Lifestyle Factors and the Microbiome in Urolithiasis: A Narrative Review
Abstract
:1. Introduction
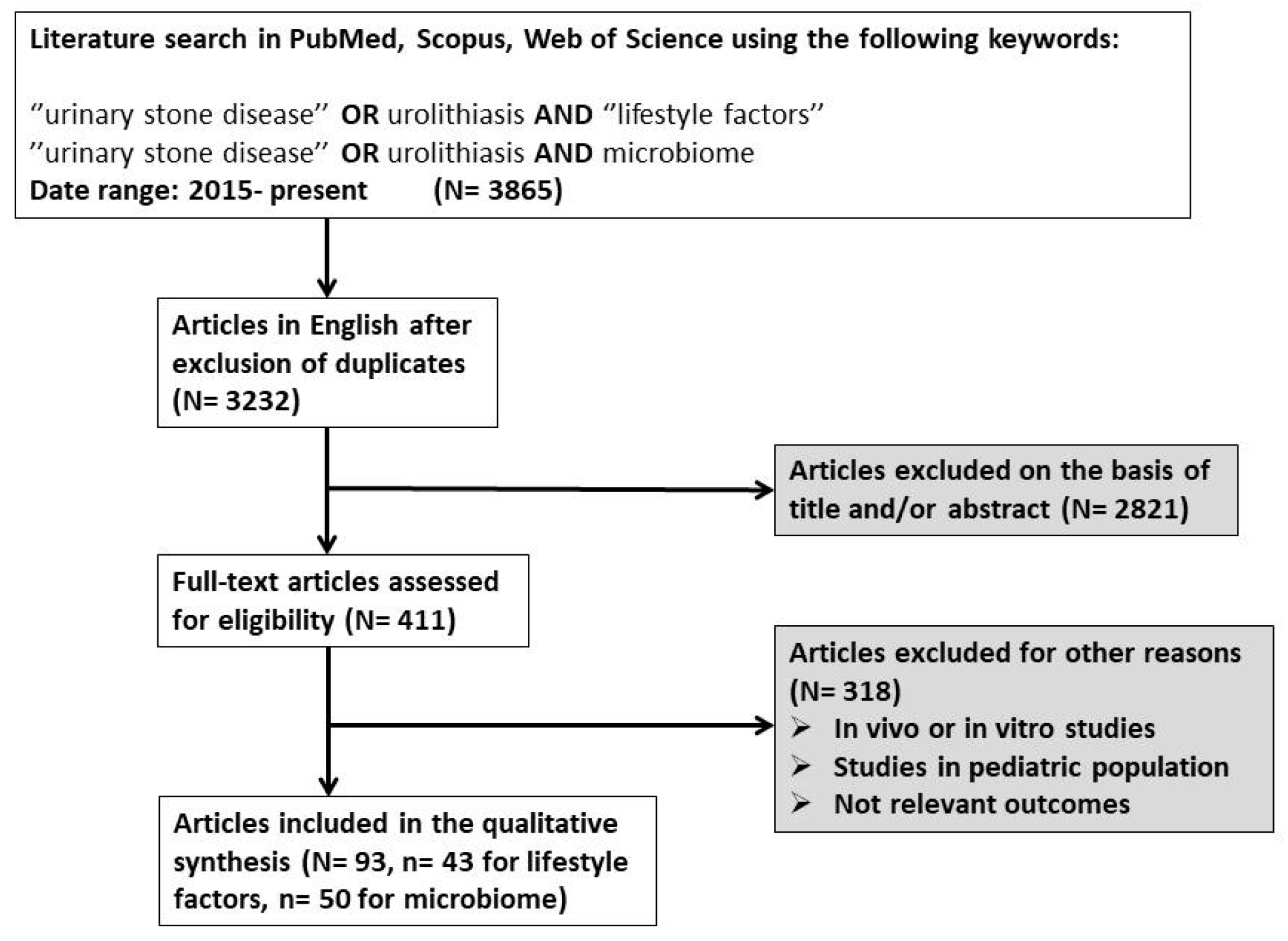
2. Materials and Methods
3. Results
3.1. Lifestyle Factors
3.1.1. Dietary Patterns
Dietary Intake of Oxalate and Calcium
Consumption of Animal-Based or Plant-Based Foods
3.1.2. Fluid Intake
Water Intake—Total Fluid Volume
Specific Liquids
Alcohol
3.1.3. Metabolism and Body Homeostasis
Factors Affecting Calcium Metabolism
Role of Metabolic Disorders
3.1.4. Environmental and Behavioral Factors
Role of Behavioral Patterns
Role of Climatic, Occupational, and Social Factors
3.2. Microbiome
3.2.1. Gut Microbiome
Studies Referring to the Oxalate-Degrading Pathway
Studies Referring to Other Alterations of the Gut Microbiome
Studies Combining Results from Gastrointestinal and Urinary Tract
3.2.2. Urinary Tract Microbiome
Studies on the Microbial Composition in Urine
Studies on the Microbial Composition in Stone Material
Studies Combining Analysis in Urine and Stone Material
3.2.3. The Role of Pharmaceutical Agents Acting on the Microbiome in Stone Formation Risk
Studies Referring to Oxalobacter formigenes Supplementation and Probiotics
Studies Referring to the Association of Microbiome Perturbation After Antibiotic Exposure and Stone Formation Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lang, J.; Narendrula, A.; El-Zawahry, A.; Sindhwani, P.; Ekwenna, O. Global trends in incidence and burden of urolithiasis from 1990 to 2019: An analysis of global burden of disease study data. Eur. Urol. Open Sci. 2022, 35, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Stamatelou, K.; Goldfarb, D.S. Epidemiology of kidney stones. Healthcare 2023, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Kittanamongkolchai, W.; Vaughan, L.E.; Enders, F.T.; Dhondup, T.; Mehta, R.A.; Krambeck, A.E.; McCollough, C.H.; Vrtiska, T.J.; Lieske, J.C.; Rule, A.D. The changing incidence and presentation of urinary stones over 3 decades. Mayo Clin. Proc. 2018, 93, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Krambeck, A.E.; Rule, A.D. Determining the true burden of kidney stone disease. Nat. Rev. Nephrol. 2020, 16, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Cicerello, E.; Mangano, M.S.; Cova, G.; Ciaccia, M. Changing in gender prevalence of nephrolithiasis. Urol. J. 2021, 88, 90–93. [Google Scholar] [CrossRef]
- Panzarino, V. Urolithiasis in children. Adv. Pediatr. 2020, 67, 105–112. [Google Scholar] [CrossRef]
- Moses, R.; Pais, V.M.; Ursiny, M.; Prien, E.L.; Miller, N.; Eisner, B.H. Changes in stone composition over two decades: Evaluation of over 10,000 stone analyses. Urolithiasis 2015, 43, 135–139. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent advances on the mechanisms of kidney stone formation. Int. J. Mol. Med. 2021, 48, 149. [Google Scholar] [CrossRef]
- Bientinesi, R.; Gandi, C.; Vaccarella, L.; Sacco, E. Lifestyle in urology: Benign diseases. Urol. J. 2021, 88, 163–174. [Google Scholar] [CrossRef]
- EAU Guidelines. (Ed.). In Proceedings of the EAU Annual Congress, Milan, Italy, 10–13 March 2023.
