Maqui and Chronic Kidney Disease: A Narrative Review on the Potential Nephroprotective Role of Anthocyanins
Abstract
1. Introduction
2. Materials and Methods
3. Complementary Treatments: Antioxidants
3.1. Definition of Antioxidants and Anthocyanins
3.2. Bioactive Compounds: Anthocyanins in Inflammatory Diseases
3.3. Foods Rich in Anthocyanins: Maqui (Aristotelia chilensis)
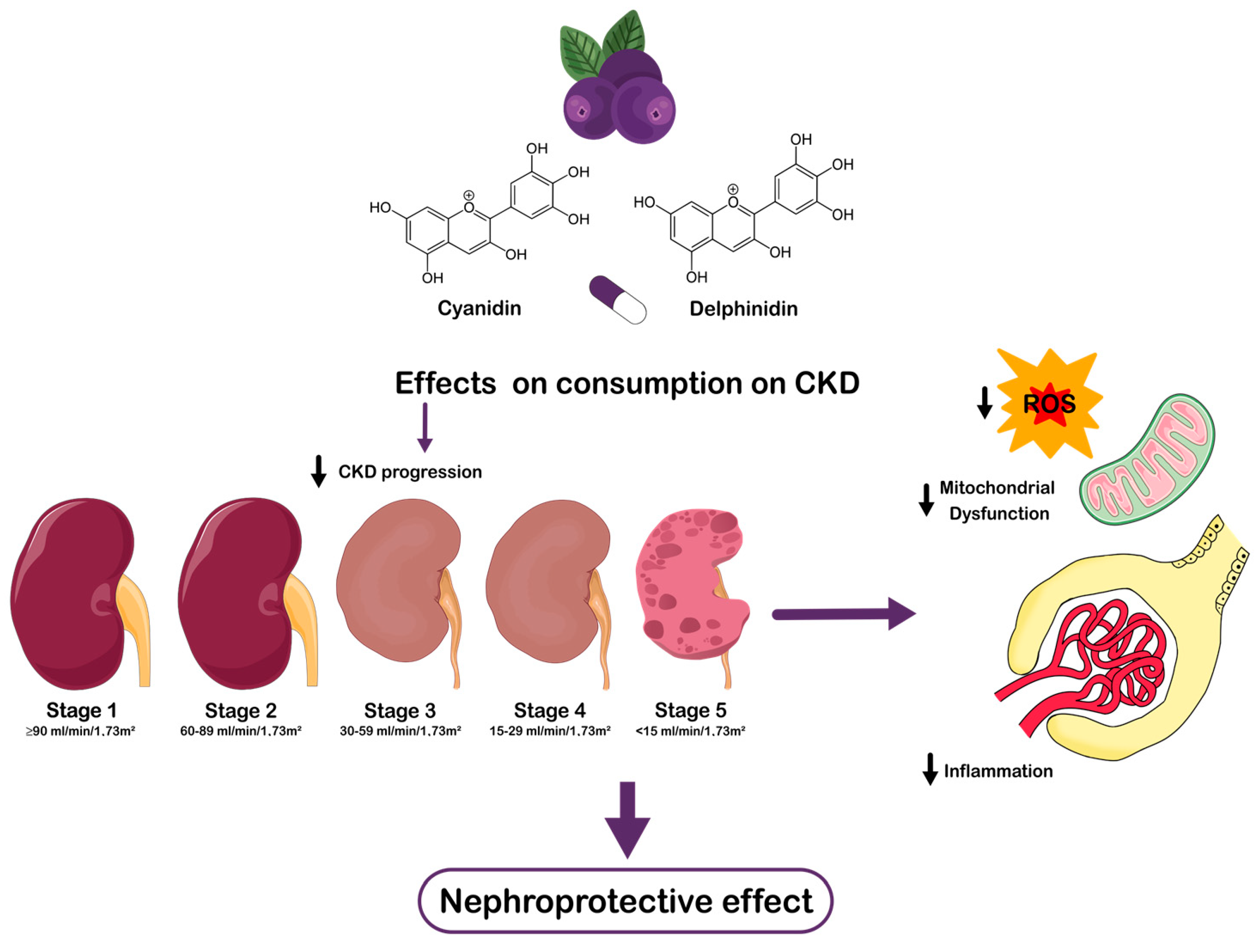
4. Properties of Anthocyanins and Their Relevance in CKD
4.1. Chemical Composition and Bioactive Properties
4.2. Antioxidant and Anti-Inflammatory Mechanism of Action
4.3. Maqui (Aristotelia chilensis) as a Rich Source of Antioxidants: The Role of Anthocyanins in Its Bioactive Profile
4.4. Optimal Dosage and Antioxidant Mechanisms of the Maqui
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| RRTs | Renal replacement therapies |
| DM | Diabetes mellitus |
| HT | Hypertension |
| ROS | Reactive oxygen species |
| DN | Diabetic nephropathy |
| CVD | Cardiovascular disease |
| ESRD | End-stage renal disease |
| GFR | Glomerular filtration rate |
| HD | Hemodialysis |
| PD | Peritoneal dialysis |
| NF-κB | Nuclear transcription factor kappa B |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1 beta |
| IS | Indoxyl sulfate |
| ADMA | Asymmetric dimethylarginine |
| NO | Nitric oxide |
| DKD | Diabetic kidney disease |
| SIRT1 | Sirtuina 1 |
