Single-Cell Analysis Dissects the Effects of Vitamin D on Genetic Senescence Signatures Across Murine Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Data Structure
2.3. Senescence-Associated Gene Sets
2.4. Preprocessing
2.4.1. Annotation
2.4.2. Integration
2.5. Functional Enrichment
2.6. Gene Regulatory Network Analysis
2.6.1. Network Processing
2.6.2. Network Analysis
2.7. Cellular Communication
3. Results
3.1. Cell Annotation UMAP Between VD and Vehicle
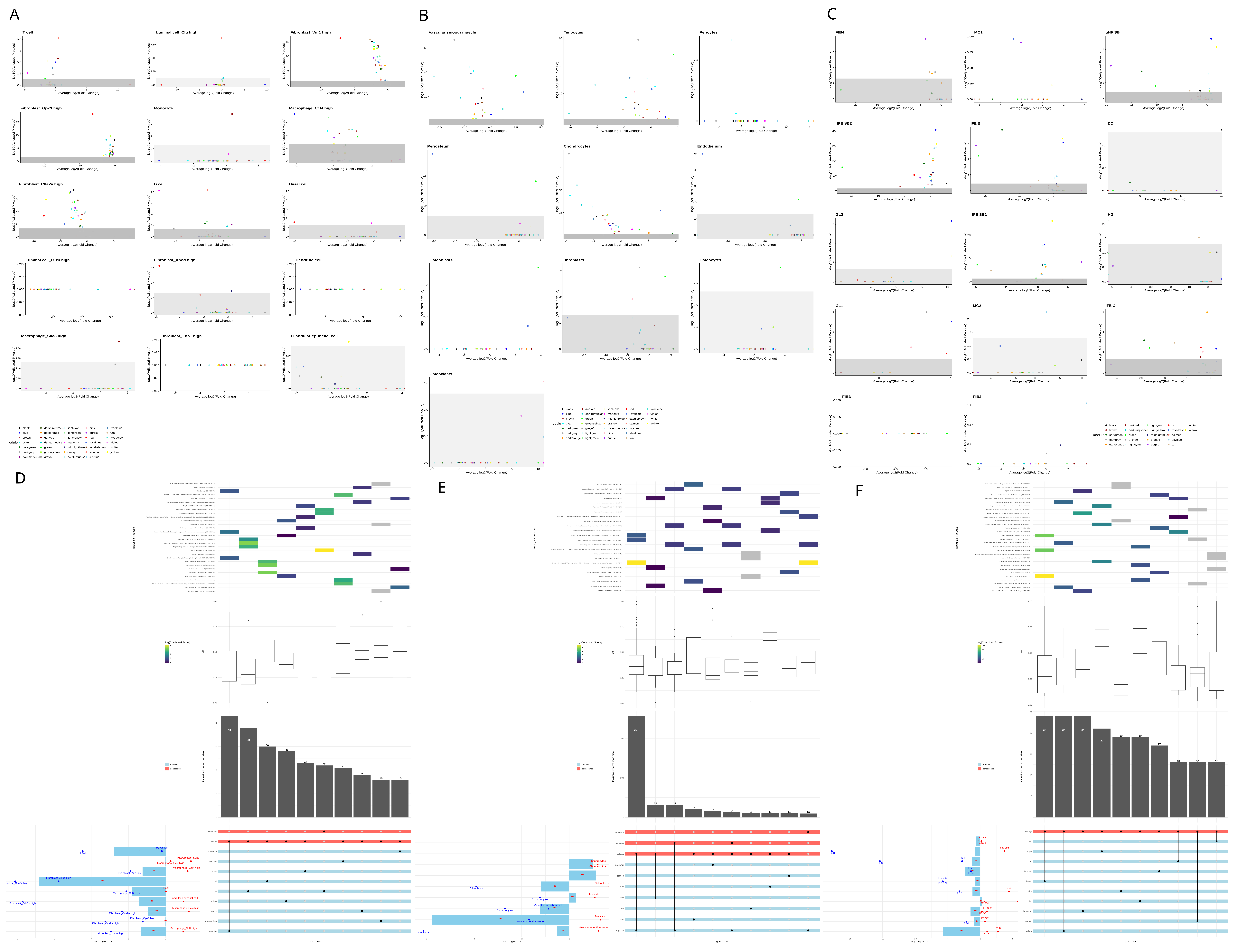
3.2. Functional Enrichment
3.3. Gene Regulatory Network Analysis
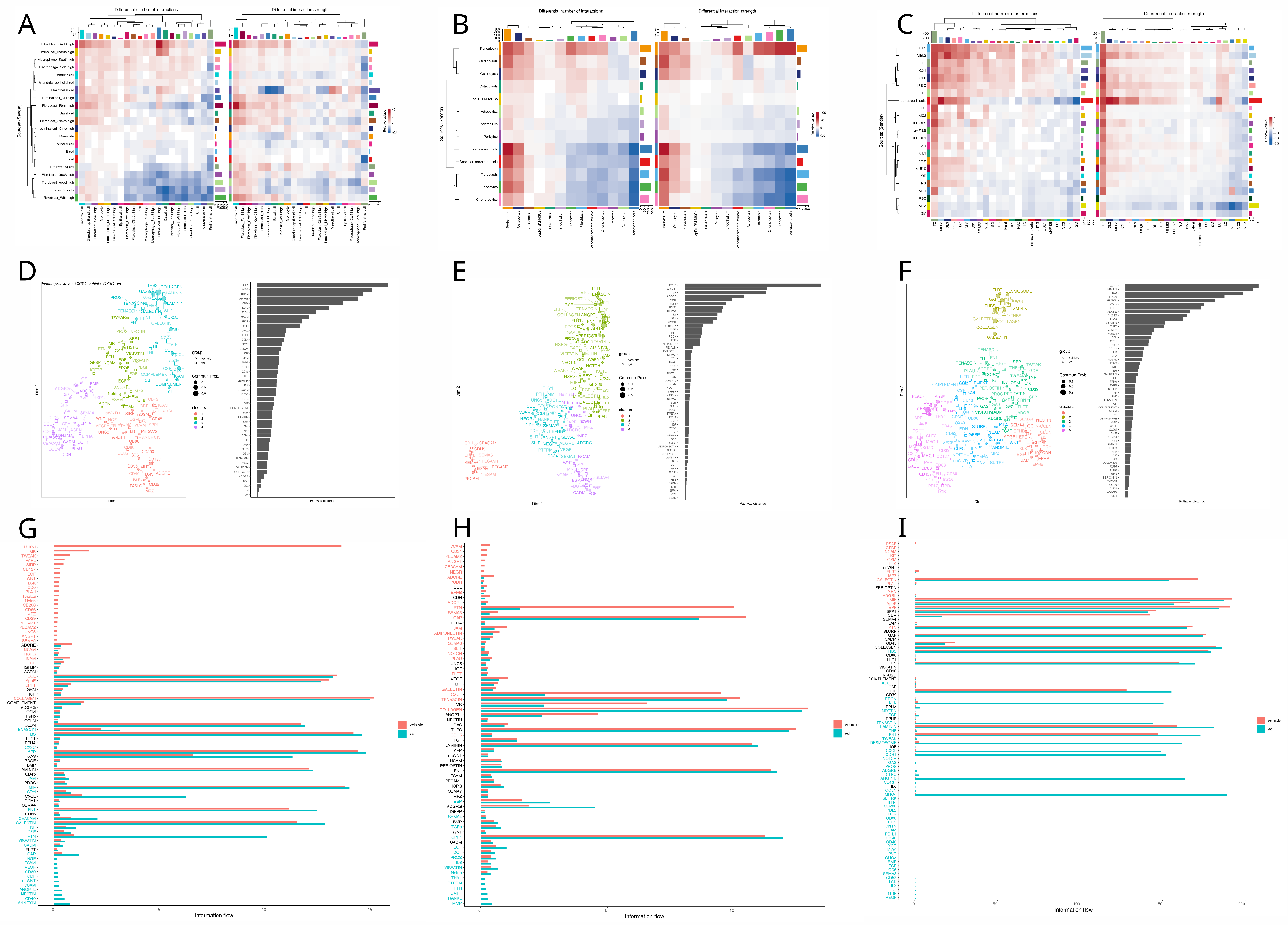
3.4. Cell Communication Analysis
4. Discussion
4.1. Preprocessing
4.2. Functional Enrichment
4.3. Gene Regulatory Network Analysis
4.4. Cell Communication
5. Conclusions
- Vitamin D seems to play a role in senescent cell expression, particularly in the SASP profile, given the most significant change in SENmayo scores and cellular communication relevance in senescent cells across the prostate, bone, and skin.
- Gene regulatory networks of the three tissues show that almost all the modules with the most senescent genes were downregulated by VD, suggesting the importance of VD in senescent profiles and in aging.
- The role of VD in the SRP pathway in bone reveals trailblazing insight into aging bone, pointing out the relevance of future investigations to further characterize these findings.
- The maintenance of cellular senescence by vitamin D via IL-6, APP reduction, matrix reorganization, and macrophage immunomodulation seems useful for early stages of prostate cancer but inconclusive for later stages.
- Senescent chondrocyte profiles triggered by VD could provide clues to the role of these cells in energy homeostasis in bone.
- Paracrine senescence seems to be inhibited in bone by vitamin D via NOTCH downregulation.