- Pearle, M.S.; Goldfarb, D.S.; Assimos, D.G.; Curhan, G.; Denu-Ciocca, C.J.; Matlaga, B.R.; Monga, M.; Penniston, K.L.; Preminger, G.M.; Turk, T.M. Medical management of kidney stones: AUA guideline. J. Urol. 2014, 192, 316–324. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Thomas-White, K.; Brady, M.; Wolfe, A.J.; Mueller, E.R. The bladder is not sterile: History and current discoveries on the urinary microbiome. Curr. Bladder Dysfunct. Rep. 2016, 11, 18–24. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Worcester, E.M.; Coe, F.L. Calcium kidney stones. N. Engl. J. Med. 2010, 363, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Patel, M.; Thomas, V.; Knight, J.; Holmes, R.P.; Mitchell, T. Dietary oxalate induces urinary nanocrystals in humans. Kidney Int. Rep. 2020, 5, 1040–1051. [Google Scholar] [CrossRef]
- Siener, R.; Hoppe, B.; Löhr, P.; Müller, S.C.; Latz, S. Metabolic profile and impact of diet in patients with primary hyperoxaluria. Int. Urol. Nephrol. 2018, 50, 1583–1589. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, dietary, and supplemental vitamin C intake and risk of incident kidney stones. Am. J. Kidney Dis. 2016, 67, 400–407. [Google Scholar] [CrossRef]
- Marić, I.; Kizivat, T.; Smolić, M.; Smolić, R.; Opačak-Bernardi, T.; Šolić, K.; Roguljić, H.; Ahić, J.M.; Tucak, A.; Mihaljević, I. Lifestyle risk factors and bone mass in recurrent stone-forming patients: A cross-sectional study in 144 subjects. Acta Clin. Croat. 2019, 58, 439. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Mandel, E.I.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Dietary protein and potassium, diet–dependent net acid load, and risk of incident kidney stones. Clin. J. Am. Soc. Nephrol. 2016, 11, 1834. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Taylor, E.N.; Curhan, G.C. Factors Associated with Sex Differences in the Risk of Kidney Stones: TH-OR30. J. Am. Soc. Nephrol. 2021, 32, 9. [Google Scholar] [CrossRef]
- Lin, B.-B.; Lin, M.-E.; Huang, R.-H.; Hong, Y.-K.; Lin, B.-L.; He, X.-J. Dietary and lifestyle factors for primary prevention of nephrolithiasis: A systematic review and meta-analysis. BMC Nephrol. 2020, 21, 267. [Google Scholar] [CrossRef] [PubMed]
- Prieto, R.M.; Rodriguez, A.; Sanchis, P.; Morey, M.; Fiol, M.; Grases, F.; Castañer, O.; Martínez-González, M.A.; Salas-Salvadó, J.; Romaguera, D. Association of adherence to the Mediterranean diet with urinary factors favoring renal lithiasis: Cross-sectional study of overweight individuals with metabolic syndrome. Nutrients 2019, 11, 1708. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Fernández-Montero, A.; de la Fuente-Arrillaga, C.; Martínez-González, M.Á.; Bertoli, S.; Battezzati, A.; Bes-Rastrollo, M. Adherence to the Mediterranean dietary pattern and incidence of nephrolithiasis in the Seguimiento Universidad de Navarra follow-up (SUN) cohort. Am. J. Kidney Dis. 2017, 70, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Dietary and lifestyle risk factors associated with incident kidney stones in men and women. J. Urol. 2017, 198, 858–863. [Google Scholar] [CrossRef]
- Zhuo, D.; Li, M.; Cheng, L.; Zhang, J.; Huang, H.; Yao, Y. A study of diet and lifestyle and the risk of urolithiasis in 1,519 patients in Southern China. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 4217. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Rossetti, S.; Friend, K.; Erickson, S.B.; Lieske, J.C. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: A systematic review and meta-analysis. J. Nephrol. 2016, 29, 211–219. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, C.; Wang, X.-L.; Liu, T.-Z.; Zeng, X.-T.; Li, S.; Duan, X.-W. Self-fluid management in prevention of kidney stones: A PRISMA-compliant systematic review and dose–response meta-analysis of observational studies. Medicine 2015, 94, e1042. [Google Scholar] [CrossRef]
- Sagy, I.; Zeldetz, V.; Halperin, D.; Abu Tailakh, M.; Novack, V. The effect of Ramadan fast on the incidence of renal colic emergency department visits. QJM Int. J. Med. 2017, 110, 571–576. [Google Scholar] [CrossRef]
- Bao, Y.; Tu, X.; Wei, Q. Water for preventing urinary stones. Cochrane Database Syst. Rev. 2020, 2, CD004292. [Google Scholar] [CrossRef]
- Littlejohns, T.J.; Neal, N.L.; Bradbury, K.E.; Heers, H.; Allen, N.E.; Turney, B.W. Fluid intake and dietary factors and the risk of incident kidney stones in UK Biobank: A population-based prospective cohort study. Eur. Urol. Focus 2020, 6, 752–761. [Google Scholar] [CrossRef]
