Adaptive Responses in Severe Acute Malnutrition: Endocrinology, Metabolomics, Mortality, and Growth
Abstract
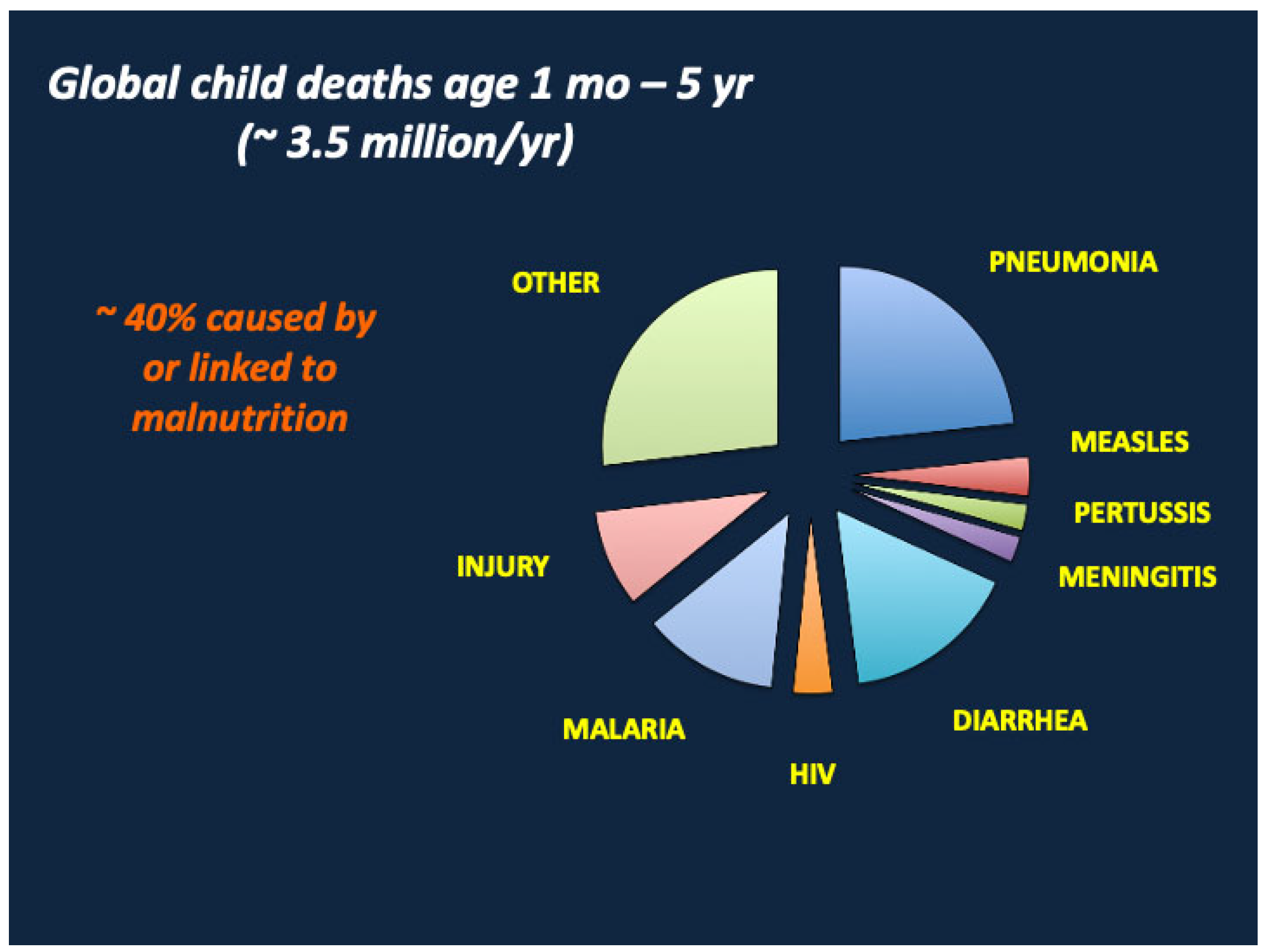
1. Introduction
- How do the endocrine and metabolic responses to nutrient deprivation promote survival and recovery from SAM?
- Why might growth suppression (“stunting”) represent an evolutionary adaptation/tradeoff that facilitates recovery from SAM?
- Can we identify biomarkers that predict mortality in SAM? What are their roles in the adaptation to SAM and the defense against life-threatening infectious diseases? And how are the circulating levels of critical biomarkers regulated by changes in body composition prior to and during treatment of SAM?
2. Methods
3. Clinical Phenotypes of Severe Acute Malnutrition
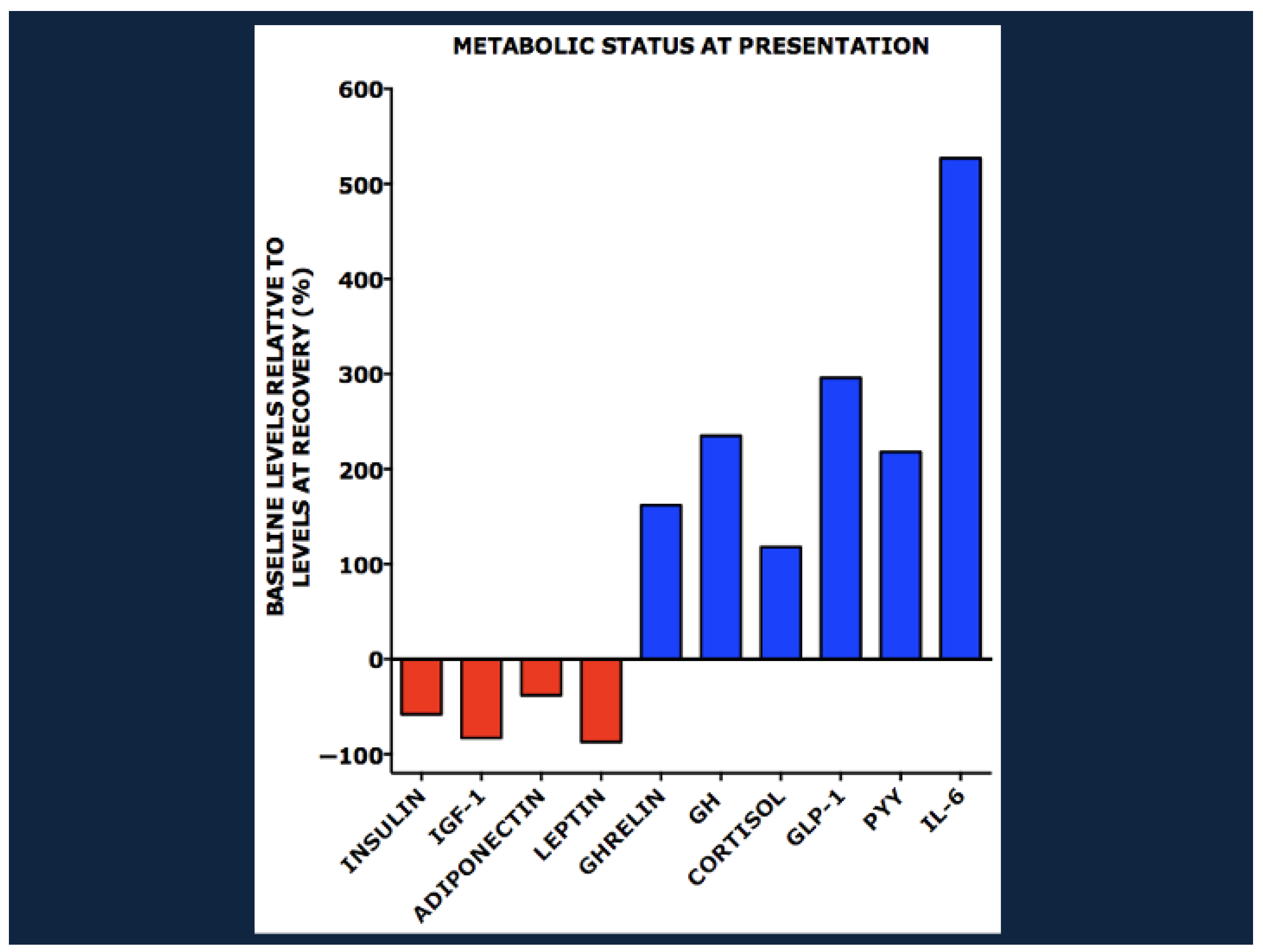
4. The Endocrinology and Metabolomics of Severe Acute Malnutrition
5. Comparative Metabolomics in Marasmus and Kwashiorkor
5.1. Protein and Lipid Metabolism
5.2. One Carbon Metabolism and the Pathogenesis of Edema
5.3. Hepatomegaly, Steatosis, and Hepatic Dysfunction
6. The Effect of Concurrent HIV Infection
7. Enteropathy and the Role of the Microbiome
8. Synthesis: How Do the Endocrine and Metabolic Responses to Nutrient Deprivation Promote Survival and Recovery from SAM?
