Abstract
Biological aging is a substantial change that leads to different diseases, including osteoporosis (OP), a condition involved in loss of bone density, deterioration of bone structure, and increased fracture risk. In old people, there is a natural decline in bone mineral density (BMD), exacerbated by hormonal changes, particularly during menopause, and it continues in the early postmenopausal years. During this transition time, hormonal alterations are linked to elevated oxidative stress (OS) and decreased antioxidant defenses, leading to a significant increase in OP. Aging is significantly associated with an abnormal ratio of oxidant/antioxidant and modified nuclear factor erythroid-derived two related factor2 (Nrf2)/Kelch-like ECH-associated protein 1 (Keap1) pathway. OS adversely affects bone health by promoting osteoclastic (bone resorbing) activity and impairing osteoblastic (bone-forming cells). Nrf2 is critical in controlling OS and various cellular processes. The expression of Nrf2 is linked to multiple age-related diseases, including OP, and Nrf2 deficiency leads to unbalanced bone formation/resorption and a consequent decline in bone mass. Various drugs are available for treating OP; however, long-term uses of these medicines are implicated in diverse illnesses such as cancer, cardiovascular, and stroke. At the same time, multiple categories of natural products, in particular flavonoids, were proposed as safe alternatives with antioxidant activity and substantial anti-osteoporotic effects.
1. Introduction
Osteoporosis (OP), the most prevalent comorbidity disease, is significantly associated with physiological aging [1]. This skeletal disorder is the primary reason for various complications, including bone fragility, fracture, disability, deterioration in life quality, and an increase in mortality rate [2,3]. As a woman progresses through menopause, the ovaries produce less estrogen, and these variations in estrogen exposure have a considerable influence on the majority of bodily tissues [4] and the lead of OP [5]. OP is one of the challenges associated with the onset of menopause and continues to increase in approximately 30–50% of all women in the postmenopausal phase [6,7]. Comorbidities are a common phenomenon among the elderly; in particular, OP is positioned as the most frequent [1]. Numerous modifications occur throughout aging, including the significant decline in osteoblast division and accelerated osteoblast and osteocyte apoptosis [8,9], elevated osteoblast aging [10], impaired osteoprogenitors [11], and increased bone marrow adipogenesis [12]. Indeed, the aged population is characterized by the inverse correlation between bone mass and bone marrow adipose tissue [13]. Even though age is the primary cause of oxidative stress (OS), other contributors, including lifestyle choices, diabetes, hypothyroidism, and prolonged chemotherapy treatment, are also considered risk factors for this skeleton impairment. At age fifty, 20% of males and 50% of women are expected to experience an osteoporotic fracture (OF) [14]. Nevertheless, the most frequent fracture among elderly people is a hip fracture, concurrent with an increased mortality rate [15].
OP is an advanced bone disorder that leads to diminished bone mass, proceeding to increased bone fragility, fractures, and death [2]. Two criteria, osteoblastogenesis, and osteoclastogenesis, are involved in OP disorders, and OP occurs when the osteoclastogenesis mechanism rate exceeds osteoblastogenesis [16]. Altered epigenetics has emerged as a crucial mechanism in the pathophysiology of OP [17,18,19], along with numerous hematopoietic and immune mediators in the bone microenvironment [20,21]. In aged people, many epigenetic changes are used as markers of OP [13], whereas DNA methylation is posited as the main epigenetic alteration [19,22]. DNA methylation can be modulated through physical exercise [23,24] and demethylating agents [25,26]. However, long-term exposure to these agents is frequently associated with severe side effects [27]. Furthermore, skeletal aging and OP are strongly linked to inheritable mutated genes [28], such as OPG, osteocalcin (OCN), SOST, OSX, RUNX2, RANKL, and Wnt10b [29].
2. Bone Composition and Metabolism
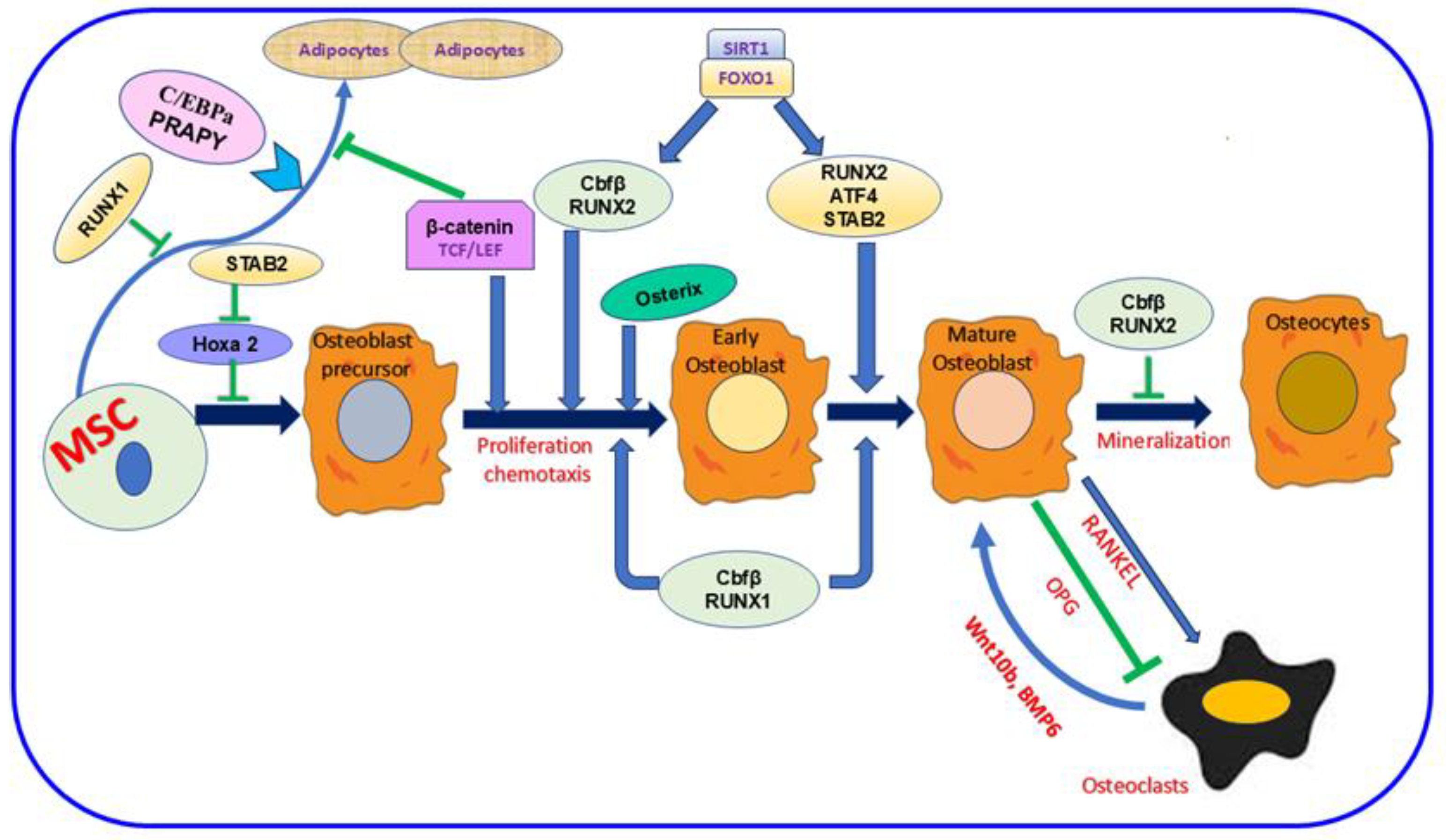
The bone comprises minerals, inorganic ions, proteins, fat, and other cellular elements [13,30,31]. This composition is essential to enhance bone function, keep the body’s structure, and shield the interior organs [13]. Four distinct cell kinds are identified in bone: osteocytes, osteoblasts, osteoclasts, and bone-lining cells [32,33]. These cell types are categorized based on their origin, abundance, location, and designated function [32,34]. In the bone, osteocyte cells are the most inner. Meanwhile, the other three cells are close to the bone surface [32,34]. Osteoblasts, the bone-making cells, are derived from mesenchymal stem cells (MSC) and are crucial in the bone-forming process [35,36]. Various proteins are involved in this mechanism, as shown in Figure 1. Inactive osteoblasts, bone-lining cells (BLC) [37] controlling bone resorption, osteoclast differentiation, regulating the synthesis of RANK, the receptor activator of nuclear factor kappa-B ligand, and osteoprotegerin (OPG) [38,39]. Osteocyte type with prevalent number and life span [40,41] orchestrate bone remodeling [42] and bone resorption [43,44,45,46]. Osteoclasts, the type of bone cell originating from the mononuclear hematopoietic stem cell, control bone resorption [16,47]. Increased osteoclast activity and production in OP and other bone illnesses promote unbalanced bone resorption/formation, decreasing bone density and increasing bone fracture risk [48,49]. Various proteins mediate bone formation, such as Runx1 and Runx2, and their major co-factor, Cbfβ. SIRT1 and FOXO1 also enhance the expression of Runx2 (Figure 1). The development of an osteoblast precursor into an early osteoblast is also promoted by Osterix and β-catenin; then, it progresses to a mature osteoblast in the presence of SATB2 and ATF4. In contrast, SATB2 slows Hoxa2 activity and the successive initial stages of osteoblast differentiation. RANKL is essential signaling during osteoclast differentiation; however, OPG inhibits this mechanism [36].
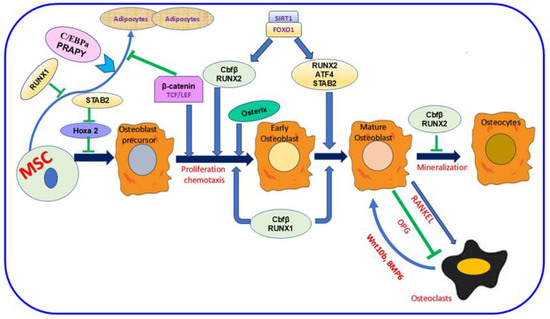
Figure 1.
The importance of mesenchymal stem cells (MSC) in the bone-formation mechanism. The figure showed the dual role of Runx2 during bone formation. This protein and Runx1 and their co-factor Cbfβ allow osteoblast differentiation. Meanwhile, Runx2 hinders the mechanism mediating osteoclast differentiation. SIRT1 and FOXO1 are crucial proteins for enhancing Runx2 expression. Various protein codings are also involved in the different stages of differentiation as follows: Osterix and β-catenin during early osteoblast formation, SATB2, and ATF4 during osteoblast maturation. In contrast, SATB2 attenuates Hoxa2 activation in the initial stage of osteoblast formation. Osteoblasts’ and osteoclasts’ interaction is vital. Meanwhile, the RANKL signaling pathway activates osteoclast differentiation, and OPG inhibits this process. In parallel, osteoclasts can also trigger osteoblast differentiation via Wnt10b, BMP6. Runx1; Runt-related 1, Runx2; Runt-related 2, Cbfβ; Core-Binding Factor Subunit Beta, Sirtuin 1; FOXO1; Forkhead Box O1, SATB2; SATB Homeobox 2, ATF4; Activating Transcription Factor 4, Hoxa2; Homeobox A2, RANKL; Receptor Activator Of Nuclear Factor Kappa-B Ligand, OPG; Osteoprotegerin, Wnt10b; Wnt Family Member 10B, BMP6; Bone Morphogenetic Protein 6.  ; inhibition.
; inhibition.
 ; inhibition.
; inhibition.
3. Bone Remodeling/Metabolism and OS
During human life, bone metabolism is controlled by bone modeling and remodeling [50,51]. In the bone modeling stage, the preformed bone transforms into a strong new bone to cope with outside factors. The remodeling mechanism maintains bone homeostasis by substituting old bone tissue with new tissue [52]. Osteoblasts and osteocytes cells are involved in the remodeling mechanism [53]. As we already know, osteocytes trigger the release of RANKL, which stimulates osteoclast activation [54]. Subsequently, the developed osteoclasts and osteoblasts form a new bone to replace the old, diminished bone [52,54,55]. Bone metabolism is also accomplished through various receptors on osteoblasts’ surface, such as estrogen, vitamin D, and other hormone receptors [54]. The multifaceted OS–bone metabolism link demonstrates the mechanism underlying OP etiology. OS has been shown to adversely affect bone health by promoting osteoclastic activity and hindering osteoblastic differentiation, initiating a net loss of bone density. Elevated reactive oxygen species (ROS) are the lead of OS [56]. Consequently, ROS accumulation enhances proteins implicated in osteoclast differentiation and activation, such as RANKL and TRAP (tartrate-resistant acid phosphatase) [57,58]. Increased ROS also leads to an elevated level of lipid peroxidation products, including malondialdehyde (MDA), which promote bone reabsorption by lowering osteoprotegerin (OPG), the typical inhibitor of osteoclastogenesis [59]. OS has been implicated in OP-linked postmenopausal complications [56].
