women of Reproductive Age in Kersa: Cross-Sectional Survey. BMC Nutr. 2021, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F.; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Agodi, A. Dietary Folate Intake and Folic Acid Supplements among Pregnant Women from Southern Italy: Evidence from the “Mamma & Bambino” Cohort. Int. J. Environ. Res. Public Health 2020, 17, 638. [Google Scholar] [CrossRef]
- Carboni, L. Active Folate Versus Folic Acid: The Role of 5-MTHF (Methylfolate) in Human Health. Integr. Med. 2022, 21, 36–41. [Google Scholar]
- Henderson, A.M.; Aleliunas, R.E.; Loh, S.P.; Khor, G.L.; Harvey-Leeson, S.; Glier, M.B.; Kitts, D.D.; Green, T.J.; Devlin, A.M. L-5-Methyltetrahydrofolate Supplementation Increases Blood Folate Concentrations to a Greater Extent than Folic Acid Supplementation in Malaysian Women. J. Nutr. 2018, 148, 885–890. [Google Scholar] [CrossRef]
- Benavides-Lara, A.; Fernández-Sánchez, O.; Barboza-Argüello, M.D.L.P.; Alfaro-Calvo, T.; Martínez, H. Integrated Surveillance Strategy to Support the Prevention of Neural Tube Defects through Food Fortification with Folic Acid: The Experience of Costa Rica. Child’s Nerv. Syst. 2023, 39, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Martinez, H.; Benavides-Lara, A.; Arynchyna-Smith, A.; Ghotme, K.A.; Arabi, M.; Arynchyn, A. Global Strategies for the Prevention of Neural Tube Defects through the Improvement of Folate Status in Women of Reproductive Age. Childs Nerv. Syst. 2023, 39, 1719–1736. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; Halsey, J.; Sherliker, P.; Pan, H.; Chen, Z.; Bennett, D.A.; Clarke, R. Global Heterogeneity in Folic Acid Fortification Policies and Implications for Prevention of Neural Tube Defects and Stroke: A Systematic Review. EClinicalMedicine 2024, 67, 102366. [Google Scholar] [CrossRef] [PubMed]
- De Wals, P.; Rusen, I.D.; Lee, N.S.; Morin, P.; Niyonsenga, T. Trend in Prevalence of Neural Tube Defects in Quebec. Birt. Defects Res. A Clin. Mol. Teratol. 2003, 67, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Hursthouse, N.A.; Gray, A.R.; Miller, J.C.; Rose, M.C.; Houghton, L.A. Folate Status of Reproductive Age Women and Neural Tube Defect Risk: The Effect of Long-Term Folic Acid Supplementation at Doses of 140 Μg and 400 Μg per Day. Nutrients 2011, 3, 49–62. [Google Scholar] [CrossRef]
- Mosley, B.S.; Cleves, M.A.; Siega-Riz, A.M.; Shaw, G.M.; Canfield, M.A.; Waller, D.K.; Werler, M.M.; Hobbs, C.A.; National Birth Defects Prevention Study. Neural Tube Defects and Maternal Folate Intake among Pregnancies Conceived after Folic Acid Fortification in the United States. Am. J. Epidemiol. 2009, 169, 9–17. [Google Scholar] [CrossRef]
- Luo, H.; Brown, K.H.; Stewart, C.P.; Beckett, L.A.; Clermont, A.; Vosti, S.A.; Guintang Assiene, J.M.; Engle-Stone, R. Review of Existing Models to Predict Reductions in Neural Tube Defects Due to Folic Acid Fortification and Model Results Using Data from Cameroon. Adv. Nutr. 2021, 12, 2401–2414. [Google Scholar] [CrossRef]
- Zadarko-Domaradzka, M.; Kruszyńska, E.; Zadarko, E. Effectiveness of Folic Acid Supplementation Recommendations among Polish Female Students from the Podkarpackie Region. Nutrients 2021, 13, 1001. [Google Scholar] [CrossRef]
- Bartošová, L. Potraviny obohatené vitamínmi a minerálmi—Sú potrebné pre naše zdravie? Trendy V Potravin. 2023, 28, 79–80. [Google Scholar]
- Czeizel, A.E.; Kökény, M. Bread Is Fortified with Folic Acid in Hungary. BMJ 2002, 325, 391. [Google Scholar] [CrossRef][Green Version]
