Celastrol Improves Preference for a Fatty Acid, and Taste Bud and Systemic Inflammation in Diet-Induced Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Diets
2.3. Diet-Induced Obesity
2.4. Determination of Pro-Inflammatory Cytokines
2.5. Liver Cholesterol and Triglyceride Determinations
2.6. Two-Bottle Preference Test
2.7. Isolation of Mouse Taste Bud Cells
2.8. mRNA Expression by Real-Time-Quantitative PCR (RT-qPCR)
2.9. Statistical Analysis of Data
3. Results
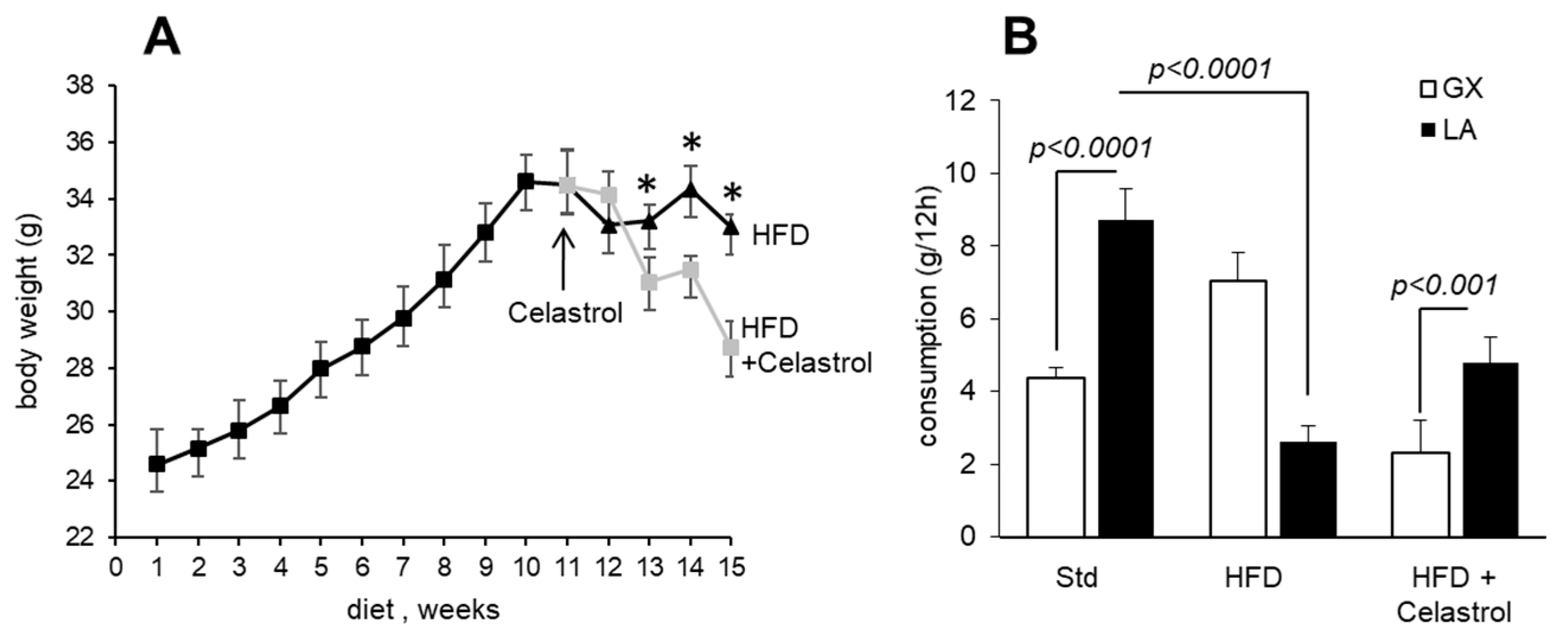
3.1. Celastrol Decreases Body Weight Gain and Modulates Fat Preference in HFD-Fed Mice
3.2. Celastrol Modulates Fat Taste Receptor and Pro-Inflammatory Cytokines mRNA Expression in HFD-Fed Mice

3.3. Celastrol Decreases the Circulating Concentrations of Pro-Inflammatory Cytokines
3.4. Celastrol Regulates Hepatic Lipid Levels and mRNA Expression of Lipid Metabolic Mediators
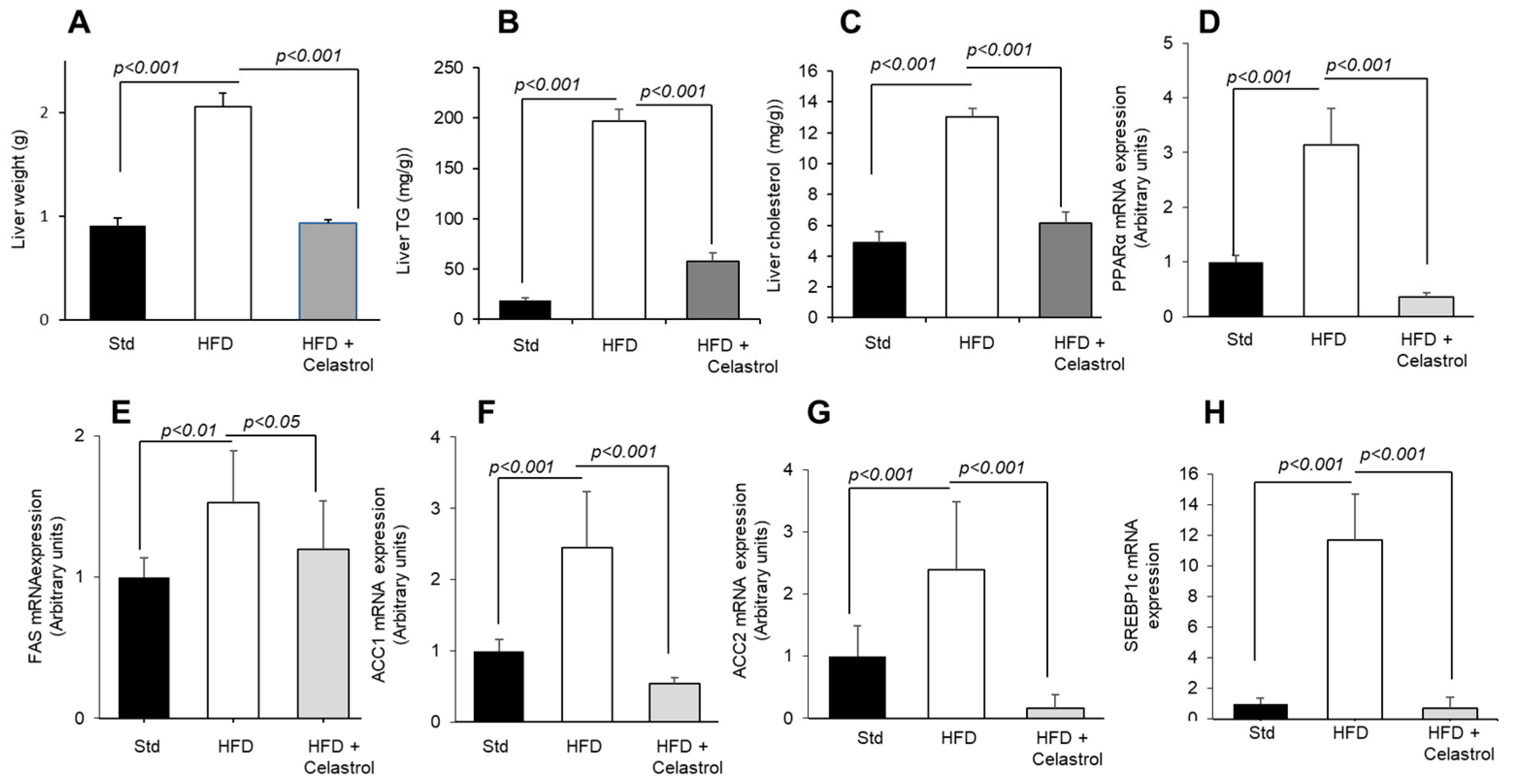
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Obesity and Overweight; WHO: Geneva, Switzerland, 2015; p. 311. [Google Scholar]
- Fukuwatari, T.; Shibata, K.; Iguchi, K.; Saeki, T.; Iwata, A.; Tani, K.; Sugimoto, E.; Fushiki, T. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol. Behav. 2003, 78, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Irmler, M.; Meyer, C.; Sachs, S.J.; Neff, F.; Hrabě de Angelis, M.; Beckers, J.; Tschöp, M.H.; Hofmann, S.M.; Ussar, S. A history of obesity leaves an inflammatory fingerprint in liver and adipose tissue. Int. J. Obes. 2018, 42, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Mela, D.J.; Sacchetti, D.A. Sensory preferences for fats: Relationships with diet and body composition. Am. J. Clin. Nutr. 1991, 53, 908–915. [Google Scholar] [CrossRef]
- Drewnowski, A.; Brunzell, J.D.; Sande, K.; Iverius, P.H.; Greenwood, M.R. Sweet tooth reconsidered: Taste responsiveness in human obesity. Physiol. Behav. 1985, 35, 617–622. [Google Scholar] [CrossRef]
- Love-Gregory, L.; Abumrad, N.A. CD36 genetics and the metabolic complications of obesity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 527–534. [Google Scholar] [CrossRef]
- Costanzo, A.; Liu, D.; Nowson, C.; Duesing, K.; Archer, N.; Bowe, S.; Keast, R. A low-fat diet up-regulates expression of fatty acid taste receptor gene FFAR4 in fungiform papillae in humans: A co-twin randomised controlled trial. Br. J. Nutr. 2019, 122, 1212–1220. [Google Scholar] [CrossRef]
- Stewart, J.E.; Feinle-Bisset, C.; Golding, M.; Delahunty, C.; Clifton, P.M.; Keast, R.S. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 2010, 104, 145–152. [Google Scholar] [CrossRef]