- ANGPTL and galectin promotion by vitamin D in senescent cells may play a crucial role in the radiated skin repair process via lipid metabolism and immune clearance.
- The senescent fibroblast SASP profile in irradiated skin appears to be relevant in extracellular matrix remodeling, and vitamin D seems to modulate it towards an anti-fibrotic type.
- Further validation of these findings with other experimental methods is crucial for further understanding the role of vitamin D in cellular senescence.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Lopez, A.G.; Kerlan, V.; Desailloud, R. Non-classical effects of vitamin D: Non-bone effects of vitamin D. Ann. D’Endocrinologie 2021, 82, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Cell senescence, rapamycin and hyperfunction theory of aging. Cell Cycle 2022, 21, 1456–1467. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, E.O.W.; Zhu, Y.; Weigand, B.M.; Tripathi, U.; Burns, T.C.; Tchkonia, T.; Kirkland, J.L. Cellular senescence in aging and age-related diseases: Implications for neurodegenerative diseases. Int. Rev. Neurobiol. 2020, 155, 203–234. [Google Scholar]
- Sosa-Díaz, E.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. The role of vitamin D on redox regulation and cellular senescence. Free Radic. Biol. Med. 2022, 193, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. An updated landscape of cellular senescence heterogeneity: Mechanisms, technologies and senotherapies. Transl. Med. Aging 2023, 7, 46–51. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat. Weight-Disord.-Stud. Anorexia Bulim. Obes. 2017, 22, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Yu, S.; Sun, H.; Chen, L.; Wang, R.; Wu, X.; Goltzman, D.; Miao, D. 1,25-Dihydroxyvitamin D insufficiency accelerates age-related bone loss by increasing oxidative stress and cell senescence. Am. J. Transl. Res. 2020, 12, 507–518. [Google Scholar]
- Stewart, L.V.; Weigel, N.L. Vitamin D and Prostate Cancer. Exp. Biol. Med. 2004, 229, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sayegh, S.; Fantecelle, C.H.; Laphanuwat, P.; Subramanian, P.; Rustin, M.; Gomes, D.C.O.; Akbar, A.N.; Chambers, E.S. Vitamin D3 inhibits p38 MAPK and senescence-associated inflammatory mediator secretion by senescent fibroblasts that impacts immune responses during ageing. Aging Cell 2024, 23, e14093. [Google Scholar] [CrossRef]
- Hedlund, E.; Deng, Q. Single-cell RNA sequencing: Technical advancements and biological applications. Mol. Asp. Med. 2018, 59, 36–46. [Google Scholar] [CrossRef]
- Hou, W.; Ji, Z.; Chen, Z.; Wherry, E.J.; Hicks, S.C.; Ji, H. A statistical framework for differential pseudotime analysis with multiple single-cell RNA-seq samples. Nat. Commun. 2023, 14, 7286. [Google Scholar] [CrossRef]
- De Simone, M.; Hoover, J.; Lau, J.; Bennett, H.M.; Wu, B.; Chen, C.; Menon, H.; Au-Yeung, A.; Lear, S.; Vaidya, S.; et al. A Comprehensive Analysis Framework for Evaluating Commercial Single-Cell RNA Sequencing Technologies. Nucleic Acids Res. 2024, gkae1186. [Google Scholar] [CrossRef] [PubMed]
- Abu el Maaty, M.A.; Grelet, E.; Keime, C.; Rerra, A.I.; Gantzer, J.; Emprou, C.; Terzic, J.; Lutzing, R.; Bornert, J.M.; Laverny, G.; et al. Single-cell analyses unravel cell type–specific responses to a vitamin D analog in prostatic precancerous lesions. Sci. Adv. 2021, 7, eabg5982. [Google Scholar] [CrossRef]
- Hanai, A.; Kawabata, A.; Nakajima, K.; Masuda, K.; Urakawa, I.; Abe, M.; Yamazaki, Y.; Fukumoto, S. Single-cell RNA sequencing identifies Fgf23-expressing osteocytes in response to 1, 25-dihydroxyvitamin D3 treatment. Front. Physiol. 2023, 14, 1102751. [Google Scholar] [CrossRef]
- Lin, Y.; Cao, Z.; Lyu, T.; Kong, T.; Zhang, Q.; Wu, K.; Wang, Y.; Zheng, J. Single-cell RNA-seq of UVB-radiated skin reveals landscape of photoaging-related inflammation and protection by vitamin D. Gene 2022, 831, 146563. [Google Scholar] [CrossRef]