8.1. Hormonal and Metabolic Responses to Nutrient Deprivation
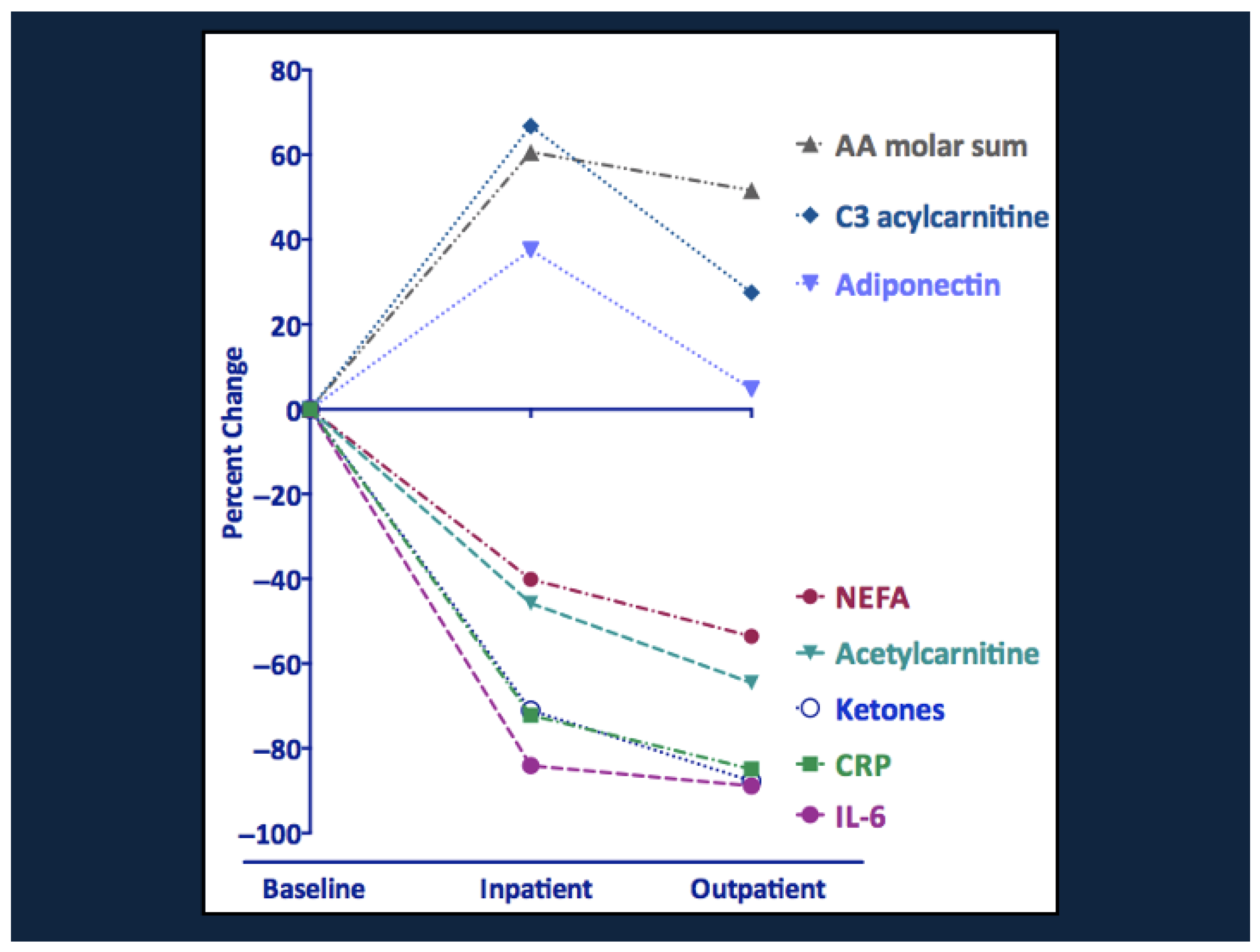
8.2. Metabolic Recovery from Malnutrition
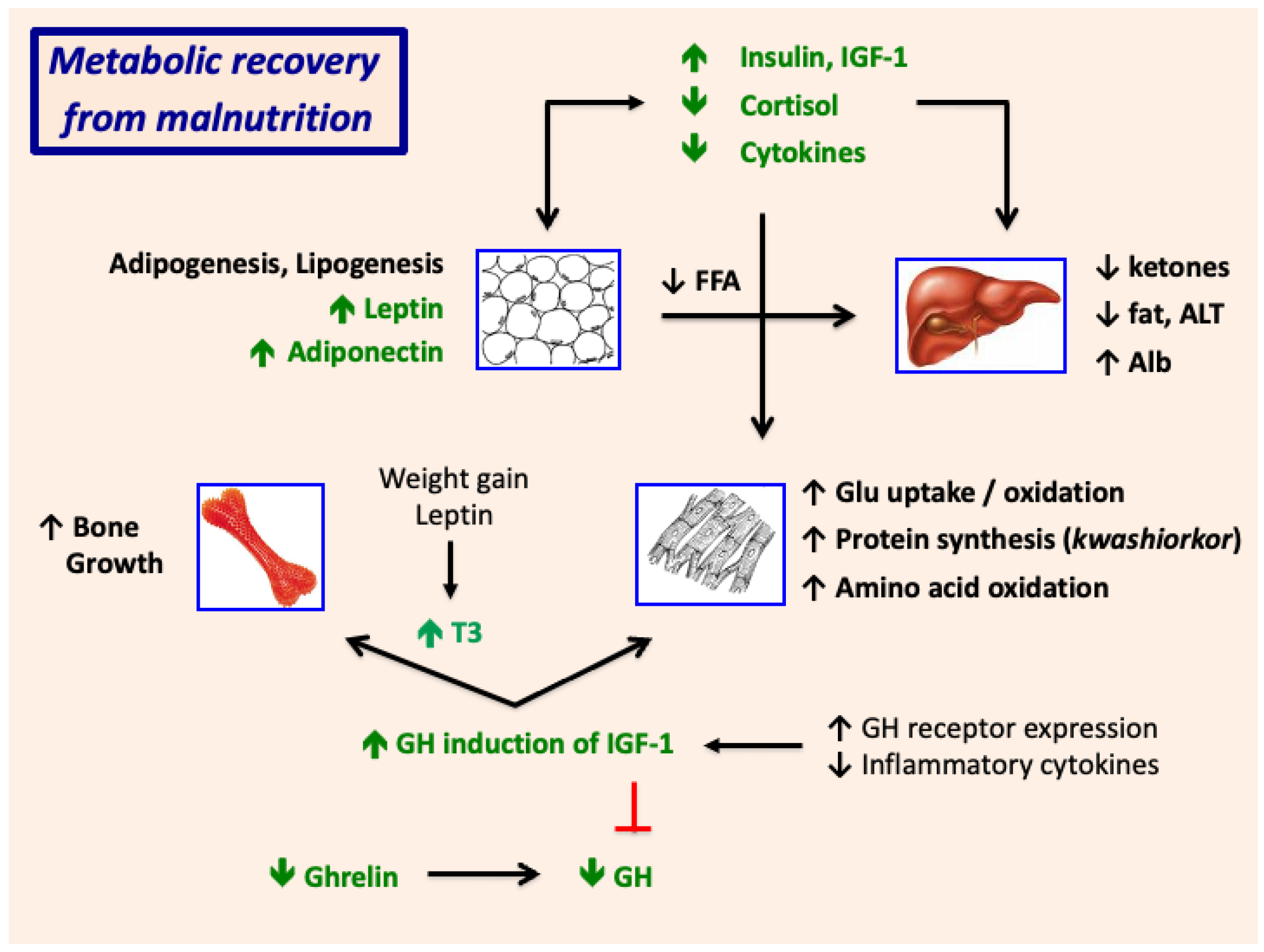
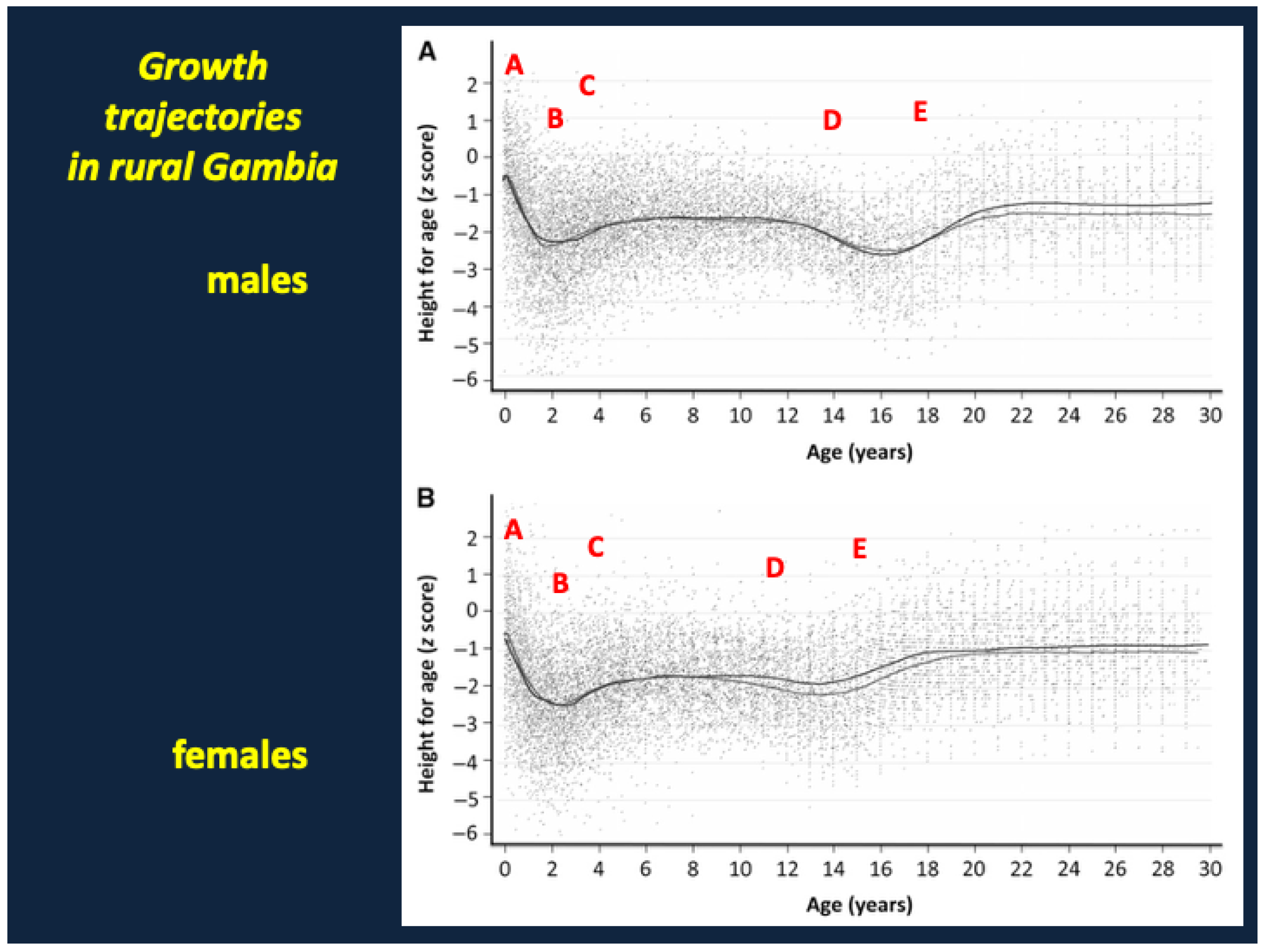
9. Growth Failure and Stunting in SAM
9.1. Why Might Growth Suppression (“Stunting”) Represent an Evolutionary Adaptation/Tradeoff That Facilitates Recovery from SAM?
9.2. Failure of Catch-Up Growth and Long-Term Metabolic Complications
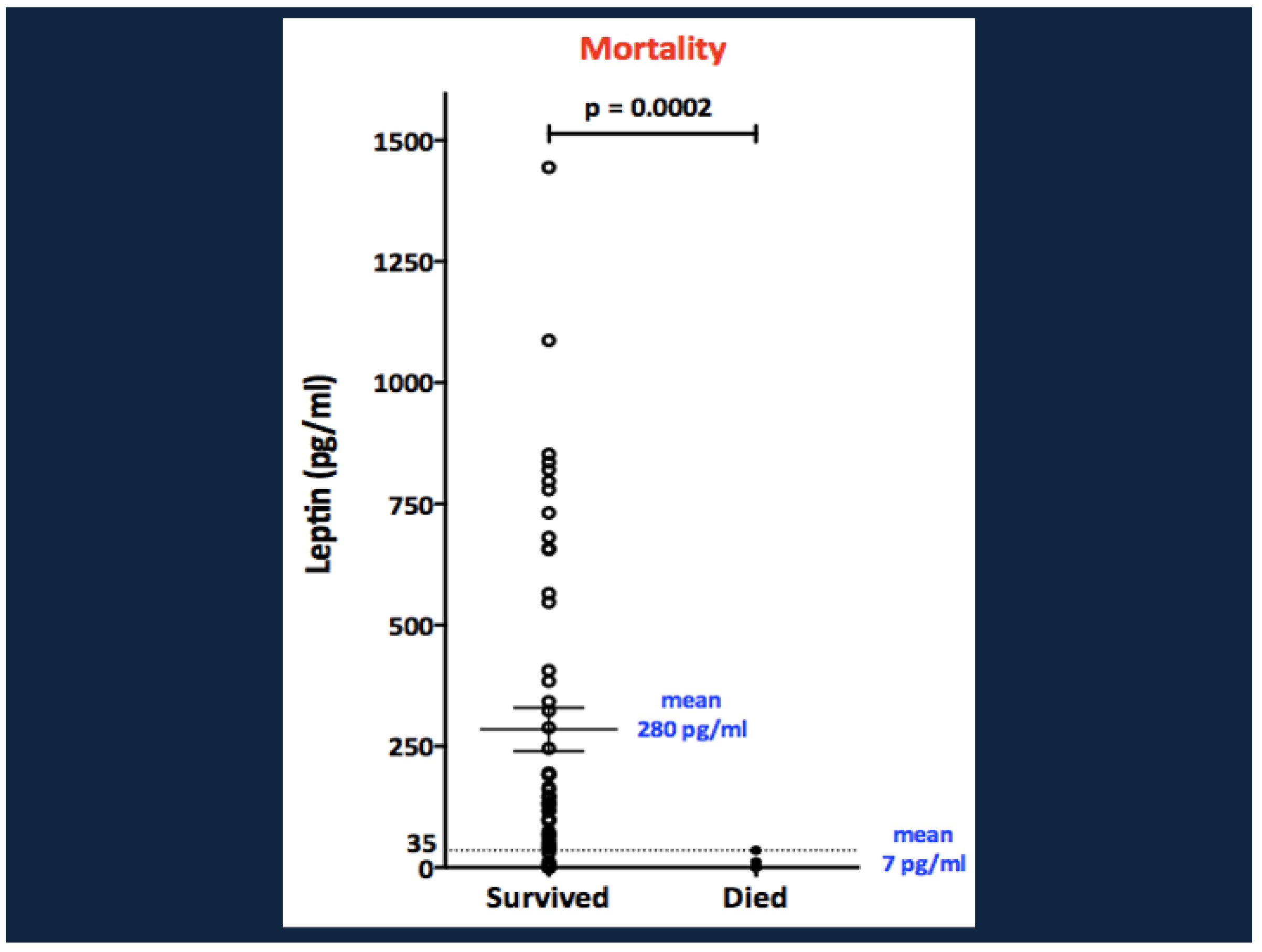
10. Biomarkers That Predict Mortality in SAM: White Adipose Tissue Energy Reserves and the Role of Leptin
10.1. Why Might Hypoleptinemia Associate with, or Predispose to, Mortality in SAM?
10.2. Role of Leptin in Innate Immunity
10.3. Role of Leptin in Adaptive Immunity
10.4. Genetic and Acquired Defects in Leptin Production or Action Increase the Risks of Morbidity & Mortality from Infectious Disease
11. Limitations
12. Conclusions, Gaps in Knowledge, and Future Investigations
- Why are prematurity, intrauterine growth restriction, and low birth weight associated with higher risks of mortality in SAM? Do they serve as markers or proxies of longstanding (and future) poverty, food insecurity, limited access to medical care, and/or lack of sanitation or education in the family and community, or might they exert epigenetic effects) that increase the susceptibility to nutrient deprivation or infection?