| HMGB1 | High Mobility Group Box 1 |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| CKD-5D | Chronic kidney disease stage 5 on dialysis |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| Nrf2 | Nuclear factor erythroid 2 |
| COX-2 | Cyclooxygenase-2 |
| C3G | Cyanidin-3-O-glucoside |
| ACR | Albumin/creatinine ratio |
| GSH | Glutathione |
| Del-3-sa-5-glu | Delphinidin-3-sambubioside-5-glucoside |
| Del-3,5-diglu | Delphinidin-3, 5-diglucoside |
| Ci-3-sa-5-glu | Cyanidine-3-sambubioside-5-glucoside |
| Ci-3,5-diglu | Cyanidin-3,5-diglucoside |
| Del-3-sa | Delphinidin-3-sambubioside |
| Del- 3-glu | Delphinidin-3-glucoside |
| Ci-3-sa | Cyanidin-3-sambubioside |
| Ci-3-glu | Cyanidin-3-glucoside |
| ORAC | Oxygen Radical Absorption Capacity |
| TPs | Total phenols |
| TFs | Total flavonoids |
| Ox-LDLs | Oxidized low-density lipoproteins |
| NOS | Newcastle-Ottawa scale |
| RoB | Risk of Bias Tool |
| RCTs | Randomized controlled trials |
References
- Guías de Práctica Clínicas Ges Prevención Secundaria de la Enfermedad Renal Crónica—2017. Available online: https://diprece.minsal.cl/wrdprss_minsal/wp-content/uploads/2018/01/2017.10.24_ENFERMEDAD-RENAL-CRONICA.pdf (accessed on 27 January 2025).
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Ammirati, A.L. Chronic Kidney Disease. Rev. Assoc. Medica Bras. (1992) 2020, 66 (Suppl. S1), s03–s09. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: The Basics Fast Facts. Available online: www.kidney.org/covid-19 (accessed on 27 January 2025).
