Biological Effect of Mycosporine-Gly-Ser (Shinorine) Against Bis-Retinoid N-Retinyl-N-Retinylidene Ethanolamine- and Blue-Light-Induced Retinal Pigment Epithelium Cell Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Cytotoxocity
2.2. Fluorescence-Activated Cell-Sorting (FACS) Assay
2.3. TUNEL Assay
2.4. Western Blot
2.5. ELISA Analysis
2.6. Immunofluorescence (IF) Analysis
2.7. Statistical Analysis
3. Results and Discussion
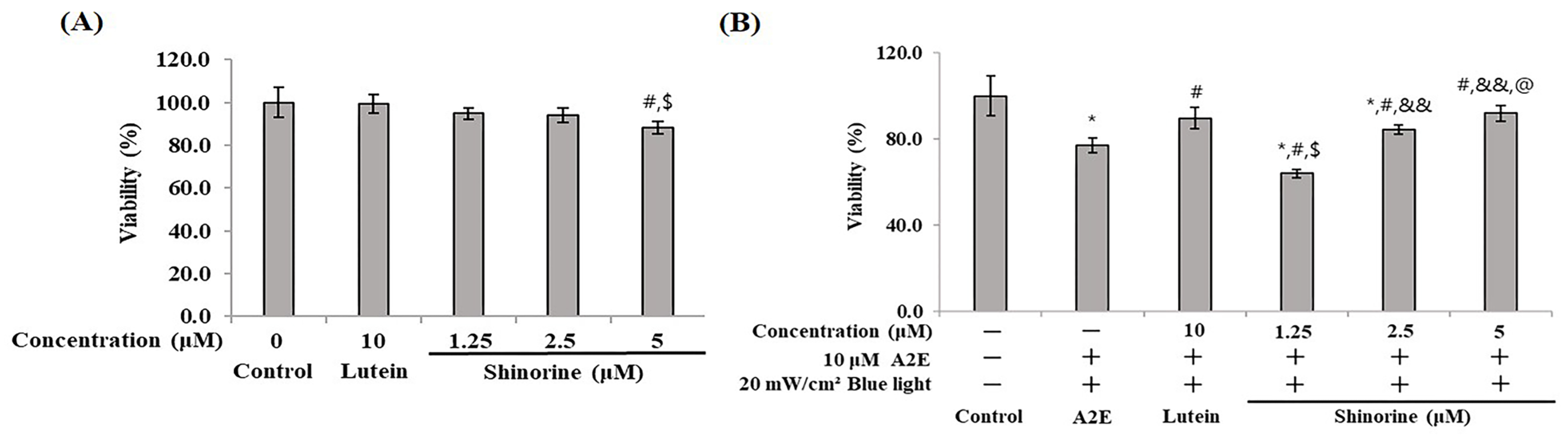
3.1. Cytotoxicity
3.2. Shinorine Regulated the Apoptotic Factors in ARPE-19
3.3. Shinorine Regulated the p38 and JNK in ARPE-19
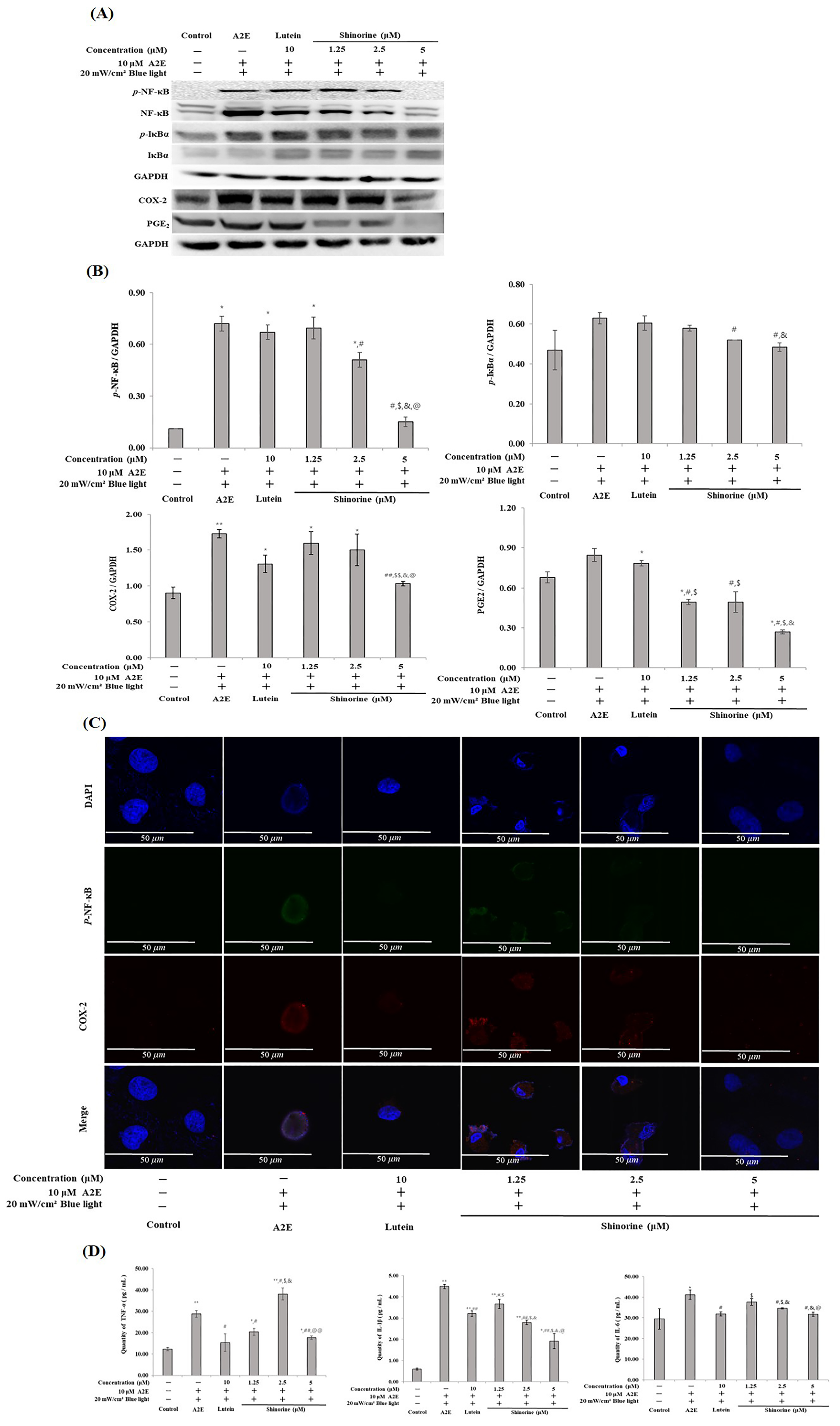
3.4. Shinorine Regulated the Inflammatory Response in ARPE-19 Cells
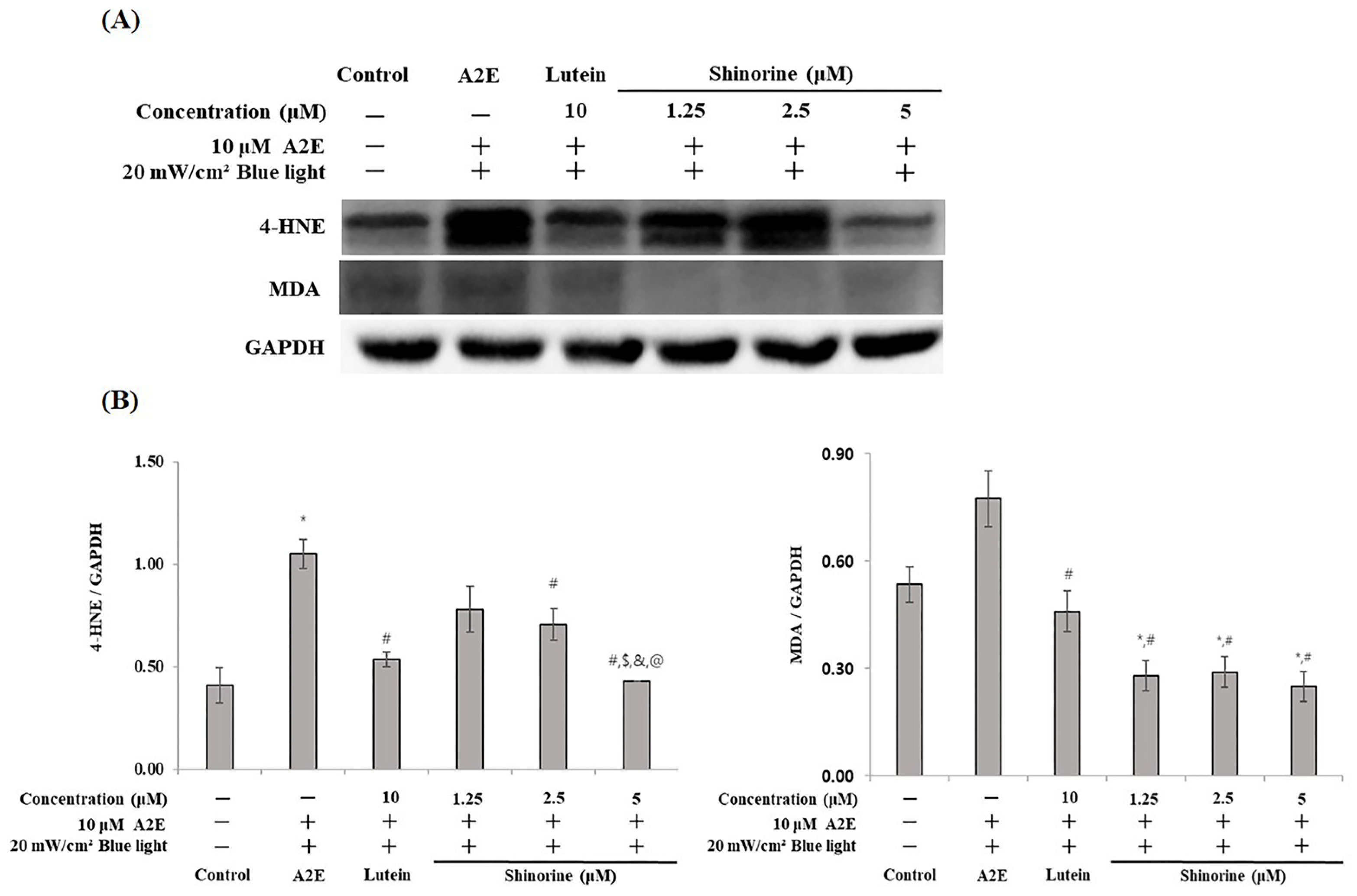
3.5. Shinorine Regulated the Carbonyl Stress Induced by A2E and BL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, J.; Guo, F.; Liu, S.; Fang, H.; Xu, Z.; Wang, T. Recent Advances and Future Prospects of Mycosporine-like Amino Acids. Molecules 2023, 28, 5588. [Google Scholar] [CrossRef] [PubMed]
- Ficek, D.; Dera, J.; Woźniak, B. UV absorption reveals mycosporine-like amino acids (MAAs) in Tatra mountain lake phytoplankton. Oceanologia 2013, 55, 599–609. [Google Scholar] [CrossRef]
- Babele, P.K.; Singh, G.; Singh, A.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. UV-B radiation and temperature stress-induced alterations in metabolic events and defense mechanisms in a bloom-forming cyanobacterium Microcystis aeruginosa. Acta Physiol. Plant. 2017, 39, 248. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of Collagenase by Mycosporine-like Amino Acids from Marine Sources. Planta Medica 2015, 81, 813–820. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.; Labidi, J.; Bulone, V. Exploiting Mycosporines as Natural Molecular Sunscreens for the Fabrication of UV-Absorbing Green Materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564. [Google Scholar] [CrossRef]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Becker, K.; Hartmann, A.; Ganzera, M.; Fuchs, D.; Gostner, J.M. Immunomodulatory Effects of the Mycosporine-Like Amino Acids Shinorine and Porphyra-334. Mar. Drugs 2016, 14, 119. [Google Scholar] [CrossRef]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 112, 321–328. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yang, D.J.; Kulkarni, A.; Moh, S.H.; Kim, K.W. Mycosporine-Like Amino Acids Promote Wound Healing through Focal Adhesion Kinase (FAK) and Mitogen-Activated Protein Kinases (MAP Kinases) Signaling Pathway in Keratinocytes. Mar. Drugs 2015, 13, 7055–7066. [Google Scholar] [CrossRef] [PubMed]
- Carignan, M.O.; Cardozo, K.H.; Oliveira-Silva, D.; Colepicolo, P.; Carreto, J.I. Palythine-threonine, a major novel mycosporine-like amino acid (MAA) isolated from the hermatypic coral Pocillopora capitata. J. Photochem. Photobiol. B Biol. 2009, 94, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P. Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol. Adv. 2009, 27, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Kim, S.; Moon, S.; Jin, H.; Hahn, J.S. Efficient production of shinorine, a natural sunscreen material, from glucose and xylose by deleting HXK2 encoding hexokinase in Saccharomyces cerevisiae. FEMS Yeast Res. 2021, 21, foab053. [Google Scholar] [CrossRef]
- Chidi-Egboka, N.C.; Jalbert, I.; Golebiowski, B. Smartphone gaming induces dry eye symptoms and reduces blinking in school-aged children. Eye 2023, 37, 1342–1349. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Zhou, Y.; Tan, G.; Li, J. Research progress about the effect and prevention of blue light on eyes. Int. J. Ophthalmol. 2018, 11, 1999–2003. [Google Scholar] [CrossRef]