- Škrečková, G.; Rimárová, K.; Takáč, P. The influence of folic acid on the aetiology of selected diseases. Hygiena 2022, 67, 101–106. [Google Scholar] [CrossRef]
- Guandalini, S.; Assiri, A. Celiac Disease: A Review. JAMA Pediatr. 2014, 168, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A Comprehensive Review and Update on Crohn’s Disease. Disease-a-Month 2018, 64, 20–57. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Guideline: Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, M.H.; Soraisham, A.; Bagga, N.; Massieu, L.A.; Maheshwari, A. Nutritional Management of Short Bowel Syndrome. Clin. Perinatol. 2022, 49, 557–572. [Google Scholar] [CrossRef]
- Tappenden, K.A. Pathophysiology of Short Bowel Syndrome: Considerations of Resected and Residual Anatomy. J. Parenter. Enter. Nutr. 2014, 38, 14S–22S. [Google Scholar] [CrossRef]
- Ami, N.; Bernstein, M.; Boucher, F.; Rieder, M.; Parker, L.; Canadian Paediatric Society; Drug Therapy and Hazardous Substances Committee. Folate and Neural Tube Defects: The Role of Supplements and Food Fortification. Paediatr. Child Health 2016, 21, 145–154. [Google Scholar] [CrossRef]
- Pravst, I.; Lavriša, Ž.; Hribar, M.; Hristov, H.; Kvarantan, N.; Seljak, B.K.; Gregorič, M.; Blaznik, U.; Gregorič, N.; Zaletel, K.; et al. Dietary Intake of Folate and Assessment of the Folate Deficiency Prevalence in Slovenia Using Serum Biomarkers. Nutrients 2021, 13, 3860. [Google Scholar] [CrossRef]
- Ji, H.J.; Kim, S.; Yon, M.; Hyun, T. Folate Content of Fast Foods and Processed Foods. Korean J. Nutr. 2009, 42, 397–405. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Recommendations for the Use of Folic Acid to Reduce the Number of Cases of Spina Bifida and Other Neural Tube Defects. Morb. Mortal. Wkly. Rep. MMWR 1992, 41, 1–7. [Google Scholar]
- Samson, K.L.I.; Loh, S.P.; Khor, G.L.; Mohd Shariff, Z.; Yelland, L.N.; Leemaqz, S.; Makrides, M.; Hutcheon, J.A.; Sulistyoningrum, D.C.; Yu, J.J.; et al. Effect of Once Weekly Folic Acid Supplementation on Erythrocyte Folate Concentrations in Women to Determine Potential to Prevent Neural Tube Defects: A Randomised Controlled Dose-Finding Trial in Malaysia. BMJ Open 2020, 10, e034598. [Google Scholar] [CrossRef] [PubMed]
- Gebremichael, T.G.; Welesamuel, T.G. Adherence to Iron-Folic Acid Supplement and Associated Factors among Antenatal Care Attending Pregnant Mothers in Governmental Health Institutions of Adwa Town, Tigray, Ethiopia: Cross-Sectional Study. PLoS ONE 2020, 15, e0227090. [Google Scholar] [CrossRef] [PubMed]
- Dolin, C.D.; Deierlein, A.L.; Evans, M.I. Folic Acid Supplementation to Prevent Recurrent Neural Tube Defects: 4 Milligrams Is Too Much. Fetal Diagn. Ther. 2018, 44, 161–165. [Google Scholar] [CrossRef]
- Bomba-Opoń, D.; Hirnle, L.; Kalinka, J.; Seremak-Mrozikiewicz, A. Folate Supplementation during the Preconception Period, Pregnancy and Puerperium. Polish Society of Gynecologists and Obstetricians Guidelines. Ginekol. Pol. 2017, 88, 633–636. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Z.; Lin, Y.; Xie, H. Risk of Excess Maternal Folic Acid Supplementation in Offspring. Nutrients 2024, 16, 755. [Google Scholar] [CrossRef]
- Wald, N.J.; Law, M.; Jordan, R. Folic Acid Food Fortification to Prevent Neural Tube Defects. Lancet 1998, 351, 834; author reply 834–835. [Google Scholar] [CrossRef]
- Scientific Opinion on the Tolerable Upper Intake Level for Folate|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/8353 (accessed on 8 December 2024).