- Small bowel enteropathy and dysbiosis have been implicated with the pathogenesis of SAM and its complications including sepsis and growth failure. What are the roles of micronutrient and protein deficiencies in the development of enteropathy and dysbiosis in SAM?
- What explains the high levels of GLP-1 and PYY in children with SAM? Do the appetite-suppressive effects of these gastrointestinal hormones have adaptive benefits in the response to nutrient deprivation? Does GLP-1 modulate the local immune responses to enteropathy?
- The roles of other gastrointestinal hormones in the adaptation to SAM are unclear. For example, Glucagon-like peptide 2 (GLP-2) regulates epithelial growth, permeability, and nutrient absorption in the gastrointestinal tract. Available studies find high circulating levels of GLP-2 in infants and young children with acute diarrhea but not in those with persistent diarrhea or SAM [197]; yet, GLP-2 levels are low in stunted infants [197,198] and, in one study, correlated negatively with mortality in SAM [199]. Thus, additional investigation of its effects on weight gain, linear growth, and survival in SAM are warranted.
- What are the roles of gastrointestinal vs. systemic inflammation in the pathogenesis of growth failure in children with SAM?
- FGF21 is thought to play a central role in the adaptation to fasting in experimental animals, while Growth Differentiation Factor 15 (GDF15) may inhibit food intake in chronic illness and cancer [200,201,202,203]. What are the roles of FGF21, GDF15, and other novel hormones and growth factors in the control of appetite, weight gain, and linear growth in SAM?
- What factors determine the rates of catch-up growth and weight gain in malnourished children with growth failure/stunting? Can final height in stunted children be predicted by parental target height independent of parental nutritional status?
- As noted previously, some studies find that adult survivors of SAM are predisposed to mild glucose intolerance, dyslipidemia, and metabolic syndrome, but increases in rates of overt diabetes or cardiovascular disease have not yet been demonstrated. Future studies should clarify the roles of central adiposity, sarcopenia, and pancreatic beta cell dysfunction in the long-term metabolic complications of SAM. It will be critical to distinguish complications of malnutrition in infancy and childhood from those of combined pre- and postnatal growth restriction.
- Leptin has important immunomodulatory roles, and a deficiency of leptin or its receptor predisposes to morbidity and mortality from severe infection. Future studies should determine if the hypoleptinemia of SAM modifies the local immune response to small bowel dysbiosis as well as the systemic response to infection.
- Finally, we considered the theoretical benefits and risks of leptin therapy in malnourished children. In children and adults with lipodystrophy, which is associated with severe hypoleptinemia, recombinant leptin has metabolic benefits including reductions in hepatic fat content, fasting glucose, HbA1c, ALT, AST, and triglyceride levels [204,205,206]. However, treatment causes weight loss and reductions in body fat mass and lean body mass owing to a decline in food intake [207]. Reductions in weight, fat mass, and lean body mass would be maladaptive in children with SAM. Moreover, leptin levels rise spontaneously and dramatically during clinical recovery from SAM. Thus, the potential risks of leptin therapy in SAM appear to outweigh its potential benefits.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Total Amino Acids |
| Alb | Albumin |
| ALT | Alanine aminotransferase |
| ATP | Adenosine Triphosphate |
| C2 | Acetylcarnitine |
| C3 | Propionylcarnitine |
| Creat | Creatinine |
| CRP | C-Reactive Protein |
| FAO | Fatty Acid Oxidation |
| FFA | Free fatty acid |
| FGF21 | Fibroblast growth factor 21 |
| FSP27 | Fat-specific protein 27 |
| GCSF | Granulocyte colony stimulating factor |
| GDF15 | Growth Differentiation Factor 15 |
| GH | Growth hormone |
| GLP-1 | Glucagon-like peptide 1 |
| GLP-2 | Glucagon-like peptide 2 |
| HAz | Height for Age z-score |
| HGP | Hepatic Glucose Production |
| IFN-γ | Interferon Gamma |
| IGF-1 | Insulin-like growth factor 1 |
| IL-1β | Interleukin-1 beta |
| IL-13 | Interleukin-13 |
| IL-15, | Interleukin-15 |
| IL-1ra | Interleukin-1 receptor antagonist |
| IL-2 | Interleukin-2 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IP10 | Also known as CXCL10 |
| Jak2 | Janus kinase 2 |
| LAz | Length for age z-score |
| LBP | Lipopolysaccharide-binding protein |
| mTORC1 | Mammalian Target of Rapamycin Complex 1 |
| MUAC | Mid-upper arm circumference |
| NEFA | Non-esterified fatty acids |
| NK cells | Natural Killer cells |
| P | Phosphorus |
| PAPPA | Pregnancy-associated plasma protein-A |
| PPAR gamma | Peroxisome proliferator-activated receptor gamma |
| PYY | Peptide YY |
| RUTF | Ready-to-Use Therapeutic Food |
| SAM | Severe Acute Malnutrition |
| SD | Standard deviation |
| SOCS2 | Suppressor of cytokine signaling 2 |
| STAT5 | Signal Transducer and Activator of Transcription 5 |
| TG | Triglycerides |
| T3 | Tri-iodothyronine |
| TNFα | Tumor Necrosis Factor alpha |
| UBEN2N | Ubiquitin conjugating enzyme E2 N |
| VLDL | Very low density lipoprotein |
| WAz | Weight for age z-score |
| WHz | Weight for Height z-score |
| ZAG | zinc-alpha-2-glycoprotein |
References
- Lichtheim, M. Volume III: The Late Period “See the translation of the Famine Stela (~200 BCE)”. In Ancient Egyptian Literature: A Book of Readings; University of California Press: Berkeley, CA, USA, 1973; Volume 3, pp. 94–100. [Google Scholar]
- Ekeng, B.; Adedokun, O.; Otu, V.; Chukwuma, S.; Okah, A.; Asemota, O.; Eshiet, U.; Akpan, U.; Nwagboso, R.; Ebiekpi, E.; et al. The Spectrum of Pathogens Associated with Infections in African Children with Severe Acute Malnutrition: A Scoping Review. Trop. Med. Infect. Dis. 2024, 9, 230. [Google Scholar] [CrossRef]
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef]
- Walker, C.L.F.; Rudan, I.; Liu, L.; Nair, H.; Theodoratou, E.; Bhutta, Z.A.; O’Brien, K.L.; Campbell, H.; Black, R.E. Global burden of childhood pneumonia and diarrhoea. Lancet 2013, 381, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; United Negro College Fund; International Bank for Reconstruction and Development; Development/The World Bank Group. Levels and Trends in Child Malnutrition: UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2023 Edition; 9789240073791; World Health Organization: Geneva, Switzerland, 2023.
- Global Nutrition Target, C. Global, regional, and national progress towards the 2030 global nutrition targets and forecasts to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2025, 404, 2543–2583. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Berkley, J.A.; Bandsma, R.H.J.; Kerac, M.; Trehan, I.; Briend, A. Severe childhood malnutrition. Nat. Rev. Dis. Primers 2017, 3, 17067. [Google Scholar] [CrossRef] [PubMed]
- David, S.M.; Pricilla, R.A.; Paul, S.S.; George, K.; Bose, A.; Prasad, J.H. Risk factors for severe acute malnutrition among children aged 6–59 months: A community-based case-control study from Vellore, Southern India. J. Fam. Med. Prim. Care 2020, 9, 2237–2243. [Google Scholar] [CrossRef]
- Owino, V.; Ahmed, T.; Freemark, M.; Kelly, P.; Loy, A.; Manary, M.; Loechl, C. Environmental Enteric Dysfunction and Growth Failure/Stunting in Global Child Health. Pediatrics 2016, 138, e20160641. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Kelly, P. Interactions between intestinal pathogens, enteropathy and malnutrition in developing countries. Curr. Opin. Infect. Dis. 2016, 29, 229–236. [Google Scholar] [CrossRef]
- Lennie, T.A. Anorexia in response to acute illness. Heart Lung 1999, 28, 386–401. [Google Scholar] [CrossRef]
- Jindal, J.; Hill, J.; Harte, J.; Dunachie, S.J.; Kronsteiner, B. Starvation and infection: The role of sickness-associated anorexia in metabolic adaptation during acute infection. Metabolism 2024, 161, 156035. [Google Scholar] [CrossRef]
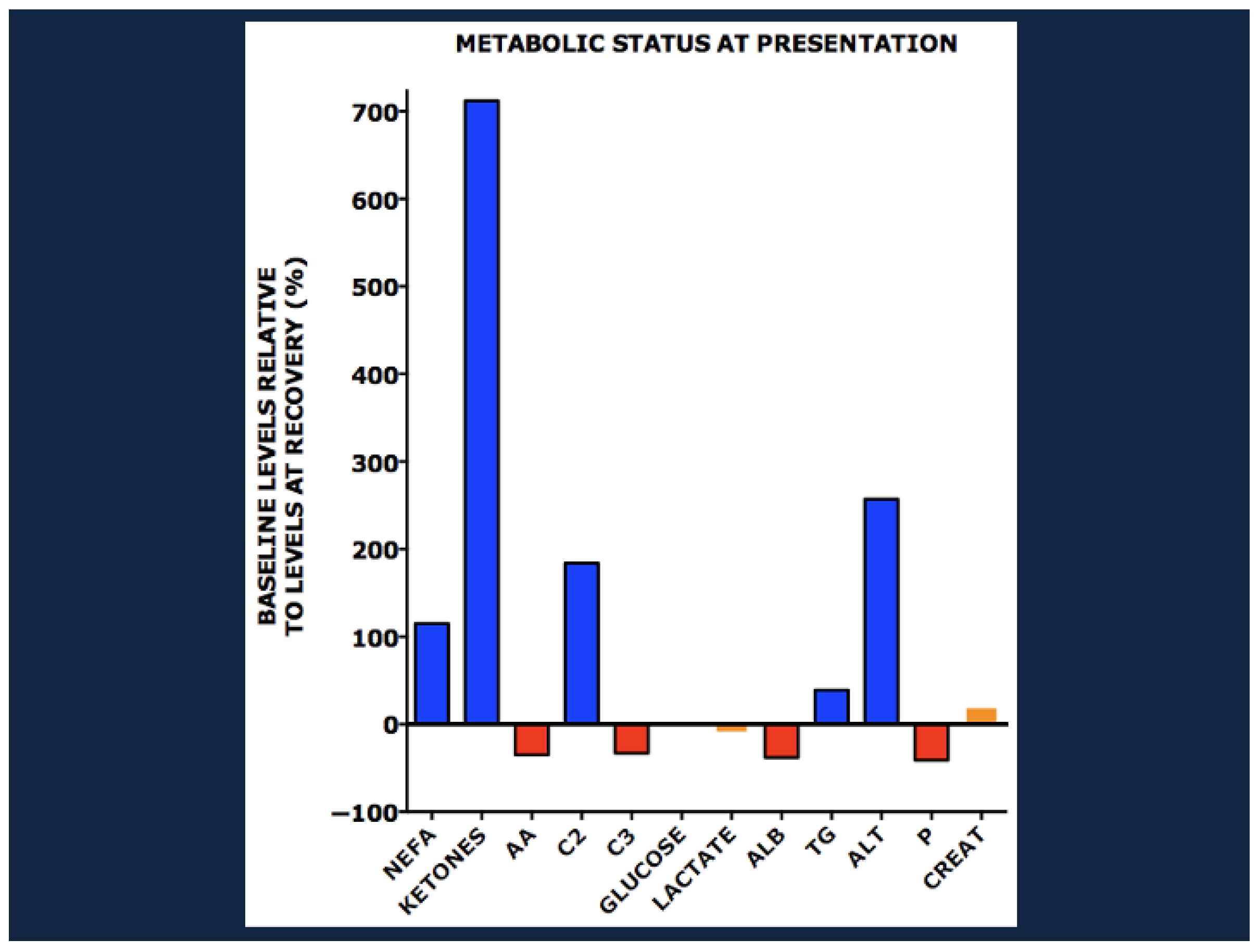
- Bartz, S.; Mody, A.; Hornik, C.; Bain, J.; Muehlbauer, M.; Kiyimba, T.; Kiboneka, E.; Stevens, R.; Bartlett, J.; St Peter, J.V.; et al. Severe acute malnutrition in childhood: Hormonal and metabolic status at presentation, response to treatment, and predictors of mortality. J. Clin. Endocrinol. Metab. 2014, 99, 2128–2137. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Cote d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef]
- Donker, A.E.; van der Staaij, H.; Swinkels, D.W. The critical roles of iron during the journey from fetus to adolescent: Developmental aspects of iron homeostasis. Blood Rev. 2021, 50, 100866. [Google Scholar] [CrossRef]
- van Niekerk, G.; Loos, B.; Nell, T.; Engelbrecht, A.M. Autophagy—A free meal in sickness-associated anorexia. Autophagy 2016, 12, 727–734. [Google Scholar] [CrossRef]
- Waterlow, J.C. Protein metabolism in human protein malnutrition. Proc. R. Soc. Lond. B Biol. Sci. 1962, 156, 345–351. [Google Scholar] [CrossRef]