4. Menopause, OS and OP
Menopause is a typical stage of aging for women [60], characterized by diminished ovarian follicular function, leading to the cessation of the menstrual cycle [61]. The number of postmenopausal women is increasing significantly with the increased life span, predicted to reach 1.2 billion by 2030 worldwide [62]. Under normal physiological conditions, natural menopause is expected in most women in their 50s. As an exception, premature or early menopause could be found in women who are 40–45 years old due to various medical interventions such as surgery or cancer therapy, leading to estrogen deficiency and increased risk for morbidity and mortality [63]. Menopause causes the alteration of sex hormones, such as the anti-Müllerian hormone, estrogen, the follicle-stimulating hormone (FSH), and insulin-like growth factors (ILGF-I) [64].
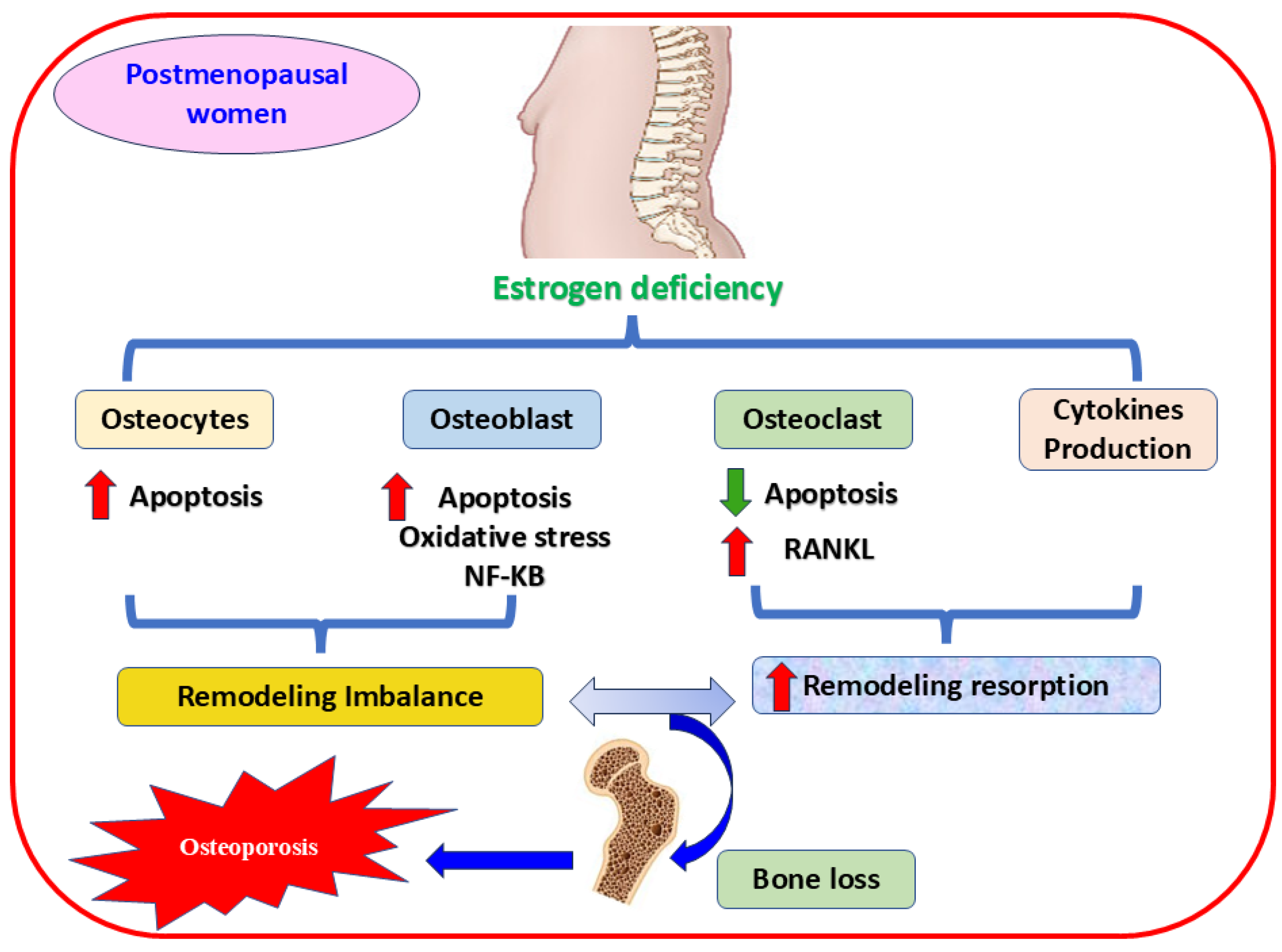
During menopause, estrogen levels decline significantly, increasing OS, bone resorption, and OP [4,5]. Estrogen and its receptors α and β sustain bone homeostasis [65], controlling bone resorption and inhibiting apoptosis in osteoblasts and osteocytes [66]. On the contrary, estrogen hindrances osteoclast differentiation [67,68] through various mechanisms, such as attenuating the expression of multiple cytokines [69] and osteoclast-linked RANKL [70], or augmenting the release of OPG, the crucial receptor of RANKL [71]. Furthermore, a deteriorated antioxidant enzymes level in osteoporotic postmenopausal women is linked to estrogen deficiency and bone loss [72,73]. This reduction in estrogen level impacts cytokine expression and leads to inflammation and elevated ROS production, subsequent excessive bone resorption, and osteoporotic fracture [74] (Figure 2). Consequently, regulating the expression of crucial genes and triggering essential proteins have become practical approaches to managing health complications in the aged population [75].
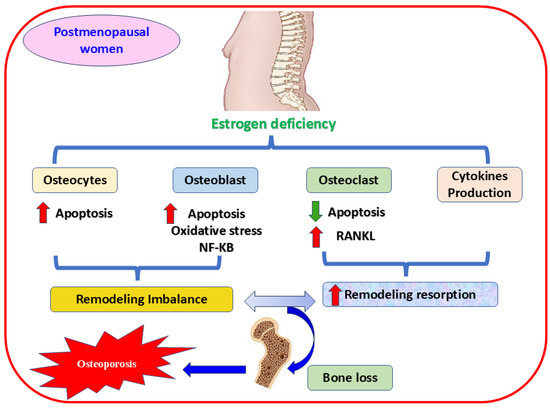
Figure 2.
The mechanism of estrogen deficiency-mediating OP in postmenopausal women. The figure shows that estrogen deficiency induces apoptosis and activates oxidative stress, the mechanism implicated in osteoporosis, by decreasing the proliferation of osteocytes and osteoblast cells. Oxidative stress and NF-κB signaling pathways are also involved in this mechanism. In contrast, activating RANKL accelerates osteoclast proliferation, and releasing proinflammatory cytokines are vital contributors in mediating bone resorption and fragility. RANKL, receptor activator of nuclear factor kappa-B ligand; NF-κB, nuclear factor kappa-B P65 Subunit,  increases,
increases,  decreases.
decreases.
 increases,
increases,  decreases.
decreases.
5. Nrf2 and OS
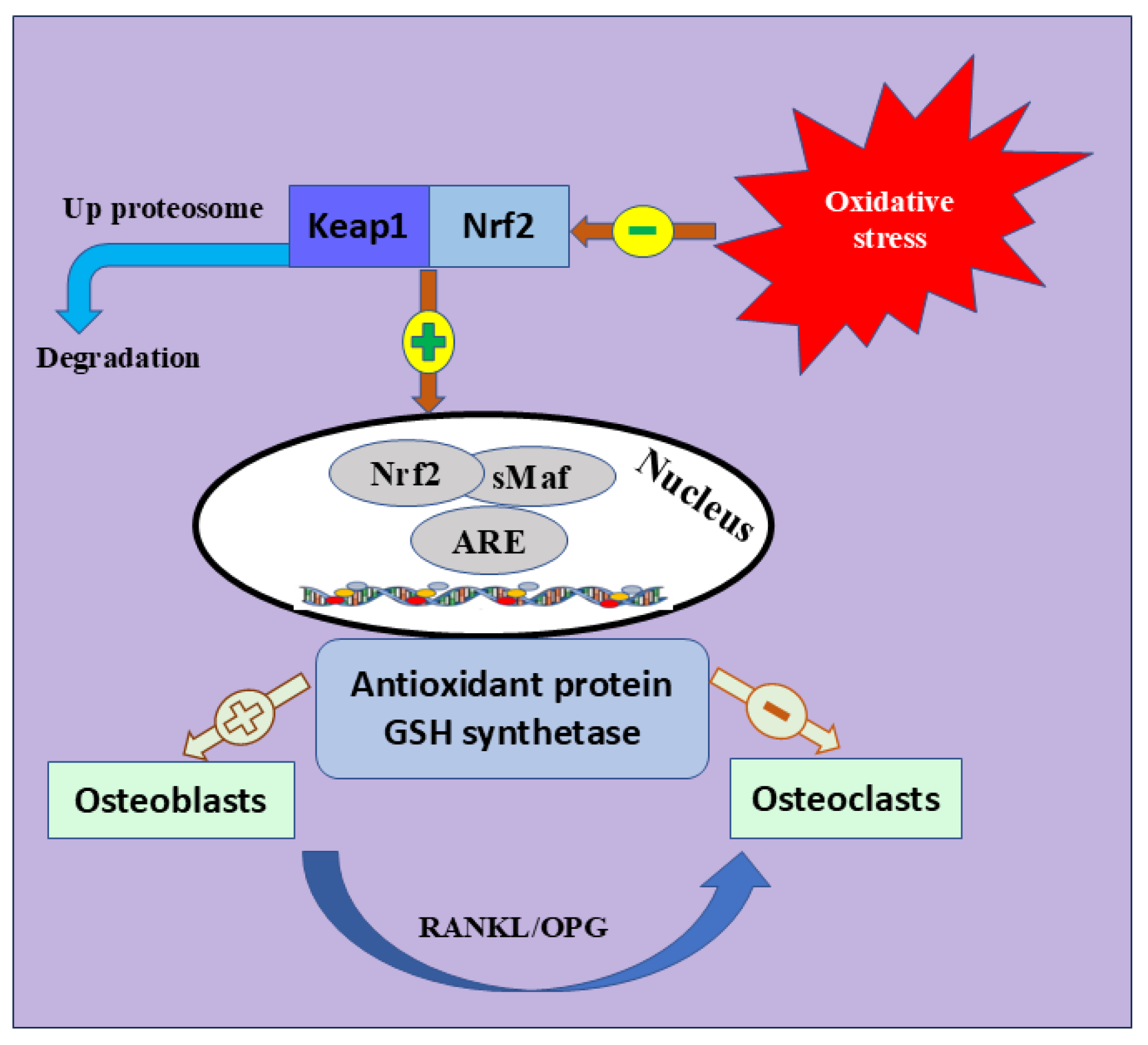
Nrf2 is the most significant intracellular defense mechanism that maintains a balance between oxidant production and elimination and mitigates OS [76,77]. Nrf2 belongs to the Cap ‘n’ Collar (CNC) subfamily [78]. It is characterized by seven functional protein homology domains (Nrf2-ECH homology, Neh). The different structures of Neh1-7 are a key factor in orchestrating the initiation, stability, and transcription activation of Nrf2 [79]. Many signaling pathways are also enhancing Nrf2 activation, including phosphatidylinositol 3-kinase (PI3K)/Ak strain transforming (Akt) pathway, epigenetics [80], mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) signaling pathway [81]. Meanwhile, Keap1 is crucial in halting the transcriptional activation of Nrf2 [82,83]. During ordinary oxidative contexts, Nrf2 is localized in the cytosol; however, its endogenous suppressor, Keap1, lowers its expression [84,85]. Once activated by OS, Nrf2 disconnects from Keap1, moves to the nucleus, and activates many endogenous antioxidants [86,87], such as glutathione sulfhydryl transferases (GSTs), catalytic enzymes of glutathione (Glutathione peroxidase, GSH-Px), peroxidase (PRDXs), superoxide dismutase (SOD), and catalase (CAT) [88]. These antioxidants mediate inflammation, OS, and identification of DNA damage [89,90] (Figure 3).
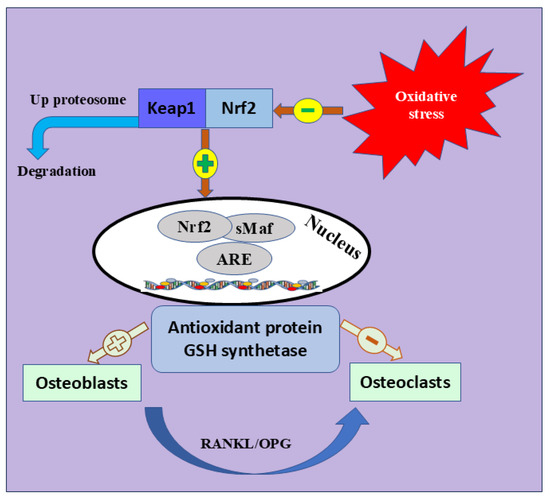
Figure 3.