- Chen, K.; Chen, D.; Lan, C.; Liang, X.; Zeng, T.; Huang, J.; Duan, X.; Kong, Z.; Li, S.; Tiselius, H.-G. Does green tea consumption increase urinary oxalate excretion? Results of a prospective trial in healthy men. Int. Urol. Nephrol. 2018, 50, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Rode, J.; Bazin, D.; Dessombz, A.; Benzerara, Y.; Letavernier, E.; Tabibzadeh, N.; Hoznek, A.; Tligui, M.; Traxer, O.; Daudon, M. Daily green tea infusions in hypercalciuric renal stone patients: No evidence for increased stone risk factors or oxalate-dependent stones. Nutrients 2019, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Wu, J.-S.; Chang, Y.-F.; Sun, Z.-J.; Chang, C.-J.; Lu, F.-H.; Yang, Y.-C. Increased amount and duration of tea consumption may be associated with decreased risk of renal stone disease. World J. Urol. 2019, 37, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Cai, H.; Xiang, Y.B.; Li, H.; Lipworth, L.; Miller, N.L.; Zheng, W.; Shu, X.O.; Hsi, R.S. Green tea intake and risk of incident kidney stones: Prospective cohort studies in middle-aged and elderly Chinese individuals. Int. J. Urol. 2019, 26, 241–246. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Sadeghian, R.; Abbasi, B. The associations between tea and coffee drinking and risk of calcium-oxalate renal stones. Plant Foods Hum. Nutr. 2021, 76, 516–522. [Google Scholar] [CrossRef]
- Barghouthy, Y.; Corrales, M.; Doizi, S.; Somani, B.K.; Traxer, O. Tea and coffee consumption and pathophysiology related to kidney stone formation: A systematic review. World J. Urol. 2021, 39, 2417–2426. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Q.; Xu, L.; Yang, X.; Lei, Y. Tea consumption, serum uric acid levels and hyperuricemia: A systematic review and meta-analysis. Clin. Rheumatol. 2024, 44, 67–80. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Wu, J.; Zhu, Y.; Lin, Y.; Zheng, X.; Xie, L. Systematic review and meta-analysis of the effect of alcohol intake on the risk of urolithiasis including dose-response relationship. Urol. Int. 2015, 94, 194–204. [Google Scholar] [CrossRef]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5, S23–S30. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Ferraro, P.M.; Folesani, G.; Lauretani, F.; Allegri, F.; Guerra, A.; Cerundolo, N.; Aloe, R.; Lippi, G. Idiopathic calcium nephrolithiasis and hypovitaminosis D: A case-control study. Urology 2016, 87, 40–45. [Google Scholar] [CrossRef]
- Johri, N.; Jaeger, P.; Ferraro, P.M.; Shavit, L.; Nair, D.; Robertson, W.G.; Gambaro, G.; Unwin, R.J. Vitamin D deficiency is prevalent among idiopathic stone formers, but does correction pose any risk? Urolithiasis 2017, 45, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Malihi, Z.; Wu, Z.; Stewart, A.W.; Lawes, C.M.; Scragg, R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 104, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Taylor, E.N.; Gambaro, G.; Curhan, G.C. Vitamin D intake and the risk of incident kidney stones. J. Urol. 2017, 197, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Malihi, Z.; Lawes, C.M.; Wu, Z.; Huang, Y.; Waayer, D.; Toop, L.; Khaw, K.-T.; Camargo, C.A., Jr.; Scragg, R. Monthly high-dose vitamin D supplementation does not increase kidney stone risk or serum calcium: Results from a randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1578–1587. [Google Scholar] [CrossRef]
- Frost, P. The Problem of Vitamin D Scarcity: Cultural and Genetic Solutions by Indigenous Arctic and Tropical Peoples. Nutrients 2022, 14, 4071. [Google Scholar] [CrossRef]
- Carlberg, C.; Haq, A. The concept of the personal vitamin D response index. J. Steroid Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef]
- Shavit, L.; Ferraro, P.M.; Johri, N.; Robertson, W.; Walsh, S.B.; Moochhala, S.; Unwin, R. Effect of being overweight on urinary metabolic risk factors for kidney stone formation. Nephrol. Dial. Transplant. 2015, 30, 607–613. [Google Scholar] [CrossRef]
- Akarken, I.; Tarhan, H.; Ekin, R.G.; Çakmak, Ö.; Koç, G.; İlbey, Y.Ö.; Zorlu, F. Visceral obesity: A new risk factor for stone disease. Can. Urol. Assoc. J. 2015, 9, E795. [Google Scholar] [CrossRef]
- Yoshimura, E.; Sawada, S.S.; Lee, I.-M.; Gando, Y.; Kamada, M.; Matsushita, M.; Kawakami, R.; Ando, R.; Okamoto, T.; Tsukamoto, K. Body mass index and kidney stones: A cohort study of Japanese men. J. Epidemiol. 2016, 26, 131–136. [Google Scholar] [CrossRef]
