Beyond the Gut: Unveiling Butyrate’s Global Health Impact Through Gut Health and Dysbiosis-Related Conditions: A Narrative Review
Abstract
1. Introduction
1.1. Introduction to Short-Chain Fatty Acids
1.2. Introduction to Butyrate
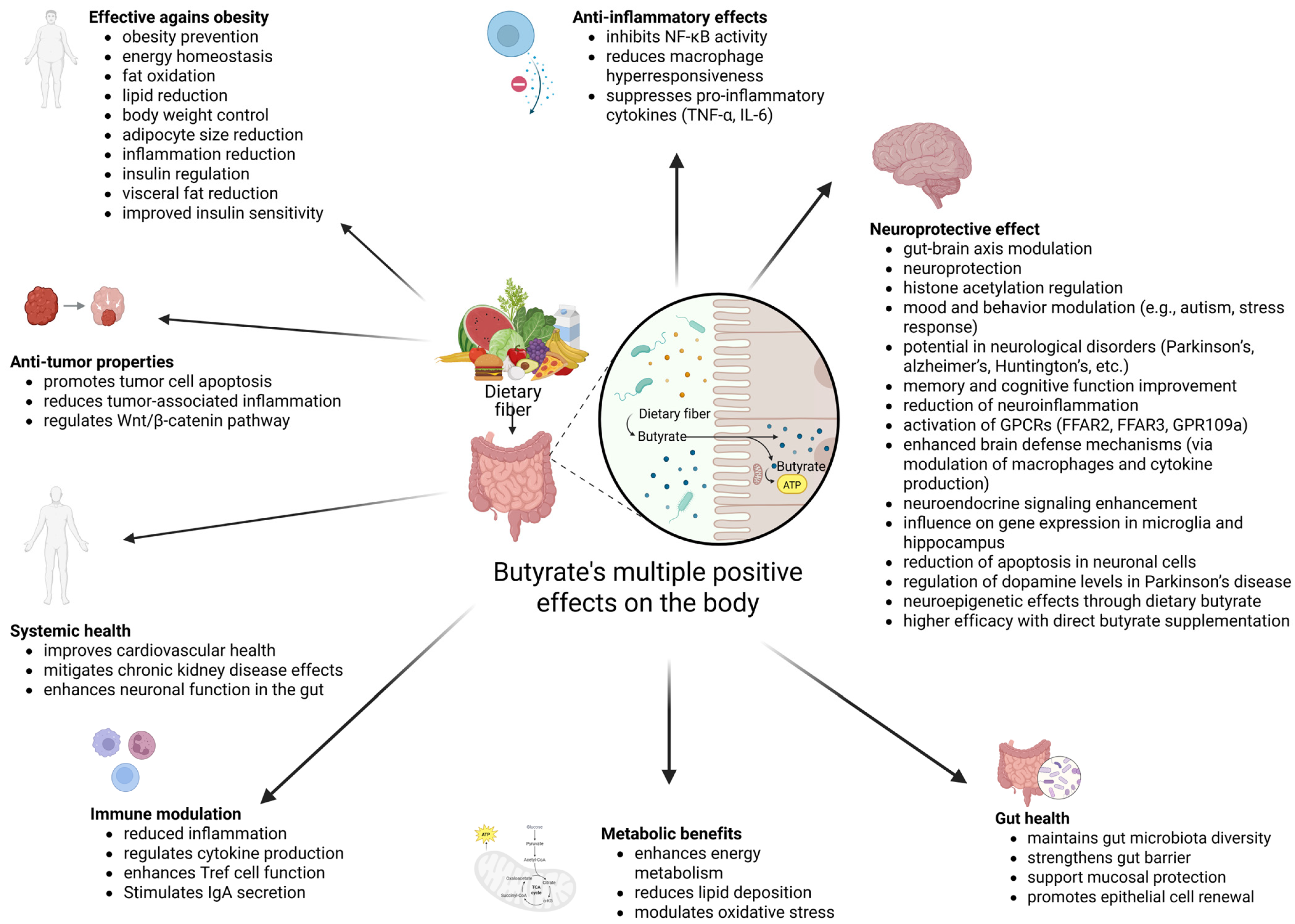
2. Butyrate and Gut Health
2.1. Gut Health
2.2. Butyrate as a Postbiotic and Butyrate–Gut Health Relationship
3. Butyrate’s Relation with Obesity
4. Butyrate as a Neurological Health Enhancer
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, J.; Xie, F.; He, H.; Johnston, L.J.; Dai, X.; Wu, C.; Ma, X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 2021, 22, e13316. [Google Scholar] [CrossRef]
- Høverstad, T. Studies of Short-Chain Fatty Acid Absorption in Man. Scand. J. Gastroenterol. 1986, 21, 257–260. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Is butyrate the link between diet, intestinal microbiota and obesity-related metabolic diseases? Obes. Rev. 2013, 14, 950–959. [Google Scholar] [CrossRef]
- Richards, J.L.; Yap, Y.-A.; McLeod, K.H.; Mackay, C.R.; Mariño, E. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clin. Transl. Immunol. 2016, 5, e82. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Ashaolu, J.O.; Adeyeye, S.A. Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: A critical review. J. Appl. Microbiol. 2021, 130, 677–687. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; Broek, T.V.D.; Schuren, F.H.J.; Steinert, R.E.; Raederstorff, D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- Fei, Y.; Chen, Z.; Han, S.; Zhang, S.; Zhang, T.; Lu, Y.; Berglund, B.; Xiao, H.; Li, L.; Yao, M. Role of prebiotics in enhancing the function of next-generation probiotics in gut microbiota. Crit. Rev. Food Sci. Nutr. 2023, 63, 1037–1054. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Elfadil, O.M.; Mundi, M.S.; Abdelmagid, M.G.; Patel, A.; Patel, N.; Martindale, R. Butyrate: More Than a Short Chain Fatty Acid. Curr. Nutr. Rep. 2023, 12, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Jacobi, S.K.; Odle, J. Nutritional factors influencing intestinal health of the neonate. Adv. Nutr. Int. Rev. J. 2012, 3, 687–696. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Barba-Ostria, C.; Simancas-Racines, D.; Guamán, L.P. Protective role of butyrate in obesity and diabetes: New insights. Front. Nutr. 2022, 9, 1067647. [Google Scholar] [CrossRef]
- USDA. Agricultural Research Service. USDA National Nutrient Database for Standard Reference. 2004. Available online: https://scholar.google.com/scholar_lookup?&journal=USDA+National+Nutrient+Database+for+Standard+Reference%2E+USDA+National+Nutrient+Database+for+Standard+Reference%2E&publication_year=2019 (accessed on 9 September 2024).
