Moringa oleifera and Blood Pressure: Evidence and Potential Mechanisms
Abstract
1. Introduction
2. Physiological Regulation of Blood Pressure
2.1. Baroreceptor Reflex
2.2. Antidiuretic Hormone
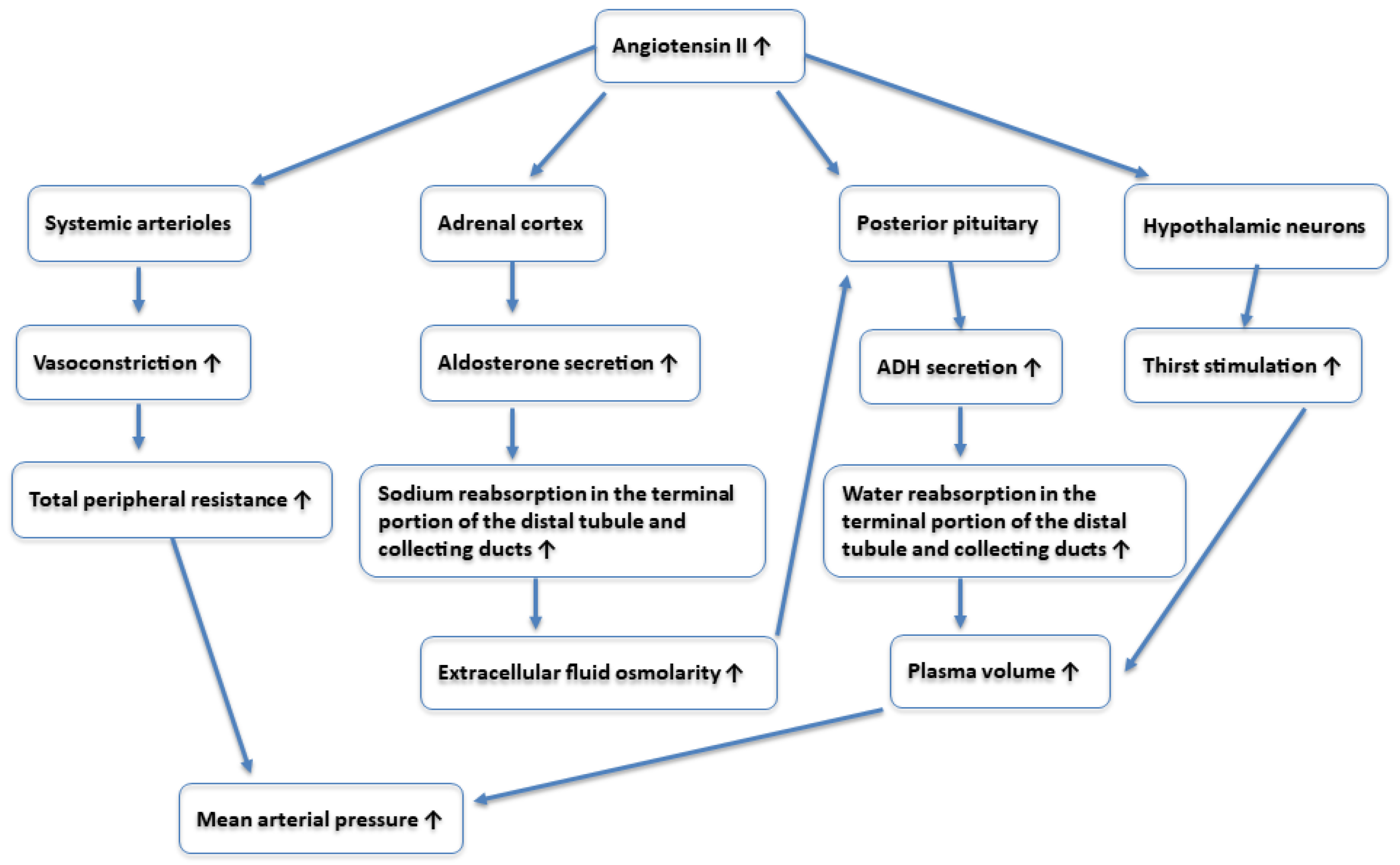
2.3. Renin–Angiotensin–Aldosterone System (RAAS)
3. Search Strategy and Study Selection
4. Effect of Moringa oleifera on Blood Pressure
4.1. Animal Studies
4.2. Human Studies
| Human Studies | |||
|---|---|---|---|
| Studies | Part of Moringa | Experimental Model | Results |
| Chan Sun et al. [42] | Cooked leaves | A total of 41 participants aged 18–65 years (14 males, 27 females) were randomized into two groups:
| Both the case and control groups showed decreased systolic blood pressure postprandially, but no significant difference was observed compared to baseline. Diastolic blood pressure decreased overall postprandially for both groups, with a significant difference noted between 2 h and baseline only in the experimental group. |
| Afiaenyi et al. [43] | Steamed leaves | A total of 40 adults (12 males, 28 females) with type 2 diabetes were split into groups and treated for 14 days: Group 1: Standard diet; Group 2: Standard diet + 20 g/day M. oleifera leaves; Group 3: Standard diet + 40 g/day M. oleifera leaves; Group 4: Standard diet + 60 g/day M. oleifera leaves. | Group 3 showed a significant decrease in SBP from 136.40 ± 7.66 mm Hg to 123.90 ± 13.82 mm Hg, with a mean decrease of 12.5 mm Hg. Group 4 did not show a significant reduction in SBP (−7.8 mm Hg). No significant reduction in DBP was observed in group 3 or 4 (−4.7 mm Hg and 3.3 mm Hg, respectively). |
| Taweerutchana et al. [45] | Leaf powder | RCT with placebo involving 32 people with type 2 diabetes. They were treated for 4 weeks with 8 g/day of M. oleifera leaf powder (experimental group) or placebo (control group). | No effect on SBP and DBP was observed. |
| Diaz-Prieto et al. [46] | Leaf powder | A double-blind RCT with placebo involving 73 patients with prediabetes. Patients were treated for 12 weeks with six capsules, each containing 400 mg of M. oleifera dry leaf powder (experimental group) or placebo (control group). | No effect on SBP and DBP was observed. |
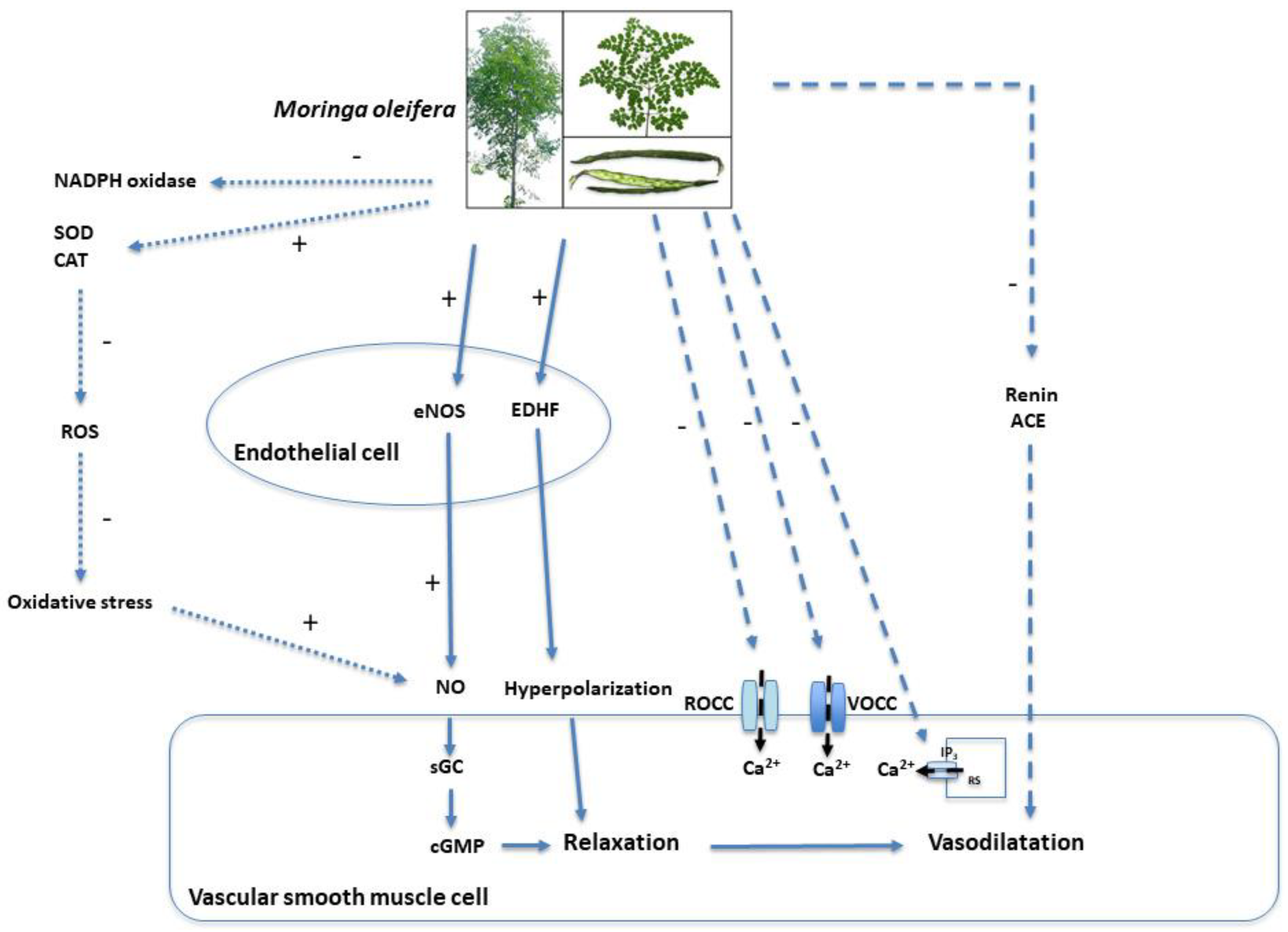
5. Possible Mechanisms of Action
6. Compounds with Hypotensive Potential
7. Limitations of Using Moringa oleifera as an Antihypertensive Therapy
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: Developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Cuspidi, C.; Tadic, M.; Grassi, G.; Mancia, G. Treatment of hypertension: The ESH/ESC guidelines recommendations. Pharmacol. Res. 2018, 128, 315–321. [Google Scholar] [CrossRef]