- Khan, A.S.; Keast, R.; Khan, N.A. Preference for dietary fat: From detection to disease. Prog. Lipid Res. 2020, 78, 101032. [Google Scholar] [CrossRef]
- Besnard, P.; Passilly-Degrace, P.; Khan, N.A. Taste of Fat: A Sixth Taste Modality? Physiol. Rev. 2016, 96, 151–176. [Google Scholar] [CrossRef]
- Subramanian, G.; Ponnusamy, V.; Vasanthakumar, K.; Panneerselvan, P.; Krishnan, V.; Subramaniam, S. The gustin gene variation at rs2274333 and PROP taster status affect dietary fat perception: A stepwise multiple regression model study. J. Nutr. Biochem. 2024, 128, 109619. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhai, Y.; Cheng, H.; Wei, W.H.; Ren, M. From Taxus to paclitaxel: Opportunities and challenges for urban agriculture to promote human health. Plant Physiol. Biochem. 2025, 220, 109502. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Wang, Y.; Zhang, X.; Yang, L.; Cai, B.; Zhang, D.; Peng, B. Characterization of global research trends and prospects on celastrol, a principal bioactive ingredient of Tripterygium wilfordii Hook F: Bibliometric analysis. Pharm. Biol. 2025, 63, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.P. Phytochemicals in Obesity Management: Mechanisms and Clinical Perspectives. Curr. Nutr. Rep. 2025, 14, 17. [Google Scholar] [CrossRef]
- Berrichi, M.; Benammar, C.; Murtaza, B.; Hichami, A.; Belarbi, M.; Khan, N.A. Zizyphus lotus L. fruit attenuates obesity-associated alterations: In vivo mechanisms. Arch. Physiol. Biochem. 2021, 127, 119–126. [Google Scholar] [CrossRef]
- Djeziri, F.Z.; Belarbi, M.; Murtaza, B.; Hichami, A.; Benammar, C.; Khan, N.A. Oleanolic acid improves diet-induced obesity by modulating fat preference and inflammation in mice. Biochimie 2018, 152, 110–120. [Google Scholar] [CrossRef]
- Murtaza, B.; Berrichi, M.; Bennamar, C.; Tordjmann, T.; Djeziri, F.Z.; Hichami, A.; Leemput, J.; Belarbi, M.; Ozdener, H.; Khan, N.A. Zizyphin modulates calcium signalling in human taste bud cells and fat taste perception in the mouse. Fundam. Clin. Pharmacol. 2017, 31, 486–494. [Google Scholar] [CrossRef]
- Bensalema, A.; Murtazaa, B.; Hichamia, A.; Khan, A.S.; Oulamarab, H.; Merlenc, G.; Berrichi, M.; Aglib, A.N.; Tordjmannc, T.; Khan, N.A. Bile acid receptor TGR5 is critically involved in preference for dietary lipids and obesity. J. Nutri. Biochem. 2020, 76, 108–298. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Zhou, D.; Li, W. Biosynthesis, total synthesis, structural modifications, bioactivity, and mechanism of action of the quinone-methide triterpenoid celastrol. Med. Res. Rev. 2021, 41, 1022–1060. [Google Scholar] [CrossRef]