- Aune, D.; Mahamat-Saleh, Y.; Norat, T.; Riboli, E. Body fatness, diabetes, physical activity and risk of kidney stones: A systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 2018, 33, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-T.; Wang, J.; Huang, J.-W.; Hung, K.-Y.; Chien, K.-L. Frailty predicts a higher risk of incident urolithiasis in 525 368 patients with diabetes mellitus: A population-based study. BMJ Open Diabetes Res. Care 2020, 8, e000755. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, S.; Yanaihara, H.; Nishimura, C.; Hayashi, T.; Asakura, H. Association of metabolic status with the presence of urinary tract stones requiring surgical intervention. SN Compr. Clin. Med. 2021, 3, 600–605. [Google Scholar] [CrossRef]
- Crivelli, J.J.; Redden, D.T.; Johnson, R.D.; Juarez, L.D.; Maalouf, N.M.; Hughes, A.E.; Wood, K.D.; Assimos, G.; Oates, G.R. Associations of obesity and neighborhood factors with urinary stone parameters. Am. J. Prev. Med. 2022, 63, S93–S102. [Google Scholar] [CrossRef]
- Siener, R.; Ernsten, C.; Bitterlich, N.; Alteheld, B.; Metzner, C. Effect of two different dietary weight loss strategies on risk factors for urinary stone formation and cardiometabolic risk profile in overweight women. Nutrients 2022, 14, 5054. [Google Scholar] [CrossRef]
- Soueidan, M.; Bartlett, S.J.; Noureldin, Y.A.; Andersen, R.E.; Andonian, S. Leisure time physical activity, smoking and risk of recent symptomatic urolithiasis: Survey of stone clinic patients. Can. Urol. Assoc. J. 2015, 9, 257. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; Sorensen, M.D.; Gambaro, G.; Taylor, E.N. Physical activity, energy intake and the risk of incident kidney stones. J. Urol. 2015, 193, 864–868. [Google Scholar] [CrossRef]
- Hess, B. Renal stone clinic survey: Calcium stone formers’ self-declared understanding of and adherence to physician’s recommendations. Urolithiasis 2017, 45, 363–370. [Google Scholar] [CrossRef]
- Arzoz-Fabregas, M.; Roca-Antonio, J.; Ibarz-Servio, L.; Jappie-Mahomed, D.; Rodgers, A. Stress–stones–stress–recurrent stones: A self-propagating cycle? Difficulties in solving this dichotomy. Urolithiasis 2017, 45, 515–524. [Google Scholar] [CrossRef]
- Park, H.K.; Bae, S.R.; Kim, S.E.; Choi, W.S.; Paick, S.H.; Ho, K.; Kim, H.G.; Lho, Y.S. The effect of climate variability on urinary stone attacks: Increased incidence associated with temperature over 18 C: A population-based study. Urolithiasis 2015, 43, 89–94. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, S.Y.; Chi, B.H.; Kim, J.W.; Kim, T.-H.; Chang, I.H. Urbanization may affect the incidence of urolithiasis in South Korea. Springerplus 2016, 5, 1891. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, X.-M.; Chen, T.-J.; Bai, M.-J. Rural-Urban Differences of Dietary Patterns, Overweight, and Bone Mineral Status in Chinese Students. Nutrients 2016, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-L.; Wang, J.-Y.; Gong, L.-L.; Gan, S.; Gu, C.-M.; Wang, S.-S. Association between cadmium exposure and urolithiasis risk: A systematic review and meta-analysis. Medicine 2018, 97, e9460. [Google Scholar] [CrossRef] [PubMed]
- Lotan, Y.; Antonelli, J.; Jiménez, I.B.; Gharbi, H.; Herring, R.; Beaver, A.; Dennis, A.; Von Merveldt, D.; Carter, S.; Cohen, A. The kidney stone and increased water intake trial in steel workers: Results from a pilot study. Urolithiasis 2017, 45, 177–183. [Google Scholar] [CrossRef]
- Bayne, D.B.; Usawachintachit, M.; Armas-Phan, M.; Tzou, D.T.; Wiener, S.; Brown, T.T.; Stoller, M.; Chi, T.L. Influence of socioeconomic factors on stone burden at presentation to tertiary referral center: Data from the registry for stones of the kidney and ureter. Urology 2019, 131, 57–63. [Google Scholar] [CrossRef]
- Suryavanshi, M.V.; Bhute, S.S.; Jadhav, S.D.; Bhatia, M.S.; Gune, R.P.; Shouche, Y.S. Hyperoxaluria leads to dysbiosis and drives selective enrichment of oxalate metabolizing bacterial species in recurrent kidney stone endures. Sci. Rep. 2016, 6, 34712. [Google Scholar] [CrossRef]
- Ticinesi, A.; Milani, C.; Guerra, A.; Allegri, F.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S. Understanding the gut–kidney axis in nephrolithiasis: An analysis of the gut microbiota composition and functionality of stone formers. Gut 2018, 67, 2097–2106. [Google Scholar] [CrossRef]