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef]
- Lo, R.; Narasaki, Y.; Lei, S.; Rhee, C.M. Management of traditional risk factors for the development and progression of chronic kidney disease. Clin. Kidney J. 2023, 16, 1737–1750. [Google Scholar] [CrossRef]
- D’andurain, J.; López, V.; Arazo-Rusindo, M.; Tiscornia, C.; Aicardi, V.; Simón, L.; Mariotti-Celis, M.S. Effect of Curcumin Consumption on Inflammation and Oxidative Stress in Patients on Hemodialysis: A Literature Review. Nutrients 2023, 15, 2239. [Google Scholar] [CrossRef] [PubMed]
- García-Maset, R.; Bover, J.; Segura de la Morena, J.; Goicoechea Diezhandino, M.; Cebollada del Hoyo, J.; Escalada San Martín, J.; Rubio, L.F.; Hamarra Ortiz, J.; Garcia-Donaire, J.A.; Guiterrez Perez, M.I.; et al. Documento de información y consenso para la detección y manejo de la enfermedad renal crónica. Nefrología 2022, 42, 233–264. [Google Scholar] [CrossRef] [PubMed]
- Pena-Polanco, J.E.; Fried, L.F. Established and Emerging Strategies in the Treatment of Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Li, P.A.; Hou, X.; Hao, S. Mitochondrial biogenesis in neurodegeneration. J. Neurosci. Res. 2017, 95, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef]
- Popov, L. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, F.; Zhang, Z.; Xing, D. Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. FEBS J. 2011, 278, 941–954. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Twig, G.; Shirihai, O.S. The Interplay Between Mitochondrial Dynamics and Mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef]
- Picard, M.; Shirihai, O.S.; Gentil, B.J.; Burelle, Y. Mitochondrial morphology transitions and functions: Implications for retrograde signaling? Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R393–R406. [Google Scholar] [CrossRef]
- Gall, J.M.; Wang, Z.; Liesa, M.; Molina, A.; Havasi, A.; Schwartz, J.H.; Shirihai, O.; Borkan, S.C.; Bonegio, R.G.B. Role of Mitofusin 2 in the Renal Stress Response. PLoS ONE 2012, 7, e31074. [Google Scholar] [CrossRef]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.J. Oxidative Stress and Renal Fi-brosis: Recent Insights for the Development of Novel Therapeutic Strategies. Front. Physiol. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Düsing, P.; Zietzer, A.; Goody, P.R.; Hosen, M.R.; Kurts, C.; Nickenig, G.; Jansen, F. Vascular pathologies in chronic kidney disease: Pathophysiological mechanisms and novel therapeutic approaches. J. Mol. Med. 2021, 99, 335–348. [Google Scholar] [CrossRef]
- Deicher, R.; Hörl, W.H. Vitamin C in chronic kidney disease and hemodialysis patients. Kidney Blood Press. Res. 2003, 26, 100–106. [Google Scholar] [CrossRef]
- Niki, E. Interaction of ascorbate and alpha-tocopherol. Ann. N. Y. Acad. Sci. 1987, 498, 186–199. [Google Scholar] [CrossRef]
- Yeung, C.K.; Billings, F.T., 4th; Claessens, A.J.; Roshanravan, B.; Linke, L.; Sundell, M.B.; Ahmad, S.; Shao, B.; Shen, D.D.; Ikizler, T.A.; et al. Coenzyme Q10 dose-escalation study in hemodialysis patients: Safety, tolerability, and effect on oxidative stress. BMC Nephrol. 2015, 16, 183. [Google Scholar] [CrossRef]
- Mehmetoglu, I.; Yerlikaya, F.H.; Kurban, S.; Erdem, S.S.; Tonbul, Z. Oxidative stress markers in hemodialysis and peritoneal dialysis patients, including coenzyme Q10 and ischemia-modified albumin. Int. J. Artif. Organs 2012, 35, 226–232. [Google Scholar] [CrossRef]
- Khajehdehi, P.; Pakfetrat, M.; Javidnia, K.; Azad, F.; Malekmakan, L.; Nasab, M.H.; Dehghanzadeh, G. Oral supplementation of turmeric attenuates proteinuria, transforming growth factor-β and interleukin-8 levels in patients with overt type 2 diabetic nephropathy: A randomized, double-blind and placebo-controlled study. Scand. J. Urol. Nephrol. 2011, 45, 365–370. [Google Scholar] [CrossRef]