- Wu, S.; Sun, C.; Wang, K.; Pan, Y. Preparative isolation and purification of celastrol from Celastrus orbiculatus Thunb. by a new counter-current chromatography method with an upright coil planet centrifuge. J. Chromatogr. A 2004, 1028, 171–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, X.; Li, W.; Chen, W.; Wang, X.; Ma, Z.; Lin, N. Tripterygium wilfordii: An inspiring resource for rheumatoid arthritis treatment. Med. Res. Rev. 2020, 14, 1337–1374. [Google Scholar] [CrossRef] [PubMed]
- Dharambir, K.; Ajay, S.; Singh, T.H.; Katrin, S.; Tapan, M.; Anupam, P. Molecular targets of celastrol in cancer: Recent trends and advancements. Crit. Rev. Oncol. Hematol. 2018, 128, 70–81. [Google Scholar]
- Liu, J.; Lee, J.; Salazar Hernandez, M.A.; Mazitschek, R.; Ozcan, U. Treatment of obesity with celastrol. Cell 2015, 161, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sureda, V.; Peinado-Onsurbe, J. A procedure for measuring triacylglyceride and cholesterol content using a small amount of tissue. Anal. Biochem. 2005, 343, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef]
- Goedeke, L.; Bates, J.; Vatner, D.F.; Perry, R.J.; Wang, T.; Ramirez, R.; Li, L.; Ellis, M.W.; Zhang, D.; Wong, K.E.; et al. Acetyl-CoA Carboxylase Inhibition Reverses NAFLD and Hepatic Insulin Resistance but Promotes Hypertriglyceridemia in Rodents. Hepatology 2018, 68, 2197–2211. [Google Scholar] [CrossRef]
- Mansouri, R.M.; Baugé, E.; Staels, B.; Gervois, P. Systemic and distal repercussions of liver-specific peroxisome proliferator-activated receptor-alpha control of the acute-phase response. Endocrinology 2008, 149, 3215–3223. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, D.H.; Bang, E.; Noh, S.G.; Chun, P.; Yokozawa, T.; Moon, H.R.; Chung, H.Y. PPARα Agonist, MHY3200, Alleviates Renal Inflammation during Aging via Regulating ROS/Akt/FoxO1 Signaling. Molecules 2021, 26, 3197. [Google Scholar] [CrossRef]
- Lan, G.C.; Zhang, J.; Ye, W.B.; Yang, F.; Li, A.; He, W.W.; Zhang, W. Celastrol as a tool for the study of the biological events of metabolic diseases. Sci. China Chem. 2019, 62, 409–416. [Google Scholar] [CrossRef]
- Hu, W.; Wang, L.; Du, G.; Guan, Q.; Dong, T.; Song, L.; Xia, Y.; Wang, X. Effects of Microbiota on the Treatment of Obesity with the Natural Product Celastrol in Rats. Diabetes Metab. J. 2020, 44, 747–763. [Google Scholar] [CrossRef]