- Batagello, C.A.; Monga, M.; Miller, A.W. Calcium oxalate urolithiasis: A case of missing microbes? J. Endourol. 2018, 32, 995–1005. [Google Scholar] [CrossRef]
- Choy, W.H. The Gut Microbiome and Metabolic Pathways of Recurrent Kidney Stone Patients and Their Non-Stone-Forming Live-in Partners; University of British Columbia: Vancouver, BC, Canada, 2018. [Google Scholar]
- Suryavanshi, M.V.; Bhute, S.S.; Gune, R.P.; Shouche, Y.S. Functional eubacteria species along with trans-domain gut inhabitants favour dysgenic diversity in oxalate stone disease. Sci. Rep. 2018, 8, 16598. [Google Scholar] [CrossRef]
- Miller, A.W.; Choy, D.; Penniston, K.L.; Lange, D. Inhibition of urinary stone disease by a multi-species bacterial network ensures healthy oxalate homeostasis. Kidney Int. 2019, 96, 180–188. [Google Scholar] [CrossRef]
- Tavasoli, S.; Alebouyeh, M.; Naji, M.; Shakiba majd, G.; Shabani Nashtaei, M.; Broumandnia, N.; Basiri, A. Association of intestinal oxalate-degrading bacteria with recurrent calcium kidney stone formation and hyperoxaluria: A case–control study. BJU Int. 2020, 125, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, Y.; Begum, R.F.; Velmurugan, R. Oxalobacter formigenes reduce the risk of kidney stones in patients exposed to oral antibiotics: A case–control study. Int. Urol. Nephrol. 2021, 53, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Bao, X.; Liu, S.; Ye, K.; Xiang, S.; Yu, L.; Xu, Q.; Zhang, Y.; Wang, X.; Zhu, X. Gut microbiota affect the formation of calcium oxalate renal calculi caused by high daily tea consumption. Appl. Microbiol. Biotechnol. 2021, 105, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Wu, J.; Gao, M.; Zhu, Z.; Chen, H. Causal relationship between kidney stones and gut microbiota contributes to the gut-kidney axis: A two-sample Mendelian randomization study. Front. Microbiol. 2023, 14, 1204311. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.M.; Moazami, S.; Qiu, Y.; Kurland, I.; Chen, Z.; Agalliu, I.; Burk, R.; Davies, K.P. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 2016, 44, 399–407. [Google Scholar] [CrossRef]
- Tang, R.; Jiang, Y.; Tan, A.; Ye, J.; Xian, X.; Xie, Y.; Wang, Q.; Yao, Z.; Mo, Z. 16S rRNA gene sequencing reveals altered composition of gut microbiota in individuals with kidney stones. Urolithiasis 2018, 46, 503–514. [Google Scholar] [CrossRef]
- Millán Rodríguez, F.; Sabiote Rubio, L.; Girón Nanne, I.; Sánchez Martín, F.; Emiliani, E.; Angerri Feu, O. The relationship between calcium oxalate lithiasis and chronic proinflammatory intestinal dysbiosis pattern: A prospective study. Urolithiasis 2020, 48, 321–328. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, X.; Hong, H.G.; Xiang, L.; Jiang, Q.; Ma, Y.; Chen, Z.; Cheng, L.; Jian, Z.; Wei, Z. The relationship between gut microbiota and short chain fatty acids in the renal calcium oxalate stones disease. FASEB J. 2020, 34, 11200–11214. [Google Scholar] [CrossRef]
- Zhao, E.; Zhang, W.; Geng, B.; You, B.; Wang, W.; Li, X. Intestinal dysbacteriosis leads to kidney stone disease. Mol. Med. Rep. 2021, 23, 180. [Google Scholar] [CrossRef]
- Xiang, L.; Jin, X.; Liu, Y.; Ma, Y.; Jian, Z.; Wei, Z.; Li, H.; Li, Y.; Wang, K. Prediction of the occurrence of calcium oxalate kidney stones based on clinical and gut microbiota characteristics. World J. Urol. 2022, 40, 221–227. [Google Scholar] [CrossRef]
- Kim, H.-N.; Kim, J.H.; Chang, Y.; Yang, D.; Joo, K.J.; Cho, Y.-S.; Park, H.J.; Kim, H.-L.; Ryu, S. Gut microbiota and the prevalence and incidence of renal stones. Sci. Rep. 2022, 12, 3732. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Jin, X.; He, Y.; Liu, Y.; Xiang, L.; Wang, K. Association of dietary patterns with gut microbiota in kidney stone and non-kidney stone individuals. Urolithiasis 2022, 50, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Xia, Y.; Li, B.; Yu, W.; Rao, T.; Ye, Z.; Yan, X.; Song, B.; Li, L.; Lin, F. Gut microbiota in patients with kidney stones: A systematic review and meta-analysis. BMC Microbiol. 2023, 23, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, W.; Zhao, R.; Zhao, Y.; Zhang, Y.; Liang, X. Causal relationship in gut microbiota and upper urinary urolithiasis using Mendelian randomization. Front. Microbiol. 2023, 14, 1170793. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Chen, H.; Chen, J.; Su, Q.; Zhuang, W. Exploring the characteristics of gut microbiome in patients of Southern Fujian with hypocitraturia urolithiasis and constructing clinical diagnostic models. Int. Urol. Nephrol. 2023, 55, 1917–1929. [Google Scholar] [CrossRef]