- McCray, T.; Pacheco, J.V.; Loitz, C.C.; Garcia, J.; Baumann, B.; Schlicht, M.J.; Valyi-Nagy, K.; Abern, M.R.; Nonn, L. Vitamin D sufficiency enhances differentiation of patient-derived prostate epithelial organoids. Iscience 2021, 24, 101974. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Abidi, Z.; Dos Santos, G.A.; Avelar, R.A.; Barardo, D.; Chatsirisupachai, K.; Clark, P.; De-Souza, E.A.; Johnson, E.J.; Lopes, I.; et al. Human Ageing Genomic Resources: Updates on key databases in ageing research. Nucleic Acids Res. 2024, 52, D900–D908. [Google Scholar] [CrossRef] [PubMed]
- Avelar, R.A.; Ortega, J.G.; Tacutu, R.; Tyler, E.J.; Bennett, D.; Binetti, P.; Budovsky, A.; Chatsirisupachai, K.; Johnson, E.; Murray, A.; et al. A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biol. 2020, 21, 1–22. [Google Scholar] [CrossRef]
- Wickham, H.; Wickham, H. Programming with ggplot2. In Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; pp. 241–253. [Google Scholar]
- Hao, Y.; Stuart, T.; Kowalski, M.H.; Choudhary, S.; Hoffman, P.; Hartman, A.; Srivastava, A.; Molla, G.; Madad, S.; Fernandez-Granda, C.; et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat. Biotechnol. 2024, 42, 293–304. [Google Scholar] [CrossRef]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R.; et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, P.; Wang, J.; Ma, L.; E, W.; Suo, S.; Jiang, M.; Li, J.; Chen, H.; Sun, H.; et al. Construction of a cross-species cell landscape at single-cell level. Nucleic Acids Res. 2023, 51, 501–516. [Google Scholar] [CrossRef]
- Joost, S.; Annusver, K.; Jacob, T.; Sun, X.; Dalessandri, T.; Sivan, U.; Sequeira, I.; Sandberg, R.; Kasper, M. The molecular anatomy of mouse skin during hair growth and rest. Cell Stem Cell 2020, 26, 441–457. [Google Scholar] [CrossRef]
- Herpelinck, T.; Ory, L.; Nasello, G.; Barzegari, M.; Bolander, J.; Luyten, F.P.; Tylzanowski, P.; Geris, L. An integrated single-cell atlas of the skeleton from development through adulthood. bioRxiv 2022. [Google Scholar] [CrossRef]
- Aibar, S.; González-Blas, C.B.; Moerman, T.; Huynh-Thu, V.A.; Imrichova, H.; Hulselmans, G.; Rambow, F.; Marine, J.C.; Geurts, P.; Aerts, J.; et al. SCENIC: Single-cell regulatory network inference and clustering. Nat. Methods 2017, 14, 1083–1086. [Google Scholar] [CrossRef]
- Borcherding, N.; Vishwakarma, A.; Voigt, A.P.; Bellizzi, A.; Kaplan, J.; Nepple, K.; Salem, A.K.; Jenkins, R.W.; Zakharia, Y.; Zhang, W. Mapping the immune environment in clear cell renal carcinoma by single-cell genomics. Commun. Biol. 2021, 4, 122. [Google Scholar] [CrossRef]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Patil, I. Visualizations with statistical details: The ’ggstatsplot’ approach. J. Open Source Softw. 2021, 6. [Google Scholar] [CrossRef]
- Morabito, S.; Reese, F.; Rahimzadeh, N.; Miyoshi, E.; Swarup, V. hdWGCNA identifies co-expression networks in high-dimensional transcriptomics data. Cell Rep. Methods 2023, 3, 100498. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 1–14. [Google Scholar] [CrossRef]
- Jin, S.; Plikus, M.V.; Nie, Q. CellChat for Systematic Analysis of Cell-Cell Communication from Single-Cell and Spatially Resolved Transcriptomics. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hawker, N.P.; Pennypacker, S.D.; Chang, S.M.; Bikle, D.D. Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J. Investig. Dermatol. 2007, 127, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Bikle, D.D.; Oda, Y. Reciprocal role of vitamin D receptor on β-catenin regulated keratinocyte proliferation and differentiation. J. Steroid Biochem. Mol. Biol. 2014, 144, 237–241. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The impact of vitamin D on skin aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Man, M.Q.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-associated alterations in epidermal function and their clinical significance. Aging 2020, 12, 5551. [Google Scholar] [CrossRef]
- Gisondi, P.; Gracia-Cazaña, T.; Kurzen, H.; Galván, J. Calcipotriol/Betamethasone Dipropionate for the Treatment of Psoriasis: Mechanism of Action and Evidence of Efficacy and Safety versus Topical Corticosteroids. J. Clin. Med. 2024, 13, 4484. [Google Scholar] [CrossRef]