- Jędrzejczak, J.; Bomba-Opoń, D.; Jakiel, G.; Kwaśniewska, A.; Mirowska-Guzel, D. Managing Epilepsy in Women of Childbearing Age—Polish Society of Epileptology and Polish Gynecological Society Guidelines. Ginekol. Pol. 2017, 88, 278–284. [Google Scholar] [CrossRef]
- Cawley, S.; Mullaney, L.; McKeating, A.; Farren, M.; McCartney, D.; Turner, M.J. A Review of European Guidelines on Periconceptional Folic Acid Supplementation. Eur. J. Clin. Nutr. 2016, 70, 143–154. [Google Scholar] [CrossRef]
- Crider, K.S.; Devine, O.; Hao, L.; Dowling, N.F.; Li, S.; Molloy, A.M.; Li, Z.; Zhu, J.; Berry, R.J. Population Red Blood Cell Folate Concentrations for Prevention of Neural Tube Defects: Bayesian Model. BMJ 2014, 349, g4554. [Google Scholar] [CrossRef]
- van Gool, J.D.; Hirche, H.; Lax, H.; De Schaepdrijver, L. Folic Acid and Primary Prevention of Neural Tube Defects: A Review. Reprod. Toxicol. 2018, 80, 73–84. [Google Scholar] [CrossRef]
- Dong, J.; Yin, L.-L.; Deng, X.-D.; Ji, C.-Y.; Pan, Q.; Yang, Z.; Peng, T.; Wu, J.-N.; Early Pregnancy Ultrasound Screening, Maternal Exposures and Congenital Malformation Risk Collaborators. Initiation and Duration of Folic Acid Supplementation in Preventing Congenital Malformations. BMC Med. 2023, 21, 292. [Google Scholar] [CrossRef] [PubMed]
- Santander Ballestín, S.; Giménez Campos, M.I.; Ballestín Ballestín, J.; Luesma Bartolomé, M.J. Is Supplementation with Micronutrients Still Necessary during Pregnancy? A Review. Nutrients 2021, 13, 3134. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.V.V.; Zhao, Y.; Binns, C.W.; Pham, N.M.; Nguyen, C.L.; Nguyen, P.T.H.; Chu, T.K.; Lee, A.H. Low Prevalence of Folic Acid Supplementation during Pregnancy: A Multicenter Study in Vietnam. Nutrients 2019, 11, 2347. [Google Scholar] [CrossRef]
- Nilsen, R.M.; Leoncini, E.; Gastaldi, P.; Allegri, V.; Agostino, R.; Faravelli, F.; Ferrazzoli, F.; Finale, E.; Ghirri, P.; Scarano, G.; et al. Prevalence and Determinants of Preconception Folic Acid Use: An Italian Multicenter Survey. Ital. J. Pediatr. 2016, 42, 65. [Google Scholar] [CrossRef]
- Ren, A.-G. Prevention of Neural Tube Defects with Folic Acid: The Chinese Experience. World J. Clin. Pediatr. 2015, 4, 41–44. [Google Scholar] [CrossRef]
- Lolowa, A.M.; Selim, N.; Alkuwari, M.; Salem Ismail, M. Knowledge and Intake of Folic Acid among Teachers of Childbearing Age in the State of Qatar: A Cross-Sectional Study. BMJ Open 2019, 9, e025005. [Google Scholar] [CrossRef]
- Halsted, C.H.; Villanueva, J.A.; Devlin, A.M.; Chandler, C.J. Metabolic Interactions of Alcohol and Folate. J. Nutr. 2002, 132, 2367S–2372S. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef]
- Tuenter, A.; Bautista Nino, P.K.; Vitezova, A.; Pantavos, A.; Bramer, W.M.; Franco, O.H.; Felix, J.F. Folate, Vitamin B12, and Homocysteine in Smoking-Exposed Pregnant Women: A Systematic Review. Matern. Child. Nutr. 2019, 15, e12675. [Google Scholar] [CrossRef]
- Otake, M.; Sakurai, K.; Watanabe, M.; Mori, C. Association Between Serum Folate Levels and Caffeinated Beverage Consumption in Pregnant Women in Chiba: The Japan Environment and Children’s Study. J. Epidemiol. 2018, 28, 414–419. [Google Scholar] [CrossRef]
- Lev, L.; Petersen, K.; Roberts, J.L.; Kupferer, K.; Werder, S. Exploring the Impact of Folic Acid Supplementation and Vitamin B12 Deficiency on Maternal and Fetal Outcomes in Pregnant Women with Celiac Disease. Nutrients 2024, 16, 3194. [Google Scholar] [CrossRef]
- Sipek, A., Jr.; Gregor, V.; Sipek, A. Primary Prevention of Congenital Anomalies and the Role of Folic Acid. Actual Gynecol. Obstet. 2013, 5, 47–51. [Google Scholar]
| Congenital Disorder | Influence of Folate Intake |
|---|---|
| Neural tube defects | Folate deficiency in pregnancy increases risk of closed and open NTDs [18]. |
| Heart defects | Research suggests that folate deficiency during pregnancy may be associated with an increased risk of congenital heart defects (non-syndromic septal, conotruncal, right or left-sided obstructive heart defect) [26]. |