Nrf2 signaling pathway and bone formation. The coexistence of Keap1 and Nrf2 inhibits the expression of Nrf2. Physiological changes in elderly and postmenopausal women trigger OS, which leads to Keap1 inhibition and segregation of Nrf2 into the nucleus to trigger different antioxidant-mediated genes. The figure also shows the positive and negative role of antioxidant protein and GSH synthetase osteoblast or osteoclast differentiation. Keap1; Kelch Like ECH Associated Protein 1, Nrf2; Nuclear Factor Erythroid 2-Related Factor 2, GSH; Glutathione peroxidase, sMaf; Small Maf, ARE; antioxidant response element, RANKL; receptor activator of nuclear factor kappa-B ligand, OPG; OPG; Osteoprotegerin.
6. Nrf2 Role in OP During Menopause
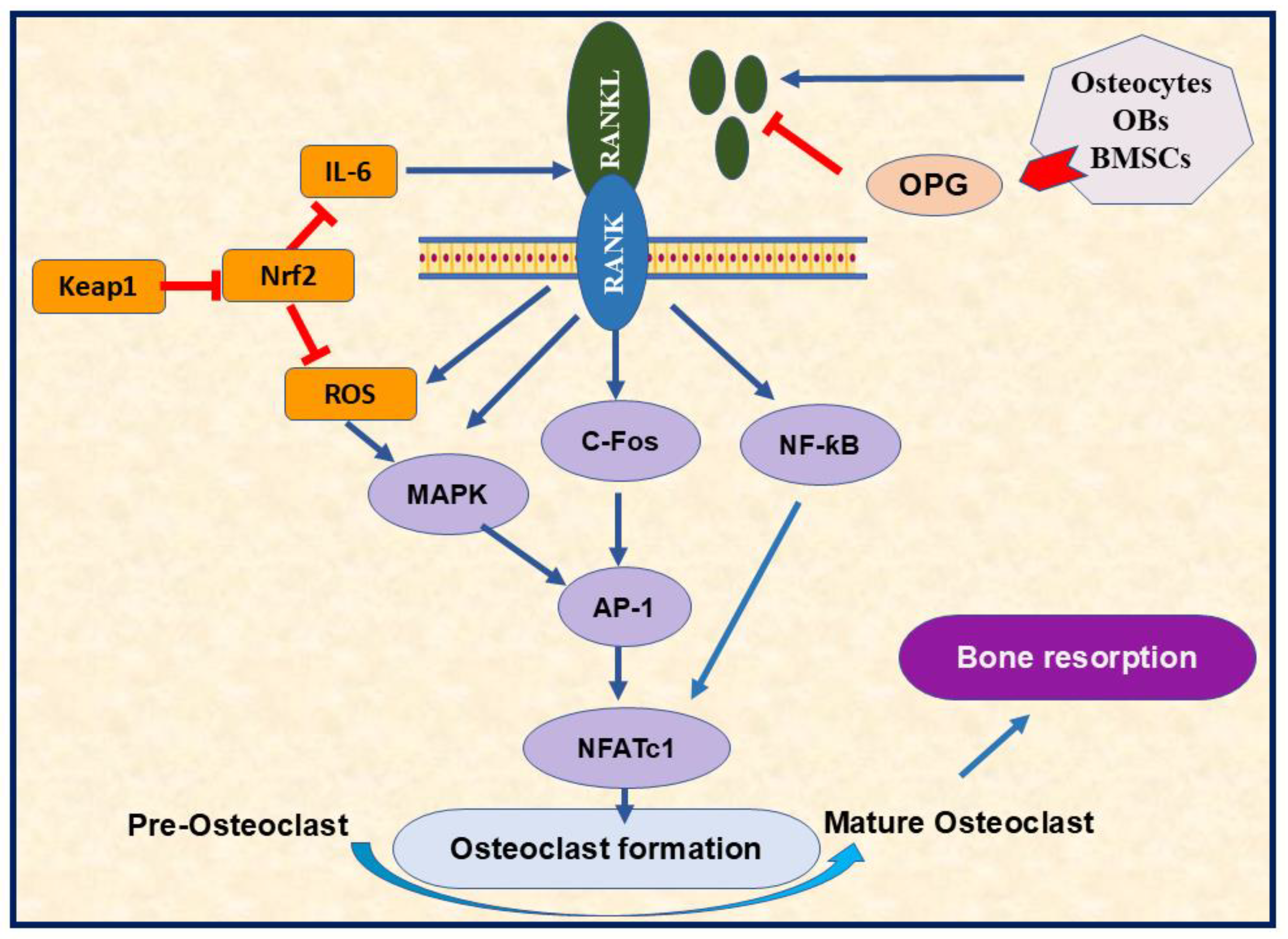
The elaborate connection between Nrf2 and menopause mainly revolves around OS and fluctuating hormones during and after the menopausal transition [91]. Inactive Nrf2 is expressed in most cells, including osteoclasts, osteoblasts, and osteocytes. Nrf2 deficiency promoted RANKL-induced osteoclast differentiation, bone resorption, and MAPK activation [92] (Figure 4). Previous research highlighted the influence of estrogen on Nrf2 activation through genomic and non-genomic avenues, the mechanism that boosts the body’s antioxidant defenses [93]. In contrast, the reduced estrogen level in postmenopausal women is implicated in decreased Nrf2 expression, increased OS markers, and imbalanced redox homeostasis, significantly targeting bone [94]. Therefore, manipulating Nrf2 expression through hormone replacement administration (HRT) may help to relieve some menopausal symptoms associated with OS [95].
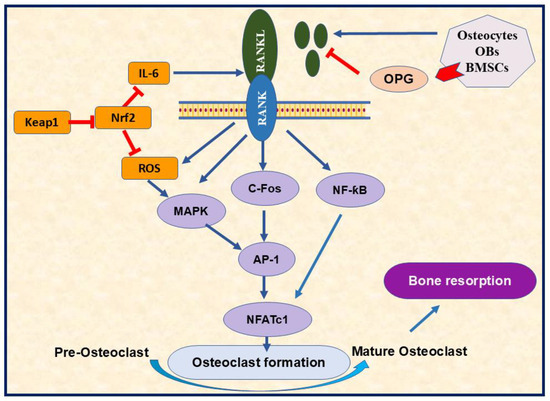
Figure 4.
The importance of RANK/RANKL signaling pathway in osteoclasts differentiation. The RANK/RANKL pathway regulates various signaling involved in osteoclastogenesis, such as MAPK, cFos, and NF-κꞵ through AP-1 and NFATc1 activation. RANK/RANKL also triggers ROS production, the mechanism that enhances osteoclastogenesis. The diagram highlighted the role of Nrf2 in decreasing osteoclastogenesis by targeting ROS formation. Furthermore, OPG can inhibit osteoclast formation by targeting RANK-RANKL binding. RANK; receptor activator of nuclear factor kappa B 2, RANKL; receptor activator of nuclear factor kappa-B ligand, OBs; osteoblasts, BMSCs; bone marrow stromal cells, OPG; osteoprotegerin, Nrf2; nuclear factor erythroid 2-related factor 2, Keap1; kelch like ECH associated protein 1, ROS; reactive oxygen species, IL-6; interleukin 6, MAPK; mitogen-activated protein kinase, C-Fos; cellular Fos proto-oncogene; NF-κB; nuclear factor kappa-B P65 Subunit, AP-1; activator protein 1, NFATc1; nuclear factor of activated T cells 1,  Inhibition.
Inhibition.
 Inhibition.
Inhibition.
7. Nrf2 and OS Role in OP
The Nrf2 signaling pathway is a fundamental mediator in orchestrating OS-linked bone homeostasis [52,96]. There is a significant association between OS and OP. Cardiovascular diseases, diabetes, and smoking are risk factors for OP that are linked to OS elevation [97,98]. ROS-mediated OS can seriously impair bone homeostasis and skeletal fragility [99,100]. ROS accumulation is a significant cause of bone loss among old people. Meanwhile, Nrf2 maintains bone homeostasis by induction of distinct genes-linked OS [101,102]. Nrf2 deficiency-mediated OS is previously demonstrated in OP complications [103], increased radiation-triggered bone fragility [104], impaired bone homeostasis, decreased bone density and strength [96], in addition to the rise in intracellular ROS levels, the mediator of bone fragility [92,104,105]. In contrast, inducing Nrf2 expression is a protective approach against OP [106,107]. Hence, attenuating the Nrf2 pathway could also initiate and enhance OP progression [108]. In osteoblasts and osteoclasts, controlling Keap1-facilitated Nrf2 activation is a critical mechanism for maintaining bone homeostasis [109]. Interestingly, antioxidant injections protected ovariectomized rats from OS via Nrf2 activation-accompanying diminished ROS concentration. Together, they support the concept that Nrf2 upregulation is an anticipated approach for maintaining bone homeostasis and managing OP [109,110].
8. Medication-Induced OP
Primary OP is an age or postmenopausal-related disease. Meanwhile, various causes can lead to secondary OP, such as hypothyroidism, diabetes, and chronic treatment with synthetic glucocorticoids [111]. Unfortunately, these drugs lead to glucocorticoid-related OP and osteonecrosis [112,113] due to increased OS, ROS, and prospective mitochondrial membrane damage [114,115,116]. Furthermore, some drugs might limit bone resorption but do not restore bone mass, ultimately decreasing bone turnover [116]. As previously mentioned, OS-related diseases downregulate Nrf2 [117], causing ROS accumulation that triggers GLOP development [118]. This mechanism emphasizes the importance of Nrf2 activation as a suggested therapeutic key for GLOP [119]. Furthermore, the degradation of superoxide dismutase (SOD) by ROS leads to substantial bone loss [73,103,110,120]. These findings highlight the crucial function of Nrf2 in osteoprotection [107].
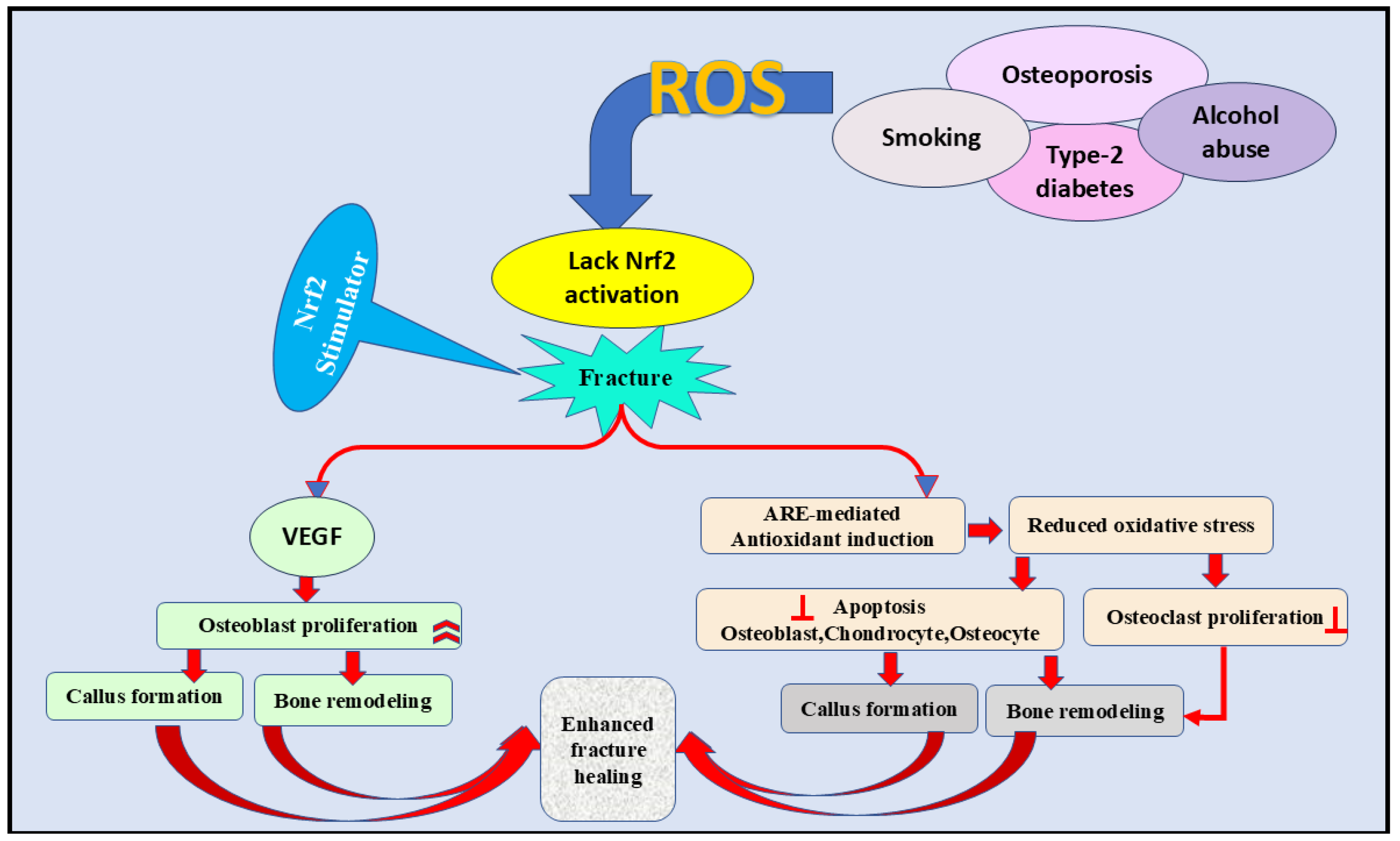
9. The Importance of the Nrf2 Signaling Pathway in Fracture Healing
Elevated expression of Nrf2 during fracture healing [121] demonstrates the significant role of this signaling pathway in managing bone formation [52]. This concept was evidenced by the slow bone-healing mechanism in Nrf2 defective mice compared with the normal [121]. In this study, the inhibition of vascular endothelial growth was the underlying process mediating the delayed bone healing [121]. Likewise, Nrf2 was also found to stimulate stem cells during tissue renewal [109]. In contrast, ROS obstruct bone homeostasis and fracture healing by stimulating osteoclasts, pausing the differentiation of osteoblasts, osteocytes, and chondrocytes [58,122,123,124,125,126]. Nrf2 signaling can also enhance fracture healing (Figure 5) by triggering antioxidant production and protecting bone cells from ROS-induced complications [109].