- Ferrer-Mayorga, G.; Niell, N.; Cantero, R.; González-Sancho, J.M.; Del Peso, L.; Muñoz, A.; Larriba, M.J. Vitamin D and Wnt3A have additive and partially overlapping modulatory effects on gene expression and phenotype in human colon fibroblasts. Sci. Rep. 2019, 9, 8085. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Swami, S.; Krishnan, A.V.; Williams, J.D.; Martin, S.; Horst, R.L.; Albertelli, M.A.; Feldman, B.J.; Feldman, D.; Diehn, M. Inhibition of mouse breast tumor-initiating cells by calcitriol and dietary vitamin D. Mol. Cancer Ther. 2015, 14, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Larriba, M.J.; González-Sancho, J.M.; Barbáchano, A.; Niell, N.; Ferrer-Mayorga, G.; Muñoz, A. Vitamin D is a multilevel repressor of Wnt/β-catenin signaling in cancer cells. Cancers 2013, 5, 1242–1260. [Google Scholar] [CrossRef]
- Tao, W.; Yu, Z.; Han, J.D.J. Single-cell senescence identification reveals senescence heterogeneity, trajectory, and modulators. Cell Metab. 2024, 36, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Li, J.; Qin, R.; Lu, N.; Goltzman, D.; Miao, D.; Yang, R. 1, 25-Dihydroxyvitamin D Deficiency accelerates aging-related osteoarthritis via downregulation of Sirt1 in mice. Int. J. Biol. Sci. 2023, 19, 610. [Google Scholar]
- Lu, H.C.; Lin, T.; Ng, M.Y.; Hsieh, C.W.; Liao, Y.W.; Chen, C.C.; Yu, C.C.; Chen, C.J. Anti-inflammaging effects of vitamin D in human gingival fibroblasts with advanced glycation end product stimulation. J. Dent. Sci. 2023, 18, 666–673. [Google Scholar] [CrossRef]
- Marampon, F.; Gravina, G.; Festuccia, C.; Popov, V.; Colapietro, E.; Sanita, P.; Musio, D.; De Felice, F.; Lenzi, A.; Jannini, E.; et al. Vitamin D protects endothelial cells from irradiation-induced senescence and apoptosis by modulating MAPK/SirT1 axis. J. Endocrinol. Investig. 2016, 39, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Valcheva, P.; Cardus, A.; Panizo, S.; Parisi, E.; Bozic, M.; Novoa, J.M.L.; Dusso, A.; Fernández, E.; Valdivielso, J.M. Lack of vitamin D receptor causes stress-induced premature senescence in vascular smooth muscle cells through enhanced local angiotensin-II signals. Atherosclerosis 2014, 235, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y. Vitamin D in the Prevention and Treatment of Osteoarthritis: From Clinical Interventions to Cellular Evidence. Nutrients 2019, 11, 243. [Google Scholar] [CrossRef]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin D in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; Von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Yeh, W.Z.; Lea, R.; Stankovich, J.; Sampangi, S.; Laverick, L.; Van der Walt, A.; Jokubaitis, V.; Gresle, M.; Butzkueven, H. Transcriptomics identifies blunted immunomodulatory effects of vitamin D in people with multiple sclerosis. Sci. Rep. 2024, 14, 1436. [Google Scholar] [CrossRef]
- Fantini, C.; Corinaldesi, C.; Lenzi, A.; Migliaccio, S.; Crescioli, C. Vitamin D as a Shield against Aging. Int. J. Mol. Sci. 2023, 24, 4546. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, R.P.; Rayego-Mateos, S.; Alique, M.; Márquez-Expósito, L.; Tejedor-Santamaria, L.; Ortiz, A.; González-Parra, E.; Ruiz-Ortega, M. Vitamin D, Cellular Senescence and Chronic Kidney Diseases: What Is Missing in the Equation? Nutrients 2023, 15, 1349. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, Y.; Li, C.; Yu, J.; Ren, D.; Qiu, S.; Nie, Y.; Yu, X.; Xu, X.; Zhu, W. 1, 25-Dihydroxyvitamin D regulates macrophage polarization and ameliorates experimental inflammatory bowel disease by suppressing miR-125b. Int. Immunopharmacol. 2019, 67, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Papaioannou, G.; Zhao, H.; Luderer, H.F.; Miller, C.; Dall’Osso, C.; Nazarian, R.M.; Wagers, A.J.; Demay, M.B. The vitamin D receptor regulates tissue resident macrophage response to injury. Endocrinology 2016, 157, 4066–4075. [Google Scholar] [CrossRef] [PubMed]