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A.; Bahonar, A.; Chifamba, J.; Dagenais, G.; Diaz, R.; et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013, 310, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; et al. Nutritional Characterization and Phenolic Profiling of Moringa oleifera Leaves Grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. Int. J. Mol. Sci. 2015, 16, 12791–12835. [Google Scholar] [CrossRef]
- Nova, E.; Redondo-Useros, N.; Martínez-García, R.M.; Gómez-Martínez, S.; Díaz-Prieto, L.E.; Marcos, A. Potential of Moringa oleifera to Improve Glucose Control for the Prevention of Diabetes and Related Metabolic Alterations: A Systematic Review of Animal and Human Studies. Nutrients 2020, 12, 2050. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Sales, J.A.; Pereira, V.S.; Castelo-Branco, D.S.C.M.; Cordeiro, R.A.; de Souza Sampaio, C.M.; de Araújo Neto Paiva, M.; Santos, J.B.F.D.; Sidrim, J.J.C.; Rocha, M.F.G. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asian Pac. J. Trop. Med. 2017, 10, 621–630. [Google Scholar] [CrossRef]
- Barichella, M.; Pezzoli, G.; Faierman, S.A.; Raspini, B.; Rimoldi, M.; Cassani, E.; Bertoli, S.; Battezzati, A.; Leone, A.; Iorio, L.; et al. Nutritional characterisation of Zambian Moringa oleifera: Acceptability and safety of short-term daily supplementation in a group of malnourished girls. Int. J. Food Sci. Nutr. 2019, 70, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Di Lello, S.; Bertoli, S.; Ravasenghi, S.; De Amicis, R.; Menichetti, F.; Fico, G.; Santagostini, L.; Mohamed-Iahdih, B.; Mohamed Lamin Saleh, S.; et al. Moringa oleifera leaf powder enhances glycemic control in sahrawi women with type 2 diabetes: Findings from a 3-month unblinded randomized controlled trial. PharmaNutrition 2025, 31, 100434. [Google Scholar] [CrossRef]
- Leone, A.; Bertoli, S.; Di Lello, S.; Bassoli, A.; Ravasenghi, S.; Borgonovo, G.; Forlani, F.; Battezzati, A. Effect of Moringa oleifera Leaf Powder on Postprandial Blood Glucose Response: In Vivo Study on Saharawi People Living in Refugee Camps. Nutrients 2018, 10, 1494. [Google Scholar] [CrossRef]
- Arora, S. Nutritional significance and therapeutic potential of Moringa oleifera: The wonder plant. J. Food Biochem. 2021, 45, e13933. [Google Scholar] [CrossRef]
- Matic, I.; Guidi, A.; Kenzo, M.; Mattei, M.; Galgani, A. Investigation of medicinal plants traditionally used as dietary supplements: A review on. J. Public. Health Afr. 2018, 9, 841. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Alia, F.; Putri, M.; Anggraeni, N.; Syamsunarno, M. The Potency of Moringa oleifera Lam. as Protective Agent in Cardiac Damage and Vascular Dysfunction. Front. Pharmacol. 2021, 12, 724439. [Google Scholar] [CrossRef]