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Matt, S.M.; Allen, J.M.; Lawson, M.A.; Mailing, L.J.; Woods, J.A.; Johnson, R.W. Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated With Aging in Mice. Front. Immunol. 2018, 9, 1832. [Google Scholar] [CrossRef]
- Brouns, F.; Kettlitz, B.; Arrigoni, E. Resistant starch and “the butyrate revolution”. Trends Food Sci. Technol. 2002, 13, 251–261. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Lærke, H.N. REVIEW: Rye Arabinoxylans: Molecular Structure, Physicochemical Properties and Physiological Effects in the Gastrointestinal Tract. Cereal Chem. 2010, 87, 353–362. [Google Scholar] [CrossRef]
- Knudsen, K.E.B. Microbial Degradation of Whole-Grain Complex Carbohydrates and Impact on Short-Chain Fatty Acids and Health. Adv. Nutr. Int. Rev. J. 2015, 6, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Langley, B.; D’Annibale, M.A.; Suh, K.; Ayoub, I.; Tolhurst, A.; Bastan, B.; Yang, L.; Ko, B.; Fisher, M.; Cho, S.; et al. Pulse Inhibition of Histone Deacetylases Induces Complete Resistance to Oxidative Death in Cortical Neurons without Toxicity and Reveals a Role for Cytoplasmic p21waf1/cip1 in Cell Cycle-Independent Neuroprotection. J. Neurosci. 2008, 28, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, C.; Kumar, C.; Gnad, F.; Nielsen, M.L.; Rehman, M.; Walther, T.C.; Olsen, J.V.; Mann, M. Lysine Acetylation Targets Protein Complexes and Co-Regulates Major Cellular Functions. Science 2009, 325, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.A.; Richon, V.M.; Rifkind, R.A. Histone deacetylase inhibitors: Inducers of differentiation or apoptosis of transformed cells. JNCI J. Natl. Cancer Inst. 2000, 92, 1210–1216. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Bischoff, S.C. ‘Gut health’: A new objective in medicine? BMC Med. 2011, 9, 24. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Reichardt, N.; Duncan, S.H.; Young, P.; Belenguer, A.; McWilliam Leitch, C.; Scott, K.P.; Flint, H.J.; Louis, P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014, 8, 1323–1335. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.R.; Davenport, E.R.; Gautam, Y.; Bhandari, D.; Tandukar, S.; Ng, K.M.; Fragiadakis, G.K.; Holmes, S.; Gautam, G.P.; Leach, J.; et al. Gut microbiome transition across a lifestyle gradient in Himalaya. PLoS Biol. 2018, 16, e2005396. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Sung, J.; Kim, S.; Cabatbat, J.J.T.; Jang, S.; Jin, Y.-S.; Jung, G.Y.; Chia, N.; Kim, P.-J. Global metabolic interaction network of the human gut microbiota for context-specific community-scale analysis. Nat. Commun. 2017, 8, 15393. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401–407. [Google Scholar] [CrossRef]
- Antharam, V.C.; Li, E.C.; Ishmael, A.; Sharma, A.; Mai, V.; Rand, K.H.; Wang, G.P. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J. Clin. Microbiol. 2013, 51, 2884–2892. [Google Scholar] [CrossRef]
- Pinto, D.; Clevers, H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp. Cell Res. 2005, 306, 357–363. [Google Scholar] [CrossRef]
- Bjerknes, M.; Cheng, H. Gastrointestinal Stem Cells. II. Intestinal stem cells. Am. J. Physiol. Liver Physiol. 2005, 289, G381–G387. [Google Scholar] [CrossRef] [PubMed]
- Shaoul, R.; Okada, Y.; Cutz, E.; Marcon, M.A. Colonic expression of MUC2, MUC5AC, and TFF1 in inflammatory bowel disease in children. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Strader, A.D.; Woods, S.C. Gastrointestinal hormones and food intake. Gastroenterology 2005, 128, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Wehkamp, J.; Fellermann, K.; Stange, E.F. Human defensins in Crohn’s disease. Chem. Immunol. Allergy 2005, 86, 42–54. [Google Scholar] [CrossRef]
- de Medina, F.S.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal Inflammation and Mucosal Barrier Function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Hering, N.A.; Fromm, M.; Schulzke, J. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J. Physiol. 2012, 590, 1035–1044. [Google Scholar] [CrossRef]
- Müller, C.A.; Autenrieth, I.B.; Peschel, A. Innate defenses of the intestinal epithelial barrier. Cell. Mol. Life Sci. 2005, 62, 1297–1307. [Google Scholar] [CrossRef]
- Kelly, P.; Bajaj-Elliott, M.; Katubulushi, M.; Zulu, I.; Poulsom, R.; Feldman, R.A.; Bevins, C.L.; Dhaliwal, W. Reduced gene expression of intestinal α-defensins predicts diarrhea in a cohort of African adults. J. Infect. Dis. 2006, 193, 1464–1470. [Google Scholar] [CrossRef]
- Schauber, J.; Rieger, D.; Weiler, F.; Wehkamp, J.; Eck, M.; Fellermann, K.; Scheppach, W.; Gallo, R.L.; Stange, E.F. Heterogeneous expression of human cathelicidin hCAP18/LL-37 in inflammatory bowel diseases. Eur. J. Gastroenterol. Hepatol. 2006, 18, 615–621. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.-Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.M.; Rauch, M.; Gilmore, M.S. The commensal microbiology of the gastrointestinal tract. Adv. Exp. Med. Biol. 2008, 635, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martin, R.; Rossi, O.; Bermudez-Humaran, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889-14. [Google Scholar] [CrossRef]
- Altemani, F.; Barrett, H.L.; Gomez-Arango, L.; Josh, P.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Tyson, G.W.; Nitert, M.D. Pregnant women who develop preeclampsia have lower abundance of the butyrate-producer Coprococcus in their gut microbiota. Pregnancy Hypertens. 2021, 23, 211–219. [Google Scholar] [CrossRef]
- Vital, M.; Penton, C.R.; Wang, Q.; Young, V.B.; Antonopoulos, D.A.; Sogin, M.L.; Morrison, H.G.; Raffals, L.; Chang, E.B.; Huffnagle, G.B.; et al. A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome 2013, 1, 8. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, Y.; Kang, S.; You, H.J.; Ji, G.E. Co-Culture with Bifidobacterium catenulatum Improves the Growth, Gut Colonization, and Butyrate Production of Faecalibacterium prausnitzii: In Vitro and In Vivo Studies. Microorganisms 2020, 8, 788. [Google Scholar] [CrossRef]