- Eichbaum, Q.; Heney, D.; Raveh, D.; Chung, M.; Davidson, M.; Epstein, J.; Ezekowitz, R.A.B. Murine macrophage mannose receptor promoter is regulated by the transcription factors PU. 1 and SP1. Blood J. Am. Soc. Hematol. 1997, 90, 4135–4143. [Google Scholar] [CrossRef]
- Zasłona, Z.; Scruggs, A.M.; Peters-Golden, M.; Huang, S.K. Protein kinase A inhibition of macrophage maturation is accompanied by an increase in DNA methylation of the colony-stimulating factor 1 receptor gene. Immunology 2016, 149, 225–237. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Mayorga, G.; Gómez-López, G.; Barbáchano, A.; Fernández-Barral, A.; Peña, C.; Pisano, D.G.; Cantero, R.; Rojo, F.; Muñoz, A.; Larriba, M.J. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut 2017, 66, 1449–1462. [Google Scholar] [CrossRef] [PubMed]
- Shany, S.; Sigal-Batikoff, I.; Lamprecht, S. Vitamin D and myofibroblasts in fibrosis and cancer: At cross-purposes with TGF-β/SMAD signaling. Anticancer. Res. 2016, 36, 6225–6234. [Google Scholar] [CrossRef]
- Soler Palacios, B.; Villares, R.; Lucas, P.; Rodríguez-Frade, J.M.; Cayuela, A.; Piccirillo, J.G.; Lombardía, M.; Delgado Gestoso, D.; Fernández-García, M.; Risco, C.; et al. Growth hormone remodels the 3D-structure of the mitochondria of inflammatory macrophages and promotes metabolic reprogramming. Front. Immunol. 2023, 14, 1200259. [Google Scholar] [CrossRef]
- Havas, A.; Yin, S.; Adams, P. The role of aging in cancer. Mol. Oncol. 2022, 16, 3213. [Google Scholar] [CrossRef] [PubMed]
- Newmark, H.; Dantoft, W.; Ghazal, P. Evolutionary origin of the interferon–immune metabolic axis: The sterol–vitamin D link. Front. Immunol. 2017, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Shi, Q.; Zhang, H.; Bao, Y.; Hu, D.; Pohl, N.; Fang, W.; Dong, H.; Xia, X.; Fan, D.; et al. c-Jun NH2-teminal kinase 1 interacts with vitamin D receptor and affects vitamin D-mediated inhibition of cancer cell proliferation. J. Steroid Biochem. Mol. Biol. 2016, 163, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.P.; Qi, X.; Pramanik, R.; Pohl, N.M.; Loesch, M.; Chen, G. Stress-induced c-Jun-dependent Vitamin D receptor (VDR) activation dissects the non-classical VDR pathway from the classical VDR activity. J. Biol. Chem. 2007, 282, 1544–1551. [Google Scholar] [CrossRef]
- Kellogg, M.K.; Tikhonova, E.B.; Karamyshev, A.L. Signal recognition particle in human diseases. Front. Genet. 2022, 13, 898083. [Google Scholar] [CrossRef]
- Kimmel, J.C.; Penland, L.; Rubinstein, N.D.; Hendrickson, D.G.; Kelley, D.R.; Rosenthal, A.Z. Murine single-cell RNA-seq reveals cell-identity-and tissue-specific trajectories of aging. Genome Res. 2019, 29, 2088–2103. [Google Scholar] [CrossRef] [PubMed]
- LeBoff, M.S.; Chou, S.H.; Ratliff, K.A.; Cook, N.R.; Khurana, B.; Kim, E.; Cawthon, P.M.; Bauer, D.C.; Black, D.; Gallagher, J.C.; et al. Supplemental vitamin D and incident fractures in midlife and older adults. N. Engl. J. Med. 2022, 387, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Segaert, S.; Degreef, H.; Bouillon, R. Vitamin D receptor expression is linked to cell cycle control in normal human keratinocytes. Biochem. Biophys. Res. Commun. 2000, 279, 89–94. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Jarrett, S.G.; D’Orazio, J.A.; Holick, M.F.; Tang, E.K.; Tuckey, R.C.; Panich, U.; Li, W.; et al. Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox Biol. 2019, 24, 101206. [Google Scholar] [CrossRef]
- Ambagaspitiya, S.S.; Appuhamillage, G.A.; Dassanayake, R.S. Impact of vitamin D on ultraviolet-induced photoaging and skin diseases. Explor. Med. 2024, 5, 363–383. [Google Scholar] [CrossRef]
- Pena, A.M.; Boulade, M.; Brizion, S.; Tissot, N.; Bornschloegl, T.; Galey, J.B.; Bernerd, F.; Planel, E. Multiphoton FLIM imaging of NADH and FAD to analyze cellular metabolic activity of reconstructed human skin in response to UVA light. In Proceedings of the Multiphoton Microscopy in the Biomedical Sciences XIX, San Francisco, CA, USA, 3–6 February 2019; Volume 10882, pp. 23–33. [Google Scholar]