- Louisa, M.; Patintingan, C.G.H.; Wardhani, B.W.K. Moringa oleifera Lam. in Cardiometabolic Disorders: A Systematic Review of Recent Studies and Possible Mechanism of Actions. Front. Pharmacol. 2022, 13, 792794. [Google Scholar] [CrossRef]
- Shahoud, J.S.; Sanvictores, T.; Aeddula, N.R. Physiology, Arterial Pressure Regulation. In StatPearls; StatPearls Publishing LLC.: Tampa, FL, USA, 2023. [Google Scholar]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Reboussin, D.M.; Allen, N.B.; Griswold, M.E.; Guallar, E.; Hong, Y.; Lackland, D.T.; Miller, E.P.R., 3rd; Polonsky, T.; Thompson-Paul, A.M.; Vupputuri, S. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e595–e616. [Google Scholar] [CrossRef]
- Aronow, W.S. Treatment of hypertensive emergencies. Ann. Transl. Med. 2017, 5, S5. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, W.A. Blood Pressure. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworth Publishers, a Division of Reed Publishing: Boston, MA, USA, 1990. [Google Scholar]
- Sanders, J.S.; Mark, A.L.; Ferguson, D.W. Importance of aortic baroreflex in regulation of sympathetic responses during hypotension. Evidence from direct sympathetic nerve recordings in humans. Circulation 1989, 79, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S. Venous function and central venous pressure: A physiologic story. Anesthesiology 2008, 108, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Malpas, S.C. What sets the long-term level of sympathetic nerve activity: Is there a role for arterial baroreceptors? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R1–R12. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E. Guyton and Hall Textobook of Medical Physiology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 14. [Google Scholar]
- Bruce, M.; Koeppen, B.A.S. Berne&Levy Fisiologia; Casa Editrice Ambrosiana: Rozzano, Italy, 2018; Volume VII, p. 943. [Google Scholar]
- McClure, J.M.; Rossi, N.F.; Chen, H.; O’Leary, D.S.; Scislo, T.J. Vasopressin is a major vasoconstrictor involved in hindlimb vascular responses to stimulation of adenosine A(1) receptors in the nucleus of the solitary tract. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1661–H1672. [Google Scholar] [CrossRef]
- Henderson, K.K.; Byron, K.L. Vasopressin-induced vasoconstriction: Two concentration-dependent signaling pathways. J. Appl. Physiol. 2007, 102, 1402–1409. [Google Scholar] [CrossRef]
- Zhang, R.; Behbehani, K.; Crandall, C.G.; Zuckerman, J.H.; Levine, B.D. Dynamic regulation of heart rate during acute hypotension: New insight into baroreflex function. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H407–H419. [Google Scholar] [CrossRef]
- Carretero, O.A.; Oparil, S. Essential hypertension. Part I: Definition and etiology. Circulation 2000, 101, 329–335. [Google Scholar] [CrossRef]