- Whitehead, R.G. The relative roles of protein and energy deficiency in the pathogenesis of protein-energy malnutrition. Z. Ernahrungswiss Suppl. 1979, 23, 72–84. [Google Scholar]
- Jahoor, F.; Badaloo, A.; Reid, M.; Forrester, T. Protein metabolism in severe childhood malnutrition. Ann. Trop. Paediatr. 2008, 28, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Golden, M. The effects of malnutrition in the metabolism of children. Trans. R. Soc. Trop. Med. Hyg. 1988, 82, 3–6. [Google Scholar] [CrossRef]
- Golden, M.H. Protein deficiency, energy deficiency, and the oedema of malnutrition. Lancet 1982, 1, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Manary, M.J.; Broadhead, R.L.; Yarasheski, K.E. Whole-body protein kinetics in marasmus and kwashiorkor during acute infection. Am. J. Clin. Nutr. 1998, 67, 1205–1209. [Google Scholar] [CrossRef]
- Pimstone, B.; Becker, D.; Kernoff, L. Growth and growth hormone in protein calorie malnutrition. S. Afr. Med. J. 1972, 46, 2102–2105. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Klish, W.; Cuellar, A.; Serrano, P.A.; Garcia, G.; Argote, R.M.; Canseco, L.; Nicholas, B.L. Energy metabolism and hormonal profile in children with edematous protein-calorie malnutrition. J. Pediatr. 1975, 87, 307–314. [Google Scholar] [CrossRef]
- Lunn, P.G.; Whitehead, R.G.; Hay, R.W.; Baker, B.A. Progressive changes in serum cortisol, insulin and growth hormone concentrations and their relationship to the distorted amino acid pattern during the development of kwashiorkor. Br. J. Nutr. 1973, 29, 399–422. [Google Scholar] [CrossRef]
- Robinson, H.; Picou, D. A comparison of fasting plasma insulin and growth hormone concentrations in marasmic, kwashiorkor, marasmic-kwashiorkor and underweight children. Pediatr. Res. 1977, 11, 637–640. [Google Scholar] [CrossRef]
- Becker, D.J.; Pimstone, B.L.; Hansen, J.D.; MacHutchon, B.; Drysdale, A. Patterns of insulin response to glucose in protein-calorie malnutrition. Am. J. Clin. Nutr. 1972, 25, 499–505. [Google Scholar] [CrossRef]
- Keusch, G.T. The history of nutrition: Malnutrition, infection and immunity. J. Nutr. 2003, 133, 336S–340S. [Google Scholar] [CrossRef]
- Mansoori, S.; Ho, M.Y.; Ng, K.K.; Cheng, K.K. Branched-chain amino acid metabolism: Pathophysiological mechanism and therapeutic intervention in metabolic diseases. Obes. Rev. 2025, 26, e13856. [Google Scholar] [CrossRef]
- McMillan, A.; Orimadegun, A.; Sumarah, M.; Renaud, J.; Muc, M.; Gloor, G.; Akinyinka, O.; Reid, G.; Allen, S. Metabolic derangements identified through untargeted metabolomics in a crosssectional study of Nigerian children with severe acute malnutrition. Metabolomics 2016, 13, 13. [Google Scholar] [CrossRef]
- Gehrig, J.L.; Venkatesh, S.; Chang, H.W.; Hibberd, M.C.; Kung, V.L.; Cheng, J.; Chen, R.Y.; Subramanian, S.; Cowardin, C.A.; Meier, M.F.; et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 2019, 365, eaau4732. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; Hassan, A.E.; Aref, M.K.; Hintz, R.L.; Rosenfeld, R.G.; Rogol, A.D. Serum insulin-like growth factors I and II concentrations and growth hormone and insulin responses to arginine infusion in children with protein-energy malnutrition before and after nutritional rehabilitation. Pediatr. Res. 1986, 20, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Kiepe, D.; Ulinski, T.; Powell, D.R.; Durham, S.K.; Mehls, O.; Tonshoff, B. Differential effects of insulin-like growth factor binding proteins-1, -2, -3, and -6 on cultured growth plate chondrocytes. Kidney Int. 2002, 62, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, A.; Nedbal, S.; Blum, W.F.; Erhard, M.; Lahm, H.; Brem, G.; Kolb, H.J.; Wanke, R.; Wolf, E. Growth inhibition in giant growth hormone transgenic mice by overexpression of insulin-like growth factor-binding protein-2. Endocrinology 2001, 142, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xi, G.; Maile, L.A.; Wai, C.; Rosen, C.J.; Clemmons, D.R. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase beta and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol. Cell Biol. 2012, 32, 4116–4130. [Google Scholar] [CrossRef]
- Fisher, M.C.; Meyer, C.; Garber, G.; Dealy, C.N. Role of IGFBP2, IGF-I and IGF-II in regulating long bone growth. Bone 2005, 37, 741–750. [Google Scholar] [CrossRef]
- Ledger, E.; Harawa, P.P.; Daniel, A.I.; Candler, T.; Prentice, A.M.; Bandsma, R.H.J. Dysglycemia in Children with Severe Acute Malnutrition: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 959–968. [Google Scholar] [CrossRef]
- Wen, B.; Brals, D.; Bourdon, C.; Erdman, L.; Ngari, M.; Chimwezi, E.; Potani, I.; Thitiri, J.; Mwalekwa, L.; Berkley, J.A.; et al. Predicting the risk of mortality during hospitalization in sick severely malnourished children using daily evaluation of key clinical warning signs. BMC Med. 2021, 19, 222. [Google Scholar] [CrossRef]
- Manary, M.J.; Yarasheski, K.E.; Berger, R.; Abrams, E.T.; Hart, C.A.; Broadhead, R.L. Whole-body leucine kinetics and the acute phase response during acute infection in marasmic Malawian children. Pediatr. Res. 2004, 55, 940–946. [Google Scholar] [CrossRef]
- Jahoor, F.; Badaloo, A.; Reid, M.; Forrester, T. Protein kinetic differences between children with edematous and nonedematous severe childhood undernutrition in the fed and postabsorptive states. Am. J. Clin. Nutr. 2005, 82, 792–800. [Google Scholar] [CrossRef]
- Badaloo, A.V.; Forrester, T.; Reid, M.; Jahoor, F. Lipid kinetic differences between children with kwashiorkor and those with marasmus. Am. J. Clin. Nutr. 2006, 83, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, V.; Bourdon, C.; Wang, D.X.; Seshadri, S.; Senga, E.; Versloot, C.J.; Voskuijl, W.; Semba, R.D.; Trehan, I.; Moaddel, R.; et al. Metabolomic Changes in Serum of Children with Different Clinical Diagnoses of Malnutrition. J. Nutr. 2016, 146, 2436–2444. [Google Scholar] [CrossRef] [PubMed]
- May, T.; de la Haye, B.; Nord, G.; Klatt, K.; Stephenson, K.; Adams, S.; Bollinger, L.; Hanchard, N.; Arning, E.; Bottiglieri, T.; et al. One-carbon metabolism in children with marasmus and kwashiorkor. EBioMedicine 2022, 75, 103791. [Google Scholar] [CrossRef]
- Golden, M.H.; Ramdath, D. Free radicals in the pathogenesis of kwashiorkor. Proc. Nutr. Soc. 1987, 46, 53–68. [Google Scholar] [CrossRef]
- Golden, M.H. Nutritional and other types of oedema, albumin, complex carbohydrates and the interstitium—A response to Malcolm Coulthard’s hypothesis: Oedema in kwashiorkor is caused by hypo-albuminaemia. Paediatr. Int. Child. Health 2015, 35, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.V.; Swaminathan, S.; Howell, S.; Jajoo, A.; Lie, N.C.; Brown, O.; Sadat, R.; Hall, N.; Zhao, L.; Marshall, K.; et al. Edematous severe acute malnutrition is characterized by hypomethylation of DNA. Nat. Commun. 2019, 10, 5791. [Google Scholar] [CrossRef]
- Davies, J.N.P. The essential pathology of kwashiorkor. Lancet 1948, 251, 317–320. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Bhattacharyya, A.K.; Mukherjee, A.M. The liver in pre-kwashiorkor and kwashiorkor-marasmus syndromes. Trans. R. Soc. Trop. Med. Hyg. 1972, 66, 258–262. [Google Scholar] [CrossRef]
- Ling, C.M.; Sheferaw, T.F.; Denno, D.M.; Chasweka, D.; Kamiza, S.B.; Ordi, J.; Moxon, C.A.; Kats, K.; Khoswe, S.; Mbale, E.; et al. Hepatic mitochondrial and peroxisomal alterations in acutely ill malnourished Malawian children: A postmortem cohort study. Glob. Pediatr. 2024, 9, 100199. [Google Scholar] [CrossRef]
- Hammond, K.D.; Tobiansky, R.; Abrahams, O.L. Serum carnitine in children with kwashiorkor. Ann. Trop. Paediatr. 1987, 7, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, F.; Uzunsel, S.; Atalay, A. Plasma free carnitine levels in children with malnutrition. Turk. J. Pediatr. 1994, 36, 133–137. [Google Scholar] [CrossRef]
- Brooks, S.E.; Doherty, J.F.; Golden, M.H. Peroxisomes and the hepatic pathology of childhood malnutrition. West. Indian. Med. J. 1994, 43, 15–17. [Google Scholar] [PubMed]
- Zhang, L.; Voskuijl, W.; Mouzaki, M.; Groen, A.K.; Alexander, J.; Bourdon, C.; Wang, A.; Versloot, C.J.; Di Giovanni, V.; Wanders, R.J.; et al. Impaired Bile Acid Homeostasis in Children with Severe Acute Malnutrition. PLoS ONE 2016, 11, e0155143. [Google Scholar] [CrossRef][Green Version]
- Cai, S.Y.; Boyer, J.L. The role of bile acids in cholestatic liver injury. Ann. Transl. Med. 2021, 9, 737. [Google Scholar] [CrossRef]
- Badaloo, A.V.; Forrester, T.; Reid, M.; Jahoor, F. Nutritional repletion of children with severe acute malnutrition does not affect VLDL apolipoprotein B-100 synthesis rate. J. Nutr. 2012, 142, 931–935. [Google Scholar] [CrossRef]
- Kerac, M.; Bunn, J.; Chagaluka, G.; Bahwere, P.; Tomkins, A.; Collins, S.; Seal, A. Follow-up of post-discharge growth and mortality after treatment for severe acute malnutrition (FuSAM study): A prospective cohort study. PLoS ONE 2014, 9, e96030. [Google Scholar] [CrossRef]