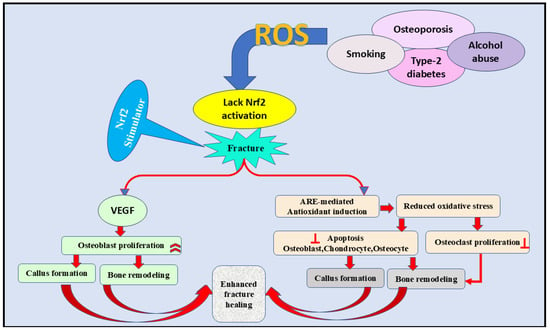
Figure 5.
The role of the Nrf2 signaling pathway in the fracture healing process by inhibiting ROS formation. Furthermore, VEGF activation and antioxidant induction are essential contributors to fracture healing through decreasing oxidative stress, increasing osteoblast proliferation, and decreasing osteoclast proliferation. VEGF; vascular endothelial growth factor, ARE; antioxidant-rsponse element, ROS; reactive oxygen species,  inhibition.
inhibition.
 inhibition.
inhibition.
10. Therapeutic Approaches for OP Treatment
Many approaches have been introduced to manage OP, such as diagnosis, fracture risk assessment, and antiresorptive anabolic drugs. The available antiresorptive agents include anti-RANKL antibodies, hormone-substitution therapy, bisphosphonates, raloxifene, and estrogen-receptor mitigators. Anabolic agents can accelerate new bone formation and stimulate bone density at low doses [127,128]. Unfortunately, various studies revealed the link between these drugs and the manifestation of serious illnesses such as stroke, cancer, and cardiovascular disorders [127,129]. Consequently, finding an alternative safe treatment is highly demanded. Natural compounds with minor side effects have been used in treating different diseases, including bone diseases. They could be projected as an alternate medicine to manage the anticipated side effects of traditional drugs [130].
11. The Possible Mechanisms of Flavonoids in Managing OP
Flavonoids are a distinguished group of natural compounds that we consume daily in food, including vegetables, fruits, cereal, wine, and tea [131,132]. Modern technology has boosted the extraction of these naturally found flavonoid compounds [133,134]. Flavonoids can be classified into three main sub-groups: iso-flavonoids (phytoestrogens), bioflavonoids, and neoflavanoids [135]. Nonetheless, these sub-groups have diverse chemical and biological properties [136]. These natural flavonoids, remarkably found in pharmaceutical manufacturing, are suggested as future therapeutic mediators in various domains [137]. Flavonoids have been used as anticancer, antioxidant, anti-inflammatory, antiresorptive, antiviral, free radical scavengers, in addition to its significant role in treating cardiovascular disorders [137,138]. Flavonoid is an alternative drug for treating OP to overcome the serious side effects associated with traditional hormone therapy [139]. These compounds have been used in treating bone loss and fracture-linked postmenopausal OP [140,141,142]. Other flavonoids such as quercetin, daidzein, kaempferol, and genistein are extensively examined. Interestingly, most investigated flavonoids have shown antioxidant properties or increased osteoblast proliferation but decreased osteoclast differentiation, and substantial anti-osteoporotic effects [47]. Soy isoflavones also revealed significant antiresorptive activity by slowing osteoclasts and advocating osteoblast differentiation [143].
Quercetin is a naturally found flavonoid in vegetables and fruits, and it has antioxidant and bone-conserving properties [130]. This flavonoid possesses several mechanisms that enhance bone, including the inhibiting of RANKL-mediated osteoclast differentiation [144] and regulating MAPK and Nrf2 signaling pathways, which are involved in osteoblast production and osteoclast differentiation [130]. Quercetin also activates the ERK signal pathway, advocating the segregation of Nrf2 from Keap1 [145] to suppress the expression of NF-κB [146]. Furthermore, quercetin augments various genes mediating the Nrf2 pathway [132,147].
Genistein is an iso-flavonoid phytoestrogen found in soybeans and exhibiting estrogenic and anti-estrogenic properties [148,149,150]. These characteristics enhance bone mineral density (BMD) in postmenopausal women with osteopenia [151]. Genistein utilizes different mechanisms to maintain bone homeostasis, such as promoting osteoblastic differentiation, suppressing tumor necrosis factor-alpha (TNF-α), the osteogenesis inhibitor [152], and preventing osteoclastogenesis and OP [153]. Furthermore, the compound upregulates ERK1/2 and protein kinase C (PKC) signaling pathways to upregulate Nrf2 mRNA and protein expression and protect cells against OS.
Kaempferol and its byproducts are generously obtained from various vegetables and fruits. Kaempferol has controlled numerous diseases, including bone disorders [154]. Earlier studies have advocated the significant role of this compound in bone formation by increasing osteogenesis while decreasing adipogenesis [155,156]. Kaempferol attenuated the TNF-α-mediate NF-κB pathway and RANKL-induced differentiation of osteoclasts [157,158,159]. Kaempferol activates the Nrf2/ARE pathway by upregulating the ERK and MAPK signaling pathways, and WNT/β-catenin [81,160,161].
Myricetin, one of the flavanol subclass, is obtained from many therapeutic plants, vegetables, fruits, and tea [162,163]. Myricetin has shown several effects, including its ability to protect osteogenesis and inhibit osteoclast differentiation [164]. Myricetin controls osteoclast differentiation and bone resorption by targeting the RANK/RANKL pathway to repress osteoclastogenic markers, inhibiting the common proinflammatory cytokines and inflammatory mediators, mainly TNF-α to suppress the NF-κB pathway [165]. Interestingly, the mechanism of NF-κB repression is facilitated by Nrf2/HO-1 upregulation [166].
The flavonoid icariin is used to moderate various bone diseases such as OP [167] by inhibiting RANKL-involved osteoclast differentiation and suppressing bone resorption by initiating osteoclast apoptosis [168]. Icariin also prevents RANKL-induced osteoclastogenesis and bone resorption by targeting MAPK and NF-κB signaling pathways [169]. This flavonoid also utilizes various mechanisms to increase osteoblasts’ mineralization and proliferation rate and maintain bone homeostasis [170]. Indeed, icariin triggers the Nrf2 signaling pathway to stimulate the antioxidative stress activity and inhibit NF-κB [171], upregulating glutathione through the PI3K/Akt/Nrf2 pathway [172].
Luteolin is also a flavonoid extracted from various herbs and used in pharmaceutical industries [173]. As exhibited by other flavonoids, luteolin has shown the ability to prevent bone loss by modulating bone resorption, inhibiting RANKL-mediated osteoclastogenesis, and eliminating OS markers [174,175], or inducing PI3K-AKT [176]. Further properties were also revealed by luteolin, such as the ability to induce the osteoblastic process and enhance collagen production [177], inhibit essential proinflammatory cytokines, and target ROS production [178].
Hesperidin belongs to the flavanones subgroup, and it is the main compound in citrus fruits [179]. This flavone has a substantial advantage in bone health [180]. Hesperidin was also revealed to stimulate Nrf2 [81] by endorsing PI3K/AKT and Wnt/b-catenin signaling pathways [80,181] and to inhibit bone resorption and NF-κB-triggered osteoclastogenesis [182]. This compound was also found to reduce OS, inflammation, p53, and manage estrogen pathway [183,184,185].
Apigenin is a member of the subgroup flavone, generously found in various vegetables and fruits [173]. This compound has a significant role in preventing bone loss [186,187,188,189,190]. Apigenin upregulates glutathione peroxidase and antioxidant enzymes to decrease ROS production. Furthermore, this flavone augments the expression of many genes mediating osteoblast differentiation, while IL-6 and NO were abolished [191]. Some signaling pathways, such as JNK, p38 MAPK, and NF-κB signaling pathways, play a crucial role during apigenin-triggered osteogenesis, demonstrating the advantages of apigenin in managing OP [191,192].
The isoflavonoid daidzein is a phytoestrogen that is found mainly in soy products. Hence, this compound has been introduced as an alternative to estrogen replacement therapy [193,194,195,196]. Daizen boosts the growth of osteoblast cells [197] and increases the expression of crucial osteogenesis genes [198]. Furthermore, daidzein increased RUNX2 expression and OPG production and reduced RANKL [199]. A substantial reduction in NF-κB and the osteoclastogenesis inducers ROS and TNF-α was also revealed in daidzein-treated OVAX mice [200,201].
Other natural flavonoids, such as capsaicin, were previously found to improve OP in OVX rats [202]. Capsaicin increases bone maturity, BMD, femoral trabecular area, and calcium and estrogen levels while reducing TNF-α and alkaline phosphatase (ALP) [202]. Fisetin, a constituent of Rhus succedanea, was previously found to prevent bone resorption and osteoclast differentiation. Fisetin prevents RANKL-involved ROS production by triggering Nrf2-linked induction of antioxidative enzymes, including HO-1, glutathione-S-transferase (GST), NQO-1, and glutamate-cysteine ligase (GCL) [203]. Alpinumisoflavone, derived from Derris eriocarpa, has considerably moderated glucocorticoid-induced OP in animal studies. This property was exhibited by inhibiting ROS level, whereas activating the Nrf2 pathway and its downstream molecules NQO-1 and HO-1, the mechanism that reverses the osteoporotic process of glucocorticoid. Neobavaisoflavone, a Chinese plant Psoralea corylifolia derivative, demonstrated an anti-osteoporotic effect in OVAX mice by inhibiting osteoclastogenesis and reducing bone loss [204,205]. This compound also protects osteoblasts against dexamethasone-generated OS by stimulating Nrf2/HO-1/NQO1 signaling pathway [206]. In consistency, the natural compounds anthocyanins have shown antioxidant properties by increasing Nrf2/HO-1 expression, whereas the ROS level was inhibited [207].
12. Clinical Trials
Meta-analyses of animal studies typically encourage more human clinical trials [208]. Flavonoids have shown a profound effect on bone health. However, only a limited number of studies have expanded beyond animal models [143]. For example, treating people with quercetin at 0.5 g/day for three months enhanced their bone health [130]. In an analogous trial, postmenopausal women who received icariin, genistein, and daidzein at a combination dosage for 24 months recognized decreased bone loss and increased BMD [140]. Many clinical trials have investigated the prospective of isoflavones to enhance (BMD). These studies have revealed the ability of soy isoflavones to lower bone loss at the lumbar spine [209,210,211]. Furthermore, the ability of genistein to manage bone metabolism in postmenopausal patients was revealed in a previous cohort study [212]. Another clinical trial also proposed curcumin as a promising natural compound for inhibiting bone loss [213]. At the same time, further human clinical trials are necessary due to the limitations of animal models in assessing the therapeutic effectiveness of icariin in treating bone diseases [140].