- Oparil, S.; Zaman, M.A.; Calhoun, D.A. Pathogenesis of hypertension. Ann. Intern. Med. 2003, 139, 761–776. [Google Scholar] [CrossRef]
- Abrogoua, D.P.; Dano, D.S.; Manda, P.; Adepo, A.J.; Kablan, B.J.; Goze, N.B.; Ehoulé, K. Effect on blood pressure of a dietary supplement containing traditional medicinal plants of Côte d’Ivoire. J. Ethnopharmacol. 2012, 141, 840–847. [Google Scholar] [CrossRef]
- Aekthammarat, D.; Tangsucharit, P.; Pannangpetch, P.; Sriwantana, T.; Sibmooh, N. Moringa oleifera leaf extract enhances endothelial nitric oxide production leading to relaxation of resistance artery and lowering of arterial blood pressure. Biomed. Pharmacother. 2020, 130, 110605. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Aftab, K.; Shaheen, F.; Gilani, A.H. Hypotensive constituents from the pods of Moringa oleifera. Planta Med. 1998, 64, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Attakpa, E.S.; Bertin, G.A.; Chabi, N.W.; Ategbo, J.M.; Seri, B.; Khan, N.A. Moringa oleifera-rich diet and T cell calcium signaling in spontaneously hypertensive rats. Physiol. Res. 2017, 66, 753–767. [Google Scholar] [CrossRef]
- Aekthammarat, D.; Pannangpetch, P.; Tangsucharit, P. Moringa oleifera leaf extract lowers high blood pressure by alleviating vascular dysfunction and decreasing oxidative stress in L-NAME hypertensive rats. Phytomedicine 2019, 54, 9–16. [Google Scholar] [CrossRef]
- Acuram, L.K.; Chichioco Hernandez, C.L. Anti-hypertensive effect of Moringa oleifera Lam. Cogent Biol. 2019, 5, 1596526. [Google Scholar] [CrossRef]
- Chen, K.H.; Chen, Y.J.; Yang, C.H.; Liu, K.W.; Chang, J.L.; Pan, S.F.; Lin, T.B.; Chen, M.J. Attenuation of the extract from Moringa oleifera on monocrotaline-induced pulmonary hypertension in rats. Chin. J. Physiol. 2012, 55, 22–30. [Google Scholar] [CrossRef]
- Randriamboavonjy, J.I.; Loirand, G.; Vaillant, N.; Lauzier, B.; Derbré, S.; Michalet, S.; Pacaud, P.; Tesse, A. Cardiac Protective Effects of Moringa oleifera Seeds in Spontaneous Hypertensive Rats. Am. J. Hypertens. 2016, 29, 873–881. [Google Scholar] [CrossRef]
- Chan Sun, M.; Ruhomally, Z.B.; Boojhawon, R.; Neergheen-Bhujun, V.S. Consumption of Moringa oleifera Lam Leaves Lowers Postprandial Blood Pressure. J. Am. Coll. Nutr. 2020, 39, 54–62. [Google Scholar] [CrossRef]
- Afiaenyi, C.I.; Ngwu, K.E.; Okafor, M.A.; Ayogu, R.N. Effects of Moringa oleifera leaves on the blood glucose, blood pressure, and lipid profile of type 2 diabetic subjects: A parallel group randomized clinical trial of efficacy. Nutr. Health 2025, 31, 281–291. [Google Scholar] [CrossRef]