- Nedjadi, T.; Moran, A.W.; Al-Rammahi, M.A.; Shirazi-Beechey, S.P. Characterization of butyrate transport across the luminal membranes of equine large intestine. Exp. Physiol. 2014, 99, 1335–1347. [Google Scholar] [CrossRef]
- Takebe, K.; Nio, J.; Morimatsu, M.; Karaki, S.-I.; Kuwahara, A.; Kato, I.; Iwanaga, T. Histochemical demonstration of a Na+-coupled transporter for short-chain fatty acids (Slc5a8) in the intestine and kidney of the mouse. Biomed. Res. 2005, 26, 213–221. [Google Scholar] [CrossRef][Green Version]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.-M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci. Rep. 2019, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Isobe, J.; Maeda, S.; Obata, Y.; Iizuka, K.; Nakamura, Y.; Fujimura, Y.; Kimizuka, T.; Hattori, K.; Kim, Y.-G.; Morita, T.; et al. Commensal-bacteria-derived butyrate promotes the T-cell-independent IgA response in the colon. Int. Immunol. 2020, 32, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.T.; Han, Y.; Shapiro, D.; West, G.; Fiocchi, C.; Cresci, G.A.M. The Postbiotic Butyrate Mitigates Gut Mucosal Disruption Caused by Acute Ethanol Exposure. Int. J. Mol. Sci. 2024, 25, 1665. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Q.; Dorfman, R.G.; Huang, X.; Fan, T.; Zhang, H.; Zhang, J.; Yu, C. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016, 16, 84. [Google Scholar] [CrossRef]
- Kibbie, J.J.; Dillon, S.M.; Thompson, T.A.; Purba, C.M.; McCarter, M.D.; Wilson, C.C. Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling. Immunobiology 2021, 226, 152126. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e437. [Google Scholar] [CrossRef]
- Jukic, A.; Bakiri, L.; Wagner, E.F.; Tilg, H.; Adolph, T.E. Calprotectin: From biomarker to biological function. Gut 2021, 70, 1978–1988. [Google Scholar] [CrossRef]
- Yin, L.; Laevsky, G.; Giardina, C. Butyrate Suppression of Colonocyte NF-κB Activation and Cellular Proteasome Activity. J. Biol. Chem. 2001, 276, 44641–44646. [Google Scholar] [CrossRef]
- Segain, J.-P.; de la Blétière, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.-P. Butyrate inhibits inflammatory responses through NFkappa B inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, B.S.; Venkataraman, S.; Srinivasan, P.; Dash, P.; Young, G.P.; Binder, H.J. Amylase-resistant starch plus oral rehydration solution for cholera. N. Engl. J. Med. 2000, 342, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Henen, M.A.; Lee, J.S.; Vögeli, B.; Colgan, S.P. Microbiota-derived butyrate is an endogenous HIF prolyl hydroxylase inhibitor. Gut Microbes 2021, 13, 1938380. [Google Scholar] [CrossRef]
- Wang, R.X.; Lee, J.S.; Campbell, E.L.; Colgan, S.P. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc. Natl. Acad. Sci. USA 2020, 117, 11648–11657. [Google Scholar] [CrossRef]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014, 99, 352–360. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Zamani, S.; Taslimi, R.; Sarabi, A.; Jasemi, S.; Sechi, L.A.; Feizabadi, M.M. Enterotoxigenic Bacteroides fragilis: A Possible Etiological Candidate for Bacterially-Induced Colorectal Precancerous and Cancerous Lesions. Front. Cell. Infect. Microbiol. 2020, 9, 449. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; Van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef]
- Meeker, S.; Seamons, A.; Paik, J.; Treuting, P.M.; Brabb, T.; Grady, W.M.; Maggio-Prince, L. Increased Dietary Vitamin D Suppresses MAPK Signaling, Colitis, and Colon Cancer. Cancer Res. 2014, 74, 4398–4408. [Google Scholar] [CrossRef]
- Perillo, F.; Amoroso, C.; Strati, F.; Giuffrè, M.R.; Díaz-Basabe, A.; Lattanzi, G.; Facciotti, F. Gut Microbiota Manipulation as a Tool for Colorectal Cancer Management: Recent Advances in Its Use for Therapeutic Purposes. Int. J. Mol. Sci. 2020, 21, 5389. [Google Scholar] [CrossRef]
- Parisi, A.; Porzio, G.; Pulcini, F.; Cannita, K.; Ficorella, C.; Mattei, V.; Monache, S.D. What Is Known about Theragnostic Strategies in Colorectal Cancer. Biomedicines 2021, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Alvandi, E.; Wong, W.K.M.; Joglekar, M.V.; Spring, K.J.; Hardikar, A.A. Short-chain fatty acid concentrations in the incidence and risk-stratification of colorectal cancer: A systematic review and meta-analysis. BMC Med. 2022, 20, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-Y.; Mei, J.-X.; Yu, G.; Lei, L.; Zhang, W.-H.; Liu, K.; Chen, X.-L.; Kołat, D.; Yang, K.; Hu, J.-K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Islam, F.; -Or-Rashid, H.; Al Mamun, A.; Rahaman, S.; Islam, M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin Degrader Akkermansia muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, S.T.N.S.J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Yi, Y.; Lu, W.; Shen, L.; Wu, Y.; Zhang, Z. The gut microbiota as a booster for radiotherapy: Novel insights into radio-protection and radiation injury. Exp. Hematol. Oncol. 2023, 12, 48. [Google Scholar] [CrossRef]
- Ding, X.; Li, Q.; Li, P.; Chen, X.; Xiang, L.; Bi, L.; Zhu, J.; Huang, X.; Cui, B.; Zhang, F. Fecal microbiota transplantation: A promising treatment for radiation enteritis? Radiother. Oncol. 2020, 143, 12–18. [Google Scholar] [CrossRef]
- Ryu, S.H.; Stappenbeck, T.S. Gut-Pancreatic Axis AMPlified in Islets of Langerhans. Immunity 2015, 43, 216–218. [Google Scholar] [CrossRef][Green Version]