13. Conclusions
OP is a significant health complication among the senior community. The progression of this bone disorder progresses to fractures. This implication, accompanied by limited mobility, is a substantial financial burden on the health care provider. Declined antioxidant enzyme levels in osteoporotic postmenopausal women are implicated in estrogen deficiency and bone loss. Long-term exposure to antiresorptive and/or anabolic agents, the traditional treatment for OP, is associated with severe health complications [130]. Therefore, finding an alternative medicine is highly demanded to limit the use of these drugs. Natural flavonoids have shown promise as a safe treatment choice in various applications, most notably in pharmaceutical and health care. Of many, natural flavonoids are characterized by antioxidant properties that propose these compounds as safe compounds for managing many diseases. As demonstrated by previous investigations, these natural drugs have the potential to boost bone formation and reduce bone resorption. Additionally, new research has illuminated the significance of the Nrf2 signaling pathway, which has become a key policy in controlling OS-mediated bone homeostasis. Thus, it is relevant to consider that Nrf2 overexpression may be a valuable approach for sustaining bone health and treating various skeleton illnesses, such as OP and the corresponding bone fracture [109].
Author Contributions
Conceptualization, S.S.M., K.F.A.S. and C.O.O.; resources, K.F.A.S.; investigation, S.S.M.; writing original draft, S.S.M.; review and editing, S.S.M., F.F.F., S.G., L.M.L., C.O.O. and K.F.A.S.; project administration K.F.A.S. and C.O.O.; funding acquisition, K.F.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Institute of Minority Health and Health Disparities of the National Institutes of Health through Grant Number U54 MD007582 and Grant Number P20 MD006738.
Data Availability Statement
No new data were generated or analyzed during this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.; Tzioupis, C.; Almalki, T.; Buckley, R. Fracture healing in osteoporotic fractures: Is it really different? A basic science perspective. Injury 2007, 38 (Suppl. S1), S90–S99. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Cong, W.N.; Ji, S.; Rothman, S.; Maudsley, S.; Martin, B. Metabolic dysfunction in Alzheimer’s disease and related neurodegenerative disorders. Curr. Alzheimer Res. 2012, 9, 5–17. [Google Scholar] [CrossRef]
- Stachenfeld, N.S. Hormonal changes during menopause and the impact on fluid regulation. Reprod. Sci. 2014, 21, 555–561. [Google Scholar] [CrossRef]
- Goh, M.; Nguyen, H.H.; Khan, N.N.; Milat, F.; Boyle, J.A.; Vincent, A.J. Identifying and addressing osteoporosis knowledge gaps in women with premature ovarian insufficiency and early menopause: A mixed-methods study. Clin. Endocrinol. 2019, 91, 498–507. [Google Scholar] [CrossRef]
- Volodymyr, S.; Marina, S.; Olexandr, D.; Anna, S.; Oleksandr, K. Management of Menopausal Osteoporosis. In Proceedings of the 8th International Scientific and Practical Conference “Priority Areas of Research in the Scientific Activity of Teachers”, Zagreb, Croatia, 27 February–1 March 2024; International Science Group: New York, NY, USA, 2024. 298p. p. 84. [Google Scholar]
- Caplan, R.M. Menopause and Osteoporosis. In Long Life Strategy: A Guide for Living a Longer, Healthier, and More Fulfilling Life; Springer: Berlin/Heidelberg, Germany, 2024; pp. 91–107. [Google Scholar]
- Jilka, R.L.; Almeida, M.; Ambrogini, E.; Han, L.; Roberson, P.K.; Weinstein, R.S.; Manolagas, S.C. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell 2010, 9, 851–867. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Haack-Sørensen, M.; Fink, T.; Kassem, M. Inhibition of osteoblast differentiation but not adipocyte differentiation of mesenchymal stem cells by sera obtained from aged females. Bone 2006, 39, 181–188. [Google Scholar] [CrossRef]
- Farr, J.N.; Fraser, D.G.; Wang, H.; Jaehn, K.; Ogrodnik, M.B.; Weivoda, M.M.; Drake, M.T.; Tchkonia, T.; LeBrasseur, N.K.; Kirkland, J.L.; et al. Identification of Senescent Cells in the Bone Microenvironment. J. Bone Miner. Res. 2016, 31, 1920–1929. [Google Scholar] [CrossRef]
- Kassem, M.; Marie, P.J. Senescence-associated intrinsic mechanisms of osteoblast dysfunctions. Aging Cell 2011, 10, 191–197. [Google Scholar] [CrossRef]
- Singh, L.; Brennan, T.A.; Russell, E.; Kim, J.H.; Chen, Q.; Brad Johnson, F.; Pignolo, R.J. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone 2016, 85, 29–36. [Google Scholar] [CrossRef]
- Chandra, A.; Rajawat, J. Skeletal Aging and Osteoporosis: Mechanisms and Therapeutics. Int. J. Mol. Sci. 2021, 22, 3553. [Google Scholar] [CrossRef] [PubMed]
- Ayub, N.; Faraj, M.; Ghatan, S.; Reijers, J.A.A.; Napoli, N.; Oei, L. The Treatment Gap in Osteoporosis. J. Clin. Med. 2021, 10, 3002. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.L.; Seeley, D.G.; Lui, L.Y.; Cauley, J.A.; Ensrud, K.; Browner, W.S.; Nevitt, M.C.; Cummings, S.R. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2003, 18, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, D.; De Luca, A.; Carina, V.; Costa, V.; Raimondi, L.; Salamanna, F.; Alessandro, R.; Fini, M.; Giavaresi, G. Deregulated miRNAs in bone health: Epigenetic roles in osteoporosis. Bone 2019, 122, 52–75. [Google Scholar] [CrossRef] [PubMed]
- Vrtačnik, P.; Marc, J.; Ostanek, B. Epigenetic mechanisms in bone. Clin. Chem. Lab Med. 2014, 52, 589–608. [Google Scholar] [CrossRef]
- Marini, F.; Cianferotti, L.; Brandi, M.L. Epigenetic Mechanisms in Bone Biology and Osteoporosis: Can They Drive Therapeutic Choices? Int. J. Mol. Sci. 2016, 17, 1329. [Google Scholar] [CrossRef]
- Letarouilly, J.G.; Broux, O.; Clabaut, A. New insights into the epigenetics of osteoporosis. Genomics 2019, 111, 793–798. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.Y. Osteoporosis: The Result of an ’Aged’ Bone Microenvironment. Trends Mol. Med. 2016, 22, 641–644. [Google Scholar] [CrossRef]
- Wang, N.; Xin, H.; Xu, P.; Yu, Z.; Shou, D. Erxian Decoction Attenuates TNF-α Induced Osteoblast Apoptosis by Modulating the Akt/Nrf2/HO-1 Signaling Pathway. Front. Pharmacol. 2019, 10, 988. [Google Scholar] [CrossRef]
- Reppe, S.; Lien, T.G.; Hsu, Y.H.; Gautvik, V.T.; Olstad, O.K.; Yu, R.; Bakke, H.G.; Lyle, R.; Kringen, M.K.; Glad, I.K.; et al. Distinct DNA methylation profiles in bone and blood of osteoporotic and healthy postmenopausal women. Epigenetics 2017, 12, 674–687. [Google Scholar] [CrossRef]
- Ferioli, M.; Zauli, G.; Maiorano, P.; Milani, D.; Mirandola, P.; Neri, L.M. Role of physical exercise in the regulation of epigenetic mechanisms in inflammation, cancer, neurodegenerative diseases, and aging process. J. Cell. Physiol. 2019, 234, 14852–14864. [Google Scholar] [CrossRef] [PubMed]
- Barrès, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Das, V.; Vyas, P.; Hajdúch, M. Nucleosidic DNA demethylating epigenetic drugs—A comprehensive review from discovery to clinic. Pharmacol. Ther. 2018, 188, 45–79. [Google Scholar] [CrossRef]
- Guan, H.; Mi, B.; Li, Y.; Wu, W.; Tan, P.; Fang, Z.; Li, J.; Zhang, Y.; Li, F. Decitabine represses osteoclastogenesis through inhibition of RANK and NF-κB. Cell Signal 2015, 27, 969–977. [Google Scholar] [CrossRef]
- Martyn-St James, M.; Carroll, S. A meta-analysis of impact exercise on postmenopausal bone loss: The case for mixed loading exercise programmes. Br. J. Sports Med. 2009, 43, 898–908. [Google Scholar] [CrossRef]
- Duncan, E.L. Gene Testing in Everyday Clinical Use: Lessons from the Bone Clinic. J. Endocr. Soc. 2021, 5, bvaa200. [Google Scholar] [CrossRef]
- Patsch, J.M.; Kohler, T.; Berzlanovich, A.; Muschitz, C.; Bieglmayr, C.; Roschger, P.; Resch, H.; Pietschmann, P. Trabecular bone microstructure and local gene expression in iliac crest biopsies of men with idiopathic osteoporosis. J. Bone Miner. Res. 2011, 26, 1584–1592. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef]
- Canalis, E. Update in new anabolic therapies for osteoporosis. J. Clin. Endocrinol. Metab. 2010, 95, 1496–1504. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Glimcher, M.J.; Cooper, R.R.; Recker, R. Bone biology. I: Structure, blood supply, cells, matrix, and mineralization. Instr. Course Lect. 1996, 45, 371–386. [Google Scholar]
- Marks, S.C., Jr.; Popoff, S.N. Bone cell biology: The regulation of development, structure, and function in the skeleton. Am. J. Anat. 1988, 183, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Schinke, T.; Karsenty, G. The osteoblast: A sophisticated fibroblast under central surveillance. Science 2000, 289, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Capulli, M.; Paone, R.; Rucci, N. Osteoblast and osteocyte: Games without frontiers. Arch. Biochem. Biophys. 2014, 561, 3–12. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.P. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024, 10, 71. [Google Scholar] [CrossRef]
- Miller, S.C.; de Saint-Georges, L.; Bowman, B.M.; Jee, W.S. Bone lining cells: Structure and function. Scanning Microsc. 1989, 3, 953–960, discussion 960–951. [Google Scholar]
- Andersen, T.L.; Sondergaard, T.E.; Skorzynska, K.E.; Dagnaes-Hansen, F.; Plesner, T.L.; Hauge, E.M.; Plesner, T.; Delaisse, J.M. A physical mechanism for coupling bone resorption and formation in adult human bone. Am. J. Pathol. 2009, 174, 239–247. [Google Scholar] [CrossRef]
- Mosley, J.R. Osteoporosis and bone functional adaptation: Mechanobiological regulation of bone architecture in growing and adult bone, a review. J. Rehabil. Res. Dev. 2000, 37, 189–199. [Google Scholar]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried alive: How osteoblasts become osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Bonewald, L.F. Osteocytes as dynamic multifunctional cells. Ann. N. Y. Acad. Sci. 2007, 1116, 281–290. [Google Scholar] [CrossRef]
- Noble, B.S.; Stevens, H.; Loveridge, N.; Reeve, J. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone 1997, 20, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.I.; Plotkin, L.I.; Stewart, S.A.; Weinstein, R.S.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 2006, 21, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I. Apoptotic osteocytes and the control of targeted bone resorption. Curr. Osteoporos. Rep. 2014, 12, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, Y.; Chen, A.; Jalali, A.; Minami, K.; Ogawa, K.; Nakshatri, H.; Li, B.Y.; Yokota, H. Osteocyte-Driven Downregulation of Snail Restrains Effects of Drd2 Inhibitors on Mammary Tumor Cells. Cancer Res. 2018, 78, 3865–3876. [Google Scholar] [CrossRef]
- Bellavia, D.; Dimarco, E.; Costa, V.; Carina, V.; De Luca, A.; Raimondi, L.; Fini, M.; Gentile, C.; Caradonna, F.; Giavaresi, G. Flavonoids in Bone Erosive Diseases: Perspectives in Osteoporosis Treatment. Trends Endocrinol. Metab. 2021, 32, 76–94. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Frost, H.M. Skeletal structural adaptations to mechanical usage (SATMU): 2. Redefining Wolff’s law: The remodeling problem. Anat. Rec. 1990, 226, 414–422. [Google Scholar] [CrossRef]