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D.; et al. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31 (Suppl. S1), S61–S78. [Google Scholar] [CrossRef]
- Taweerutchana, R.; Lumlerdkij, N.; Vannasaeng, S.; Akarasereenont, P.; Sriwijitkamol, A. Effect of Moringa oleifera Leaf Capsules on Glycemic Control in Therapy-Naïve Type 2 Diabetes Patients: A Randomized Placebo Controlled Study. Evid. Based Complement. Altern. Med. 2017, 2017, 6581390. [Google Scholar] [CrossRef]
- Díaz-Prieto, L.E.; Gómez-Martínez, S.; Vicente-Castro, I.; Heredia, C.; González-Romero, E.A.; Martín-Ridaura, M.D.C.; Ceinos, M.; Picón, M.J.; Marcos, A.; Nova, E. Effects of Moringa oleifera Lam. Supplementation on Inflammatory and Cardiometabolic Markers in Subjects with Prediabetes. Nutrients 2022, 14, 1937. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.C.; Tan, C.S.; Ch’ng, Y.S.; Yeap, Z.Q.; Ng, C.H.; Yam, M.F. Overview of the Microenvironment of Vasculature in Vascular Tone Regulation. Int. J. Mol. Sci. 2018, 19, 120. [Google Scholar] [CrossRef]
- Randriamboavonjy, J.I.; Heurtebise, S.; Pacaud, P.; Loirand, G.; Tesse, A. Moringa oleifera seeds Improve Aging-Related Endothelial Dysfunction in Wistar Rats. Oxid. Med. Cell. Longev. 2019, 2019, 2567198. [Google Scholar] [CrossRef] [PubMed]
- Kukongviriyapan, U.; Kukongviriyapan, V.; Pannangpetch, P.; Donpunha, W.; Sripui, J.; Sae-Eaw, A.; Boonla, O. Mamao Pomace Extract Alleviates Hypertension and Oxidative Stress in Nitric Oxide Deficient Rats. Nutrients 2015, 7, 6179–6194. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zou, M.H. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation 2009, 120, 1266–1286. [Google Scholar] [CrossRef]
- Randriamboavonjy, J.I.; Rio, M.; Pacaud, P.; Loirand, G.; Tesse, A. Moringa oleifera seeds Attenuate Vascular Oxidative and Nitrosative Stresses in Spontaneously Hypertensive Rats. Oxid. Med. Cell. Longev. 2017, 2017, 4129459. [Google Scholar] [CrossRef]
- Aekthammarat, D.; Pannangpetch, P.; Tangsucharit, P. Moringa oleifera leaf extract induces vasorelaxation via endothelium-dependent hyperpolarization and calcium channel blockade in mesenteric arterial beds isolated from L-NAME hypertensive rats. Clin. Exp. Hypertens. 2020, 42, 490–501. [Google Scholar] [CrossRef]
- Aekthammarat, D.; Tangsucharit, P.; Pannangpetch, P. Hydrogen sulfide as a mediator of endothelium-dependent relaxation evoked by Moringa oleifera leaf extract in mesenteric arterial beds isolated from L-NAME hypertensive rats. J. Complement. Integr. Med. 2020, 18, 287–293. [Google Scholar] [CrossRef]