- Jaime-Lara, R.B.; Brooks, B.E.; Vizioli, C.; Chiles, M.; Nawal, N.; Ortiz-Figueroa, R.S.E.; Livinski, A.A.; Agarwal, K.; Colina-Prisco, C.; Iannarino, N.; et al. A systematic review of the biological mediators of fat taste and smell. Physiol. Rev. 2023, 103, 855–918. [Google Scholar] [CrossRef] [PubMed]
- Ahart, Z.C.; Martin, L.E.; Kemp, B.R.; Dutta Banik, D.; Roberts, S.G.E.; Torregrossa, A.M.; Medler, K.F. Differential Effects of Diet and Weight on Taste Responses in Diet-Induced Obese Mice. Obesity 2020, 28, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Boothe, K.; He, L.; Shi, Y.; McCluskey, L.P. Altered peripheral taste function in a mouse model of inflammatory bowel disease. Sci. Rep. 2023, 13, 18895. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Hichami, A.; Murtaza, B.; Louillat-Habermeyer, M.L.; Ramseyer, C.; Azadi, M.; Yesylevskyy, S.; Mangin, F.; Lirussi, F.; Leemput, J.; et al. Novel Fat Taste Receptor Agonists Curtail Progressive Weight Gain in Obese Male Mice. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 633–663. [Google Scholar] [CrossRef]
- Feng, P.; Chai, J.; Yi, H.; Redding, K.; Margolskee, R.F.; Huang, L.; Wang, H. Aggravated gut inflammation in mice lacking the taste signaling protein α-gustducin. Brain Behav. Immun. 2018, 71, 23–27. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, M.; Brand, J.; Huang, L. Inflammation and taste disorders: Mechanisms in taste buds. Ann. NY Acad. Sci. 2009, 1170, 596–603. [Google Scholar] [CrossRef]
- Hichami, A.; Saidi, H.; Khan, A.S.; Degbeni, P.; Khan, N.A. In Vitro Functional Characterization of Type-I Taste Bud Cells as Monocytes/Macrophages-like Which Secrete Proinflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 10325. [Google Scholar] [CrossRef]
- Cohn, Z.J.; Kim, A.; Huang, L.; Brand, J.; Wang, H. Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells. BMC Neurosci. 2010, 11, 72. [Google Scholar] [CrossRef]
- Feng, P.; Zhao, H.; Chai, J.; Huang, L.; Wang, H. Expression and secretion of TNF-α in mouse taste buds: A novel function of a specific subset of type II taste cells. PLoS ONE 2012, 7, e35588. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.H.; Yu, G.Y.; He, G.; Ali, S.R.; Holzer, R.G.; Osterreicher, C.H.; Takahashi, H.; Karin, M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adiposevexpression of tumor necrosis factor- in human obesity and insulin resistance. J. Clin. Invest. 1995, 95, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Noh, H.M.; Choi, B.; Park, J.E.; Kim, J.E.; Jang, Y.; Lee, H.K.; Chang, E.