- Theruveethi, N.; Bui, B.V.; Joshi, M.B.; Valiathan, M.; Ganeshrao, S.B.; Gopalakrishnan, S.; Kabekkodu, S.P.; Bhat, S.S.; Surendran, S. Blue Light-Induced Retinal Neuronal Injury and Amelioration by Commercially Available Blue Light-Blocking Lenses. Life 2022, 12, 243. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Zhang, D.; Wen, Y.; Zhang, L.; Xia, Y.; Chen, J.; Xie, C.; Zhu, H.; Tong, J.; et al. Long-term blue light exposure impairs mitochondrial dynamics in the retina in light-induced retinal degeneration in vivo and in vitro. J. Photochem. Photobiol. B Biol. 2023, 240, 112654. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, S.Y.; Kim, H.G.; Jeong, J.C.; Batara, D.C.; Kim, S.H.; Cho, J.Y. Shinorine and porphyra-334 isolated from laver (Porphyra dentata) inhibit adipogenesis in 3T3-L1 cells. Food Sci. Biotechnol. 2022, 31, 617–625. [Google Scholar] [CrossRef]
- Song, S.Y.; Park, D.H.; Lee, S.H.; Lim, H.K.; Park, J.W.; Seo, J.W.; Cho, S.S. Protective Effects of 7S,15R-Dihydroxy-16S,17S-Epoxy-Docosapentaenoic Acid (diHEP-DPA) against Blue Light-Induced Retinal Damages in A2E-Laden ARPE-19 Cells. Antioxidants 2024, 13, 982. [Google Scholar] [CrossRef]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical damage of the retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Agrón, E.; Mares, J.; Clemons, T.E.; Swaroop, A.; Chew, E.Y.; Keenan, T.D.L. Dietary Nutrient Intake and Progression to Late Age-Related Macular Degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology 2021, 128, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-D.; Kim, J.; Choung, S.-Y. Protective effects of quercetin-3-O-α-l-arabinopyranoside against UVA induced apoptosis via regulating inflammatory pathways in ARPE-19 cells and Balb/c mice. J. Funct. Foods 2019, 62, 103541. [Google Scholar] [CrossRef]