- Ma, K.; Wang, Y.; Wang, M.; Wang, Z.; Wang, X.; Ju, X.; He, R. Antihypertensive activity of the ACE–renin inhibitory peptide derived from Moringa oleifera protein. Food Funct. 2021, 12, 8994–9006. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.-u.-H. Isolation and structure elucidation of novel hypotensive agents, niazinin A, niazinin B, niazimicin and niaziminin A + B from Moringa oleifera: The first naturally occurring thiocarbamates. J. Chem. Soc. Perkin Trans. 1992, 1, 3237–3241. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.H. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J. Nat. Prod. 1994, 57, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.-u.-H. Novel hypotensive agents, niazimin A, niazimin B, Niazicin A and Niazicin B from Moringa oleifera: Isolation of first naturally occurring carbamates. J. Chem. Soc. Perkin Trans. 1994, 1, 3035–3040. [Google Scholar] [CrossRef]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.H. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry 1995, 38, 957–963. [Google Scholar] [CrossRef]
- Gilani, A.H.; Aftab, K.; Suria, A.; Siddiqui, S.; Salem, R.; Siddiqui, B.S.; Faizi, S. Pharmacological studies on hypotensive and spasmolytic activities of pure compounds from Moringa oleifera. Phytother. Res. 1994, 8, 87–91. [Google Scholar] [CrossRef]
- Foster, M.C.; Hwang, S.J.; Massaro, J.M.; Hoffmann, U.; DeBoer, I.H.; Robins, S.J.; Vasan, R.S.; Fox, C.S. Association of subcutaneous and visceral adiposity with albuminuria: The Framingham Heart Study. Obesity 2011, 19, 1284–1289. [Google Scholar] [CrossRef]

| Studies | Part of Moringa | Experimental Model | Results |
|---|---|---|---|
| Abrogoua et al. [34] | Aqueous extract of Bidens pilosa and fresh leaves of M. oleifera | Healthy rabbits were treated with increasing doses of the traditional dietary supplement (5 × 10−8 to 5 × 10−2 mg/kg bw) or acetylcholine. | The extract produced a dose-dependent hypotensive effect, reducing blood pressure by 7.14 ± 4% to 100 ± 7.5% compared to normal levels in rabbits. |
| Aekthammarat et al. [35] | Aqueous leaf extract | Six male Wistar rats received acetylcholine (control), followed by leaf extract (1, 3, 10, 30 mg/kg). Six male Wistar rats were pretreated with L-NAME, followed by the same leaf extract doses. | Acute intravenous injection of M. oleifera extract caused a dose-dependent reduction in the mean arterial pressure in the normotensive rats, with reductions of 12.87 ± 1.45%, 21.52 ± 1.27%, 27.83 ± 0.64%, and 29.87 ± 2.34%, respectively. Significant differences were observed at all doses compared to 1 mg/kg. L-NAME pretreatment significantly reduced the BP-lowering effect of the extract, with reductions of 2.31 ± 0.61%, 6.09 ± 0.78%, 8.43 ± 1.23%, and 8.57 ± 1.43% at the respective doses. |