- Zampini, A.; Nguyen, A.H.; Rose, E.; Monga, M.; Miller, A.W. Defining dysbiosis in patients with urolithiasis. Sci. Rep. 2019, 9, 5425. [Google Scholar] [CrossRef]
- Kachroo, N.; Lange, D.; Penniston, K.L.; Stern, J.; Tasian, G.; Bajic, P.; Wolfe, A.J.; Suryavanshi, M.; Ticinesi, A.; Meschi, T. Meta-analysis of clinical microbiome studies in urolithiasis reveal age, stone composition, and study location as the predominant factors in urolithiasis-associated microbiome composition. MBio 2021, 12, e02007–e02021. [Google Scholar] [CrossRef]
- Suryavanshi, M.; Agudelo, J.; Miller, A. Rare phylotypes in stone, stool, and urine microbiomes are associated with urinary stone disease. Front. Mol. Biosci. 2023, 10, 1210225. [Google Scholar] [CrossRef]
- Al, K.F.; Joris, B.R.; Daisley, B.A.; Chmiel, J.A.; Bjazevic, J.; Reid, G.; Gloor, G.B.; Denstedt, J.D.; Razvi, H.; Burton, J.P. Multi-site microbiota alteration is a hallmark of kidney stone formation. Microbiome 2023, 11, 263. [Google Scholar] [CrossRef]
- Flannigan, R.K.; Battison, A.; De, S.; Humphreys, M.R.; Bader, M.; Lellig, E.; Monga, M.; Chew, B.H.; Lange, D. Evaluating factors that dictate struvite stone composition: A multi-institutional clinical experience from the EDGE Research Consortium. Can. Urol. Assoc. J. 2018, 12, 131. [Google Scholar] [CrossRef]
- Amimanan, P.; Tavichakorntrakool, R.; Fong-Ngern, K.; Sribenjalux, P.; Lulitanond, A.; Prasongwatana, V.; Wongkham, C.; Boonsiri, P.; Umka Welbat, J.; Thongboonkerd, V. Elongation factor Tu on Escherichia coli isolated from urine of kidney stone patients promotes calcium oxalate crystal growth and aggregation. Sci. Rep. 2017, 7, 2953. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Huang, J.-S.; Huang, X.-J.; Peng, J.-M.; Yu, Z.; Yuan, Y.-Q.; Xiao, K.-F.; Guo, J.-N. Profiling the urinary microbiome in men with calcium-based kidney stones. BMC Microbiol. 2020, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, N.; Jiang, P.; Zhai, Q.; Li, C.; Yu, D.; Wu, Y.; Zhang, Y.; Lv, L.; Xu, X. Characteristics of the urinary microbiome in kidney stone patients with hypertension. J. Transl. Med. 2020, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Goloshchapov, E.T.; Chetverikov, A.V. Microbial load of the urine in patients with recurrent urolithiasis and its correction. Urol. Rep. 2020, 10, 51–55. [Google Scholar] [CrossRef]
- Shen, C.; Zhu, Q.; Dong, F.; Wang, W.; Fan, B.; Li, K.; Chen, J.; Hu, S.; He, Z.; Li, X. Identifying two novel clusters in calcium oxalate stones with urinary tract infection using 16S rDNA sequencing. Front. Cell. Infect. Microbiol. 2021, 11, 723781. [Google Scholar] [CrossRef]
- Yang, Y.; Hong, S.; Xu, J.; Li, C.; Wang, S.; Xun, Y. Enterobacter cloacae: A villain in CaOx stone disease? Urolithiasis 2022, 50, 177–188. [Google Scholar] [CrossRef]
- Kachroo, N.; Monga, M.; Miller, A.W. Comparative functional analysis of the urinary tract microbiome for individuals with or without calcium oxalate calculi. Urolithiasis 2022, 50, 303–317. [Google Scholar] [CrossRef]
- Gao, H.; Lin, J.; Xiong, F.; Yu, Z.; Pan, S.; Huang, Y. Urinary Microbial and Metabolomic Profiles in Kidney Stone Disease. Front. Cell. Infect. Microbiol. 2022, 1297. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Yang, Y.-Y.; Xu, J.-Z.; Xia, Q.-D.; Wang, S.-G.; Xun, Y. The renal pelvis urobiome in the unilateral kidney stone patients revealed by 2bRAD-M. J. Transl. Med. 2022, 20, 431. [Google Scholar] [CrossRef]
- Ansari, H.; Sepahi, A.A.; Sepahi, M.A. Different Approaches to Detect “Nanobacteria” in Patients with Kidney Stones: An Infectious Cause or a Subset of Life? Urol. J. 2017, 14, 5001–5007. [Google Scholar]
- Saw, J.J.; Sivaguru, M.; Wilson, E.M.; Dong, Y.; Sanford, R.A.; Fields, C.J.; Cregger, M.A.; Merkel, A.C.; Bruce, W.J.; Weber, J.R. In Vivo entombment of bacteria and fungi during calcium oxalate, brushite, and struvite urolithiasis. Kidney360 2021, 2, 298. [Google Scholar] [CrossRef] [PubMed]
