Lion’s Mane Mushroom (Hericium erinaceus): A Neuroprotective Fungus with Antioxidant, Anti-Inflammatory, and Antimicrobial Potential—A Narrative Review
Abstract
:1. Introduction
2. Methods
3. Characteristics, Habitat, and Chemical Composition of H. erinaceus
3.1. Taxonomy and Morphology
3.2. Habitat and Cultivation Methods of H. erinaceus
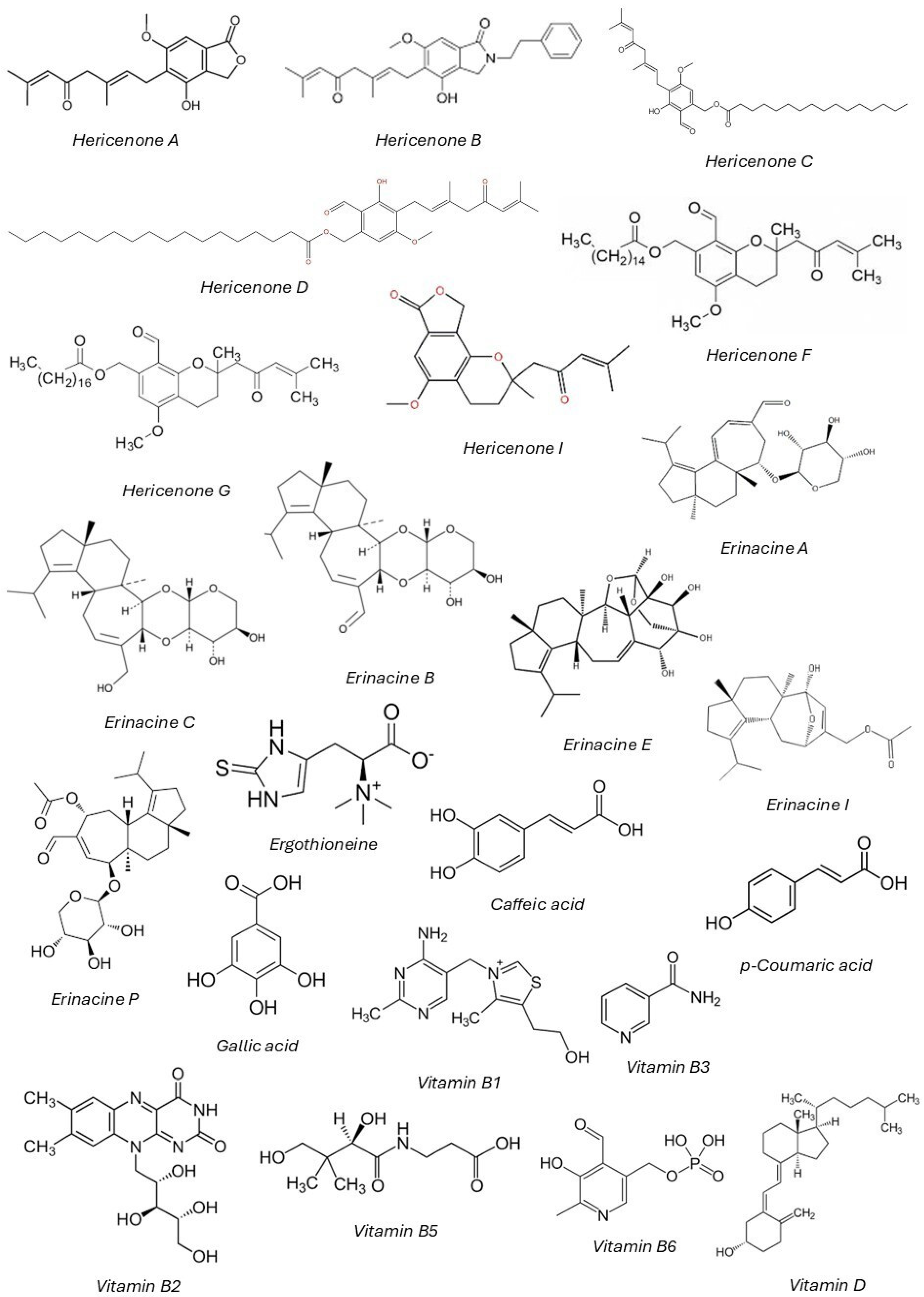
3.3. General Chemical Composition of H. erinaceus
4. Biological Properties and Mechanisms of Action
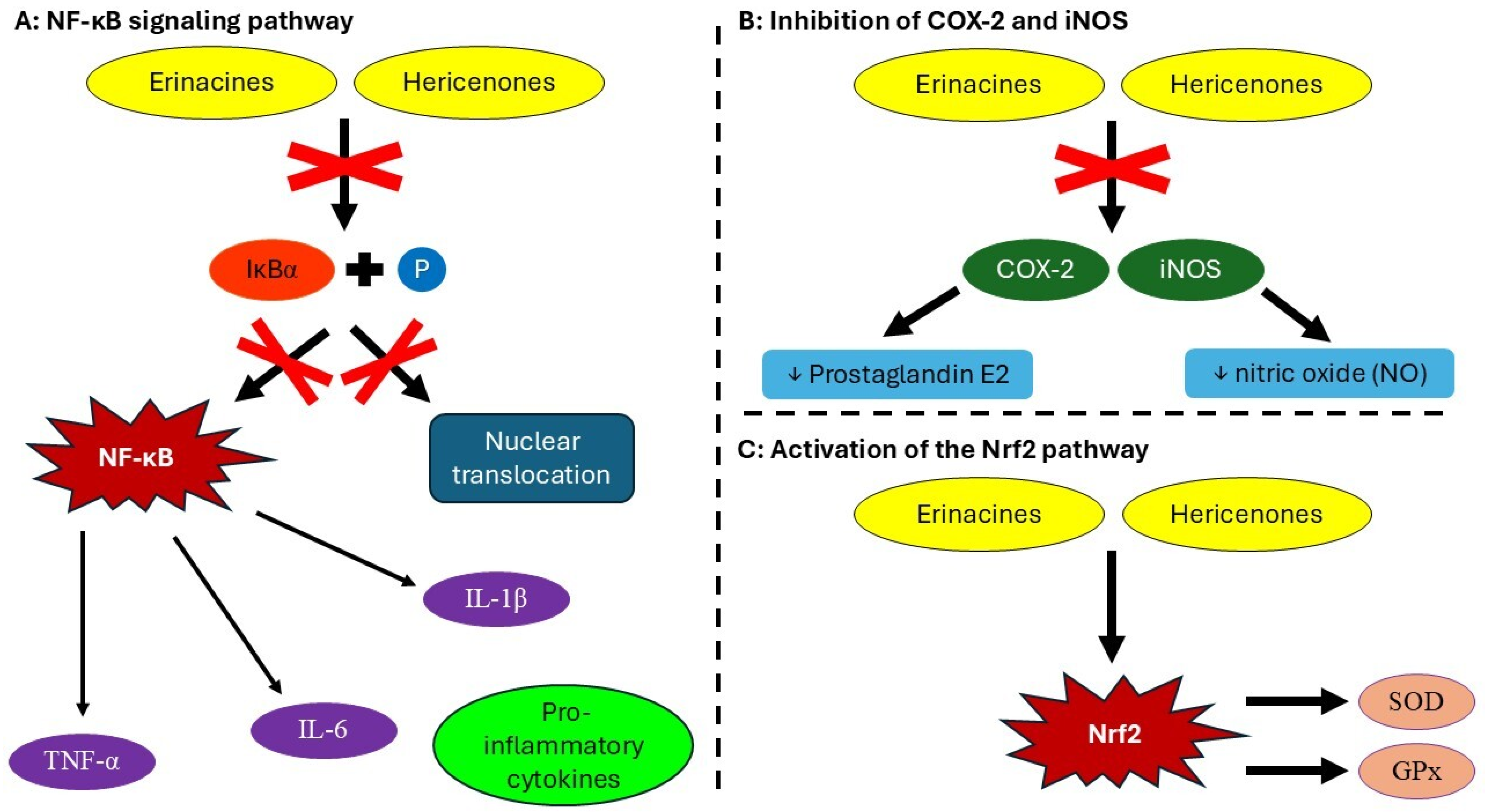
4.1. Anti-Inflammatory Activity
4.2. Clinical Trials
4.3. Bioavailability and Blood–Brain Barrier Penetration
4.4. Antioxidant Activity
- Induction of Antioxidant Enzymes: H. erinaceus extracts have been reported to upregulate the activity of antioxidant enzymes, including superoxide dismutase (SOD—converts superoxide radicals into less harmful molecules); catalase (CAT—breaks down hydrogen peroxide into water and oxygen, reducing cellular toxicity); and glutathione peroxidase (GPx—protects cells from oxidative damage by reducing peroxides) [145].
- Inhibition of Lipid Peroxidation: Studies have demonstrated that H. erinaceus extracts prevent the peroxidation of lipids, reducing malondialdehyde (MDA) levels, a significant factor in the aging process and the development of cardiovascular diseases [129].