| Faizi et al. [36] | Ethanolic and hot water extracts and relative fractions and isolates of pods, seeds, pulp, and leaves | Normotensive Wistar rats were treated with fractions (10 and 30 mg/kd) and isolates (3 and 10 mg/kg) of M. oleifera pod, seed, pulp and leaf extract. | Ethanolic extracts of M. oleifera pods and seeds reduced blood pressure by 41% and 43% at 30 mg/kg. The aqueous fraction was inactive. The neutral and acidic sub-fractions of the ethyl acetate phase reduced pressure by 37.6% and 25.5%. Isolated compounds also lowered blood pressure. Hot water extracts of pods and leaves showed similar effects at 30 mg/kg. |
| Attakpa et al. [37] | Aqueous leaf extract | A total of 20 Wistar–Kyoto normotensive rats (WKYs) and 20 spontaneously hypertensive rats (SHRs) followed a control diet for 16 weeks, then were divided into four groups and treated for 8 weeks. Group 1 was treated only with diet. Groups 2 to 4 received a control diet + M. oleifera leaf extract (200, 400, and 600 mg/kg). | The extract reduced BP in the SHR rats in a dose-dependent manner. Significant differences were found between group 2 and group 3, but not between group 3 and group 4. No effects were seen in the normotensive rats. |
| Aekthammarat et al. [38] | Aqueous leaf extract | A total of 48 male Wistar rats were split into 6 groups. Groups 1 and 2 were normal rats, while groups 3 to 6 were L-NAME-induced hypertensive rats. They were treated daily for 3 weeks with the following:
| Daily intragastrically administration of M. oleifera leaf extract decreased the SBP in a dose-dependent manner in the L-NAME hypertensiverats. The highest dose resulted in a lowering of pressure as early as one week into treatment. M. oleifera extract (60 mg/kg/day) did not affect blood pressure or heart rate in the normal rats. |
| Acuram et al. [39] | Methanolic and ethyl acetate leaf extracts | A total of 35 female ICR mice were divided into 7 groups and treated for 25 days:
| Methanolic extract reduced SBP from 102.35 ± 1.55 mm Hg to 90.97 ± 0.80 mm Hg. The ethanolic extract significantly reduced SBP to values comparable to those in the control group. No significant difference in BP was observed at either dosage (0.01 g/kg/day and 0.3 g/kg/day) |
| Chen et al. [40] | Leaf extract | Acute study: A total of 48 male Wistar rats (26 control and 22 with monocrotaline-induced pulmonary hypertension) were treated for 3 weeks with 1.5, 4.5, or 15 mg/kg of M. oleifera extract. Chronic study: A total of 22 male Wistar rats (7 control and 15 with monocrotaline-induced pulmonary hypertension) were treated for 3 weeks. On days 14–20, the control group received saline injection, and half of the monocrotaline-induced hypertensive rats received 4.5 mg/kg of M. oleifera extract. | Acute study: In the control rats, no significant difference was found in pulmonary arterial pressure at any dose. In the