-J. Interleukin-22 induces the infiltration of visceral fat tissue by a discrete subset of Duffy antigen receptor for chemokine-positive M2-like macrophages in response to a high fat diet. Cells 2019, 8, 587. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Budavari, A.; Murray, D.; Spiegelman, B.M. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J. Clin. Investig. 1994, 94, 1543–1549. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous adipose tissue releases interleukin-6 but not tumour necrosis factor-α, in vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar]
- Bastard, J.P.; Maachi, M.; Van Nhieu, J.T.; Jardel, C.; Bruckert, E.; Grimaldi, A.; Robert, J.-J.; Capeau, J.; Hainque, B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J. Clin. Endocrinol. Metab. 2000, 87, 2084–2089. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Gu, J.; Chen, C.; Duanmu, J.; Miao, J.; Yao, W.; Tao, J.; Tu, M.; Xiong, B.; et al. Celastrol exerts anti-inflammatory effect in liver fibrosis via activation of AMPK-SIRT3 signalling. J. Cell Mol. Med. 2020, 24, 941–953. [Google Scholar] [CrossRef]
- Inoue, M.; Ohtake, T.; Motomura, W.; Takahashi, N.; Hosoki, Y.; Miyoshi, S.; Suzuki, Y.; Saito, H.; Kohgo, Y.; Okumura, T. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem. Biophys. Res. Commun. 2005, 336, 215–222. [Google Scholar] [CrossRef]
- Knight, B.L.; Hebbachi, A.; Hauton, D.; Brown, A.M.; Wiggins, D.; Patel, D.D.; Gibbons, G.F. A role for PPARalpha in the control of SREBP activity and lipid synthesis in the liver. Biochem. J. 2005, 89, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Fan, N.; Zhang, X.; Ngo, F.Y.; Zhao, J.; Zhao, W.; Huang, M.; Li, D.; Wang, Y.; Rong, J. Covalent inhibition of endoplasmic reticulum chaperone GRP78 disconnects the transduction of ER stress signals to inflammation and lipid accumulation in diet-induced obese mice. Elife 2022, 11, e72182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.; Shi, C.; Yang, X.; Yang, M.; Sun, H.; Wang, C. Celastrol suppresses obesity process via increasing antioxidant capacity and improving lipid metabolism. Eur. J. Pharmacol. 2014, 744, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.H.; Nor Shahril, N.S.; Mohamad Khalid, M.S.F.; Mohammad, S.; Shariff, K.A.; Karunakaran, T.; Mohd Salleh, R.; Mohamad Rosdi, M.N. Celastrol alleviates high-fat diet-induced obesity via enhanced muscle glucose utilization and mitochondrial oxidative metabolism-mediated upregulation of pyruvate dehydrogenase complex. Toxicol. Appl. Pharmacol. 2022, 449, 116099. [Google Scholar] [CrossRef]
| Fatty Acids (g/100 g) | STD | HFD |