4.5. Antimicrobial Activity
- Cell Membrane Disruption: Several bioactive compounds in H. erinaceus, particularly terpenoids and phenolic compounds, have been shown to interfere with bacterial and fungal cell membranes [29,74]. These compounds disrupt membrane integrity by altering lipid bilayer stability, leading to increased permeability, the leakage of intracellular contents, and eventual cell death. This mechanism is particularly relevant against Gram-positive bacteria, which have a thick peptidoglycan layer that is more susceptible to membrane-targeting agents [154].
- Inhibition of Biofilm Formation: Biofilms are protective structures formed by microbial communities that enhance resistance to antibiotics and immune responses [155]. Polysaccharides and terpenoids from H. erinaceus have demonstrated the ability to inhibit biofilm formation by interfering with quorum sensing pathways, the bacterial communication system that regulates biofilm development [93]. By preventing biofilm maturation, H. erinaceus compounds enhance the susceptibility of bacteria to antimicrobial agents and host immune defenses [155].
- Enzyme Inhibition and Metabolic Disruption: Phenolic compounds in H. erinaceus have been reported to inhibit key bacterial enzymes involved in cell wall synthesis, DNA replication, and energy metabolism [69,156]. For example, some erinacines and hericenones have been shown to interfere with bacterial ATP production, disrupting essential metabolic pathways and leading to growth inhibition [93,145].
- Induction of Oxidative Stress: Some bioactive compounds in H. erinaceus promote the generation of reactive oxygen species in microbial cells [157]. Excess ROS accumulation leads to oxidative damage to proteins, lipids, and DNA, ultimately resulting in cell death [7]. This mechanism is particularly effective against antibiotic-resistant bacteria, which often rely on antioxidant defense systems to survive in hostile environments [142].
- Modulation of Host Immune Responses: Polysaccharides, especially β-glucans, play a crucial role in enhancing the host’s immune response against infections [67]. These compounds stimulate macrophages, dendritic cells, and NK cells, boosting antimicrobial activity and facilitating the clearance of bacterial and fungal pathogens [110].
4.6. Calcium Binding Activity and Other Functional Properties
5. Nutritional and Therapeutic Applications
6. Future Perspectives and Challenges
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allegra, M. Antioxidant and anti-inflammatory properties of plants extract. Antioxidants 2019, 8, 549. [Google Scholar] [CrossRef]
- Rodríguez-Yoldi, M.J. Anti-inflammatory and antioxidant properties of plant extracts. Antioxidants 2021, 10, 921. [Google Scholar] [CrossRef] [PubMed]
- Rybczyńska-Tkaczyk, K.; Grenda, A.; Jakubczyk, A.; Kiersnowska, K.; Bik-Małodzińska, M. Natural compounds with antimicrobial properties in cosmetics. Pathogens 2023, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Contato, A.G.; Aranha, G.M.; de Abreu Filho, B.A.; Peralta, R.M.; de Souza, C.G.M. Evaluation of the antioxidant and antimicrobial activity of the culinary-medicinal mushroom Lentinula boryana (Agaricomycetes) from Brazil. Int. J. Med. Mushrooms 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.; Liu, J.; Aleksandrova, Y.; Klochkov, S.; Fan, R. Therapeutic influence on important targets associated with chronic inflammation and oxidative stress in cancer treatment. Cancers 2021, 13, 6062. [Google Scholar] [CrossRef]
- Contato, A.G.; Brugnari, T.; Sibin, A.P.A.; Buzzo, A.J.D.R.; de Sá-Nakanishi, A.B.; Bracht, L.; Bersani-Amado, C.A.; Peralta, R.M.; de Souza, C.G.M. Biochemical properties and effects on mitochondrial respiration of aqueous extracts of Basidiomycete mushrooms. Cell Biochem. Biophys. 2020, 78, 111–119. [Google Scholar] [CrossRef]
- Blagov, A.V.; Summerhill, V.I.; Sukhorukov, V.N.; Zhigmitova, E.B.; Postnov, A.Y.; Orekhov, A.N. Potential use of antioxidants for the treatment of chronic inflammatory diseases. Front. Pharmacol. 2024, 15, 1378335. [Google Scholar] [CrossRef]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and Alzheimer’s disease: The role of antioxidants in prevention and treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef]