monocrotaline-induced hypertensive group, pulmonary arterial pressure decreased after Moringa extract administration. The 4.5 mg/kg dose resulted in an 80% reduction in pulmonary arterial pressure. Chronic study: Compared to the control group, MCT administration increased pulmonary arterial pressure. Repeated administrations of Moringa extract during the last week significantly reversed the monocrotaline-induced hypertension to a level similar to that of the control group. |
| Randriamboavonjy et al. [41] | Seed powder | A total of 12 spontaneous hypertensive rats were treated for 8 weeks. The experimental group received M. oleifera seed powder mixed into food (750 mg/d/rat), while the control group received normal food. | The treatment had no effect on diurnal and nocturnal SBP and DBP in the hypertensive rats. The treatment reduced the nocturnal heart rate during wakefulness. |
| Category | Mechanism | Effect | Final Result | |
|---|---|---|---|---|
| Endothelium-Dependent | (+) eNOS | (+) NO biosynthesis | (+) Vasorelaxation | |
| (+) Protein kinase B (AKT) | ||||
| (-) Arginase-1 | ||||
| (-) Reactive oxygen species (ROS) | (-) oxidative stress | (+) NO availability | ||
| (+) Superoxide dismutase (SOD) | ||||
| (+) Catalase (CAT) | ||||
| (-) NADPH oxidase | ||||
| (+) Endothelium-derived hyperpolarizing factors (EDHF) bioavailability | Hyperpolarization of VSMC | |||
| Non-Endothelium-Dependent | (-) Receptor-operated calcium channels (ROCCs) | (-) Intracellular Ca2+ in VSMC | ||
| (-) Voltage-operated calcium channels (VOCCs) | ||||
| (-) Inositol trisphosphate (IP3) receptor | ||||
| Systemic Regulatory Mechanisms | Inhibition of the renin–angiotensin–aldosterone system (RAAS) | (-) Systemic vascular resistance | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menichetti, F.; Berteotti, C.; Schirinzi, V.; Poli, C.; Arrighi, R.; Leone, A. Moringa oleifera and Blood Pressure: Evidence and Potential Mechanisms. Nutrients 2025, 17, 1258. https://doi.org/10.3390/nu17071258
Menichetti F, Berteotti C, Schirinzi V, Poli C, Arrighi R, Leone A. Moringa oleifera and Blood Pressure: Evidence and Potential Mechanisms. Nutrients. 2025; 17(7):1258. https://doi.org/10.3390/nu17071258
Chicago/Turabian StyleMenichetti, Francesca, Chiara Berteotti, Vittoria Schirinzi, Carolina Poli, Roberta Arrighi, and Alessandro Leone. 2025. "Moringa oleifera and Blood Pressure: Evidence and Potential Mechanisms" Nutrients 17, no. 7: 1258. https://doi.org/10.3390/nu17071258
APA StyleMenichetti, F., Berteotti, C., Schirinzi, V., Poli, C., Arrighi, R., & Leone, A. (2025). Moringa oleifera and Blood Pressure: Evidence and Potential Mechanisms. Nutrients, 17(7), 1258. https://doi.org/10.3390/nu17071258