|---|---|---|
| SFA | 0.56 | 15.42 |
| MUFA | 0.78 | 13.46 |
| PUFA | 1.62 | 4.75 |
| Composition (g/100 g) | STD | HFD |
|---|---|---|
| Starch | 66.8 | 40.07 |
| Proteins | 16.10 | 14.6 |
| Fats | 3.10 | 35.3 |
| Cholesterol | - | 0.03 |
| Cellulose | 3.9 | 2.7 |
| Vitamins | 5.0 | 3.4 |
| Minerals | 5.1 | 3.9 |
| Energy (Kcal 100 g) | 359.5 | 536.65 |
| Fat Energy (% of total Energy) | 8.0 | 60.0 |
| Gene | Primer Sequence |
|---|---|
| Beta-Actin | Forward: TGTTACCAACTGGGACGACA |
| Reverse: CTGGGTCATCTTTTCACGGT | |
| Gustducin | Forward: ACACATTGCAGTCCATCCTAGC |
| Reverse: ATCACCATCTTCTAGTGTATTTGCC | |
| CD36 | Forward: ATGGGCTGTGATCGGAACTG |
| Reverse: TTTGCCACGTCATCTGGGTTT | |
| GPR120 | Forward: GTGCCGGGACTGGTCATTGTG |
| Reverse: TTGTTGGGACACTCGGATCTGG | |
| IL-1β | Forward: CACAGCAGCACATCAACAAG |
| Reverse: GTGCTCATGTCCTCATCCTG | |
| IL-6 | Forward: CCGCTATGAAGTTCCTCTCTGC |
| Reverse: ATCCTCTGTGAAGTCTCCTCTCC | |
| TNF-α | Forward: CCCTCACACTCAGATCATCTTCT |
| Reverse: GCTACGACGTGGGCTACAG | |
| PPARα | Forward: AGAGCCCCATCTGTCCTCTC |
| Reverse: ACTGGTAGTCTGCAAAACCAAA | |
| SREBP1c | Forward: CCCACCTCAAACCTGGATCT |
| Reverse: AAGCAGCAAGATGTCCTCCT | |
| FAS | Forward: GGCTCTATGGATTACCCAAGC |
| Reverse: CCAGTGTTCGTTCCTCGGA | |
| ACC1 | Forward: CGGACCTTTGAAGATTTTGTGAGG |
| Reverse: GCTTTATTCTGCTGGGTGAACTCTC | |
| ACC2 | Forward: GGAAGCAGGCACACATCAAGA |
| Reverse: CGGGAGGAGTTCTGGAAGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benmouna, M.; Benammar, C.; Khan, A.S.; Djeziri, F.Z.; Hichami, A.; Khan, N.A. Celastrol Improves Preference for a Fatty Acid, and Taste Bud and Systemic Inflammation in Diet-Induced Obese Mice. Nutrients 2025, 17, 1308. https://doi.org/10.3390/nu17081308
Benmouna M, Benammar C, Khan AS, Djeziri FZ, Hichami A, Khan NA. Celastrol Improves Preference for a Fatty Acid, and Taste Bud and Systemic Inflammation in Diet-Induced Obese Mice. Nutrients. 2025; 17(8):1308. https://doi.org/10.3390/nu17081308
Chicago/Turabian StyleBenmouna, Manal, Chahid Benammar, Amira Sayed Khan, Fatima Zohra Djeziri, Aziz Hichami, and Naim A. Khan. 2025. "Celastrol Improves Preference for a Fatty Acid, and Taste Bud and Systemic Inflammation in Diet-Induced Obese Mice" Nutrients 17, no. 8: 1308. https://doi.org/10.3390/nu17081308
APA StyleBenmouna, M., Benammar, C., Khan, A. S., Djeziri, F. Z., Hichami, A., & Khan, N. A. (2025). Celastrol Improves Preference for a Fatty Acid, and Taste Bud and Systemic Inflammation in Diet-Induced Obese Mice. Nutrients, 17(8), 1308. https://doi.org/10.3390/nu17081308