- Yang, C.; Hu, T.; Xue, X.; Su, X.; Zhang, X.; Fan, Y.; Shen, X.; Dong, X. Multi-omics analysis of fecal microbiota transplantation’s impact on functional constipation and comorbid depression and anxiety. BMC Microbiol. 2023, 23, 389. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.M.; Kahn, S.A.; Delmont, T.O.; Shaiber, A.; Esen, Ö.C.; Hubert, N.A.; Morrison, H.G.; Antonopoulos, D.A.; Rubin, D.T.; Eren, A.M. Tracking microbial colonization in fecal microbiota transplantation experiments via genome-resolved metagenomics. Microbiome 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hu, J.; Yi, X.; Lv, J.; Yao, J.; Tang, W.; Zhang, S.; Wan, M. Gut microbiota interacts with inflammatory responses in acute pancreatitis. Ther. Adv. Gastroenterol. 2023, 16, 17562848231202133. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Patel, K.H.; Bhatia, M.; Iyer, S.G.; Madhavan, K.; Moochhala, S.M. Gut microbiome in acute pancreatitis: A review based on current literature. World J. Gastroenterol. 2021, 27, 5019–5036. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Yang, M.; Zhang, M.; Xiao, M.; Li, X. Butyrate mitigates TNF-α-induced attachment of monocytes to endothelial cells. J. Bioenerg. Biomembr. 2020, 52, 247–256. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Su, C.; Xi, M.; Zhang, X.; Jiang, Z.; Wang, L.; Hong, B. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br. J. Pharmacol. 2020, 177, 1754–1772. [Google Scholar] [CrossRef]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef]
- Vernia, P.; Marcheggiano, A.; Caprilli, R.; Frieri, G.; Corrao, G.; Valpiani, D.; DI Paolo, M.C.; Paoluzi, P.; Torsoli, A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment. Pharmacol. Ther. 1995, 9, 309–313. [Google Scholar] [CrossRef]
- Vernia, P.; Annese, V.; Bresci, G.; Giaccari, S.; Ingrosso, M.; Mansi, C.; Riegler, G.; Valpiani, D.; Caprilli, R.; GISC. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: Results of a multicentre trial. Eur. J. Clin. Investig. 2003, 33, 244–248. [Google Scholar] [CrossRef]
- Senagore, A.J.; MacKeigan, J.M.; Scheider, M.; Ebrom, S.J. Short-chain fatty acid enemas: A cost-effective alternative in the treatment of nonspecific proctosigmoiditis. Dis. Colon Rectum 1992, 35, 923–927. [Google Scholar] [CrossRef]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.-M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology 1992, 103, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Lührs, H.; Gerke, T.; Müller, J.G.; Melcher, R.; Schauber, J.; Boxberger, F.; Scheppach, W.; Menzel, T. Butyrate Inhibits NF-κB Activation in Lamina Propria Macrophages of Patients with Ulcerative Colitis. Scand. J. Gastroenterol. 2002, 37, 458–466. [Google Scholar] [CrossRef] [PubMed]
- D’incà, R.; Dal Pont, E.; Di Leo, V.; Benazzato, L.; Martinato, M.; Lamboglia, F.; Oliva, L.; Sturniolo, G.C. Can Calprotectin Predict Relapse Risk in Inflammatory Bowel Disease? Off. J. Am. Coll. Gastroenterol. 2008, 103, 2007–2014. Available online: https://journals.lww.com/ajg/fulltext/2008/08000/can_calprotectin_predict_relapse_risk_in.19.aspx?casa_token=zXA_CRzeMJwAAAAA:sOfDYgFk63i3JSlNCadAsnRUECWE3XQI-Hp60bpjG5LkfeuqW6Z_2t3rzT_n2Vj0oZ7HMa44zEaMzcGGW23zi7ofdi68M-IXZA&casa_token=Y_0TFtiN5KkAAAAA:TAmsIjzRjE-7Ez9waTSqTtirRg-1xfCjX8LmHiddlRHEW5aoWGvyZZsAzWIOdpiy2JbO3dueGbKh0XkQgZzkwJ8xag4kwjKi1g (accessed on 9 January 2025). [CrossRef] [PubMed]
- Caviglia, G.P.; Ribaldone, D.G.; Rosso, C.; Saracco, G.M.; Astegiano, M.; Pellicano, R. Fecal calprotectin: Beyond intestinal organic diseases. Panminerva Medica 2018, 60, 29–34. [Google Scholar] [CrossRef]
- Correction. J. Crohn’s Colitis 2023, 17, 149. [CrossRef]
- Ozturk, O.; Celebi, G.; Duman, U.G.; Kupcuk, E.; Uyanik, M.; Sertoglu, E. Short-chain fatty acid levels in stools of patients with inflammatory bowel disease are lower than those in healthy subjects. Eur. J. Gastroenterol. Hepatol. 2024, 36, 890–896. [Google Scholar] [CrossRef]
- Caetano, M.A.F.; Magalhães, H.I.R.; Duarte, J.R.L.; Conceição, L.B.; Castelucci, P. Butyrate Protects Myenteric Neurons Loss in Mice Following Experimental Ulcerative Colitis. Cells 2023, 12, 1672. [Google Scholar] [CrossRef]
- Li, H.-B.; Xu, M.-L.; Xu, X.-D.; Tang, Y.-Y.; Jiang, H.-L.; Li, L.; Xia, W.-J.; Cui, N.; Bai, J.; Dai, Z.-M.; et al. Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis. Circ. Res. 2022, 131, E120–E134. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- James, W.P.T. WHO recognition of the global obesity epidemic. Int. J. Obes. 2008, 32, S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Kilpi, F.; Webber, L.; Musaigner, A.; Aitsi-Selmi, A.; Marsh, T.; Rtveladze, K.; McPherson, K.; Brown, M. Alarming predictions for obesity and non-communicable diseases in the Middle East. Public Health Nutr. 2014, 17, 1078–1086. [Google Scholar] [CrossRef]
- Bessesen, D.H. Update on Obesity. J. Clin. Endocrinol. Metab. 2008, 93, 2027–2034. [Google Scholar] [CrossRef]
- Coppola, S.; Avagliano, C.; Calignano, A.; Canani, R.B. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules 2021, 26, 682. [Google Scholar] [CrossRef]
- Isolauri, E. Microbiota and Obesity. In Nestlé Nutrition Institute Workshop Series; Isolauri, E., Sherman, P.M., Walker, W.A., Eds.; S. Karger AG: Basel, Switzerland, 2017; Volume 88, pp. 95–106. [Google Scholar] [CrossRef]
- Roda, A.; Simoni, P.; Magliulo, M.; Nanni, P.; Baraldini, M.; Roda, G.; Roda, E. A new oral formulation for the release of sodium butyrate in the ileo-cecal region and colon. World J. Gastroenterol. 2007, 13, 1079–1084. [Google Scholar] [CrossRef]
- Weng, H.; Endo, K.; Li, J.; Kito, N.; Iwai, N. Induction of peroxisomes by butyrate-producing probiotics. PLoS ONE 2015, 10, e0117851. [Google Scholar] [CrossRef]
- Banasiewicz, T.; Domagalska, D.; Borycka-Kiciak, K.; Rydzewska, G. Determination of butyric acid dosage based on clinical and experimental studies—A literature review. Gastroenterol. Rev. 2020, 15, 119–125. [Google Scholar] [CrossRef]