| Cleft lip and palate | Some studies suggest that sufficient folate intake may reduce the risk of cleft lip and palate in newborns [27]. |
| Limb deformities | Folate is important for proper limb development; its deficiency may be associated with a variety of limb reduction defects [28]. |
| Food | Folate Content (µg/100 g) | Choline Content (mg/100 g) |
|---|---|---|
| High folate content (≥100 µg/100 g) | ||
| Beef liver (raw) | 290 | 333 |
| Spinach (raw) | 194 | 19.3 |
| Lentils (cooked) | 181 | 32.7 |
| Chickpeas (cooked) | 172 | 42.8 |
| Asparagus (cooked) | 149 | 26.1 |
| Moderate folate content (30–99 µg/100 g) | ||
| Avocado (raw) | 89 | 14.2 |
| Peas (raw) | 65 | 28.4 |
| Broccoli (raw) | 63 | 18.7 |
| Kimchi | 52 | 15.5 |
| Red bell pepper (raw) | 47 | 5.6 |
| Eggs (whole, cooked) | 44 | 294 |
| Mango (raw) | 43 | 7.6 |
| Bread (whole wheat) | 42 | 27.2 |
| Low folate content (<30 µg/100 g) | ||
| Salmon (Atlantic, farmed, raw) | 26 | 78.5 |
| Orange (raw) | 25 | 8.4 |
| Sauerkraut (fermented cabbage) | 23 | 10.4 |
| Carrots (raw) | 19 | 8.8 |
| Tofu | 15 | 28.8 |
| Potatoes (baked) | 9 | 14.5 |
| Chicken breast (cooked) | 4 | 35 |
| White rice (cooked) | 1 | 2.1 |
| Country | Recommending Society | Folic Acid Dosage | Common Recommendations | ||
|---|---|---|---|---|---|
| Low Risk * | Intermediate Risk ** | High Risk *** | |||
| Slovakia | The American Society of Gynaecologists and Obstetricians [54] | 400 µg/day 2–3 months prior to pregnancy and throughout the 1st trimester; 600 µg/day is recommended during the 2nd and 3rd trimester and throughout lactation | 1000 µg/day 3 months prior to pregnancy and throughout the 1st trimester | 4000 µg/day 3 months prior to pregnancy and the entire 1st trimester | A diet rich in folate is recommended for women of reproductive age. Vitamin B12 supplementation is recommended along with folate. The dosage of folic acid is based on the risk of NTDs. |
| Czechia | The Czech Society of Gynaecologists and Obstetricians [51] | 400–800 µg/day one month prior to pregnancy and throughout the 1st trimester | 4000 µg/day in case of previous NTD pregnancy, BMI > 30, or genetic mutations in folate metabolism | N/A | |
| Poland | The Polish Society of Gynaecologists and Obstetricians [89] | 400 µg/day 3 months prior to pregnancy, during pregnancy, and lactation | 800 µg/day 3 months prior to pregnancy, during pregnancy, and lactation in case of pre-existing type 1 or 2 diabetes mellitus, use of antiepileptic drugs, or bariatric surgery | 5000 µg/day 3 months prior to pregnancy and throughout 1st trimester, 800 µg/day throughout 2nd and 3rd trimester and lactation | |
| Hungary | The National Institute for Health Promotion in Hungary and The National Council of Hungarian Gynaecologists [94] | 400 µg/day 3 months prior to and during pregnancy | N/A | N/A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rísová, V.; Saade, R.; Jakuš, V.; Gajdošová, L.; Varga, I.; Záhumenský, J. Preconceptional and Periconceptional Folic Acid Supplementation in the Visegrad Group Countries for the Prevention of Neural Tube Defects. Nutrients 2025, 17, 126. https://doi.org/10.3390/nu17010126
Rísová V, Saade R, Jakuš V, Gajdošová L, Varga I, Záhumenský J. Preconceptional and Periconceptional Folic Acid Supplementation in the Visegrad Group Countries for the Prevention of Neural Tube Defects. Nutrients. 2025; 17(1):126. https://doi.org/10.3390/nu17010126
Chicago/Turabian StyleRísová, Vanda, Rami Saade, Vladimír Jakuš, Lívia Gajdošová, Ivan Varga, and Jozef Záhumenský. 2025. "Preconceptional and Periconceptional Folic Acid Supplementation in the Visegrad Group Countries for the Prevention of Neural Tube Defects" Nutrients 17, no. 1: 126. https://doi.org/10.3390/nu17010126
APA StyleRísová, V., Saade, R., Jakuš, V., Gajdošová, L., Varga, I., & Záhumenský, J. (2025). Preconceptional and Periconceptional Folic Acid Supplementation in the Visegrad Group Countries for the Prevention of Neural Tube Defects. Nutrients, 17(1), 126. https://doi.org/10.3390/nu17010126