- Lee, Y.T.; Yunus, M.H.M.; Ugusman, A.; Yazid, M.D. Natural compounds affecting inflammatory pathways of osteoarthritis. Antioxidants 2022, 11, 1722. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Lee, S.F.; Cheng, Z.; Davidson, R.; Rupasinghe, H.V. Carvacrol exhibits rapid bactericidal activity against Streptococcus pyogenes through cell membrane damage. Sci. Rep. 2021, 11, 1487. [Google Scholar] [CrossRef]
- Shinde, S.; Lee, L.H.; Chu, T. Inhibition of biofilm formation by the synergistic action of EGCG-S and antibiotics. Antibiotics 2021, 10, 102. [Google Scholar] [CrossRef]
- Perry, E.K.; Meirelles, L.A.; Newman, D.K. From the soil to the clinic: The impact of microbial secondary metabolites on antibiotic tolerance and resistance. Nat. Rev. Microbiol. 2022, 20, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, A.; Mallard, B.; Tiralongo, E.; Tiralongo, J. Mushroom natural products in neurodegenerative disease drug discovery. Cells 2022, 11, 3938. [Google Scholar] [CrossRef] [PubMed]
- You, S.W.; Hoskin, R.T.; Komarnytsky, S.; Moncada, M. Mushrooms as functional and nutritious food ingredients for multiple applications. ACS Food Sci. Technol. 2022, 2, 1184–1195. [Google Scholar] [CrossRef]
- Macoris, J.D.M.; Brugnari, T.; Boer, C.G.; Contato, A.G.; Peralta, R.M.; de Souza, C.G.M. Antioxidant properties and antimicrobial potential of aqueous extract of basidioma from Lentinus edodes (BerK) Sing (Shiitake). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3757–3767. [Google Scholar] [CrossRef]
- Contato, A.G.; Conte-Junior, C.A. Mushrooms in innovative food products: Challenges and potential opportunities as meat substitutes, snacks and functional beverages. Trends Food Sci. Technol. 2025, 156, 104868. [Google Scholar] [CrossRef]
- Contato, A.G.; de Araújo, C.A.V.; Zanzarin, D.M.; Aranha, G.M.; Sybuia, P.A.; Pilau, E.J.; Castoldi, R.; Peralta, R.M.; de Souza, C.G.M. Biological characterization and antimicrobial bioactives of mycelium extracts from medicinal mushrooms Phellinus linteus and Pleurotus albidus (Agaricomycetes). Int. J. Med. Mushrooms 2022, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Aranha, G.M.; Contato, A.G.; Salgado, J.C.D.S.; de Oliveira, T.B.; Retamiro, K.M.; Ortolan, G.G.; Crevelin, E.J.; Nakamura, C.V.; de Moraes, L.A.B.; Peralta, R.M.; et al. Biochemical characterization and biological properties of mycelium extracts from Lepista sordida GMA-05 and Trametes hirsuta GMA-01: New mushroom strains isolated in Brazil. Braz. J. Microbiol. 2022, 53, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Brugnari, T.; da Silva, P.H.A.; Contato, A.G.; Inácio, F.D.; Nolli, M.M.; Kato, C.G.; Peralta, R.M.; de Souza, C.G.M. Effects of cooking and in vitro digestion on antioxidant properties and cytotoxicity of the culinary-medicinal mushroom Pleurotus ostreatoroseus (Agaricomycetes). Int. J. Med. Mushrooms 2018, 20, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Saini, R.K.; Kumar, A.; Chawla, P.; Kaushik, R. Mushrooms as Nutritional Powerhouses: A Review of Their Bioactive Compounds, Health Benefits, and Value-Added Products. Foods 2025, 14, 741. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Sousa, A.S.; Relvas, J.B.; Tavaria, F.K.; Pintado, M. An overview on mushroom polysaccharides: Health-promoting properties, prebiotic and gut microbiota modulation effects and structure-function correlation. Carbohydr. Polym. 2024, 333, 121978. [Google Scholar] [CrossRef]
- Ma, G.; Du, H.; Hu, Q.; Yang, W.; Pei, F.; Xiao, H. Health benefits of edible mushroom polysaccharides and associated gut microbiota regulation. Crit. Rev. Food Sci. Nutr. 2021, 62, 6646–6663. [Google Scholar] [CrossRef]
- Moura, M.A.F.E.; Martins, B.D.A.; Oliveira, G.P.D.; Takahashi, J.A. Alternative protein sources of plant, algal, fungal and insect origins for dietary diversification in search of nutrition and health. Crit. Rev. Food Sci. Nutr. 2023, 63, 10691–10708. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, G.; Liu, W.; Zhang, F.; Linhardt, R.J.; Wang, X.; Zhang, A. Bioactive substances in Hericium erinaceus and their biological properties: A review. Food Sci. Hum. Wellness 2024, 13, 1825–1844. [Google Scholar] [CrossRef]