- Mody, A.; Bartz, S.; Hornik, C.P.; Kiyimba, T.; Bain, J.; Muehlbauer, M.; Kiboneka, E.; Stevens, R.; St Peter, J.V.; Newgard, C.B.; et al. Effects of HIV infection on the metabolic and hormonal status of children with severe acute malnutrition. PLoS ONE 2014, 9, e102233. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Njunge, J.M.; Gichuki, B.M.; Wen, B.; Potani, I.; Voskuijl, W.; Bandsma, R.H.J.; Berkley, J.A. Plasma proteomics reveals markers of metabolic stress in HIV infected children with severe acute malnutrition. Sci. Rep. 2020, 10, 11235. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.D.; et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef]
- Kumar, M.; Ji, B.; Babaei, P.; Das, P.; Lappa, D.; Ramakrishnan, G.; Fox, T.E.; Haque, R.; Petri, W.A.; Backhed, F.; et al. Gut microbiota dysbiosis is associated with malnutrition and reduced plasma amino acid levels: Lessons from genome-scale metabolic modeling. Metab. Eng. 2018, 49, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.I.; Yatsunenko, T.; Manary, M.J.; Trehan, I.; Mkakosya, R.; Cheng, J.; Kau, A.L.; Rich, S.S.; Concannon, P.; Mychaleckyj, J.C.; et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013, 339, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, aad3311. [Google Scholar] [CrossRef]
- Chen, R.Y.; Mostafa, I.; Hibberd, M.C.; Das, S.; Mahfuz, M.; Naila, N.N.; Islam, M.M.; Huq, S.; Alam, M.A.; Zaman, M.U.; et al. A Microbiota-Directed Food Intervention for Undernourished Children. N. Engl. J. Med. 2021, 384, 1517–1528. [Google Scholar] [CrossRef]
- Farras, M.; Chandwe, K.; Mayneris-Perxachs, J.; Amadi, B.; Louis-Auguste, J.; Besa, E.; Zyambo, K.; Guerrant, R.; Kelly, P.; Swann, J.R. Characterizing the metabolic phenotype of intestinal villus blunting in Zambian children with severe acute malnutrition and persistent diarrhea. PLoS ONE 2018, 13, e0192092. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Begum, S.; Rahman, M.M.; Parvez, M.; Mazumder, R.N.; Sarker, S.A.; Hasan, M.M.; Fahim, S.M.; Gazi, M.A.; Das, S.; et al. Environmental enteric dysfunction and small intestinal histomorphology of stunted children in Bangladesh. PLoS Negl. Trop. Dis. 2023, 17, e0010472. [Google Scholar] [CrossRef]
- Jamil, Z.; VanBuskirk, K.; Mweetwa, M.; Mouksassi, S.; Smith, G.; Ahmed, T.; Chandwe, K.; Denno, D.M.; Fahim, S.M.; Kelly, P.; et al. Anthropometry relationship with duodenal histologic features of children with environmental enteric dysfunction: A multicenter cross-sectional study. Am. J. Clin. Nutr. 2024, 120 (Suppl. S1), S65–S72. [Google Scholar] [CrossRef] [PubMed]
- Mayneris-Perxachs, J.; Bolick, D.T.; Leng, J.; Medlock, G.L.; Kolling, G.L.; Papin, J.A.; Swann, J.R.; Guerrant, R.L. Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am. J. Clin. Nutr. 2016, 104, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Lelijveld, N.; Seal, A.; Wells, J.C.; Kirkby, J.; Opondo, C.; Chimwezi, E.; Bunn, J.; Bandsma, R.; Heyderman, R.S.; Nyirenda, M.J.; et al. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): A cohort study. Lancet Glob. Health 2016, 4, e654–e662. [Google Scholar] [CrossRef]
- Hoeldtke, R.D.; Wurtman, R.J. Excretion of catecholamines and catecholamine metabolites in kwashiorkor. Am. J. Clin. Nutr. 1973, 26, 205–210. [Google Scholar] [CrossRef]
- Ramirez, A.; Fletes, L.; Mizrahi, L.; Parra, A. Daily urinary catecholamine profile in marasmus and kwashiorkor. Am. J. Clin. Nutr. 1978, 31, 41–45. [Google Scholar] [CrossRef]
- Kopchick, J.J.; Berryman, D.E.; Puri, V.; Lee, K.Y.; Jorgensen, J.O.L. The effects of growth hormone on adipose tissue: Old observations, new mechanisms. Nat. Rev. Endocrinol. 2020, 16, 135–146. [Google Scholar] [CrossRef]
- Karki, S. FSP27 and Links to Obesity and Diabetes Mellitus. Curr. Obes. Rep. 2019, 8, 255–261. [Google Scholar] [CrossRef]
- Ottosson, M.; Vikman-Adolfsson, K.; Enerback, S.; Elander, A.; Bjorntorp, P.; Eden, S. Growth hormone inhibits lipoprotein lipase activity in human adipose tissue. J. Clin. Endocrinol. Metab. 1995, 80, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Samra, J.S.; Clark, M.L.; Humphreys, S.M.; MacDonald, I.A.; Bannister, P.A.; Frayn, K.N. Effects of physiological hypercortisolemia on the regulation of lipolysis in subcutaneous adipose tissue. J. Clin. Endocrinol. Metab. 1998, 83, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Stimson, R.H.; Anderson, A.J.; Ramage, L.E.; Macfarlane, D.P.; de Beaux, A.C.; Mole, D.J.; Andrew, R.; Walker, B.R. Acute physiological effects of glucocorticoids on fuel metabolism in humans are permissive but not direct. Diabetes Obes. Metab. 2017, 19, 883–891. [Google Scholar] [CrossRef]
- Perry, R.J.; Shulman, G.I. The Role of Leptin in Maintaining Plasma Glucose During Starvation. Postdoc J. 2018, 6, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.J.; Wang, Y.; Cline, G.W.; Rabin-Court, A.; Song, J.D.; Dufour, S.; Zhang, X.M.; Petersen, K.F.; Shulman, G.I. Leptin Mediates a Glucose-Fatty Acid Cycle to Maintain Glucose Homeostasis in Starvation. Cell 2018, 172, 234–248.e17. [Google Scholar] [CrossRef]
- Perry, R.J.; Resch, J.M.; Douglass, A.M.; Madara, J.C.; Rabin-Court, A.; Kucukdereli, H.; Wu, C.; Song, J.D.; Lowell, B.B.; Shulman, G.I. Leptin’s hunger-suppressing effects are mediated by the hypothalamic-pituitary-adrenocortical axis in rodents. Proc. Natl. Acad. Sci. USA 2019, 116, 13670–13679. [Google Scholar] [CrossRef]
- Chan, J.L.; Heist, K.; DePaoli, A.M.; Veldhuis, J.D.; Mantzoros, C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 2003, 111, 1409–1421. [Google Scholar] [CrossRef]
- Bandsma, R.H.; Mendel, M.; Spoelstra, M.N.; Reijngoud, D.J.; Boer, T.; Stellaard, F.; Brabin, B.; Schellekens, R.; Senga, E.; Heikens, G.T. Mechanisms behind decreased endogenous glucose production in malnourished children. Pediatr. Res. 2010, 68, 423–428. [Google Scholar] [CrossRef]
- Robinson, H.M.; Seakins, A. Fasting pancreatic glucagon in Jamaican children during malnutrition and subsequent recovery. Pediatr. Res. 1982, 16, 1011–1015. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Muller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Yada, T.; Dezaki, K.; Iwasaki, Y. GLP-1 and ghrelin inversely regulate insulin secretion and action in pancreatic islets, vagal afferents, and hypothalamus for controlling glycemia and feeding. Am. J. Physiol. Cell Physiol. 2025, 328, C1793–C1807. [Google Scholar] [CrossRef]
- Liu, W.W.; Reicher, N.; Alway, E.; Rupprecht, L.E.; Weng, P.; Schaefgen, C.; Klein, M.E.; Villalobos, J.A.; Puerto-Hernandez, C.; Kiesling Altun, Y.G.; et al. A gut sense for a microbial pattern regulates feeding. Nature 2025. [Google Scholar] [CrossRef]
- Sharma, R.; Kopchick, J.J.; Puri, V.; Sharma, V.M. Effect of growth hormone on insulin signaling. Mol. Cell. Endocrinol. 2020, 518, 111038. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Wu, Y.; Sun, H.; Li, X.; Jeddy, Z.; Liu, C.; Schwartz, G.J.; Yakar, S.; LeRoith, D. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes 2012, 61, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Moller, N.; Christiansen, J.S.; Jorgensen, J.O. Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes 2001, 50, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Segerlantz, M.; Bramnert, M.; Manhem, P.; Laurila, E.; Groop, L.C. Inhibition of the rise in FFA by Acipimox partially prevents GH-induced insulin resistance in GH-deficient adults. J. Clin. Endocrinol. Metab. 2001, 86, 5813–5818. [Google Scholar] [CrossRef] [PubMed]
- Moller, N.; Jorgensen, J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009, 30, 152–177. [Google Scholar] [CrossRef]
- Nellemann, B.; Vendelbo, M.H.; Nielsen, T.S.; Bak, A.M.; Hogild, M.; Pedersen, S.B.; Bienso, R.S.; Pilegaard, H.; Moller, N.; Jessen, N.; et al. Growth hormone-induced insulin resistance in human subjects involves reduced pyruvate dehydrogenase activity. Acta Physiol. 2014, 210, 392–402. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Fryburg, D.A.; Gelfand, R.A.; Barrett, E.J. Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am. J. Physiol. 1991, 260, E499–E504. [Google Scholar] [CrossRef]
- Haymond, M.W.; Horber, F.F. The effects of human growth hormone and prednisone on whole body estimates of protein metabolism. Horm. Res. 1992, 38 (Suppl. S2), 44–46. [Google Scholar] [CrossRef]
- Moller, N.; Vendelbo, M.H.; Kampmann, U.; Christensen, B.; Madsen, M.; Norrelund, H.; Jorgensen, J.O. Growth hormone and protein metabolism. Clin. Nutr. 2009, 28, 597–603. [Google Scholar] [CrossRef]
- Sherwin, R.S.; Hendler, R.G.; Felig, P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J. Clin. Investig. 1975, 55, 1382–1390. [Google Scholar] [CrossRef]
- Tessari, P.; Nissen, S.L.; Miles, J.M.; Haymond, M.W. Inverse relationship of leucine flux and oxidation to free fatty acid availability in vivo. J. Clin. Investig. 1986, 77, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.S.; Welle, S.L.; Halliday, D.; Campbell, R.G. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J. Clin. Investig. 1988, 82, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Norrelund, H.; Nair, K.S.; Nielsen, S.; Frystyk, J.; Ivarsen, P.; Jorgensen, J.O.; Christiansen, J.S.; Moller, N. The decisive role of free fatty acids for protein conservation during fasting in humans with and without growth hormone. J. Clin. Endocrinol. Metab. 2003, 88, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Poudel, S.B.; Yakar, S. Effects of GH/IGF axis on bone and cartilage. Mol. Cell. Endocrinol. 2021, 519, 111052. [Google Scholar] [CrossRef]