- Karimi, G.; Vahabzadeh, M. Butyric Acid. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 597–601. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; Bleeker, A.; Gerding, A.; Van Eunen, K.; Havinga, R.; Van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet–Induced Obesity via a PPARgamma-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, C.-X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, C.; Li, P.; Lu, Y.; Chang, X.; Qi, K. Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Sci. Rep. 2016, 6, 37589. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.-D.; Li, H.-F.; Tian, M.-L.; Pan, J.-Q.; Shu, G.; Jiang, Q.-Y.; Yin, Y.-L.; Zhang, L. LSD1 mediates microbial metabolite butyrate-induced thermogenesis in brown and white adipose tissue. Metabolism 2020, 102, 154011. [Google Scholar] [CrossRef]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071–56082. [Google Scholar] [CrossRef]
- Khan, S.; Jena, G. Sodium butyrate reduces insulin-resistance, fat accumulation and dyslipidemia in type-2 diabetic rat: A comparative study with metformin. Chem. Biol. Interact. 2016, 254, 124–134. [Google Scholar] [CrossRef]
- Arnoldussen, I.A.C.; Wiesmann, M.; Pelgrim, C.E.; Wielemaker, E.M.; Van Duyvenvoorde, W.; Amaral-Santos, P.L.; Verschuren, L.; Keijser, B.J.F.; Heerschap, A.; Kleemann, R.; et al. Butyrate restores HFD-induced adaptations in brain function and metabolism in mid-adult obese mice. Int. J. Obes. 2017, 41, 935–944. [Google Scholar] [CrossRef]
- Pelgrim, C.E.; Franx, B.A.A.; Snabel, J.; Kleemann, R.; Arnoldussen, I.A.C.; Kiliaan, A.J. Butyrate Reduces HFD-Induced Adipocyte Hypertrophy and Metabolic Risk Factors in Obese LDLr-/-.Leiden Mice. Nutrients 2017, 9, 714. [Google Scholar] [CrossRef]
- Matheus, V.A.; Monteiro, L.; Oliveira, R.B.; Maschio, D.A.; Collares-Buzato, C.B. Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice. Exp. Biol. Med. 2017, 242, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Cleophas, M.C.P.; Ratter, J.M.; Bekkering, S.; Quintin, J.; Schraa, K.; Stroes, E.S.; Netea, M.G.; Joosten, L.A.B. Effects of oral butyrate supplementation on inflammatory potential of circulating peripheral blood mononuclear cells in healthy and obese males. Sci. Rep. 2019, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4·4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- Zhang, W.-Q.; Zhao, T.-T.; Gui, D.-K.; Gao, C.-L.; Gu, J.-L.; Gan, W.-J.; Huang, W.; Xu, Y.; Zhou, H.; Chen, W.-N.; et al. Sodium Butyrate Improves Liver Glycogen Metabolism in Type 2 Diabetes Mellitus. J. Agric. Food Chem. 2019, 67, 7694–7705. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Gao, C.-L.; Guo, H.-L.; Zhang, W.-Q.; Huang, W.; Tang, S.-S.; Gan, W.-J.; Xu, Y.; Zhou, H.; Zhu, Q. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J. Endocrinol. 2018, 238, 231–244. [Google Scholar] [CrossRef]
- Bulpitt, C.J.; Palmer, A.J.; Battersby, C.; Fletcher, A.E. Association of Symptoms of Type 2 Diabetic Patients With Severity of Disease, Obesity, and Blood Pressure. Diabetes Care 1998, 21, 111–115. [Google Scholar] [CrossRef]
- Sanna, S.; Van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vila, A.V.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Reitmeier, S.; Kiessling, S.; Clavel, T.; List, M.; Almeida, E.L.; Ghosh, T.S.; Neuhaus, K.; Grallert, H.; Linseisen, J.; Skurk, T.; et al. Arrhythmic Gut Microbiome Signatures Predict Risk of Type 2 Diabetes. Cell Host Microbe 2020, 28, 258–272.e6. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef]
- Noureldein, M.H.; Bitar, S.; Youssef, N.; Azar, S.; Eid, A.A. Butyrate modulates diabetes-linked gut dysbiosis: Epigenetic and mechanistic modifications. J. Mol. Endocrinol. 2020, 64, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.X.; Lee, J. Dietary Regulation of Histone Acetylases and Deacetylases for the Prevention of Metabolic Diseases. Nutrients 2012, 4, 1868–1886. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jena, G.; Tikoo, K. Sodium valproate ameliorates diabetes-induced fibrosis and renal damage by the inhibition of histone deacetylases in diabetic rat. Exp. Mol. Pathol. 2015, 98, 230–239. [Google Scholar] [CrossRef]
- Chen, J.; Wang, N.; Dong, M.; Guo, M.; Zhao, Y.; Zhuo, Z.; Zhang, C.; Chi, X.; Pan, Y.; Jiang, J.; et al. The Metabolic Regulator Histone Deacetylase 9 Contributes to Glucose Homeostasis Abnormality Induced by Hepatitis C Virus Infection. Diabetes 2015, 64, 4088–4098. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- Gao, F.; Lv, Y.-W.; Long, J.; Chen, J.-M.; He, J.-M.; Ruan, X.-Z.; Zhu, H.-B. Butyrate Improves the Metabolic Disorder and Gut Microbiome Dysbiosis in Mice Induced by a High-Fat Diet. Front. Pharmacol. 2019, 10, 1040. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Li, J.; Wang, X.; Zhang, M.; Du, M.; Jiang, W.; Li, C. Butyrate and Propionate are Negatively Correlated with Obesity and Glucose Levels in Patients with Type 2 Diabetes and Obesity. Diabetes Metab. Syndr. Obesity Targets Ther. 2024, 17, 1533–1541. [Google Scholar] [CrossRef]
- Tilves, C.; Yeh, H.; Maruthur, N.; Juraschek, S.P.; Miller, E.; White, K.; Appel, L.J.; Mueller, N.T. Increases in Circulating and Fecal Butyrate are Associated With Reduced Blood Pressure and Hypertension: Results From the SPIRIT Trial. J. Am. Heart Assoc. 2022, 11, e024763. [Google Scholar] [CrossRef]
- Lastra, G.; Syed, S.; Kurukulasuriya, L.R.; Manrique, C.; Sowers, J.R. Type 2 Diabetes Mellitus and Hypertension. Endocrinol. Metab. Clin. N. Am. 2014, 43, 103–122. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Sun, M.-F.; Shen, Y.-Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Selkrig, J.; Wong, P.; Zhang, X.; Pettersson, S. Metabolic tinkering by the gut microbiome: Implications for brain development and function. Gut Microbes 2014, 5, 369–380. [Google Scholar] [CrossRef]