- Frost, H.M. Skeletal structural adaptations to mechanical usage (SATMU): 1. Redefining Wolff’s law: The bone modeling problem. Anat. Rec. 1990, 226, 403–413. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, A.H.; Yang, Y.; Li, J. Role of Nrf2 in bone metabolism. J. Biomed. Sci. 2015, 22, 101. [Google Scholar] [CrossRef]
- Crockett, J.C.; Rogers, M.J.; Coxon, F.P.; Hocking, L.J.; Helfrich, M.H. Bone remodelling at a glance. J. Cell Sci. 2011, 124, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G. The interaction of biological factors with mechanical signals in bone adaptation: Recent developments. Curr. Osteoporos. Rep. 2012, 10, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.F.; Tirapeli, K.G.; Chaves-Neto, A.H.; da Silva Brasilino, M.; da Rocha, C.Q.; Belló-Klein, A.; Llesuy, S.F.; Dornelles, R.C.M.; Nakamune, A. Ilex paraguariensis supplementation may be an effective nutritional approach to modulate oxidative stress during perimenopause. Exp. Gerontol. 2017, 90, 14–18. [Google Scholar] [CrossRef]
- Garnero, P.; Sornay-Rendu, E.; Claustrat, B.; Delmas, P.D. Biochemical markers of bone turnover, endogenous hormones and the risk of fractures in postmenopausal women: The OFELY study. J. Bone Miner. Res. 2000, 15, 1526–1536. [Google Scholar] [CrossRef]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef]
- Huang, M.S.; Morony, S.; Lu, J.; Zhang, Z.; Bezouglaia, O.; Tseng, W.; Tetradis, S.; Demer, L.L.; Tintut, Y. Atherogenic phospholipids attenuate osteogenic signaling by BMP-2 and parathyroid hormone in osteoblasts. J. Biol. Chem. 2007, 282, 21237–21243. [Google Scholar] [CrossRef]
- Ilankoon, I.; Samarasinghe, K.; Elgán, C. Menopause is a natural stage of aging: A qualitative study. BMC Women’s Health 2021, 21, 47. [Google Scholar] [CrossRef]
- Goodman, N.F.; Cobin, R.H.; Ginzburg, S.B.; Katz, I.A.; Woode, D.E. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause. Endocr. Pract. 2011, 17 (Suppl. S6), 1–25. [Google Scholar] [CrossRef]
- Afshari, F.; Bahri, N.; Sajjadi, M.; Mansoorian, M.R.; Tohidinik, H.R. Menopause uncertainty: The impact of two educational interventions among women during menopausal transition and beyond. Prz. Menopauzalny 2020, 19, 18–24. [Google Scholar] [CrossRef]
- Shuster, L.T.; Rhodes, D.J.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Premature menopause or early menopause: Long-term health consequences. Maturitas 2010, 65, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.R.; Eyvazzadeh, A.D.; McConnell, D.; Yosef, M.; Jannausch, M.L.; Zhang, D.; Harlow, S.; Randolph, J.F., Jr. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J. Clin. Endocrinol. Metab. 2008, 93, 3478–3483. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef]
- Emerton, K.B.; Hu, B.; Woo, A.A.; Sinofsky, A.; Hernandez, C.; Majeska, R.J.; Jepsen, K.J.; Schaffler, M.B. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone 2010, 46, 577–583. [Google Scholar] [CrossRef]
- Faloni, A.P.; Sasso-Cerri, E.; Rocha, F.R.; Katchburian, E.; Cerri, P.S. Structural and functional changes in the alveolar bone osteoclasts of estrogen-treated rats. J. Anat. 2012, 220, 77–85. [Google Scholar] [CrossRef]
- Faloni, A.P.; Sasso-Cerri, E.; Katchburian, E.; Cerri, P.S. Decrease in the number and apoptosis of alveolar bone osteoclasts in estrogen-treated rats. J. Periodontal Res. 2007, 42, 193–201. [Google Scholar] [CrossRef]
- Cenci, S.; Weitzmann, M.N.; Roggia, C.; Namba, N.; Novack, D.; Woodring, J.; Pacifici, R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J. Clin. Investig. 2000, 106, 1229–1237. [Google Scholar] [CrossRef]
- Robinson, L.J.; Yaroslavskiy, B.B.; Griswold, R.D.; Zadorozny, E.V.; Guo, L.; Tourkova, I.L.; Blair, H.C. Estrogen inhibits RANKL-stimulated osteoclastic differentiation of human monocytes through estrogen and RANKL-regulated interaction of estrogen receptor-alpha with BCAR1 and Traf6. Exp. Cell Res. 2009, 315, 1287–1301. [Google Scholar] [CrossRef]
- Li, M.; Xu, D. Antiresorptive activity of osteoprotegerin requires an intact heparan sulfate-binding site. Proc. Natl. Acad. Sci. USA 2020, 117, 17187–17194. [Google Scholar] [CrossRef]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig. 2003, 112, 915–923. [Google Scholar] [CrossRef]
- Muthusami, S.; Ramachandran, I.; Muthusamy, B.; Vasudevan, G.; Prabhu, V.; Subramaniam, V.; Jagadeesan, A.; Narasimhan, S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta 2005, 360, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Pfeilschifter, J. Role of cytokines in postmenopausal bone loss. Curr. Osteoporos. Rep. 2003, 1, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Moskot, M.; Jakóbkiewicz-Banecka, J.; Kloska, A.; Smolińska, E.; Mozolewski, P.; Malinowska, M.; Rychłowski, M.; Banecki, B.; Węgrzyn, G.; Gabig-Cimińska, M. Modulation of expression of genes involved in glycosaminoglycan metabolism and lysosome biogenesis by flavonoids. Sci. Rep. 2015, 5, 9378. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Yeo, D. Oxidative stress: An evolving definition. Fac. Rev. 2021, 10, 13. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Demir Cetinkaya, B.; Biray Avci, C. Molecular perspective on targeted therapy in breast cancer: A review of current status. Med. Oncol. 2022, 39, 149. [Google Scholar] [CrossRef]
- Lin, L.; Wu, Q.; Lu, F.; Lei, J.; Zhou, Y.; Liu, Y.; Zhu, N.; Yu, Y.; Ning, Z.; She, T.; et al. Nrf2 signaling pathway: Current status and potential therapeutic targetable role in human cancers. Front. Oncol. 2023, 13, 1184079. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, J.; Bai, T.; Sachleben, L.R., Jr.; Cai, L.; Zheng, Y. Potential drugs which activate nuclear factor E2-related factor 2 signaling to prevent diabetic cardiovascular complications: A focus on fumaric acid esters. Life Sci. 2015, 134, 56–62. [Google Scholar] [CrossRef]
- Cimino, F.; Speciale, A.; Anwar, S.; Canali, R.; Ricciardi, E.; Virgili, F.; Trombetta, D.; Saija, A. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013, 8, 391–399. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim Biophys Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Paramasivan, P.; Kankia, I.H.; Langdon, S.P.; Deeni, Y.Y. Emerging role of nuclear factor erythroid 2-related factor 2 in the mechanism of action and resistance to anticancer therapies. Cancer Drug Resist. 2019, 2, 490–515. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, S.; Zhang, C.; Kong, A.N. Epigenetic regulation of Keap1-Nrf2 signaling. Free Radic. Biol. Med. 2015, 88, 337–349. [Google Scholar] [CrossRef]
- Aspera-Werz, R.H.; Ehnert, S.; Heid, D.; Zhu, S.; Chen, T.; Braun, B.; Sreekumar, V.; Arnscheidt, C.; Nussler, A.K. Nicotine and Cotinine Inhibit Catalase and Glutathione Reductase Activity Contributing to the Impaired Osteogenesis of SCP-1 Cells Exposed to Cigarette Smoke. Oxid. Med. Cell. Longev. 2018, 2018, 3172480. [Google Scholar] [CrossRef]
- Hannon Barroeta, P.; O’Sullivan, M.J.; Zisterer, D.M. The role of the Nrf2/GSH antioxidant system in cisplatin resistance in malignant rhabdoid tumours. J. Cancer Res. Clin. Oncol. 2023, 149, 8379–8391. [Google Scholar] [CrossRef]
- Yi, M.; Cruz Cisneros, L.; Cho, E.J.; Alexander, M.; Kimelman, F.A.; Swentek, L.; Ferrey, A.; Tantisattamo, E.; Ichii, H. Nrf2 pathway and oxidative stress as a common target for treatment of diabetes and its comorbidities. Int. J. Mol. Sci. 2024, 25, 821. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef]
- Gao, X.; Wang, B.; Huang, Y.; Wu, M.; Li, Y.; Li, Y.; Zhu, X.; Wu, M. Role of the Nrf2 Signaling Pathway in Ovarian Aging: Potential Mechanism and Protective Strategies. Int. J. Mol. Sci. 2023, 24, 13327. [Google Scholar] [CrossRef]
- Hyeon, S.; Lee, H.; Yang, Y.; Jeong, W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic. Biol. Med. 2013, 65, 789–799. [Google Scholar] [CrossRef]
- Samy, D.M.; Mostafa, D.K.; Saleh, S.R.; Hassaan, P.S.; Zeitoun, T.M.; Ammar, G.A.G.; Elsokkary, N.H. Carnosic Acid Mitigates Depression-Like Behavior in Ovariectomized Mice via Activation of Nrf2/HO-1 Pathway. Mol. Neurobiol. 2023, 60, 610–628. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Pérez, L.G.; Calzada-Mendoza, C.C.; Hernández-Campos, M.E.; López-Sánchez, P.; Anguiano-Robledo, L. Hormonal Replacement Therapy Modulates the Expression of Nrf2 and the Activity of Antioxidant Enzymes in Vascular Organs in a Model of Ovariectomy. Available online: https://www.jstage.jst.go.jp/article/jpssuppl/WCP2018/0/WCP2018_PO4-2-46/_article (accessed on 8 January 2025).
- Sun, Y.X.; Li, L.; Corry, K.A.; Zhang, P.; Yang, Y.; Himes, E.; Mihuti, C.L.; Nelson, C.; Dai, G.; Li, J. Deletion of Nrf2 reduces skeletal mechanical properties and decreases load-driven bone formation. Bone 2015, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Nacamuli, R.P.; Morgan, E.F.; Giaccia, A.J.; Longaker, M.T. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J. Biol. Chem. 2004, 279, 40007–40016. [Google Scholar] [CrossRef] [PubMed]
- Arnett, T.R.; Gibbons, D.C.; Utting, J.C.; Orriss, I.R.; Hoebertz, A.; Rosendaal, M.; Meghji, S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell. Physiol. 2003, 196, 2–8. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef]
- Pellegrini, G.G.; Cregor, M.; McAndrews, K.; Morales, C.C.; McCabe, L.D.; McCabe, G.P.; Peacock, M.; Burr, D.; Weaver, C.; Bellido, T. Nrf2 regulates mass accrual and the antioxidant endogenous response in bone differently depending on the sex and age. PLoS ONE 2017, 12, e0171161. [Google Scholar] [CrossRef]
- Park, C.K.; Lee, Y.; Kim, K.H.; Lee, Z.H.; Joo, M.; Kim, H.H. Nrf2 is a novel regulator of bone acquisition. Bone 2014, 63, 36–46. [Google Scholar] [CrossRef]
- Ibáñez, L.; Ferrándiz, M.L.; Brines, R.; Guede, D.; Cuadrado, A.; Alcaraz, M.J. Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxid. Med. Cell. Longev. 2014, 2014, 726590. [Google Scholar] [CrossRef]
- Rana, T.; Schultz, M.A.; Freeman, M.L.; Biswas, S. Loss of Nrf2 accelerates ionizing radiation-induced bone loss by upregulating RANKL. Free Radic. Biol. Med. 2012, 53, 2298–2307. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Myeloma bone disease: From biology findings to treatment approaches. Blood 2019, 133, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, B.; Pan, X.; Huang, H.; Xie, Z.; Ma, Y.; Hu, B.; Wang, J.; Chen, Z.; Shi, P. Octyl itaconate inhibits osteoclastogenesis by suppressing Hrd1 and activating Nrf2 signaling. FASEB J. 2019, 33, 12929–12940. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xie, Z.; Hu, B.; Zhang, B.; Ma, Y.; Pan, X.; Huang, H.; Wang, J.; Zhao, X.; Jie, Z.; et al. The Nrf2 activator RTA-408 attenuates osteoclastogenesis by inhibiting STING dependent NF-κb signaling. Redox Biol. 2020, 28, 101309. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Wei, A.; Chen, F.; Gao, Q.; Lu, K.; Jiang, Q.; Cao, W. Nrf2 epigenetic derepression induced by running exercise protects against osteoporosis. Bone Res. 2021, 9, 15. [Google Scholar] [CrossRef]
- Kubo, Y.; Wruck, C.J.; Fragoulis, A.; Drescher, W.; Pape, H.C.; Lichte, P.; Fischer, H.; Tohidnezhad, M.; Hildebrand, F.; Pufe, T.; et al. Role of Nrf2 in Fracture Healing: Clinical Aspects of Oxidative Stress. Calcif. Tissue Int. 2019, 105, 341–352. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein Suppresses Oxidative Stress and Inflammation by Nrf2 Activation in an Osteoporosis Rat Model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar] [CrossRef]