- Racine, H.L.; Serrat, M.A. The Actions of IGF-1 in the Growth Plate and Its Role in Postnatal Bone Elongation. Curr. Osteoporos. Rep. 2020, 18, 210–227. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; De Luca, F. Insulin-Like Growth Factor-Independent Effects of Growth Hormone on Growth Plate Chondrogenesis and Longitudinal Bone Growth. Endocrinology 2015, 156, 2541–2551. [Google Scholar] [CrossRef]
- Swarbrick, M.M.; Havel, P.J. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab. Syndr. Relat. Disord. 2008, 6, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Straub, L.G.; Scherer, P.E. Metabolic Messengers: Adiponectin. Nat. Metab. 2019, 1, 334–339. [Google Scholar] [CrossRef]
- Modan-Moses, D.; Stein, D.; Pariente, C.; Yaroslavsky, A.; Ram, A.; Faigin, M.; Loewenthal, R.; Yissachar, E.; Hemi, R.; Kanety, H. Modulation of adiponectin and leptin during refeeding of female anorexia nervosa patients. J. Clin. Endocrinol. Metab. 2007, 92, 1843–1847. [Google Scholar] [CrossRef]
- Lubbers, E.R.; List, E.O.; Jara, A.; Sackman-Sala, L.; Cordoba-Chacon, J.; Gahete, M.D.; Kineman, R.D.; Boparai, R.; Bartke, A.; Kopchick, J.J.; et al. Adiponectin in mice with altered GH action: Links to insulin sensitivity and longevity? J. Endocrinol. 2013, 216, 363–374. [Google Scholar] [CrossRef]
- Nilsson, L.; Binart, N.; Bohlooly, Y.M.; Bramnert, M.; Egecioglu, E.; Kindblom, J.; Kelly, P.A.; Kopchick, J.J.; Ormandy, C.J.; Ling, C.; et al. Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem. Biophys. Res. Commun. 2005, 331, 1120–1126. [Google Scholar] [CrossRef]
- Chikani, V.; Ho, K.K. Action of GH on skeletal muscle function: Molecular and metabolic mechanisms. J. Mol. Endocrinol. 2014, 52, R107–R123. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R. Thyroid hormone actions in cartilage and bone. Eur. Thyroid. J. 2013, 2, 3–13. [Google Scholar] [CrossRef]
- Wang, L.; Shao, Y.Y.; Ballock, R.T. Thyroid hormone-mediated growth and differentiation of growth plate chondrocytes involves IGF-1 modulation of beta-catenin signaling. J. Bone Min. Res. 2010, 25, 1138–1146. [Google Scholar] [CrossRef]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Matarese, G.; Lord, G.M.; Keogh, J.M.; Lawrence, E.; Agwu, C.; Sanna, V.; Jebb, S.A.; Perna, F.; Fontana, S.; et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig. 2002, 110, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Murphy, E.M.; Heymsfield, S.B.; Matthews, D.E.; Leibel, R.L. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J. Clin. Endocrinol. Metab. 2002, 87, 2391–2394. [Google Scholar] [CrossRef]
- Chan, J.L.; Williams, C.J.; Raciti, P.; Blakeman, J.; Kelesidis, T.; Kelesidis, I.; Johnson, M.L.; Thorner, M.O.; Mantzoros, C.S. Leptin does not mediate short-term fasting-induced changes in growth hormone pulsatility but increases IGF-I in leptin deficiency states. J. Clin. Endocrinol. Metab. 2008, 93, 2819–2827. [Google Scholar] [CrossRef]
- Beghini, M.; Brandt, S.; Korber, I.; Kohlsdorf, K.; Vollbach, H.; Lennerz, B.; Denzer, C.; Shalitin, S.; Santini, F.; Blum, W.F.; et al. Serum IGF1 and linear growth in children with congenital leptin deficiency before and after leptin substitution. Int. J. Obes. 2021, 45, 1448–1456. [Google Scholar] [CrossRef]
- Tiwari, R.; Ausman, L.M.; Agho, K.E. Determinants of stunting and severe stunting among under-fives: Evidence from the 2011 Nepal Demographic and Health Survey. BMC Pediatr. 2014, 14, 239. [Google Scholar] [CrossRef]
- Sania, A.; Spiegelman, D.; Rich-Edwards, J.; Hertzmark, E.; Mwiru, R.S.; Kisenge, R.; Fawzi, W.W. The contribution of preterm birth and intrauterine growth restriction to childhood undernutrition in Tanzania. Matern. Child. Nutr. 2015, 11, 618–630. [Google Scholar] [CrossRef]
- Christian, P.; Lee, S.E.; Donahue Angel, M.; Adair, L.S.; Arifeen, S.E.; Ashorn, P.; Barros, F.C.; Fall, C.H.; Fawzi, W.W.; Hao, W.; et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int. J. Epidemiol. 2013, 42, 1340–1355. [Google Scholar] [CrossRef]
- Mertens, A.; Benjamin-Chung, J.; Colford, J.M.J.; Coyle, J.; van der Laan, M.J.; Hubbard, A.E.; Rosete, S.; Malenica, I.; Hejazi, N.; Sofrygin, O.; et al. Causes and consequences of child growth faltering in low-resource settings. Nature 2023, 621, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J.; Humphrey, J.H. The stunting syndrome in developing countries. Paediatr. Int. Child. Health 2014, 34, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Rajpal, S.; Joe, W.; Corsi, D.J.; Sankar, R.; Kumar, A.; Subramanian, S.V. Assessing associational strength of 23 correlates of child anthropometric failure: An econometric analysis of the 2015–2016 National Family Health Survey, India. Soc. Sci. Med. 2019, 238, 112374. [Google Scholar] [CrossRef]
- Subramanian, S.V.; Ackerson, L.K.; Davey Smith, G.; John, N.A. Association of maternal height with child mortality, anthropometric failure, and anemia in India. JAMA 2009, 301, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Schoenbuchner, S.M.; Dolan, C.; Mwangome, M.; Hall, A.; Richard, S.A.; Wells, J.C.; Khara, T.; Sonko, B.; Prentice, A.M.; Moore, S.E. The relationship between wasting and stunting: A retrospective cohort analysis of longitudinal data in Gambian children from 1976 to 2016. Am. J. Clin. Nutr. 2019, 110, 498–507. [Google Scholar] [CrossRef] [PubMed]
- de Onis, M.; Branca, F. Childhood stunting: A global perspective. Matern. Child. Nutr. 2016, 12 (Suppl. S1), 12–26. [Google Scholar] [CrossRef]
- Perumal, N.; Bassani, D.G.; Roth, D.E. Use and Misuse of Stunting as a Measure of Child Health. J. Nutr. 2018, 148, 311–315. [Google Scholar] [CrossRef]
- Scheffler, C.; Hermanussen, M.; Bogin, B.; Liana, D.S.; Taolin, F.; Cempaka, P.; Irawan, M.; Ibbibah, L.F.; Mappapa, N.K.; Payong, M.K.E.; et al. Stunting is not a synonym of malnutrition. Eur. J. Clin. Nutr. 2020, 74, 377–386. [Google Scholar] [CrossRef]
- Southon, S.; Gee, J.M.; Bayliss, C.E.; Wyatt, G.M.; Horn, N.; Johnson, I.T. Intestinal microflora, morphology and enzyme activity in zinc-deficient and Zn-supplemented rats. Br. J. Nutr. 1986, 55, 603–611. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical manifestations of zinc deficiency. Annu. Rev. Nutr. 1985, 5, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Herring, C.M.; Bazer, F.W.; Johnson, G.A.; Wu, G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp. Biol. Med. 2018, 243, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D. The Rise and Fall of Protein Malnutrition in Global Health. Ann. Nutr. Metab. 2016, 69, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Endrinikapoulos, A.; Afifah, D.N.; Mexitalia, M.; Andoyo, R.; Hatimah, I.; Nuryanto, N. Study of the importance of protein needs for catch-up growth in Indonesian stunted children: A narrative review. SAGE Open Med. 2023, 11, 20503121231165562. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Nguyen, P.; Martorell, R. Effects of micronutrients on growth of children under 5 y of age: Meta-analyses of single and multiple nutrient interventions. Am. J. Clin. Nutr. 2009, 89, 191–203. [Google Scholar] [CrossRef]
- Liu, E.; Pimpin, L.; Shulkin, M.; Kranz, S.; Duggan, C.P.; Mozaffarian, D.; Fawzi, W.W. Effect of Zinc Supplementation on Growth Outcomes in Children under 5 Years of Age. Nutrients 2018, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Gera, T.; Shah, D.; Sachdev, H.S. Zinc Supplementation for Promoting Growth in Children Under 5 years of age in Low- and Middle-income Countries: A Systematic Review. Indian. Pediatr. 2019, 56, 391–406. [Google Scholar] [CrossRef]
- De Benedetti, F.; Alonzi, T.; Moretta, A.; Lazzaro, D.; Costa, P.; Poli, V.; Martini, A.; Ciliberto, G.; Fattori, E. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J. Clin. Investig. 1997, 99, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Sederquist, B.; Fernandez-Vojvodich, P.; Zaman, F.; Savendahl, L. Recent research on the growth plate: Impact of inflammatory cytokines on longitudinal bone growth. J. Mol. Endocrinol. 2014, 53, T35–T44. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Rukobo, S.; Chasekwa, B.; Mutasa, K.; Ntozini, R.; Mbuya, M.N.; Jones, A.; Moulton, L.H.; Stoltzfus, R.J.; Humphrey, J.H. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS ONE 2014, 9, e86928. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Cho, S.; Blenis, J. mTORC1, the maestro of cell metabolism and growth. Genes Dev. 2025, 39, 109–131. [Google Scholar] [CrossRef]
- Roberson, P.A.; Jefferson, L.S.; Kimball, S.R. Convergence of signaling pathways in mediating actions of leucine and IGF-1 on mTORC1 in L6 myoblasts. Am. J. Physiol. Cell Physiol. 2022, 323, C804–C812. [Google Scholar] [CrossRef]
- Jux, C.; Leiber, K.; Hugel, U.; Blum, W.; Ohlsson, C.; Klaus, G.; Mehls, O. Dexamethasone impairs growth hormone (GH)-stimulated growth by suppression of local insulin-like growth factor (IGF)-I production and expression of GH- and IGF-I-receptor in cultured rat chondrocytes. Endocrinology 1998, 139, 3296–3305. [Google Scholar] [CrossRef]