- Stilling, R.M.; Dinan, T.G.; Cryan, J.F. Microbial genes, brain & behaviour—Epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar] [CrossRef]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L., Jr. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Rasmussen, H.E.; Hamaker, B.R. Potential of Prebiotic Butyrogenic Fibers in Parkinson’s Disease. Front. Neurol. 2019, 10, 663. [Google Scholar] [CrossRef]
- D’souza, W.N.; Douangpanya, J.; Mu, S.; Jaeckel, P.; Zhang, M.; Maxwell, J.R.; Rottman, J.B.; Labitzke, K.; Willee, A.; Beckmann, H.; et al. Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses. PLoS ONE 2017, 12, e0180190. [Google Scholar] [CrossRef] [PubMed]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef]
- Thangaraju, M.; Cresci, G.A.; Liu, K.; Ananth, S.; Gnanaprakasam, J.P.; Browning, D.D.; Mellinger, J.D.; Smith, S.B.; Digby, G.J.; Lambert, N.A.; et al. GPR109A Is a G-protein–Coupled Receptor for the Bacterial Fermentation Product Butyrate and Functions as a Tumor Suppressor in Colon. Cancer Res. 2009, 69, 2826–2832. [Google Scholar] [CrossRef]
- Wakade, C.; Chong, R.; Bradley, E.; Thomas, B.; Morgan, J. Upregulation of GPR109A in Parkinson’s Disease. PLoS ONE 2014, 9, e109818. [Google Scholar] [CrossRef]
- Fu, S.-P.; Wang, J.-F.; Xue, W.-J.; Liu, H.-M.; Liu, B.-R.; Zeng, Y.-L.; Li, S.-N.; Huang, B.-X.; Lv, Q.-K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflammation 2015, 12, 9. [Google Scholar] [CrossRef]
- Won, Y.-J.; Lu, V.B.; Puhl, H.L.; Ikeda, S.R. β-Hydroxybutyrate Modulates N-Type Calcium Channels in Rat Sympathetic Neurons by Acting as an Agonist for the G-Protein-Coupled Receptor FFA3. J. Neurosci. 2013, 33, 19314–19325. [Google Scholar] [CrossRef]
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 2007, 62, 55–64. [Google Scholar] [CrossRef]
- Braat, S.; Kooy, R.F. The GABAA Receptor as a Therapeutic Target for Neurodevelopmental Disorders. Neuron 2015, 86, 1119–1130. [Google Scholar] [CrossRef]
- Ni, Y.-F.; Wang, J.; Yan, X.-L.; Tian, F.; Zhao, J.-B.; Wang, Y.-J.; Jiang, T. Histone deacetylase inhibitor, butyrate, attenuates lipopolysaccharide-induced acute lung injury in mice. Respir. Res. 2010, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Dalgard, C.L.; Wynder, C.; Doughty, M.L. Histone deacetylase inhibitors SAHA and sodium butyrate block G1-to-S cell cycle progression in neurosphere formation by adult subventricular cells. BMC Neurosci. 2011, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Kratsman, N.; Getselter, D.; Elliott, E. Sodium butyrate attenuates social behavior deficits and modifies the transcription of inhibitory/excitatory genes in the frontal cortex of an autism model. Neuropharmacology 2016, 102, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, H.; Delgado-Morales, R.; Sanz-Garcia, A.; Armario, A. High doses of the histone deacetylase inhibitor sodium butyrate trigger a stress-like response. Neuropharmacology 2014, 79, 75–82. [Google Scholar] [CrossRef]
- Cuervo-Zanatta, D.; Syeda, T.; Sánchez-Valle, V.; Irene-Fierro, M.; Torres-Aguilar, P.; Torres-Ramos, M.A.; Shibayama-Salas, M.; Silva-Olivares, A.; Noriega, L.G.; Torres, N.; et al. Dietary Fiber Modulates the Release of Gut Bacterial Products Preventing Cognitive Decline in an Alzheimer’s Mouse Model. Cell. Mol. Neurobiol. 2023, 43, 1595–1618. [Google Scholar] [CrossRef]
- Kilgore, M.; Miller, C.A.; Fass, D.M.; Hennig, K.M.; Haggarty, S.J.; Sweatt, J.D.; Rumbaugh, G. Inhibitors of Class 1 Histone Deacetylases Reverse Contextual Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar] [CrossRef]
- Govindarajan, N.; Agis-Balboa, R.C.; Walter, J.; Sananbenesi, F.; Fischer, A. Sodium Butyrate Improves Memory Function in an Alzheimer’s Disease Mouse Model When Administered at an Advanced Stage of Disease Progression. J. Alzheimer’s Dis. 2011, 26, 187–197. [Google Scholar] [CrossRef]
- Sharma, S.; Taliyan, R.; Singh, S. Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: Modulation of histone deacetylase activity. Behav. Brain Res. 2015, 291, 306–314. [Google Scholar] [CrossRef]
- Paiva, I.; Pinho, R.; Pavlou, M.A.; Hennion, M.; Wales, P.; Schütz, A.-L.; Rajput, A.; Szegő, É.M.; Kerimoglu, C.; Gerhardt, E.; et al. Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum. Mol. Genet. 2017, 26, 2231–2246. [Google Scholar] [CrossRef]
- Drottar, M.; Liberman, M.C.; Ratan, R.R.; Roberson, D.W. The Histone Deacetylase Inhibitor Sodium Butyrate Protects Against Cisplatin-Induced Hearing Loss in Guinea Pigs. Laryngoscope 2006, 116, 292–296. [Google Scholar] [CrossRef]
- Gardian, G.; Browne, S.E.; Choi, D.-K.; Klivenyi, P.; Gregorio, J.; Kubilus, J.K.; Ryu, H.; Langley, B.; Ratan, R.R.; Ferrante, R.J.; et al. Neuroprotective Effects of Phenylbutyrate in the N171-82Q Transgenic Mouse Model of Huntington’s Disease. J. Biol. Chem. 2005, 280, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, R.J.; Kubilus, J.K.; Lee, J.; Ryu, H.; Beesen, A.; Zucker, B.; Smith, K.; Kowall, N.W.; Ratan, R.R.; Luthi-Carter, R.; et al. Histone Deacetylase Inhibition by Sodium Butyrate Chemotherapy Ameliorates the Neurodegenerative Phenotype in Huntington’s Disease Mice. J. Neurosci. 2003, 23, 9418–9427. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Borges, J.; Valvassori, S.S.; Varela, R.B.; Tonin, P.T.; Vieira, J.S.; Gonçalves, C.L.; Streck, E.L.; Quevedo, J. Histone deacetylase inhibitors reverse manic-like behaviors and protect the rat brain from energetic metabolic alterations induced by ouabain. Pharmacol. Biochem. Behav. 2015, 128, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Valvassori, S.S.; Calixto, K.V.; Budni, J.; Resende, W.R.; Varela, R.B.; de Freitas, K.V.; Gonçalves, C.L.; Streck, E.L.; Quevedo, J. Sodium butyrate reverses the inhibition of Krebs cycle enzymes induced by amphetamine in the rat brain. J. Neural Transm. 2013, 120, 1737–1742. [Google Scholar] [CrossRef]