- Parkhomenko, E.; De Fazio, A.; Tran, T.; Thai, J.; Blum, K.; Gupta, M. A multi-institutional study of struvite stones: Patterns of infection and colonization. J. Endourol. 2017, 31, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Dornbier, R.A.; Bajic, P.; Van Kuiken, M.; Jardaneh, A.; Lin, H.; Gao, X.; Knudsen, B.; Dong, Q.; Wolfe, A.J.; Schwaderer, A.L. The microbiome of calcium-based urinary stones. Urolithiasis 2020, 48, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Dornbier, R.A.; Doshi, C.P.; Desai, S.C.; Bajic, P.; Van Kuiken, M.; Khemmani, M.; Farooq, A.V.; Bresler, L.; Turk, T.M.; Wolfe, A.J. Metabolic syndrome and the urinary microbiome of patients undergoing percutaneous nephrolithotomy. Asian J. Urol. 2024, 2, 316–323. [Google Scholar] [CrossRef]
- Lemberger, U.; Pjevac, P.; Hausmann, B.; Berry, D.; Moser, D.; Jahrreis, V.; Özsoy, M.; Shariat, S.F.; Veser, J. The microbiome of kidney stones and urine of patients with nephrolithiasis. Urolithiasis 2023, 51, 27. [Google Scholar] [CrossRef]
- Hoppe, B.; Niaudet, P.; Salomon, R.; Harambat, J.; Hulton, S.-A.; Van’t Hoff, W.; Moochhala, S.H.; Deschênes, G.; Lindner, E.; Sjögren, A.; et al. A randomised Phase I/II trial to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Pediatr. Nephrol. 2017, 32, 781–790. [Google Scholar] [CrossRef]
- Milliner, D.; Hoppe, B.; Groothoff, J. A randomised Phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 2018, 46, 313–323. [Google Scholar] [CrossRef]
- Lieske, J.C. Probiotics for prevention of urinary stones. Ann. Transl. Med. 2017, 5, 29. [Google Scholar] [CrossRef]
- Tavasoli, S.; Jalali, S.; Naji, M.; Borumandnia, N.; Majd, G.S.; Basiri, A.; Darani, K.K.; Karamad, D.; Tajabadi-Ebrahimi, M.; Taheri, M. Effect of a probiotic supplement containing lactobacillus acidophilus and bifidobacterium animalis lactis on urine oxalate in calcium stone formers with hyperoxaluria: A randomized, placebo-controlled, double-blind and In-Vitro trial. Urol. J. 2022, 19, 179–188. [Google Scholar]
- Tasian, G.E.; Jemielita, T.; Goldfarb, D.S.; Copelovitch, L.; Gerber, J.S.; Wu, Q.; Denburg, M.R. Oral Antibiotic Exposure and Kidney Stone Disease. J. Am. Soc. Nephrol. 2018, 29, 1731–1740. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Antibiotic Use and Risk of Incident Kidney Stones in Female Nurses. Am. J. Kidney Dis. 2019, 74, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, L.; Francois, F.; Henderson, N.; Liu, M.; Li, H.; Koh, H.; Wang, C.; Gao, Z.; Perez, G.P.; Asplin, J.R.; et al. Effect of antibiotic treatment on Oxalobacter formigenes colonization of the gut microbiome and urinary oxalate excretion. Sci. Rep. 2021, 11, 16428. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Vaughan, L.E.; Barreto, E.F.; Mehta, R.A.; Koo, K.; Schulte, P.J.; Lieske, J.C.; Rule, A.D. Outpatient Antibiotic Use is Not Associated with an Increased Risk of First-Time Symptomatic Kidney Stones. J. Am. Soc. Nephrol. 2023, 34, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Scales Jr, C.D.; Tasian, G.E.; Schwaderer, A.L.; Goldfarb, D.S.; Star, R.A.; Kirkali, Z. Urinary stone disease: Advancing knowledge, patient care, and population health. Clin. J. Am. Soc. Nephrol. 2016, 11, 1305. [Google Scholar] [CrossRef]
- Rodgers, A.; Trinchieri, A. Fifty years of basic and clinical renal stone research: Have we achieved major breakthroughs? A debate. Curr. Opin. Nephrol. Hypertens. 2023, 32, 177–182. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Romanidou, G.; Voidarou, C.; Bezirtzoglou, E. Focus on the gut–kidney axis in health and disease. Front. Med. 2021, 7, 620102. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Microbiome-based therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Todor, S.B.; Anderco, P.; Popa, M.L. The Importance of Microbiota and Fecal Microbiota Transplantation in Pancreatic Disorders. Diagnostics 2024, 14, 861. [Google Scholar] [CrossRef]
- Brusnic, O.; Onisor, D.; Boicean, A.; Hasegan, A.; Ichim, C.; Guzun, A.; Chicea, R.; Todor, S.B.; Vintila, B.I.; Anderco, P. Fecal Microbiota Transplantation: Insights into Colon Carcinogenesis and Immune Regulation. J. Clin. Med. 2024, 13, 6578. [Google Scholar] [CrossRef]
- Hunthai, S.; Usawachintachit, M.; Taweevisit, M.; Srisa-Art, M.; Anegkamol, W.; Tosukhowong, P.; Rattanachaisit, P.; Chuaypen, N.; Dissayabutra, T. Unraveling the role of gut microbiota by fecal microbiota transplantation in rat model of kidney stone disease. Sci. Rep. 2024, 14, 21924. [Google Scholar]