- Inagaki, T.; Lin, V.Y.; Goetz, R.; Mohammadi, M.; Mangelsdorf, D.J.; Kliewer, S.A. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 2008, 8, 77–83. [Google Scholar] [CrossRef]
- Wu, S.; Levenson, A.; Kharitonenkov, A.; De Luca, F. Fibroblast growth factor 21 (FGF21) inhibits chondrocyte function and growth hormone action directly at the growth plate. J. Biol. Chem. 2012, 287, 26060–26067. [Google Scholar] [CrossRef]
- Arndt, M.B.; Richardson, B.A.; Mahfuz, M.; Ahmed, T.; Haque, R.; Gazi, M.A.; John-Stewart, G.C.; Denno, D.M.; Scarlett, J.M.; Walson, J.L.; et al. Plasma Fibroblast Growth Factor 21 Is Associated with Subsequent Growth in a Cohort of Underweight Children in Bangladesh. Curr. Dev. Nutr. 2019, 3, nzz024. [Google Scholar] [CrossRef]
- Lauer, J.M.; Pyykko, J.; Chembe, M.; Billima-Mulenga, T.; Sikazwe, D.; Chibwe, B.; Henderson, S.; Parkerson, D.; Leppanen, J.M.; Fink, G.; et al. Markers of Environmental Enteric Dysfunction are Associated with Poor Growth and Developmental Outcomes among Young Children in Lusaka, Zambia. J. Pediatr. 2025, 277, 114408. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M.; Ward, K.A.; Goldberg, G.R.; Jarjou, L.M.; Moore, S.E.; Fulford, A.J.; Prentice, A. Critical windows for nutritional interventions against stunting. Am. J. Clin. Nutr. 2013, 97, 911–918. [Google Scholar] [CrossRef]
- Francis-Emmanuel, P.M.; Thompson, D.S.; Barnett, A.T.; Osmond, C.; Byrne, C.D.; Hanson, M.A.; Gluckman, P.D.; Forrester, T.E.; Boyne, M.S. Glucose metabolism in adult survivors of severe acute malnutrition. J. Clin. Endocrinol. Metab. 2014, 99, 2233–2240. [Google Scholar] [CrossRef]
- Stein, A.D.; Wang, M.; Martorell, R.; Norris, S.A.; Adair, L.S.; Bas, I.; Sachdev, H.S.; Bhargava, S.K.; Fall, C.H.; Gigante, D.P.; et al. Growth patterns in early childhood and final attained stature: Data from five birth cohorts from low- and middle-income countries. Am. J. Hum. Biol. 2010, 22, 353–359. [Google Scholar] [CrossRef]
- Coly, A.N.; Milet, J.; Diallo, A.; Ndiaye, T.; Benefice, E.; Simondon, F.; Wade, S.; Simondon, K.B. Preschool stunting, adolescent migration, catch-up growth, and adult height in young senegalese men and women of rural origin. J. Nutr. 2006, 136, 2412–2420. [Google Scholar] [CrossRef] [PubMed]
- Bwakura-Dangarembizi, M.; Dumbura, C.; Ngosa, D.; Majo, F.D.; Piper, J.D.; Sturgeon, J.P.; Nathoo, K.J.; Amadi, B.; Norris, S.; Chasekwa, B.; et al. Fat and lean mass predict time to hospital readmission or mortality in children treated for complicated severe acute malnutrition in Zimbabwe and Zambia. Br. J. Nutr. 2023, 130, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K. Body composition of children with moderate and severe undernutrition and after treatment: A narrative review. BMC Med. 2019, 17, 215. [Google Scholar] [CrossRef]
- Gizaw, G.; Wells, J.C.; Argaw, A.; Olsen, M.F.; Abdissa, A.; Asres, Y.; Challa, F.; Berhane, M.; Abera, M.; Sadler, K.; et al. Associations of early childhood exposure to severe acute malnutrition and recovery with cardiometabolic risk markers in later childhood: 5-year prospective matched cohort study in Ethiopia. Am. J. Clin. Nutr. 2025, 121, 343–354. [Google Scholar] [CrossRef]
- Thompson, D.S.; Bourdon, C.; Massara, P.; Boyne, M.S.; Forrester, T.E.; Gonzales, G.B.; Bandsma, R.H.J. Childhood severe acute malnutrition is associated with metabolomic changes in adulthood. JCI Insight 2020, 5, e141316. [Google Scholar] [CrossRef] [PubMed]
- Mwene-Batu, P.; Bisimwa, G.; Ngaboyeka, G.; Dramaix, M.; Macq, J.; Hermans, M.P.; Lemogoum, D.; Donnen, P. Severe acute malnutrition in childhood, chronic diseases, and human capital in adulthood in the Democratic Republic of Congo: The Lwiro Cohort Study. Am. J. Clin. Nutr. 2021, 114, 70–79. [Google Scholar] [CrossRef]
- Mwene-Batu, P.; Bisimwa, G.; Donnen, P.; Bisimwa, J.; Tshongo, C.; Dramaix, M.; Hermans, M.P.; Briend, A. Risk of Chronic Disease after an Episode of Marasmus, Kwashiorkor or Mixed-Type Severe Acute Malnutrition in the Democratic Republic of Congo: The Lwiro Follow-Up Study. Nutrients 2022, 14, 2465. [Google Scholar] [CrossRef]
- Thompson, D.S.; Francis-Emmanuel, P.M.; Barnett, A.T.; Osmond, C.; Hanson, M.A.; Byrne, C.D.; Gluckman, P.D.; Forrester, T.E.; Boyne, M.S. The effect of wasting and stunting during severe acute malnutrition in infancy on insulin sensitivity and insulin clearance in adult life. J. Dev. Orig. Health Dis. 2022, 13, 750–756. [Google Scholar] [CrossRef]
- Harder, T.; Rodekamp, E.; Schellong, K.; Dudenhausen, J.W.; Plagemann, A. Birth weight and subsequent risk of type 2 diabetes: A meta-analysis. Am. J. Epidemiol. 2007, 165, 849–857. [Google Scholar] [CrossRef]
- Whincup, P.H.; Kaye, S.J.; Owen, C.G.; Huxley, R.; Cook, D.G.; Anazawa, S.; Barrett-Connor, E.; Bhargava, S.K.; Birgisdottir, B.E.; Carlsson, S.; et al. Birth weight and risk of type 2 diabetes: A systematic review. JAMA 2008, 300, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.; Gamborg, M.; Sorensen, T.I.; Baker, J.L. Sex Differences in the Association Between Birth Weight and Adult Type 2 Diabetes. Diabetes 2015, 64, 4220–4225. [Google Scholar] [CrossRef]
- Yoshida-Montezuma, Y.; Stone, E.; Iftikhar, S.; De Rubeis, V.; Andreacchi, A.T.; Keown-Stoneman, C.; Mbuagbaw, L.; Brown, H.K.; de Souza, R.J.; Anderson, L.N. The association between late preterm birth and cardiometabolic conditions across the life course: A systematic review and meta-analysis. Paediatr. Perinat. Epidemiol. 2022, 36, 264–275. [Google Scholar] [CrossRef]
- Bwakura-Dangarembizi, M.; Dumbura, C.; Amadi, B.; Ngosa, D.; Majo, F.D.; Nathoo, K.J.; Mwakamui, S.; Mutasa, K.; Chasekwa, B.; Ntozini, R.; et al. Risk factors for postdischarge mortality following hospitalization for severe acute malnutrition in Zimbabwe and Zambia. Am. J. Clin. Nutr. 2021, 113, 665–674. [Google Scholar] [CrossRef]
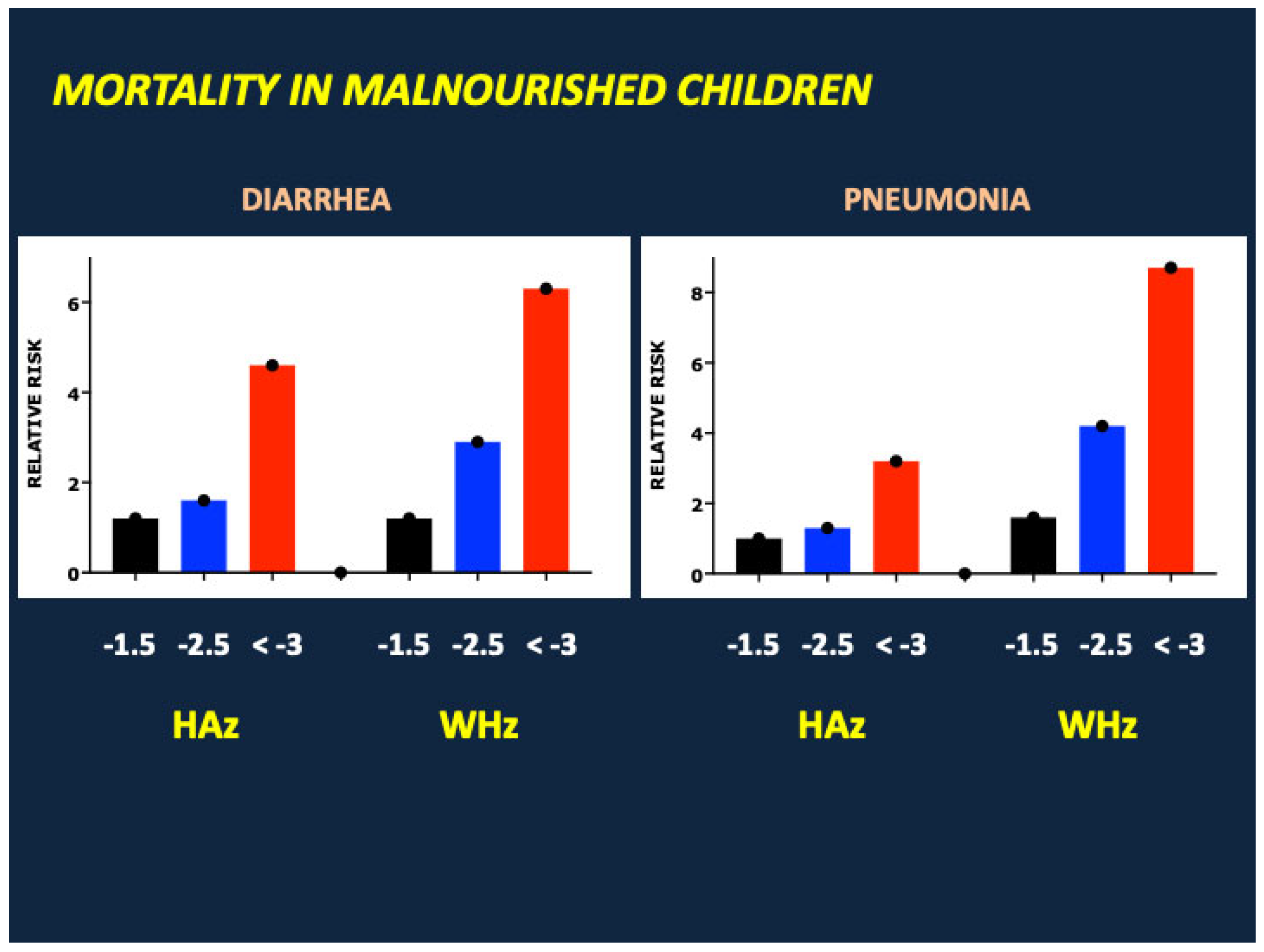
- Karunaratne, R.; Sturgeon, J.P.; Patel, R.; Prendergast, A.J. Predictors of inpatient mortality among children hospitalized for severe acute malnutrition: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2020, 112, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, J.P.; Mufukari, W.; Tome, J.; Dumbura, C.; Majo, F.D.; Ngosa, D.; Chandwe, K.; Kapoma, C.; Mutasa, K.; Nathoo, K.J.; et al. Risk factors for inpatient mortality among children with severe acute malnutrition in Zimbabwe and Zambia. Eur. J. Clin. Nutr. 2023, 77, 895–904. [Google Scholar] [CrossRef]
- Sonego, M.; Pellegrin, M.C.; Becker, G.; Lazzerini, M. Risk factors for mortality from acute lower respiratory infections (ALRI) in children under five years of age in low and middle-income countries: A systematic review and meta-analysis of observational studies. PLoS ONE 2015, 10, e0116380. [Google Scholar] [CrossRef]
- Attia, S.; Versloot, C.J.; Voskuijl, W.; van Vliet, S.J.; Di Giovanni, V.; Zhang, L.; Richardson, S.; Bourdon, C.; Netea, M.G.; Berkley, J.A.; et al. Mortality in children with complicated severe acute malnutrition is related to intestinal and systemic inflammation: An observational cohort study. Am. J. Clin. Nutr. 2016, 104, 1441–1449. [Google Scholar] [CrossRef]
- Njunge, J.M.; Gwela, A.; Kibinge, N.K.; Ngari, M.; Nyamako, L.; Nyatichi, E.; Thitiri, J.; Gonzales, G.B.; Bandsma, R.H.J.; Walson, J.L.; et al. Biomarkers of post-discharge mortality among children with complicated severe acute malnutrition. Sci. Rep. 2019, 9, 5981. [Google Scholar] [CrossRef] [PubMed]
- Dailey-Chwalibog, T.; Freemark, M.; Muehlbauer, M.; Roberfroid, D.; Kemokai, I.A.; Mostak, M.R.; Alim, M.A.; Khan, M.; Khan, M.A.H.; Bawo, L.; et al. Clinical and Biochemical Markers of Risk in Uncomplicated Severe Acute Malnutrition. Pediatrics 2021, 147, e2020027003. [Google Scholar] [CrossRef]
- Deng, L.; Argaw, A.; Guesdon, B.; Freemark, M.; Roberfroid, D.; Kemokai, I.A.; Mostak, M.R.; Alim, M.A.; Khan, M.A.H.; Muehlbauer, M.; et al. Clinical and biochemical responses to treatment of uncomplicated severe acute malnutrition: A multicenter observational cohort from the OptiDiag study. Am. J. Clin. Nutr. 2024, 120, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Nalwanga, D.; Musiime, V.; Kiguli, S.; Olupot-Olupot, P.; Alaroker, F.; Opoka, R.; Tagoola, A.; Mnjalla, H.; Mogaka, C.; Nabawanuka, E.; et al. Is fat mass a better predictor of 6-month survival than muscle mass among African children aged 6-59 months with severe pneumonia? BMC Nutr. 2024, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, J.; Eeckels, R.; Hokken-Koelega, A. Fatness and muscularity as risk indicators of child mortality in rural Congo. Int. J. Epidemiol. 1998, 27, 840–844. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gomez-Reino, J.J.; Mera, A.; Lago, F.; Gomez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Kiernan, K.; MacIver, N.J. The Role of the Adipokine Leptin in Immune Cell Function in Health and Disease. Front. Immunol. 2020, 11, 622468. [Google Scholar] [CrossRef]
- Wrann, C.D.; Laue, T.; Hubner, L.; Kuhlmann, S.; Jacobs, R.; Goudeva, L.; Nave, H. Short-term and long-term leptin exposure differentially affect human natural killer cell immune functions. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E108–E116. [Google Scholar] [CrossRef]