- Kasanah, N.; Ulfah, M.; Imania, O.; Hanifah, A.N.; Marjan, M.I.D. Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application. Molecules 2022, 27, 7788. [Google Scholar] [CrossRef]
- Marie, M.; Gondouin, P.; Pagan, D.; Barrau, C.; Villette, T.; Sahel, J.; Picaud, S. Blue-violet light decreases VEGFa production in an in vitro model of AMD. PLoS ONE 2019, 14, e0223839. [Google Scholar] [CrossRef]
- Kyosseva, S.V. Targeting MAPK Signaling in Age-Related Macular Degeneration. Ophthalmol. Eye Dis. 2016, 8, 23–30. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Roduit, R.; Schorderet, D.F. MAP kinase pathways in UV-induced apoptosis of retinal pigment epithelium ARPE19 cells. Apoptosis 2008, 13, 343–353. [Google Scholar] [CrossRef]
- Tsao, Y.P.; Ho, T.C.; Chen, S.L.; Cheng, H.C. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sci. 2006, 79, 545–550. [Google Scholar] [CrossRef]
- Sawyer, R.T. Thrombolytics and Anticoagulants from Leeches. Bio/Technology 1991, 9, 513–518. [Google Scholar] [CrossRef]
- Jürgensmeier, J.M.; Xie, Z.; Deveraux, Q.; Ellerby, L.; Bredesen, D.; Reed, J.C. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 1998, 95, 4997–5002. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Zhou, J.; Ben-Shabat, S.; Vollmer, H.; Itagaki, Y.; Nakanishi, K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1222–1227. [Google Scholar]
- Fang, Y.; Su, T.; Qiu, X.; Mao, P.; Xu, Y.; Hu, Z.; Zhang, Y.; Zheng, X.; Xie, P.; Liu, Q. Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death. Sci. Rep. 2016, 6, 21018. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, D.; Wang, V.M.; Yu, C.R.; Wang, R.X.; Tuo, J.; Chan, C.C. Enhanced apoptosis in retinal pigment epithelium under inflammatory stimuli and oxidative stress. Apoptosis 2012, 17, 1144–1155. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Guillonneau, X.; Eandi, C.M.; Paques, M.; Sahel, J.A.; Sapieha, P.; Sennlaub, F. On phagocytes and macular degeneration. Prog. Retin. Eye Res. 2017, 61, 98–128. [Google Scholar] [CrossRef]