- Wang, Y.; Sun, J.; Xie, S.; Zhou, Y.; Wang, T.; Liu, Z.; Li, C.; Gao, L.; Pan, T. Increased abundance of bacteria of the family Muribaculaceae achieved by fecal microbiome transplantation correlates with the inhibition of kidney calcium oxalate stone deposition in experimental rats. Front. Cell. Infect. Microbiol. 2023, 13, 1145196. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Ingram, C.; Scovell, J.M.; Link, R.E.; Mayer, W.A. The microbiome and urolithiasis: Current advancements and future challenges. Curr. Urol. Rep. 2022, 23, 47–56. [Google Scholar] [CrossRef] [PubMed]
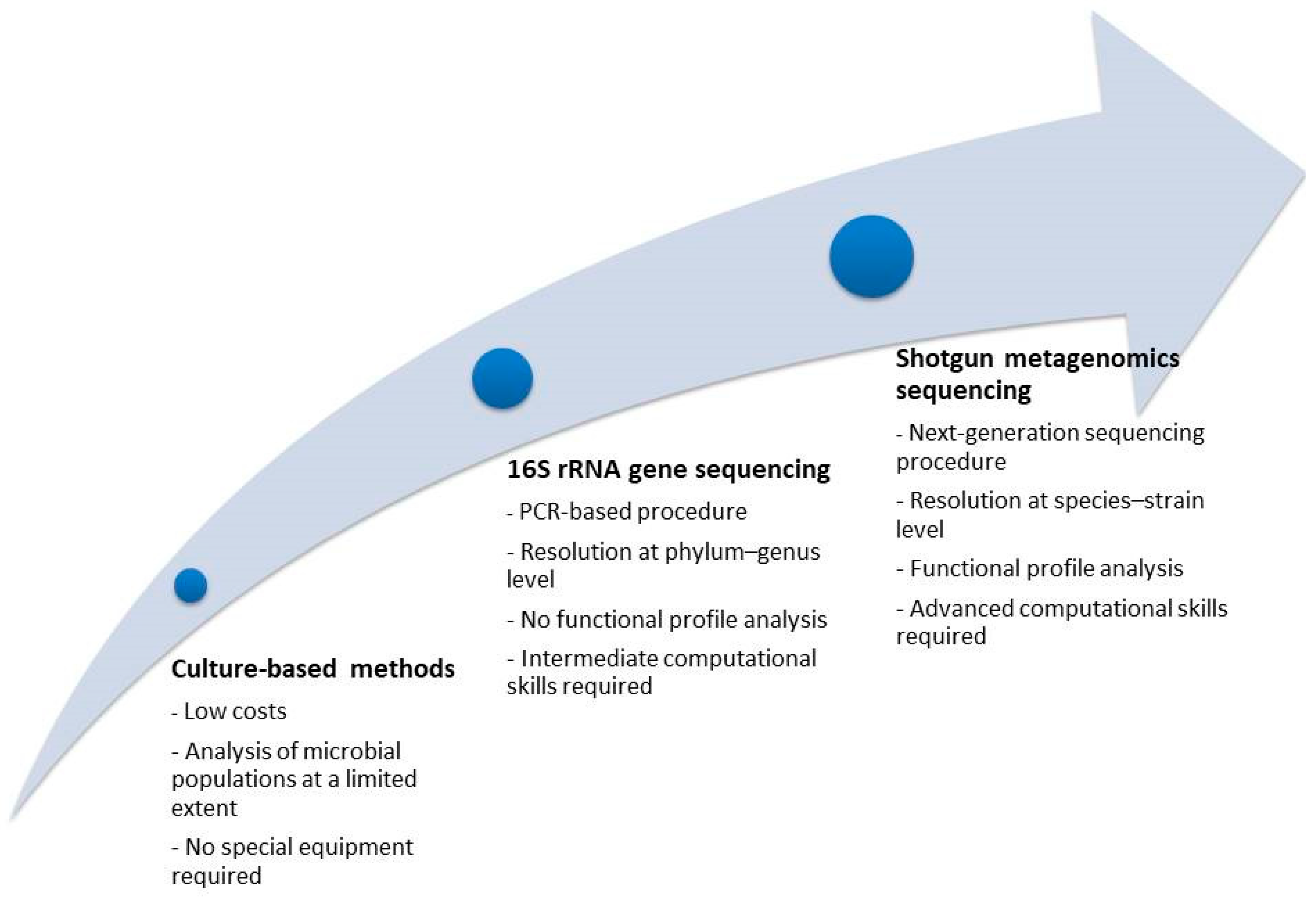
- Bharti, R.; Grimm, D.G. Current challenges and best-practice protocols for microbiome analysis. Brief. Bioinform. 2021, 22, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, N.; Lange, D.; Penniston, K.L.; Stern, J.; Tasian, G.; Bajic, P.; Wolfe, A.J.; Suryavanshi, M.; Ticinesi, A.; Meschi, T. Standardization of microbiome studies for urolithiasis: An international consensus agreement. Nat. Rev. Urol. 2021, 18, 303–311. [Google Scholar] [CrossRef]
- Brubaker, L.; Gourdine, J.-P.F.; Siddiqui, N.Y.; Holland, A.; Halverson, T.; Limeria, R.; Pride, D.; Ackerman, L.; Forster, C.S.; Jacobs, K.M. Forming consensus to advance urobiome research. Msystems 2021, 6, e0137120. [Google Scholar] [CrossRef]
- Sharon, I.; Quijada, N.M.; Pasolli, E.; Fabbrini, M.; Vitali, F.; Agamennone, V.; Dötsch, A.; Selberherr, E.; Grau, J.H.; Meixner, M. The core human microbiome: Does it exist and how can we find It? A critical review of the concept. Nutrients 2022, 14, 2872. [Google Scholar] [CrossRef]

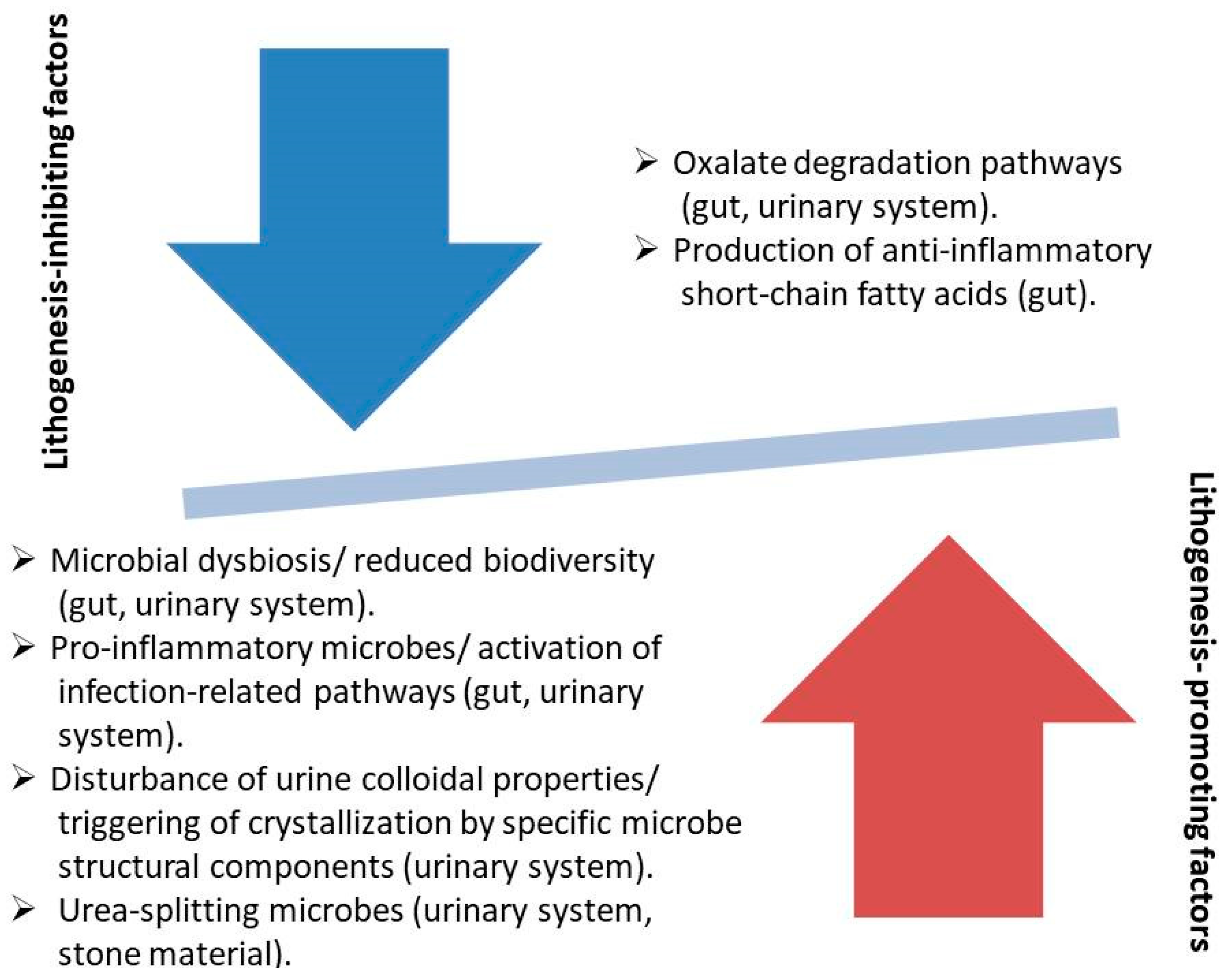

| Microbes Acting as Lithogenesis Inhibitors | Microbes Acting as Lithogenesis Promoters | |
|---|---|---|
| Gut | Prevotella (species) Ruminococcus (genus) Lactobacillus (genus) Faecalibacterium (genus) Bifidobacterium (genus) Oscillospira (genus) Akkermansia (genus) Lachnoclostridium (genus) | Bacteroidetes (phylum) |
| Urinary system | Lactobacillus (genus) Gardnerella (genus) Corynebacterium (genus) Prevotella (species) | Enterobacteriaceae (family) Urea-splitting microbes |
| Standard Recommendations | Protective Effect on Urine Chemistry | Quantitative Limit a |
|---|---|---|
| Reduction in dietary NaCl uptake | Decreased calcium concentration in urine Increased citric acid concentration in urine | ≤4–5 g/day |
| Reduction in sodas and calorie-rich beverages | Increased urinary pH Decreased calcium concentration in urine | Not defined |
| Reduction in dietary oxalate uptake | Decreased oxalate concentration in urine | Not defined |
| Reduction in non-dairy animal protein uptake | Increased urinary pH Decreased uric acid concentration in urine | ≤0.8–1.0 g/kg/day |
| Preservation of dietary calcium uptake | Decreased oxalate concentration in urine | 1–1.2 g/day |
| Increase in vegetable and fiber uptake | Increased urinary pH Increased citric acid concentration in urine | Not defined |
| Increase in fluid uptake (preferred fluid: water, increased fluid compensation under exposure to high temperatures) | Dilution of lithogenic components | Fluid volume: 2.5–3 L/day (diuresis volume: 2.0–2.5 L/day) |
| Dietary interventions for further investigation | Protective effect on urine chemistry | |
| Vitamin D supplementation in individuals with vitamin D deficiency | Unclear | |
| Tea consumption | Unclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koudonas, A.; Tsiakaras, S.; Tzikoulis, V.; Papaioannou, M.; de la Rosette, J.; Anastasiadis, A.; Dimitriadis, G. Lifestyle Factors and the Microbiome in Urolithiasis: A Narrative Review. Nutrients 2025, 17, 465. https://doi.org/10.3390/nu17030465
Koudonas A, Tsiakaras S, Tzikoulis V, Papaioannou M, de la Rosette J, Anastasiadis A, Dimitriadis G. Lifestyle Factors and the Microbiome in Urolithiasis: A Narrative Review. Nutrients. 2025; 17(3):465. https://doi.org/10.3390/nu17030465
Chicago/Turabian StyleKoudonas, Antonios, Stavros Tsiakaras, Vasileios Tzikoulis, Maria Papaioannou, Jean de la Rosette, Anastasios Anastasiadis, and Georgios Dimitriadis. 2025. "Lifestyle Factors and the Microbiome in Urolithiasis: A Narrative Review" Nutrients 17, no. 3: 465. https://doi.org/10.3390/nu17030465
APA StyleKoudonas, A., Tsiakaras, S., Tzikoulis, V., Papaioannou, M., de la Rosette, J., Anastasiadis, A., & Dimitriadis, G. (2025). Lifestyle Factors and the Microbiome in Urolithiasis: A Narrative Review. Nutrients, 17(3), 465. https://doi.org/10.3390/nu17030465