- Mori, T.A.; Beilin, L.J. Omega-3 fatty acids and inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef]
- Saldanha, J.F.; Leal, V.d.O.; Stenvinkel, P.; Carraro-Eduardo, J.C.; Mafra, D. Resveratrol: Why is it a promising therapy for chronic kidney disease patients? Oxid. Med. Cell Longev. 2013, 2013, 963217. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Uddin, M.J.; Lee, G.; Jiang, S.; Cho, A.; Lee, J.H.; Lee, S.R.; Bae, Y.S.; Moon, S.H.; Lee, S.J.; et al. A novel pan-Nox inhibitor, APX-115, protects kidney injury in streptozotocin-induced diabetic mice: Possible role of peroxisomal and mitochondrial biogenesis. Oncotarget 2017, 8, 74217–74232. [Google Scholar] [CrossRef]
- Cha, J.J.; Min, H.S.; Kim, K.T.; Kim, J.E.; Ghee, J.Y.; Kim, H.W.; Lee, J.E.; Han, J.Y.; Lee, G.; Ha, H.J.; et al. APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury. Lab. Investig. 2017, 97, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.P.; Bakris, G.L.; Block, G.A.; Chertow, G.M.; Goldsberry, A.; Inker, L.A.; Heerspink, H.J.L.; O’Grady, M.; Pergola, P.E.; Wanner, C.; et al. Bardoxolone Methyl Improves Kidney Function in Patients with Chronic Kidney Disease Stage 4 and Type 2 Diabetes: Post-Hoc Analyses from Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Study. Am. J. Nephrol. 2018, 47, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Flores, J.C.; Alvo, M.; Borja Hernán, M.; Vega, J.; Zúñiga, C.; Müller, H.; Münzenmayer, J. Clinical guidelines on identitication, management and complications of chronic kidney disease. Rev. Méd. Chile 2009, 137, 137–177. [Google Scholar]
- Encuesta Nacional de Salud 2016–2017 Primeros Resultados. Available online: https://redsalud.ssmso.cl/wp-content/uploads/2018/02/ENS-2016-17_PRIMEROS-RESULTADOS-ilovepdf-compressed.pdf (accessed on 27 January 2025).
- Orozco, R.B. Recognition and prevention of chronic kidney disease (CKD). Rev. Medica Clin. Las Condes 2010, 21, 779–789. [Google Scholar]
- Afsar, B.; Afsar, R.E.; Ertuglu, L.A.; Covic, A.; Kanbay, M. Nutrition, Immunology, and Kidney: Looking Beyond the Horizons. Curr. Nutr. Rep. 2022, 11, 69–81. [Google Scholar] [CrossRef]
- Galli, F.; Piroddi, M.; Annetti, C.; Arosio, B.; Franconi, F.; Matucci Cerinic, M. Oxidative stress and reactive oxygen species. Cardiovasc. Disord. Hemodial. 2005, 149, 240–260. [Google Scholar]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Del Bò, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Panchal, S.K.; John, O.D.; Mathai, M.L.; Brown, L. Anthocyanins in Chronic Diseases: The Power of Purple. Nutrients 2022, 14, 2161. [Google Scholar] [CrossRef]
- García-Milla, P.; Peñalver, R.; Nieto, G. A Review of the Functional Characteristics and Applications of Aristotelia chilensis (Maqui Berry), in the Food Industry. Foods 2024, 13, 838. [Google Scholar] [CrossRef]
- Akagi, T.; Ikegami, A.; Tsujimoto, T.; Kobayashi, S.; Sato, A.; Kono, A.; Yonemori, K. DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol. 2009, 151, 2028–2045. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Jiménez-Aspee, F.; Theoduloz, C.; Ladio, A. Patagonian berries as native food and medicine. J. Ethnopharmacol. 2019, 241, 111979. [Google Scholar] [CrossRef]
- Quispe-Fuentes, I.; Vega-Gálvez, A.; Campos-Requena, V.H. Antioxidant Compound Extraction from Maqui (Aristotelia chilensis [Mol] Stuntz) Berries: Optimization by Response Surface Methodology. Antioxidants 2017, 6, 10. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Davidge, S.T.; Wu, J. Biotransformation of anthocyanins by gut microflora and their effects on gut and cardiovascular health. J. Nutr. Biochem. 2013, 24, 1508–1518. [Google Scholar]
- Salinas, J.; Caballé, G. Maqui el Fruto Silvestre de Mayor Importancia en Chile—Santiago: Instituto Forestal. 2020. Available online: https://hdl.handle.net/20.500.14001/67594 (accessed on 27 January 2025).