- Schäcke, H.; Döcke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Han, D.; Gu, X.; Gao, J.; Wang, Z.; Liu, G.; Barkema, H.W.; Han, B. Chlorogenic acid promotes the Nrf2/HO-1 anti-oxidative pathway by activating p21(Waf1/Cip1) to resist dexamethasone-induced apoptosis in osteoblastic cells. Free Radic. Biol.Med. 2019, 137, 1–12. [Google Scholar] [CrossRef]
- Xu, W.N.; Zheng, H.L.; Yang, R.Z.; Jiang, L.S.; Jiang, S.D. HIF-1α Regulates Glucocorticoid-Induced Osteoporosis Through PDK1/AKT/mTOR Signaling Pathway. Front. Endocrinol. 2019, 10, 922. [Google Scholar] [CrossRef]
- Li, H.; Qian, W.; Weng, X.; Wu, Z.; Li, H.; Zhuang, Q.; Feng, B.; Bian, Y. Glucocorticoid receptor and sequential P53 activation by dexamethasone mediates apoptosis and cell cycle arrest of osteoblastic MC3T3-E1 cells. PLoS ONE 2012, 7, e37030. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.F.; Wang, G.D.; Zhu, L.Q.; Tan, S.P.; Zhang, F.Y.; Zhou, X.Z.; Wang, X.D. P53 dependent mitochondrial permeability transition pore opening is required for dexamethasone-induced death of osteoblasts. J. Cell. Physiol. 2014, 229, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, S.L.; Xie, J.; Yan, D.Y.; Weng, S.J.; Tang, J.H.; Wang, B.Z.; Xie, Z.J.; Wu, Z.Y.; Yang, L. Proanthocyanidins-Mediated Nrf2 Activation Ameliorates Glucocorticoid-Induced Oxidative Stress and Mitochondrial Dysfunction in Osteoblasts. Oxid. Med. Cell. Longev. 2020, 2020, 9102012. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kim, I.S.; More, S.V.; Kim, B.W.; Choi, D.K. Natural product-derived pharmacological modulators of Nrf2/ARE pathway for chronic diseases. Nat. Prod. Rep. 2014, 31, 109–139. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, R.H.; Weston, C.J.; Curbishley, S.M.; Adams, D.H.; Afford, S.C. Autophagy: A cyto-protective mechanism which prevents primary human hepatocyte apoptosis during oxidative stress. Autophagy 2012, 8, 545–558. [Google Scholar] [CrossRef]
- Zhao, C.; Gillette, D.D.; Li, X.; Zhang, Z.; Wen, H. Nuclear factor E2-related factor-2 (Nrf2) is required for NLRP3 and AIM2 inflammasome activation. J. Biol. Chem. 2014, 289, 17020–17029. [Google Scholar] [CrossRef]
- Shuid, A.N.; Mohamad, S.; Muhammad, N.; Fadzilah, F.M.; Mokhtar, S.A.; Mohamed, N.; Soelaiman, I.N. Effects of α-tocopherol on the early phase of osteoporotic fracture healing. J. Orthop. Res. 2011, 29, 1732–1738. [Google Scholar] [CrossRef]
- Lippross, S.; Beckmann, R.; Streubesand, N.; Ayub, F.; Tohidnezhad, M.; Campbell, G.; Kan, Y.W.; Horst, F.; Sönmez, T.T.; Varoga, D.; et al. Nrf2 deficiency impairs fracture healing in mice. Calcif. Tissue Int. 2014, 95, 349–361. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef]
- Morita, K.; Miyamoto, T.; Fujita, N.; Kubota, Y.; Ito, K.; Takubo, K.; Miyamoto, K.; Ninomiya, K.; Suzuki, T.; Iwasaki, R.; et al. Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J. Exp. Med. 2007, 204, 1613–1623. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Parfitt, A.M. What old means to bone. Trends Endocrinol. Metab. 2010, 21, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.M. Overview of the fracture healing cascade. Injury 2005, 36 (Suppl. S3), S5–S7. [Google Scholar] [CrossRef]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative stress in bone remodelling and disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Chiarito, M.; D’Amato, G.; Colaianni, G.; Colucci, S.; Grano, M.; Brunetti, G. Monoclonal antibodies for treating osteoporosis. Expert Opin. Biol. Ther. 2018, 18, 149–157. [Google Scholar] [CrossRef]
- Kling, J.M.; Clarke, B.L.; Sandhu, N.P. Osteoporosis prevention, screening, and treatment: A review. J. Womens Health 2014, 23, 563–572. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. Quercetin as an Agent for Protecting the Bone: A Review of the Current Evidence. Int. J. Mol. Sci. 2020, 21, 6448. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018, 261, 75–86. [Google Scholar] [CrossRef]
- Braun, K.F.; Ehnert, S.; Freude, T.; Egaña, J.T.; Schenck, T.L.; Buchholz, A.; Schmitt, A.; Siebenlist, S.; Schyschka, L.; Neumaier, M.; et al. Quercetin protects primary human osteoblasts exposed to cigarette smoke through activation of the antioxidative enzymes HO-1 and SOD-1. Sci. World J. 2011, 11, 2348–2357. [Google Scholar] [CrossRef]
- Hassan, A.R.; Amer, K.F.; El-Toumy, S.A.; Nielsen, J.; Christensen, S.B. A new flavonol glycoside and other flavonoids from the aerial parts of Taverniera aegyptiaca. Nat. Prod. Res. 2019, 33, 1135–1139. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol. 2013, 19, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Hardcastle, A.C. The effects of flavonoids on bone. Curr. Osteoporos. Rep. 2014, 12, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Chen, H.; Lin, H.; Xie, S.; Huang, B.; Qian, Y.; Chen, K.; Niu, Y.; Shen, H.M.; Cai, J.; Li, P.; et al. Myricetin inhibits NLRP3 inflammasome activation via reduction of ROS-dependent ubiquitination of ASC and promotion of ROS-independent NLRP3 ubiquitination. Toxicol. Appl. Pharmacol. 2019, 365, 19–29. [Google Scholar] [CrossRef]
- Al-Anazi, A.F.; Qureshi, V.F.; Javaid, K.; Qureshi, S. Preventive effects of phytoestrogens against postmenopausal osteoporosis as compared to the available therapeutic choices: An overview. J. Nat. Sci. Biol. Med. 2011, 2, 154–163. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef]
- Zhao, B.J.; Wang, J.; Song, J.; Wang, C.F.; Gu, J.F.; Yuan, J.R.; Zhang, L.; Jiang, J.; Feng, L.; Jia, X.B. Beneficial Effects of a Flavonoid Fraction of Herba Epimedii on Bone Metabolism in Ovariectomized Rats. Planta Med. 2016, 82, 322–329. [Google Scholar] [CrossRef]
- Jiang, J.; Xiao, S.; Xu, X.; Ma, H.; Feng, C.; Jia, X. Isomeric flavonoid aglycones derived from Epimedii Folium exerted different intensities in anti-osteoporosis through OPG/RANKL protein targets. Int. Immunopharmacol. 2018, 62, 277–286. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid intake and bone health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, J.; Wu, W.; Ma, P.; Ren, L.; Yi, Z.; Wu, J. Quercetin Prevents Oxidative Stress-Induced Injury of Periodontal Ligament Cells and Alveolar Bone Loss in Periodontitis. Drug Des. Devel. Ther. 2021, 15, 3509–3522. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Ci, X.; Huang, J.; Liu, Q.; Yu, Q.; Zhou, J.; Deng, X. Asiatic acid enhances Nrf2 signaling to protect HepG2 cells from oxidative damage through Akt and ERK activation. Biomed. Pharmacother. 2017, 88, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef]
- Vimalraj, S.; Rajalakshmi, S.; Raj Preeth, D.; Vinoth Kumar, S.; Deepak, T.; Gopinath, V.; Murugan, K.; Chatterjee, S. Mixed-ligand copper(II) complex of quercetin regulate osteogenesis and angiogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 187–194. [Google Scholar] [CrossRef]
- Dixon, R.A.; Ferreira, D. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Albertazzi, P.; Steel, S.A.; Bottazzi, M. Effect of pure genistein on bone markers and hot flushes. Climacteric 2005, 8, 371–379. [Google Scholar] [CrossRef]
- Inpan, R.; Dukaew, N.; Na Takuathung, M.; Teekachunhatean, S.; Koonrungsesomboon, N. Effects of isoflavone interventions on bone turnover markers and factors regulating bone metabolism in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Arch. Osteoporos. 2024, 20, 2. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Intern Med. 2007, 146, 839–847. [Google Scholar] [CrossRef]
- Fanti, P.; Monier-Faugere, M.C.; Geng, Z.; Schmidt, J.; Morris, P.E.; Cohen, D.; Malluche, H.H. The phytoestrogen genistein reduces bone loss in short-term ovariectomized rats. Osteoporos. Int. 1998, 8, 274–281. [Google Scholar] [CrossRef]
- Ha, H.; Lee, H.Y.; Lee, J.H.; Jung, D.; Choi, J.; Song, K.Y.; Jung, H.J.; Choi, J.S.; Chang, S.I.; Kim, C. Formononetin prevents ovariectomy-induced bone loss in rats. Arch. Pharm. Res. 2010, 33, 625–632. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. The Osteoprotective Effects of Kaempferol: The Evidence from In Vivo and In Vitro Studies. Drug Des. Devel. Ther. 2019, 13, 3497–3514. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Kumar, S.; Kumar, A.; Siddiqui, J.A.; Swarnkar, G.; Gupta, V.; Kendurker, A.; Dwivedi, A.K.; Romero, J.R.; Chattopadhyay, N. Kaempferol has osteogenic effect in ovariectomized adult Sprague-Dawley rats. Mol. Cell. Endocrinol. 2008, 289, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tang, H.; Zhang, Z.; Zhang, Y.; Qiu, C.; Zhang, L.; Huang, P.; Li, F. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int. Immunopharmacol. 2017, 43, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liao, Y.; Xie, H.; Liao, Y.; Zeng, Y.; Li, N.; Sun, G.; Wu, Q.; Zhou, G. Effects of combined treatment with ibandronate and pulsed electromagnetic field on ovariectomy-induced osteoporosis in rats. Bioelectromagnetics 2017, 38, 31–40. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z.; Zhou, J. Tumor necrosis factor α in the onset and progression of leukemia. Exp. Hematol. 2017, 45, 17–26. [Google Scholar] [CrossRef]
- Pang, J.L.; Ricupero, D.A.; Huang, S.; Fatma, N.; Singh, D.P.; Romero, J.R.; Chattopadhyay, N. Differential activity of kaempferol and quercetin in attenuating tumor necrosis factor receptor family signaling in bone cells. Biochem. Pharmacol. 2006, 71, 818–826. [Google Scholar] [CrossRef]
- Chiou, W.F.; Lee, C.H.; Liao, J.F.; Chen, C.C. 8-Prenylkaempferol accelerates osteoblast maturation through bone morphogenetic protein-2/p38 pathway to activate Runx2 transcription. Life Sci. 2011, 88, 335–342. [Google Scholar] [CrossRef]
- Sharma, A.R.; Nam, J.S. Kaempferol stimulates WNT/β-catenin signaling pathway to induce differentiation of osteoblasts. J. Nutr. Biochem. 2019, 74, 108228. [Google Scholar] [CrossRef]
- Huang, J.; Wu, C.; Tian, B.; Zhou, X.; Ma, N.; Qian, Y. Myricetin Prevents Alveolar Bone Loss in an Experimental Ovariectomized Mouse Model of Periodontitis. Int. J. Mol. Sci. 2016, 17, 422. [Google Scholar] [CrossRef]
- Gupta, G.; Siddiqui, M.A.; Khan, M.M.; Ajmal, M.; Ahsan, R.; Rahaman, M.A.; Ahmad, M.A.; Arshad, M.; Khushtar, M. Current Pharmacological Trends on Myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef]
- Fu, Y.X.; Wang, Y.H.; Tong, X.S.; Gong, Z.; Sun, X.M.; Yuan, J.C.; Zheng, T.T.; Li, C.; Niu, D.Q.; Dai, H.G.; et al. EDACO, a derivative of myricetin, inhibits the differentiation of Gaoyou duck embryonic osteoclasts in vitro. Br. Poult. Sci. 2019, 60, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, W.; Tian, B.; Liu, X.; Qu, X.; Zhai, Z.; Li, H.; Liu, F.; Fan, Q.; Tang, T.; et al. Myricetin prevents titanium particle-induced osteolysis in vivo and inhibits RANKL-induced osteoclastogenesis in vitro. Biochem. Pharmacol. 2015, 93, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, T.; Zhang, Z.; Chen, X.; Chen, C.; Chen, L.; Wang, X.; Ying, X. Activation of Nrf2/HO-1 signal with Myricetin for attenuating ECM degradation in human chondrocytes and ameliorating the murine osteoarthritis. Int. Immunopharmacol. 2019, 75, 105742. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Wang, X.L.; Zheng, L.Z.; Dai, Y.; Zhang, J.Y.; Guo, B.L.; Yang, Z.J.; Yao, X.S.; Qin, L. Comparative study of two types of herbal capsules with different Epimedium species for the prevention of ovariectomised-induced osteoporosis in rats. J. Orthop. Translat. 2016, 4, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.G.; Chen, K.M.; Xian, C.J. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J. Cell. Physiol. 2013, 228, 513–521. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, G.; Liu, X.; Dai, M.; Zhang, B. Icariin inhibits RANKL-induced osteoclastogenesis via modulation of the NF-κB and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2019, 508, 902–906. [Google Scholar] [CrossRef]