- Demay, M.B.; MacDonald, P.N.; Skorija, K.; Dowd, D.R.; Cianferotti, L.; Cox, M. Role of the vitamin D receptor in hair follicle biology. J. Steroid Biochem. Mol. Biol. 2007, 103, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.i.; Tsutsumi, S.; Suzuki, T.; Horie-Inoue, K.; Ikeda, K.; Kaneshiro, K.; Fujimura, T.; Kumagai, J.; Urano, T.; Sakaki, Y.; et al. Amyloid precursor protein is a primary androgen target gene that promotes prostate cancer growth. Cancer Res. 2009, 69, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Amyloid precursor protein regulates migration and metalloproteinase gene expression in prostate cancer cells. Biochem. Biophys. Res. Commun. 2014, 452, 828–833. [Google Scholar] [CrossRef]
- Culig, Z.; Pencik, J.; Merkel, O.; Kenner, L. Breaking a paradigm: IL-6/STAT3 signaling suppresses metastatic prostate cancer upon ARF expression. Mol. Cell. Oncol. 2016, 3, e1090048. [Google Scholar] [CrossRef]
- Chen, J.; Kuang, S.; Cen, J.; Zhang, Y.; Shen, Z.; Qin, W.; Huang, Q.; Wang, Z.; Gao, X.; Huang, F.; et al. Multiomics profiling reveals VDR as a central regulator of mesenchymal stem cell senescence with a known association with osteoporosis after high-fat diet exposure. Int. J. Oral Sci. 2024, 16, 41. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- yu Song, Z.; Yao, Q.; Zhuo, Z.; Ma, Z.; Chen, G. Circulating vitamin D level and mortality in prostate cancer patients: A dose–response meta-analysis. Endocr. Connect. 2018, 7, R294–R303. [Google Scholar] [CrossRef]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of Vitamin D3 Supplements on Development of Advanced Cancer: A Secondary Analysis of the VITAL Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef]
- Banerjee, S.; Ghoshal, S.; Girardet, C.; DeMars, K.M.; Yang, C.; Niehoff, M.L.; Nguyen, A.D.; Jayanth, P.; Hoelscher, B.A.; Xu, F.; et al. Adropin correlates with aging-related neuropathology in humans and improves cognitive function in aging mice. NPJ Aging Mech. Dis. 2021, 7, 23. [Google Scholar] [CrossRef]
- Yang, R.; Fang, W.; Liang, J.; Lin, C.; Wu, S.; Yan, S.; Hu, C.; Ke, X. Apelin/APJ axis improves angiotensin II-induced endothelial cell senescence through AMPK/SIRT1 signaling pathway. Arch. Med Sci. 2018, 14, 725–734. [Google Scholar] [CrossRef]
- Chow, E.C.Y.; Quach, H.P.; Vieth, R.; Pang, K.S. Temporal changes in tissue 1α,25-dihydroxyvitamin D3, vitamin D receptor target genes, and calcium and PTH levels after 1,25(OH)2D3 treatment in mice. Am. J. Physiol.-Endocrinol. Metab. 2013, 304, E977–E989. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.V.; Rattanavirotkul, N.; Olova, N.; Salzano, A.; Quintanilla, A.; Tarrats, N.; Kiourtis, C.; Müller, M.; Green, A.R.; Adams, P.D.; et al. Notch signaling mediates secondary senescence. Cell Rep. 2019, 27, 997–1007. [Google Scholar] [CrossRef]
- Cao, H.; Yang, P.; Liu, J.; Shao, Y.; Li, H.; Lai, P.; Wang, H.; Liu, A.; Guo, B.; Tang, Y.; et al. MYL3 protects chondrocytes from senescence by inhibiting clathrin-mediated endocytosis and activating of Notch signaling. Nat. Commun. 2023, 14, 6190. [Google Scholar] [CrossRef] [PubMed]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: A randomized clinical trial. JAMA 2019, 322, 736–745. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Udagawa, N.; Suda, T.; Takahashi, N. Mechanisms involved in bone resorption regulated by vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 177, 70–76. [Google Scholar] [CrossRef]
- Liu, L.; Tong, X.; Huang, M.; Zhu, C.; Chen, X.; Yuan, Y.; Bennett, S.; Xu, J.; Zou, J. The effects of cGAS-Sting pathway on bone mineral density. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Pan, J.X.; Tang, F.; Xiong, F.; Xiong, L.; Zeng, P.; Wang, B.; Zhao, K.; Guo, H.; Shun, C.; Xia, W.F.; et al. APP promotes osteoblast survival and bone formation by regulating mitochondrial function and preventing oxidative stress. Cell Death Dis. 2018, 9, 1077. [Google Scholar] [CrossRef]