- Supriyadi, R.; Koswara, M.I.A.; Soelaeman, M.A.; Huang, I. The effect of antioxidants supplementation on oxidative stress and proinflammatory biomarkers in patients with chronic kidney disease: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1413–1426. [Google Scholar]
- Céspedes, C.L.; El-Hafidi, M.; Pavon, N.; Alarcon, J. Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), Maqui. Food Chem. 2008, 107, 860–867. [Google Scholar] [CrossRef]
- Nikbakht, E.; Singh, I.; Vider, J. Potential of anthocyanin as an anti-inflammatory agent: A human clinical trial on type 2 diabetic, diabetic at-risk and healthy adults. Inflamm. Res. 2021, 70, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Quiñones, M.; Miguel, M.; Aleixandre, A. Los polifenoles, compuestos con efectos beneficiosos sobre la salud. Nutr. Hosp. 2012, 27, 7–19. [Google Scholar]
- Surai, P.F. Polyphenol compounds in the chicken/animal diet: From the past to the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef]
- Li, Y.X.; Lu, Y.P.; Tang, D.; Hu, B.; Zhang, Z.Y.; Wu, H.W.; Fan, L.J.; Cai, K.W.; Tang, C.; Zhang, Y.Q.; et al. Anthocyanin improves kidney function in diabetic kidney disease by regulating amino acid metabolism. Transl. Med. 2022, 20, 510. [Google Scholar] [CrossRef]
- Madduma-Hewage, S.; Prashar, S.; Debnath, S.C.; O, K.; Siow, Y.L. Inhibition of Inflammatory Cytokine Expression Prevents High-Fat Diet-Induced Kidney Injury: Role of Lingonberry Supplementation. Front. Med. 2020, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhai, Q.; Li, Y.; Cao, M.; Xu, Y.; Zhao, K.; Wang, T. Cyanidin-3-O-glucoside ameliorates diabetic nephropathy through regulation of glutathione pool. Biomed. Pharmacother. 2018, 103, 1223–1230. [Google Scholar] [CrossRef]
- Alvarado, J.L.; Leschot, A.; Olivera-Nappa, Á.; Salgado, A.M.; Rioseco, H.; Lyon, C.; Vigil, P. Delphinidin-Rich Maqui Berry Extract (Delphinol®) Lowers Fasting and Postprandial Glycemia and Insulinemia in Prediabetic Individuals during Oral Glucose Tolerance Tests. Biomed Res. Int. 2016, 2016, 9070537. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Bertoglio, J.C.; Zarrelli, A.; Pina, R.; Scapagnini, G. A Randomized Clinical Trial Evaluating the Efficacy of an Anthocyanin-Maqui Berry Extract (Delphinol®) on Oxidative Stress Biomarkers. Am. Coll. Nutr. 2015, 34 (Suppl. S1), 28–33. [Google Scholar] [CrossRef]
- Varghese, R.T.; Jialal, I. Diabetic Nephropathy; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Crisóstomo-Ayala, K.A.; Sabater-Jara, A.B.; Pérez Manriquez, C.; Ferreres, F.; Gil-Izquierdo, Á.; Pedreño, M.Á.; Hernández de la Torre, M.; Sanchez-Olate, M.; Ríos Leal, D.G. Comparative Study of Metabolomic Profile and Antioxidant Content of Adult and In Vitro Leaves of Aristotelia chilensis. Plants 2021, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, C.L.; Valdez-Morales, M.; Avila, J.G.; El-Hafidi, M.; Alarcón, J.; Paredes-López, O. Phytochemical profile and the antioxidant activity of Chilean wild black-berry fruits, Aristotelia chilensis (Mol) Stuntz (Elaeocarpaceae). Food Chem. 2010, 119, 886–895. [Google Scholar] [CrossRef]
- Brauch, J.E.; Buchweitz, M.; Schweiggert, R.M.; Carle, R. Detailed analyses of fresh and dried maqui (Aristotelia chilensis (Mol.) Stuntz) berries and juice. Food Chem. 2016, 190, 308–316. [Google Scholar] [CrossRef]
- Zapata, L.M.; Heredia, A.M.; Quinteros, C.F.; Malleret, A.D.; Clemente, G.; Cárcel, J.A. Optimización de la extracción de antocianinas de arándanos. Cienc. Docencia Tecnol. 2014, 49, 166–192. [Google Scholar]
- Rubio Ochoa, E.; Pérez Sánchez, R.E.; Ávila Val, T.C.; Gómez Leyva, J.F.; García Saucedo, P.A. Propiedades fisicoquímicas de frutos silvestres de Rubus con potencial nutracéutico y alimenticio. Rev. Mex. Cienc. Agric. 2019, 10, 291–301. [Google Scholar] [CrossRef]