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H.; Liu, M.H. Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine 2010, 17, 414–423. [Google Scholar] [CrossRef]
- Hwang, E.; Lin, P.; Ngo, H.T.T.; Gao, W.; Wang, Y.S.; Yu, H.S.; Yi, T.H. Icariin and icaritin recover UVB-induced photoaging by stimulating Nrf2/ARE and reducing AP-1 and NF-κB signaling pathways: A comparative study on UVB-irradiated human keratinocytes. Photochem. Photobiol. Sci. 2018, 17, 1396–1408. [Google Scholar] [CrossRef]
- Song, Y.H.; Cai, H.; Zhao, Z.M.; Chang, W.J.; Gu, N.; Cao, S.P.; Wu, M.L. Icariin attenuated oxidative stress induced-cardiac apoptosis by mitochondria protection and ERK activation. Biomed. Pharmacother. 2016, 83, 1089–1094. [Google Scholar] [CrossRef]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef]
- Kim, T.H.; Jung, J.W.; Ha, B.G.; Hong, J.M.; Park, E.K.; Kim, H.J.; Kim, S.Y. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J. Nutr. Biochem. 2011, 22, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M. Modulatory effects of luteolin on osteoblastic function and inflammatory mediators in osteoblastic MC3T3-E1 cells. Cell Biol. Int. 2007, 31, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Yang, Y.; Wei, L.; Cao, Y.; Ma, J.; Zheng, X.; Teng, J.; Qin, N. Luteolin rescues postmenopausal osteoporosis elicited by OVX through alleviating osteoblast pyroptosis via activating PI3K-AKT signaling. Phytomedicine 2024, 128, 155516. [Google Scholar] [CrossRef] [PubMed]
- Nash, L.A.; Sullivan, P.J.; Peters, S.J.; Ward, W.E. Rooibos flavonoids, orientin and luteolin, stimulate mineralization in human osteoblasts through the Wnt pathway. Mol. Nutr. Food Res. 2015, 59, 443–453. [Google Scholar] [CrossRef]
- Chen, C.Y.; Peng, W.H.; Tsai, K.D.; Hsu, S.L. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007, 81, 1602–1614. [Google Scholar] [CrossRef]
- Trzeciakiewicz, A.; Habauzit, V.; Mercier, S.; Barron, D.; Urpi-Sarda, M.; Manach, C.; Offord, E.; Horcajada, M.N. Molecular mechanism of hesperetin-7-O-glucuronide, the main circulating metabolite of hesperidin, involved in osteoblast differentiation. J. Agric. Food Chem. 2010, 58, 668–675. [Google Scholar] [CrossRef]
- Trzeciakiewicz, A.; Habauzit, V.; Mercier, S.; Lebecque, P.; Davicco, M.J.; Coxam, V.; Demigne, C.; Horcajada, M.N. Hesperetin stimulates differentiation of primary rat osteoblasts involving the BMP signalling pathway. J. Nutr. Biochem. 2010, 21, 424–431. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.Y.; Park, Y.D.; Kang, K.L.; Lee, J.C.; Heo, J.S. Hesperetin alleviates the inhibitory effects of high glucose on the osteoblastic differentiation of periodontal ligament stem cells. PLoS ONE 2013, 8, e67504. [Google Scholar] [CrossRef]
- Horcajada, M.N.; Habauzit, V.; Trzeciakiewicz, A.; Morand, C.; Gil-Izquierdo, A.; Mardon, J.; Lebecque, P.; Davicco, M.J.; Chee, W.S.; Coxam, V.; et al. Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J. Appl. Physiol. 2008, 104, 648–654. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, X.; Chen, X.; Jiang, R.; Peng, K.; Tang, X.; Liu, Z. Antiosteoporotic effect of hesperidin against ovariectomy-induced osteoporosis in rats via reduction of oxidative stress and inflammation. J. Biochem. Mol. Toxicol. 2021, 35, e22832. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, D.; Zeng, N.; Liu, Z.; Chen, X.; Xiao, H.; Xiao, L.; Liu, Z.; Dong, Y.; Zheng, J. Hesperidin Ameliorates Dexamethasone-Induced Osteoporosis by Inhibiting p53. Front Cell Dev. Biol. 2022, 10, 820922. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Zhang, Z.Z.; Jiang, X.Y.; Duan, T.H.; Feng, W.; Wang, X.G. Hesperidin Anti-Osteoporosis by Regulating Estrogen Signaling Pathways. Molecules 2023, 28, 6987. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Hagiwara, K.; Shirai, N.; Yoshida, K.; Hagiwara, H. Apigenin inhibits osteoblastogenesis and osteoclastogenesis and prevents bone loss in ovariectomized mice. Cytotechnology 2015, 67, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, M.; Li, N.; Li, Q.; Li, Y.; Zhai, Y. Total Flavonoids Isolated from the Leaves of Eucommia ulmoides Augment Peak Bone Mass in Female Rats and Show no Side Effects in Other Organs. Curr. Pharm. Des. 2024, 30, 2410–2423. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yincang, W.; Jiazhe, D.; Xilin, X.; Zhang, X. Pharmacology and mechanisms of apigenin in preventing osteoporosis. Front. Pharmacol. 2024, 15, 1486646. [Google Scholar] [CrossRef]
- Huai, Y.; Wang, X.; Mao, W.; Wang, X.; Zhao, Y.; Chu, X.; Huang, Q.; Ru, K.; Zhang, L.; Li, Y.; et al. HuR-positive stress granules: Potential targets for age-related osteoporosis. Aging Cell 2024, 23, e14053. [Google Scholar] [CrossRef]
- Ali, D.; Okla, M.; Abuelreich, S.; Vishnubalaji, R.; Ditzel, N.; Hamam, R.; Kowal, J.M.; Sayed, A.; Aldahmash, A.; Alajez, N.M.; et al. Apigenin and Rutaecarpine reduce the burden of cellular senescence in bone marrow stromal stem cells. Front. Endocrinol. 2024, 15, 1360054. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, C.; Zha, X.; Xu, Z.; Li, L.; Liu, Y.; Xu, L.; Cui, L.; Xu, D.; Zhu, B. Apigenin promotes osteogenic differentiation of human mesenchymal stem cells through JNK and p38 MAPK pathways. Mol. Cell. Biochem. 2015, 407, 41–50. [Google Scholar] [CrossRef]
- Choi, E.M. Apigenin increases osteoblastic differentiation and inhibits tumor necrosis factor-alpha-induced production of interleukin-6 and nitric oxide in osteoblastic MC3T3-E1 cells. Pharmazie 2007, 62, 216–220. [Google Scholar]
- Dang, Z.; Löwik, C.W. The balance between concurrent activation of ERs and PPARs determines daidzein-induced osteogenesis and adipogenesis. J. Bone Miner. Res. 2004, 19, 853–861. [Google Scholar] [CrossRef]
- Harahap, I.A.; Schmidt, M.; Pruszyńska-Oszmałek, E.; Sassek, M.; Suliburska, J. Impact of Lactobacillus acidophilus and Its Combination with Isoflavone Products on Calcium Status, Calcium Transporters, and Bone Metabolism Biomarkers in a Post-Menopausal Osteoporotic Rat Model. Nutrients 2024, 16, 2524. [Google Scholar] [CrossRef] [PubMed]
- Harahap, I.A.; Olejnik, A.; Kowalska, K.; Suliburska, J. Effects of Daidzein, Tempeh, and a Probiotic Digested in an Artificial Gastrointestinal Tract on Calcium Deposition in Human Osteoblast-like Saos-2 Cells. Int. J. Mol. Sci. 2024, 25, 1008. [Google Scholar] [CrossRef] [PubMed]
- Harahap, I.A.; Kuligowski, M.; Cieslak, A.; Kołodziejski, P.A.; Suliburska, J. Effect of Tempeh and Daidzein on Calcium Status, Calcium Transporters, and Bone Metabolism Biomarkers in Ovariectomized Rats. Nutrients 2024, 16, 651. [Google Scholar] [CrossRef]
- Jia, T.L.; Wang, H.Z.; Xie, L.P.; Wang, X.Y.; Zhang, R.Q. Daidzein enhances osteoblast growth that may be mediated by increased bone morphogenetic protein (BMP) production. Biochem. Pharmacol. 2003, 65, 709–715. [Google Scholar] [CrossRef]
- Yu, B.; Tang, D.Z.; Li, S.Y.; Wu, Y.; Chen, M. Daidzein promotes proliferation and differentiation in osteoblastic OCT1 cells via activation of the BMP-2/Smads pathway. Pharmazie 2017, 72, 35–40. [Google Scholar] [CrossRef]
- De Wilde, A.; Lieberherr, M.; Colin, C.; Pointillart, A. A low dose of daidzein acts as an ERbeta-selective agonist in trabecular osteoblasts of young female piglets. J. Cell. Physiol. 2004, 200, 253–262. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Srivastava, K.; Sharan, K.; Yadav, D.; Maurya, R.; Singh, D. Daidzein prevents the increase in CD4+CD28null T cells and B lymphopoesis in ovariectomized mice: A key mechanism for anti-osteoclastogenic effect. PLoS ONE 2011, 6, e21216. [Google Scholar] [CrossRef]
- Karieb, S.; Fox, S.W. Phytoestrogens directly inhibit TNF-α-induced bone resorption in RAW264.7 cells by suppressing c-fos-induced NFATc1 expression. J. Cell. Biochem. 2011, 112, 476–487. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, B.; Ma, G.; Cao, H. Sensory nerve regulation of bone homeostasis: Emerging therapeutic opportunities for bone-related diseases. Ageing Res. Rev. 2024, 99, 102372. [Google Scholar] [CrossRef]
- Sakai, E.; Shimada-Sugawara, M.; Yamaguchi, Y.; Sakamoto, H.; Fumimoto, R.; Fukuma, Y.; Nishishita, K.; Okamoto, K.; Tsukuba, T. Fisetin inhibits osteoclastogenesis through prevention of RANKL-induced ROS production by Nrf2-mediated up-regulation of phase II antioxidant enzymes. J. Pharmacol. Sci. 2013, 121, 288–298. [Google Scholar] [CrossRef]
- Xu, X.K.; Chen, Z.Y.; Li, Z.Q.; Zhang, Y.M.; Liao, L.P.; Zhou, Q.; Zhang, Z.J. [Absorption mechanism of neobavaisoflavone in Caco-2 cell monolayer mode]. Zhongguo Zhong Yao Za Zhi 2016, 41, 2922–2926. [Google Scholar] [CrossRef]
- Chen, H.; Fang, C.; Zhi, X.; Song, S.; Gu, Y.; Chen, X.; Cui, J.; Hu, Y.; Weng, W.; Zhou, Q.; et al. Neobavaisoflavone inhibits osteoclastogenesis through blocking RANKL signalling-mediated TRAF6 and c-Src recruitment and NF-κB, MAPK and Akt pathways. J. Cell. Mol. Med. 2020, 24, 9067–9084. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, X.; Wang, Z.; Zhao, Z.; Zhou, P.; Gao, X. Neobavaisoflavone protects osteoblasts from dexamethasone-induced oxidative stress by upregulating the CRNDE-mediated Nrf2/HO-1 signaling pathway. Drug Dev. Res. 2021, 82, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- van Luijk, J.; Leenaars, M.; Hooijmans, C.; Wever, K.; de Vries, R.; Ritskes-Hoitinga, M. Towards evidence-based translational research: The pros and cons of conducting systematic reviews of animal studies. Altex 2013, 30, 256–257. [Google Scholar] [CrossRef]
- Taku, K.; Melby, M.K.; Takebayashi, J.; Mizuno, S.; Ishimi, Y.; Omori, T.; Watanabe, S. Effect of soy isoflavone extract supplements on bone mineral density in menopausal women: Meta-analysis of randomized controlled trials. Asia Pac. J. Clin. Nutr. 2010, 19, 33–42. [Google Scholar]
- Ma, D.F.; Qin, L.Q.; Wang, P.Y.; Katoh, R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: Meta-analysis of randomized controlled trials. Clin. Nutr. 2008, 27, 57–64. [Google Scholar] [CrossRef]
- Liu, J.; Ho, S.C.; Su, Y.X.; Chen, W.Q.; Zhang, C.X.; Chen, Y.M. Effect of long-term intervention of soy isoflavones on bone mineral density in women: A meta-analysis of randomized controlled trials. Bone 2009, 44, 948–953. [Google Scholar] [CrossRef]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J. Bone Miner. Res. 2008, 23, 715–720. [Google Scholar] [CrossRef]
- Daily, J.W.; Yang, M.; Park, S. Efficacy of Turmeric Extracts and Curcumin for Alleviating the Symptoms of Joint Arthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Med. Food 2016, 19, 717–729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).