- Hsu, A.; Aronoff, D.M.; Phipps, J.; Goel, D.; Mancuso, P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin. Exp. Immunol. 2007, 150, 332–339. [Google Scholar] [CrossRef]
- Maurya, R.; Bhattacharya, P.; Dey, R.; Nakhasi, H.L. Leptin Functions in Infectious Diseases. Front. Immunol. 2018, 9, 2741. [Google Scholar] [CrossRef]
- Tsiotra, P.C.; Boutati, E.; Dimitriadis, G.; Raptis, S.A. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. Biomed. Res. Int. 2013, 2013, 487081. [Google Scholar] [CrossRef]
- Moraes-Vieira, P.M.; Larocca, R.A.; Bassi, E.J.; Peron, J.P.; Andrade-Oliveira, V.; Wasinski, F.; Araujo, R.; Thornley, T.; Quintana, F.J.; Basso, A.S.; et al. Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur. J. Immunol. 2014, 44, 794–806. [Google Scholar] [CrossRef]
- Lo, C.K.; Lam, Q.L.; Yang, M.; Ko, K.H.; Sun, L.; Ma, R.; Wang, S.; Xu, H.; Tam, S.; Wu, C.Y.; et al. Leptin signaling protects NK cells from apoptosis during development in mouse bone marrow. Cell Mol. Immunol. 2009, 6, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Najib, S.; Sanchez-Margalet, V. Human leptin promotes survival of human circulating blood monocytes prone to apoptosis by activation of p42/44 MAPK pathway. Cell Immunol. 2002, 220, 143–149. [Google Scholar] [CrossRef]
- Sun, Z.; Dragon, S.; Becker, A.; Gounni, A.S. Leptin inhibits neutrophil apoptosis in children via ERK/NF-kappaB-dependent pathways. PLoS ONE 2013, 8, e55249. [Google Scholar] [CrossRef][Green Version]
- Conus, S.; Bruno, A.; Simon, H.U. Leptin is an eosinophil survival factor. J. Allergy Clin. Immunol. 2005, 116, 1228–1234. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lim, J.H.; Choi, S.W.; Kim, M.; Kim, S.T.; Kim, M.S.; Cho, Y.S.; Chun, E.; Lee, K.Y. Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem. Biophys. Res. Commun. 2010, 394, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Gruver, A.L.; Ventevogel, M.S.; Sempowski, G.D. Leptin receptor is expressed in thymus medulla and leptin protects against thymic remodeling during endotoxemia-induced thymus involution. J. Endocrinol. 2009, 203, 75–85. [Google Scholar] [CrossRef]
- Rytter, M.J.H.; Cichon, B.; Fabiansen, C.; Yameogo, C.W.; Windinmi, S.Z.; Michaelsen, K.F.; Filteau, S.; Jeppesen, D.L.; Friis, H.; Briend, A.; et al. Thymus size in children with moderate malnutrition: A cohort study from Burkina Faso. Pediatr. Res. 2021, 89, 1732–1741. [Google Scholar] [CrossRef]
- Nabukeera-Barungi, N.; Lanyero, B.; Grenov, B.; Friis, H.; Namusoke, H.; Mupere, E.; Michaelsen, K.F.; Molgaard, C.; Wiese, M.; Nielsen, D.S.; et al. Thymus size and its correlates among children admitted with severe acute malnutrition: A cross-sectional study in Uganda. BMC Pediatr. 2021, 21, 1. [Google Scholar] [CrossRef]
- Watts, T. Thymus weights in malnourished children. J. Trop. Pediatr. 1969, 15, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.K.; Lord, G.M.; Matarese, G.; Vendetti, S.; Ghatei, M.A.; Ritter, M.A.; Lechler, R.I.; Bloom, S.R. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J. Clin. Investig. 1999, 104, 1051–1059. [Google Scholar] [CrossRef]
- Hick, R.W.; Gruver, A.L.; Ventevogel, M.S.; Haynes, B.F.; Sempowski, G.D. Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J. Immunol. 2006, 177, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Gollapudi, S.; Su, H.; Gupta, S. Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 2011, 31, 472–478. [Google Scholar] [CrossRef]
- Saeed, S.; Khanam, R.; Janjua, Q.M.; Manzoor, J.; Ning, L.; Hanook, S.; Canouil, M.; Ali, M.; Ayesha, H.; Khan, W.I.; et al. High morbidity and mortality in children with untreated congenital deficiency of leptin or its receptor. Cell Rep. Med. 2023, 4, 101187. [Google Scholar] [CrossRef]
- Ozata, M.; Ozdemir, I.C.; Licinio, J. Human leptin deficiency caused by a missense mutation: Multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 1999, 84, 3686–3695. [Google Scholar] [CrossRef]
- Fernandez-Pombo, A.; Sanchez-Iglesias, S.; Castro-Pais, A.I.; Ginzo-Villamayor, M.J.; Cobelo-Gomez, S.; Prado-Morana, T.; Diaz-Lopez, E.J.; Casanueva, F.F.; Loidi, L.; Araujo-Vilar, D. Natural history and comorbidities of generalised and partial lipodystrophy syndromes in Spain. Front. Endocrinol. 2023, 14, 1250203. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Asi, N.; Farah, W.; Almasri, J.; Barrionuevo, P.; Alsawas, M.; Wang, Z.; Haymond, M.W.; Brown, R.J.; Murad, M.H. Clinical Features and Management of Non-HIV-Related Lipodystrophy in Children: A Systematic Review. J. Clin. Endocrinol. Metab. 2017, 102, 363–374. [Google Scholar] [CrossRef]
- Lima, J.G.; Nobrega, L.H.C.; Lima, N.N.; Dos Santos, M.C.F.; Silva, P.H.D.; Baracho, M.F.P.; Lima, D.N.; de Melo Campos, J.T.A.; Ferreira, L.C.; Freire Neto, F.P.; et al. Causes of death in patients with Berardinelli-Seip congenital generalized lipodystrophy. PLoS ONE 2018, 13, e0199052. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Oral, E.A.; Neidert, A.; Rus, D.; Cheng, W.Y.; Thompson-Leduc, P.; Cheung, H.C.; Bradt, P.; Foss de Freitas, M.C.; Montenegro, R.M.; et al. Comorbidities and Survival in Patients With Lipodystrophy: An International Chart Review Study. J. Clin. Endocrinol. Metab. 2019, 104, 5120–5135. [Google Scholar] [CrossRef]
- Yildirim Simsir, I.; Tuysuz, B.; Ozbek, M.N.; Tanrikulu, S.; Celik Guler, M.; Karhan, A.N.; Denkboy Ongen, Y.; Gunes, N.; Soyaltin, U.E.; Altay, C.; et al. Clinical features of generalized lipodystrophy in Turkey: A cohort analysis. Diabetes Obes. Metab. 2023, 25, 1950–1963. [Google Scholar] [CrossRef]
- Besa, E.C.; Chandwe, K.; Banda, R.; Munalula, L.; Kalomo, L.; Amadi, B.; Kelly, P. Glucagon-like Peptide 2 Concentrations Vary in Zambian Children During Diarrhoea, in Malnutrition and Seasonally. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 513–520. [Google Scholar] [CrossRef]
- Besa, E.; Tembo, M.J.; Mulenga, C.; Mweetwa, M.; Choudhry, N.; Chandwe, K.; Storer, C.; Head, R.; Amadi, B.; Haritunians, T.; et al. Potential determinants of low circulating glucagon-like peptide 2 concentrations in Zambian children with non-responsive stunting. Exp. Physiol. 2023, 108, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, J.P.; Tome, J.; Dumbura, C.; Majo, F.D.; Ngosa, D.; Mutasa, K.; Zyambo, K.; Besa, E.; Chandwe, K.; Kapoma, C.; et al. Inflammation and epithelial repair predict mortality, hospital readmission, and growth recovery in complicated severe acute malnutrition. Sci. Transl. Med. 2024, 16, eadh0673. [Google Scholar] [CrossRef] [PubMed]
- Chrysafi, P.; Valenzuela-Vallejo, L.; Stefanakis, K.; Kelesidis, T.; Connelly, M.A.; Mantzoros, C.S. Total and H-specific GDF-15 levels increase in caloric deprivation independently of leptin in humans. Nat. Commun. 2024, 15, 5190. [Google Scholar] [CrossRef]
- Rostami, N.; Fabre-Estremera, B.; Buno-Soto, A.; Banegas, J.R.; Rodriguez-Artalejo, F.; Ortola, R. Growth differentiation factor 15 and malnutrition in older adults. J. Nutr. Health Aging 2024, 28, 100230. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Sigvardsen, C.M.; Richter, M.M.; Engelbeen, S.; Kleinert, M.; Richter, E.A. GDF15 is still a mystery hormone. Trends Endocrinol. Metab. 2025, 36, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.A.; Simha, V.; Ruiz, E.; Andewelt, A.; Premkumar, A.; Snell, P.; Wagner, A.J.; DePaoli, A.M.; Reitman, M.L.; Taylor, S.I.; et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 2002, 346, 570–578. [Google Scholar] [CrossRef]
- Ozalkak, S.; Demiral, M.; Unal, E.; Tas, F.F.; Onay, H.; Demirbilek, H.; Ozbek, M.N. Metreleptin Treatment in a Boy with Congenital Generalized Lipodystrophy due to Homozygous c.465_468delGACT (p.T156Rfs*8) Mutation in the BSCL2 Gene: Results from the First-year. J. Clin. Res. Pediatr. Endocrinol. 2023, 15, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Meehan, C.A.; Cochran, E.; Rother, K.I.; Kleiner, D.E.; Walter, M.; Gorden, P. Effects of Metreleptin in Pediatric Patients With Lipodystrophy. J. Clin. Endocrinol. Metab. 2017, 102, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Moran, S.A.; Patten, N.; Young, J.R.; Cochran, E.; Sebring, N.; Reynolds, J.; Premkumar, A.; Depaoli, A.M.; Skarulis, M.C.; Oral, E.A.; et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism 2004, 53, 513–519. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page, L.; McCain, E.; Freemark, M. Adaptive Responses in Severe Acute Malnutrition: Endocrinology, Metabolomics, Mortality, and Growth. Nutrients 2025, 17, 2864. https://doi.org/10.3390/nu17172864
Page L, McCain E, Freemark M. Adaptive Responses in Severe Acute Malnutrition: Endocrinology, Metabolomics, Mortality, and Growth. Nutrients. 2025; 17(17):2864. https://doi.org/10.3390/nu17172864
Chicago/Turabian StylePage, Laura, Elizabeth McCain, and Michael Freemark. 2025. "Adaptive Responses in Severe Acute Malnutrition: Endocrinology, Metabolomics, Mortality, and Growth" Nutrients 17, no. 17: 2864. https://doi.org/10.3390/nu17172864
APA StylePage, L., McCain, E., & Freemark, M. (2025). Adaptive Responses in Severe Acute Malnutrition: Endocrinology, Metabolomics, Mortality, and Growth. Nutrients, 17(17), 2864. https://doi.org/10.3390/nu17172864