- Mitic, V.; Stankov Jovanovic, V.; Dimitrijevic, M.; Cvetkovic, J.; Simonovic, S.; Nikolic Mandic, S. Chemometric analysis of antioxidant activity and anthocyanin content of selected wild and cultivated small fruit from Serbia. Fruits 2014, 69, 413–422. [Google Scholar] [CrossRef]
- Romero Román, M.E.; Noriega Vásquez, F.; Farías Villagra, M.; Jara Zapata, P.; Vera Flores, B.; López Belchi, M.D. Nuevas fuentes de antioxidantes naturales: Caracterización de compuestos bioactivos en cinco frutos nativos de chile. Perfiles 2020, 2, 34–41. [Google Scholar] [CrossRef]
- Base de Datos de Actividad Antioxidante (ORAC) y de Contenido de Polifenoles Totales (PFT) en Frutas. Portal Antioxidantes—2021. Available online: https://portalantioxidantes.com/base-de-datos-de-actividad-antioxidante-orac-y-de-contenido-de-polifenoles-totales-pft-en-frutas/ (accessed on 27 January 2025).
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind. Crops Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Biodisponibilidad—Diccionario Médico: Clínica Universidad de Navarra. Available online: https://www.cun.es/diccionario-medico/terminos/biodisponibilidad (accessed on 27 January 2025).
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of maqui (Aristotelia chilensis) protect against hydrogen peroxide-induced oxidative stress in cultured neuronal cells. Biol. Res. 2011, 44, 109–115. [Google Scholar]
- Guo, Y.; Zhang, P.; Liu, Y.; Zha, L.; Ling, W.; Guo, H. A dose-response evaluation of purified anthocyanins on inflammatory and oxidative biomarkers and metabolic risk factors in healthy young adults: A randomized controlled trial. Nutrition 2020, 74, 110745. [Google Scholar] [CrossRef]
| Authors | Type of Source | Aim | Principal Findings |
|---|---|---|---|
| Kwon, et al., 2017 [34] | Research Article | To evaluate APX-115’s efficacy in mitigating diabetic kidney injury. | APX-115 prevented kidney injury, oxidative stress, and organelle dysfunction in diabetic mice, comparable to losartan, suggesting its potential as a therapeutic agent for diabetic kidney disease. |
| Cha, et al., 2017 [35] | Research Article | To evaluate the therapeutic efficacy of APX-115 in diabetic nephropathy. | APX-115 reduced oxidative stress, improved renal function, and attenuated mesangial expansion in diabetic mice. It demonstrated superior or comparable efficacy to GKT137831, suggesting pan-Nox inhibition as a potential treatment for diabetic nephropathy. |
| Céspedes, et al., 2008 [59] | Research Article | To evaluate antioxidant and cardioprotective effects of Aristotelia chilensis fruit extract. | The methanol extract of Aristotelia chilensis demonstrated significant antioxidant activity, cardioprotective effects against ischemia/reperfusion injury, and reduced lipid peroxidation, correlating with high polyphenol content. |
| Nikbakht, et al., 2021 [60] | Clinical Trial | To evaluate the anti-inflammatory effects of dietary anthocyanin in type 2 diabetic, at-risk, and healthy individuals. | Dietary anthocyanin significantly reduced pro-inflammatory biomarkers in type 2 diabetic participants and improved select biochemical parameters in at-risk individuals. |
| Li, et al., 2015 [61] | Randomized Controlled Trial | To evaluate anthocyanins’ effects on dyslipidaemia, oxidative status, and insulin sensitivity in type 2 diabetes patients. | Anthocyanin supplementation improved lipid profiles, enhanced antioxidant capacity, reduced oxidative stress markers, and improved insulin sensitivity and glucose metabolism in type 2 diabetes patients. |
| Li, et al., 2022 [64] | Research Article | To investigate anthocyanins’ effects on diabetic kidney disease via metabolic pathways. | Anthocyanins significantly improved renal function, reduced blood glucose, and alleviated glomerular lesions in DKD mice by regulating amino acid metabolism, particularly taurine, hypotaurine, tryptophan, and tyrosine pathways. |