- Yang, H.J.; Hu, R.; Sun, H.; Bo, C.; Li, X.; Chen, J.B. 4-HNE induces proinflammatory cytokines of human retinal pigment epithelial cells by promoting extracellular efflux of HSP70. Exp. Eye Res. 2019, 188, 107792. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.-Y.; Cho, J.-Y.; Park, D.-H.; Song, S.-H.; Lee, S.-H.; Park, J.-W.; Lim, H.-K.; Cho, S.-S. Biological Effect of Mycosporine-Gly-Ser (Shinorine) Against Bis-Retinoid N-Retinyl-N-Retinylidene Ethanolamine- and Blue-Light-Induced Retinal Pigment Epithelium Cell Damage. Nutrients 2025, 17, 1363. https://doi.org/10.3390/nu17081363
Song S-Y, Cho J-Y, Park D-H, Song S-H, Lee S-H, Park J-W, Lim H-K, Cho S-S. Biological Effect of Mycosporine-Gly-Ser (Shinorine) Against Bis-Retinoid N-Retinyl-N-Retinylidene Ethanolamine- and Blue-Light-Induced Retinal Pigment Epithelium Cell Damage. Nutrients. 2025; 17(8):1363. https://doi.org/10.3390/nu17081363
Chicago/Turabian StyleSong, Seung-Yub, Jeong-Yong Cho, Dae-Hun Park, Si-Hun Song, Sung-Ho Lee, Jin-Woo Park, Han-Kyu Lim, and Seung-Sik Cho. 2025. "Biological Effect of Mycosporine-Gly-Ser (Shinorine) Against Bis-Retinoid N-Retinyl-N-Retinylidene Ethanolamine- and Blue-Light-Induced Retinal Pigment Epithelium Cell Damage" Nutrients 17, no. 8: 1363. https://doi.org/10.3390/nu17081363
APA StyleSong, S.-Y., Cho, J.-Y., Park, D.-H., Song, S.-H., Lee, S.-H., Park, J.-W., Lim, H.-K., & Cho, S.-S. (2025). Biological Effect of Mycosporine-Gly-Ser (Shinorine) Against Bis-Retinoid N-Retinyl-N-Retinylidene Ethanolamine- and Blue-Light-Induced Retinal Pigment Epithelium Cell Damage. Nutrients, 17(8), 1363. https://doi.org/10.3390/nu17081363