- Moretti, M.; Valvassori, S.S.; Varela, R.B.; Ferreira, C.L.; Rochi, N.; Benedet, J.; Scaini, G.; Kapczinski, F.; Streck, E.L.; Zugno, A.I.; et al. Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behav. Pharmacol. 2011, 22, 766–772. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Lærke, H.N.; Theil, P.K.; Sørensen, J.F.; Saarinen, M.; Forssten, S.; Knudsen, K.E.B. Diets high in resistant starch and arabinoxylan modulate digestion processes and SCFA pool size in the large intestine and faecal microbial composition in pigs. Br. J. Nutr. 2014, 112, 1837–1849. [Google Scholar] [CrossRef]
- Ingerslev, A.K.; Theil, P.K.; Hedemann, M.S.; Lærke, H.N.; Knudsen, K.E.B. Resistant starch and arabinoxylan augment SCFA absorption, but affect postprandial glucose and insulin responses differently. Br. J. Nutr. 2014, 111, 1564–1576. [Google Scholar] [CrossRef]
- Ghadirian, S.; Karbasi, S.; Kharazi, A.Z.; Setayeshmehr, M. Evaluation of the Effects of Halloysite Nanotubes on Physical, Mechanical, and Biological Properties of Polyhydroxy Butyrate Electrospun Scaffold for Cartilage Tissue Engineering Applications. J. Polym. Environ. 2024, 32, 1170–1187. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Evon, P.; Espinach, F.X.; Raynaud, C.; Méndez, J.A. Polyhydroxy-3-Butyrate (PHB)-Based Composite Materials Reinforced with Cellulosic Fibers, Obtained from Barley Waste Straw, to Produce Pieces for Agriculture Applications: Production, Characterization and Scale-Up Analysis. Materials 2024, 17, 1901. [Google Scholar] [CrossRef]
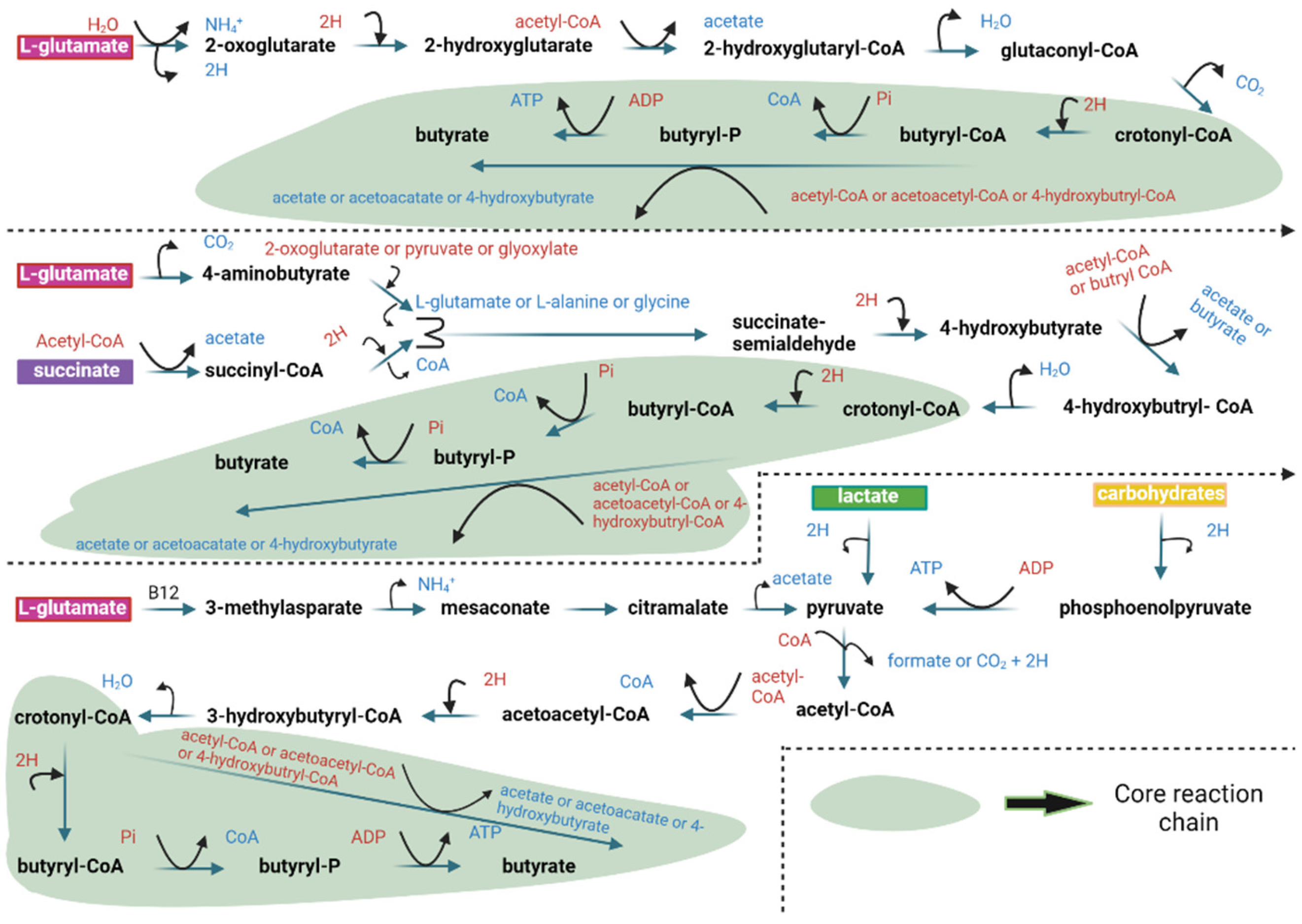
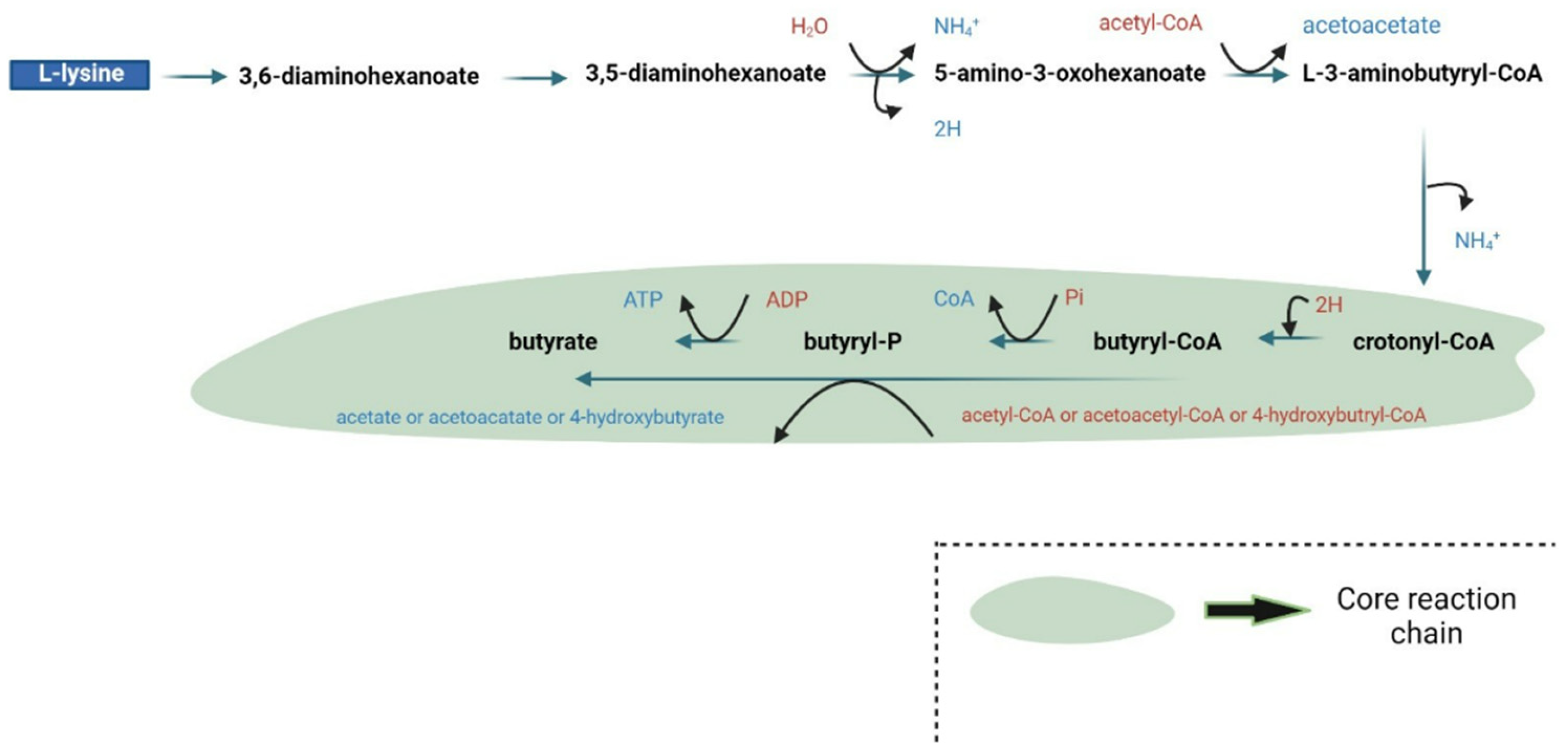
| Supplement | Microorganism | Outcome(s) | Reference |
|---|---|---|---|
| Natural butyrate producers analyzed | Firmicutes (dominant), Fusobacteria, Bacteroidetes | Identification of four main butyrate production pathways (acetyl-CoA, glutarate, 4-aminobutyrate, lysine), conservation of specific genes, and functional predictions for microbial butyrate synthesis. | [55] |
| Butyrate | Coprococcus, Roseburia, Lachnospira, Butyricimonas | Reduced circulating butyrate levels and decreased butyrate-producing bacteria abundance in developed late-onset preeclampsia women. | [56] |
| SCFA | Coprococcus (correlated with SCFA levels in controls) | Lower acetate and propionate levels trend; significant decrease in butyrate. | |
| None | Coprococcus, Bifidobacterium | Significant negative correlation between butyrate abundance and fasting triglyceride levels. | |
| Butyrate-producing community analysis | F. prausnitzii, Roseburia sp., E. rectale, Acidaminococcus sp. | Demonstrated high abundance of butyrate-producing communities (5–26%) in patients after ileostomy takedown. | [57] |
| 16S rRNA gene analysis | E. hallii, E. rectale, Subdoligranulum sp., Anaerostipes sp. | Supported functional gene data but showed discrepancies in detecting certain butyrate producers, like Eubacterium sp. | |
| F. prausnitzii alone or co-culture with Bifidobacterium | F. prausnitzii | Improved viability and gut colonization of F. prausnitzii, alleviated colitis symptoms in DSS (dextran sodium sulfate)-induced colitis model. | [58] |
| Bifidobacterium (B. catenulatum, B. animalis strains) | Bifidobacterium strains (B. catenulatum, B. animalis) | Enhanced growth and butyrate production of F. prausnitzii, improved intestinal delivery in co-culture. | |
| F. prausnitzii and Bifidobacterium co-culture | F. prausnitzii, Bifidobacterium, A. muciniphila | Increased gut colonization, particularly of F. prausnitzii, and altered microbiome composition (higher A. muciniphila abundance). | |
| Butyrate | - | Transported across equine colonic luminal membrane via MCT1 (Monocarboxylate transporter 1) by an electroneutral, H+-symport mechanism; upregulated by increased luminal butyrate concentrations. | [59] |
| Acetate, Propionate | - | Inhibited butyrate transport via competition or shared transport protein MCT1; may affect SCFA absorption under conditions of excessive lactic acid in the gut. | |
| SCFAs | - | Affect colonic cell proliferation and function as an energy source for intestinal epithelial cells. | [60] |
| Butyrate | Ruminoclostridium, Roseburia | Activation of the Aryl hydrocarbon receptor (AhR) pathway in intestinal epithelial cells. | [62] |
| Butyrylated high-amylose maize starch diet | Increased IgA production and enhanced mucosal barrier function in the colon during inflammation | Fecal microbiota from SPF (specific pathogen-free) mice (via transplantation). | [63] |
| n-Butyrate | - | Reduced secretion of IL-6, IL-12p40, and nitric oxide (NO) in bone marrow-derived macrophages (BMDM) and colonic lamina propria macrophages. | [64] |
| Tributyrin | Citrobacter rodentium | Mitigated acute antibiotic- and ethanol-induced gut microbial disturbances; reduced Gram-negative bacterial growth. | [65] |
| Roseburia hominis | Increased abundance of Roseburia hominis in antibiotic and ethanol-exposed mice. | ||
| Butyrate-treated rats | - | Ameliorated weight loss, increased colon inflammation, and lower Neurath scores. | [66] |
| Butyrate | - | Human UC patients: Significantly lower net concentration of butyric acid compared to healthy controls. TNBS-treated rats: Lower butyric acid and total SCFA concentrations compared to control rats. Butyrate administration in rats: Increased fecal concentration of butyric acid, total SCFA, and percentage of butyric acid. | [67] |
| Butyrate | C. rodentium, Salmonella | Enhanced bactericidal function of colonic macrophages and reduced systemic bacterial dissemination in C. rodentium and Salmonella infections. | [67] |
| Butyrate | - | Suppresses NF-κB activation in HT-29 cells by stabilizing IκB-α and increasing p100 levels, potentially through reduced proteasome activity. | [68] |
| Butyrate | - | Suppressed mucosal inflammation and constitutive NF-κB p50 dimer activity in HT-29 cells. | [71] |