- Ge, Y.; Luo, J.; Li, D.; Li, C.; Huang, J.; Yu, H.; Lin, X.; Li, Y.; Man, M.; Zhang, J.; et al. Deficiency of vitamin D receptor in keratinocytes augments dermal fibrosis and inflammation in a mouse model of HOCl-induced scleroderma. Biochem. Biophys. Res. Commun. 2022, 591, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Joko, Y.; Yamamoto, Y.; Kato, S.; Takemoto, T.; Abe, M.; Matsumoto, T.; Fukumoto, S.; Sawatsubashi, S. VDR is an essential regulator of hair follicle regression through the progression of cell death. Life Sci. Alliance 2023, 6, e202302014. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, C.; Le, Y.; Gong, W.; Ju, J.; Zhang, G.; Ji, P.; Zuo, R.; Liu, Z.; Zhang, P.; et al. Angiopoietin-like 4 promotes epidermal stem cell proliferation and migration and contributes to cutaneous wound re-epithelialization: ANGPTL4 promotes EpSC proliferation and skin wound healing. Acta Biochim. Biophys. Sin. 2023, 55, 1265. [Google Scholar] [CrossRef]
- Karlsson, A.; Christenson, K.; Matlak, M.; Björstad, Å.; Brown, K.L.; Telemo, E.; Salomonsson, E.; Leffler, H.; Bylund, J. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology 2009, 19, 16–20. [Google Scholar] [CrossRef]
- Correa, S.G.; Sotomayor, C.E.; Aoki, M.P.; Maldonado, C.A.; Rabinovich, G.A. Opposite effects of galectin-1 on alternative metabolic pathways of L-arginine in resident, inflammatory, and activated macrophages. Glycobiology 2003, 13, 119–128. [Google Scholar] [CrossRef]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.; et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef]
- Dahlhoff, M.; Camera, E.; Picardo, M.; Zouboulis, C.C.; Schneider, M.R. Angiopoietin-like 4, a protein strongly induced during sebocyte differentiation, regulates sebaceous lipogenesis but is dispensable for sebaceous gland function in vivo. J. Dermatol. Sci. 2014, 75, 148–150. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Yan, Y. Vitamin D status and efficacy of vitamin D supplementation in acne patients: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2021, 20, 3802–3807. [Google Scholar] [CrossRef]
- Micheli, A.J.D.; Spector, J.A.; Elemento, O.; Cosgrove, B.D. A reference single-cell transcriptomic atlas of human skeletal muscle tissue reveals bifurcated muscle stem cell populations. Skelet. Muscle 2020, 10, 19. [Google Scholar] [CrossRef]
- Westoby, J.; Artemov, P.; Hemberg, M.; Ferguson-Smith, A.C. Obstacles to detecting isoforms using full-length scRNA-seq data. Genome Biol. 2020, 21, 74. [Google Scholar] [CrossRef]
- Kharchenko, P.V. The triumphs and limitations of computational methods for scRNA-seq. Nat. Methods 2021, 18, 723–732. [Google Scholar] [CrossRef]
- Majo, F.D.; Hegenbarth, J.C.; Rühle, F.; Bär, C.; Thum, T.; de Boer, M.; Duncker, D.J.; Schroen, B.; Armand, A.S.; Stoll, M.; et al. Dichotomy between the transcriptomic landscape of naturally versus accelerated aged murine hearts. Sci. Rep. 2020, 10, 8136. [Google Scholar]
- Provost, P. Interpretation and applicability of microRNA data to the context of Alzheimer’s and age-related diseases. Aging 2010, 2, 166–169. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sosa-Díaz, E.; Reyes-Gopar, H.; de Anda-Jáuregui, G.; Hernández-Lemus, E. Single-Cell Analysis Dissects the Effects of Vitamin D on Genetic Senescence Signatures Across Murine Tissues. Nutrients 2025, 17, 429. https://doi.org/10.3390/nu17030429
Sosa-Díaz E, Reyes-Gopar H, de Anda-Jáuregui G, Hernández-Lemus E. Single-Cell Analysis Dissects the Effects of Vitamin D on Genetic Senescence Signatures Across Murine Tissues. Nutrients. 2025; 17(3):429. https://doi.org/10.3390/nu17030429
Chicago/Turabian StyleSosa-Díaz, Emilio, Helena Reyes-Gopar, Guillermo de Anda-Jáuregui, and Enrique Hernández-Lemus. 2025. "Single-Cell Analysis Dissects the Effects of Vitamin D on Genetic Senescence Signatures Across Murine Tissues" Nutrients 17, no. 3: 429. https://doi.org/10.3390/nu17030429
APA StyleSosa-Díaz, E., Reyes-Gopar, H., de Anda-Jáuregui, G., & Hernández-Lemus, E. (2025). Single-Cell Analysis Dissects the Effects of Vitamin D on Genetic Senescence Signatures Across Murine Tissues. Nutrients, 17(3), 429. https://doi.org/10.3390/nu17030429