| Qin, et al., 2018 [66] | Research Article | To investigate the effects of cyanidin 3-glucoside on diabetic nephropathy in db/db mice. | Cyanidin 3-glucoside ameliorates diabetic nephropathy by reducing glucose metabolic dysfunction, renal inflammation, fibrosis, and oxidative stress, while enhancing glutathione synthesis in db/db mice. |
| Alvarado, et al., 2016 [67] | Clinical Trial | To evaluate Delphinol®’s effects on glucose metabolism and lipid profiles in prediabetic subjects. | Delphinol® significantly reduced HbA1c and LDL levels, increased HDL, and improved glucose metabolism over three months, with no adverse effects observed. Fasting insulin and glucose changes were non-significant. |
| Davinelli, et al., 2015 [68] | Randomized Controlled Trial | To evaluate maqui berry extract’s impact on lipid peroxidation biomarkers | Delphinol® supplementation reduced Ox-LDL and urinary F2-isoprostanes at 4 weeks, but effects diminished by 40 days. No significant changes in anthropometrics, blood pressure, or lipid profile were observed. |
| Crisóstomo-Ayala, et al., 2021 [70] | Research Article | To compare bioactive compounds in maqui leaves from in vitro and ex vitro sources across developmental stages and seasons. | In vitro maqui leaves exhibited higher total phenolic content, while winter basal leaves had higher flavonoid content. Spring basal leaves were enriched in quercetin, catechin, kaempferol, and 3-caffeoyl quinic acids, whereas in vitro leaves contained α-tocopherol and β-sitosterol. Adult leaves showed elevated linolenic and linoleic acids, indicating potential antioxidant and nutraceutical applications. |
| Lucas-González, et al., 2016 [78] | Research Article | To evaluate the impact of in vitro gastrointestinal digestion on maqui berry polyphenolic stability and antioxidant activity. | Gastrointestinal digestion significantly reduced polyphenolic concentrations, particularly anthocyanins, and decreased antioxidant scavenging properties. However, chelating activity increased, and phenolic and flavonoid bioaccessibility remained at 78.19% and 14.20%, respectively, indicating retained antioxidant potential. |
| Guo, et al., 2020 [83] | Randomized Controlled Trial | To evaluate the dose-response relationship of anthocyanins on metabolic and inflammatory biomarkers. | Anthocyanin supplementation (>80 mg/d) significantly reduced fasting plasma glucose, increased interleukin-10 levels, and decreased 8-iso-prostaglandin F2α, demonstrating antioxidant and anti-inflammatory effects in healthy adults. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiscornia, C.; Tapia, V.; Águila, D.; Lorca-Ponce, E.; Aicardi, V.; Vásquez, F. Maqui and Chronic Kidney Disease: A Narrative Review on the Potential Nephroprotective Role of Anthocyanins. Nutrients 2025, 17, 1058. https://doi.org/10.3390/nu17061058
Tiscornia C, Tapia V, Águila D, Lorca-Ponce E, Aicardi V, Vásquez F. Maqui and Chronic Kidney Disease: A Narrative Review on the Potential Nephroprotective Role of Anthocyanins. Nutrients. 2025; 17(6):1058. https://doi.org/10.3390/nu17061058
Chicago/Turabian StyleTiscornia, Caterina, Violeta Tapia, Daniela Águila, Enrique Lorca-Ponce, Valeria Aicardi, and Fabián Vásquez. 2025. "Maqui and Chronic Kidney Disease: A Narrative Review on the Potential Nephroprotective Role of Anthocyanins" Nutrients 17, no. 6: 1058. https://doi.org/10.3390/nu17061058
APA StyleTiscornia, C., Tapia, V., Águila, D., Lorca-Ponce, E., Aicardi, V., & Vásquez, F. (2025). Maqui and Chronic Kidney Disease: A Narrative Review on the Potential Nephroprotective Role of Anthocyanins. Nutrients, 17(6), 1058. https://doi.org/10.3390/nu17061058