| Butyrate | - | Enhances intestinal epithelial barrier formation (increased transepithelial electrical resistance over 72 h) | [73] |
| Butyrate | Microbiota-derived butyrate | Stabilizes hypoxia-inducible factor-1, regulates gut homeostasis genes, increases 2-OG (2-oxoglutarate) levels. | [75] |
| Butyrate | - | Significant promotion of intestinal barrier function, increased transepithelial electrical resistance, accelerated wound closure, and upregulation of synaptopodin expression (both mRNA and protein levels). | [76] |
| Focus Area | Model | Findings | Reference |
|---|---|---|---|
| Energy Expenditure and Thermogenesis | HFD mice (5% butyrate diet) | Increased AMPK activity, improved insulin sensitivity, higher energy expenditure | [123] |
| Diabetes Management | Rat models | Reduced fat accumulation, improved glucose management, lowered HDAC activity | [131] |
| Obesity and Metabolic Disorders | HFD in LDLR-KO mice | Reduced body weight, smaller adipocytes, lower inflammation, insulin regulation | [133] |
| Liver and Pancreatic Health | HFD animal models | Reduced liver steatosis, less pancreatic fat, improved β-cell function and insulin stability | [134] |
| Inflammation and Atherosclerosis | Obese individuals | Enhanced immune memory, reduced oxidized LDL, potential in vascular inflammation treatment | [135] |
| Type 2 Diabetes and Genetics | Human metagenomic and genomic data | High butyrate levels linked to better insulin response | [141] |
| Type 2 Diabetes | Human microbiota study | Disrupted bacterial rhythms in diabetic patients; potential predictive marker | [142] |
| Type 2 Diabetes and Epigenetics | Various animal models | Butyrate altered HDAC activity, reduced oxidative stress and improved gut permeability | [143,144] |
| Glucose Metabolism and Obesity | HFD mice | Improved glucose tolerance, increased AMPK and GLUT-4 expression | [149] |
| Obesity and Type 2 Diabetes | Obese and diabetic individuals | Higher butyrate levels linked to lower BMI, visceral fat, and better glucose metabolism | [150] |
| Focus Area | Model | Findings | Reference |
|---|---|---|---|
| Neuroprotection in Stroke | Mouse models | Sodium butyrate mitigated brain damage and preserved brain integrity in stroke models, although toxicity was a challenge. | [23] |
| Autism Spectrum Disorder | BTBR mice | Low dose of sodium butyrate (100 mg/kg) showed no significant histone acetylation changes but alleviated social impairments. | [173] |
| Autism Spectrum Disorder (Gene Expression) | Autistic mouse models | Sodium butyrate increased inhibitory gene expression in the frontal cortex, but low doses (100 mg/kg) had minimal effect on social impairments. | [173] |
| HPA Axis Stress Response | Mouse models | Higher dose of sodium butyrate (1200 mg/kg) induced a stress-like response in the HPA axis; lower dose (200 mg/kg) showed no effect. | [174] |
| Cognitive and Memory Improvement (Alzheimer’s) | Mouse models with a fiber-rich diet (fructans) | Increased butyrate-producing bacteria and enhanced cognitive and spatial memory with reduced anxiety. | [175] |
| Alzheimer’s Disease (HDAC Regulation) | Transgenic and wild-type mouse models | Sodium butyrate supplementation improved histone acetylation, promoted learning-related genes expression, and improved memory in transgenic models. | [176,177] |
| Parkinson’s Disease | Mouse models | Sodium butyrate alleviated motor impairments, increased dopamine levels, and reduced neuroinflammation. | [178] |
| Huntington’s Disease (Histone Acetylation) | Animal models (Huntington’s disease) | Sodium butyrate restored histone acetylation and reduced apoptosis in neuronal cells, leading to increased life expectancy. | [181,182] |
| Mitochondrial Function and Brain Activity | Animal models exhibiting mania | Sodium butyrate replenished mitochondrial complexes and counteracted Krebs cycle inhibition. | [183,184,185] |
| Gut Microbiota and Inflammation | Pigs fed a diet rich in arabinoxylan | Increased beneficial butyrate-producing bacteria (e.g., Faecalibacterium prausnitzii), indicating a positive impact on gut health. | [186,187] |
| Neuroinflammation and Cognitive Impairment | Low-density lipoprotein-receptor knockout mice (HFD) | Butyrate reduced neuroinflammation, linked to decreased brain connectivity, especially in middle-aged mice. | [132] |
| Neuroepigenetics and Diet | Animal models with high-fiber diets | Sodium butyrate from direct supplementation showed superior neuroprotective effects compared to dietary butyrate. | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalkan, A.E.; BinMowyna, M.N.; Raposo, A.; Ahmad, M.F.; Ahmed, F.; Otayf, A.Y.; Carrascosa, C.; Saraiva, A.; Karav, S. Beyond the Gut: Unveiling Butyrate’s Global Health Impact Through Gut Health and Dysbiosis-Related Conditions: A Narrative Review. Nutrients 2025, 17, 1305. https://doi.org/10.3390/nu17081305
Kalkan AE, BinMowyna MN, Raposo A, Ahmad MF, Ahmed F, Otayf AY, Carrascosa C, Saraiva A, Karav S. Beyond the Gut: Unveiling Butyrate’s Global Health Impact Through Gut Health and Dysbiosis-Related Conditions: A Narrative Review. Nutrients. 2025; 17(8):1305. https://doi.org/10.3390/nu17081305
Chicago/Turabian StyleKalkan, Arda Erkan, Mona N. BinMowyna, António Raposo, Md Faruque Ahmad, Faiyaz Ahmed, Abdullah Y. Otayf, Conrado Carrascosa, Ariana Saraiva, and Sercan Karav. 2025. "Beyond the Gut: Unveiling Butyrate’s Global Health Impact Through Gut Health and Dysbiosis-Related Conditions: A Narrative Review" Nutrients 17, no. 8: 1305. https://doi.org/10.3390/nu17081305
APA StyleKalkan, A. E., BinMowyna, M. N., Raposo, A., Ahmad, M. F., Ahmed, F., Otayf, A. Y., Carrascosa, C., Saraiva, A., & Karav, S. (2025). Beyond the Gut: Unveiling Butyrate’s Global Health Impact Through Gut Health and Dysbiosis-Related Conditions: A Narrative Review. Nutrients, 17(8), 1305. https://doi.org/10.3390/nu17081305