- Bizjak, M.Č.; Pražnikar, Z.J.; Kenig, S.; Hladnik, M.; Bandelj, D.; Gregori, A.; Kranjc, K. Effect of erinacine A-enriched Hericium erinaceus supplementation on cognition: A randomized, double-blind, placebo-controlled pilot study. J. Funct. Foods 2024, 115, 106120. [Google Scholar] [CrossRef]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Valu, M.V.; Soare, L.C.; Ducu, C.; Moga, S.; Negrea, D.; Vamanu, E.; Balseanu, T.A.; Carradori, S.; Hritcu, L.; Boiangiu, R.S. Hericium erinaceus (Bull.) Pers. ethanolic extract with antioxidant properties on scopolamine-induced memory deficits in a zebrafish model of cognitive impairment. J. Fungi 2021, 7, 477. [Google Scholar] [CrossRef]
- Sevindik, M.; Gürgen, A.; Khassanov, V.T.; Bal, C. Biological activities of ethanol extracts of Hericium erinaceus obtained as a result of optimization analysis. Foods 2024, 13, 1560. [Google Scholar] [CrossRef]
- Xie, G.; Tang, L.; Xie, Y.; Xie, L. Secondary metabolites from Hericium erinaceus and their anti-inflammatory activities. Molecules 2022, 27, 2157. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Geng, Y.; Lu, Z.; Xu, H.Y.; Shi, J.S.; Xu, X.; Xu, Z.H. Anti-inflammatory effects of ethanol extract of Lion’s Mane medicinal mushroom, Hericium erinaceus (Agaricomycetes, in mice with ulcerative colitis. Int. J. Med. Mushrooms 2016, 18, 227–234. [Google Scholar] [CrossRef]
- Lins, M.; Puppin Zandonadi, R.; Raposo, A.; Ginani, V.C. Food waste on foodservice: An overview through the perspective of sustainable dimensions. Foods 2021, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Jahedi, A.; Ahmadifar, S.; Mohammadigoltapeh, E. Revival of wild edible-medicinal mushroom (Hericium erinaceus) based on organic agro-industrial waste-achieving a commercial protocol with the highest yield; optimum reuse of organic waste. Sci. Hortic. 2024, 323, 112510. [Google Scholar] [CrossRef]
- Boddy, L.; Crockatt, M.E.; Ainsworth, A.M. Ecology of Hericium cirrhatum, H. coralloides and H. erinaceus in the UK. Fungal Ecol. 2011, 4, 163–173. [Google Scholar] [CrossRef]
- Persoon, C.H. Neuer versuch einer systematischen einteilung der schwämme. Neues Mag. Bot. 1794, 1, 63. [Google Scholar]
- Almjalawi, B.S.A.; Chechan, R.A.; Alhesnawi, A.S.M. Some applications of Hericium erinaceus mushrooms: A review. South Asian Res. J. Agric. Fish. 2024, 6, 92–100. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Larou, E.; Mountzouris, K.C.; Zervakis, G.I. Detoxification of olive mill wastewater and bioconversion of olive crop residues into high-value-added biomass by the choice edible mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. 2016, 180, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Jozífek, M.; Praus, L.; Matějka, J.; Jablonský, I.; Koudela, M. Selenium uptake by Hericium erinaceus basidiocarps on various substrates and their effect on growth and yield. Agriculture 2025, 15, 460. [Google Scholar] [CrossRef]
- Russell, B. Field Guide to Wild Mushrooms of Pennsylvania and the Mid-Atlantic: Revised and Expanded Edition; Penn State Press: University Park, PA, USA, 2017. [Google Scholar]
- Atila, F. Lignocellulosic and proximate based compositional changes in substrates during cultivation of Hericium erinaceus mushroom. Sci. Hortic. 2019, 258, 108779. [Google Scholar] [CrossRef]
- Cesaroni, V.; Brusoni, M.; Cusaro, C.M.; Girometta, C.; Perini, C.; Picco, A.M.; Salerni, E.; Savino, E. Phylogenetic comparison between Italian and worldwide Hericium species (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 943–954. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef]
- Kunca, V.; Čiliak, M. Habitat preferences of Hericium erinaceus in Slovakia. Fungal Ecol. 2017, 27, 189–192. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, J.S.; Kim, M.C.; Balasundaram, C.; Heo, M.S. Hericium erinaceum enriched diets enhance the immune response in Paralichthys olivaceus and protect from Philasterides dicentrarchi infection. Aquaculture 2011, 318, 48–53. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Xu, D.; Konishi, T.; Gao, Q. Hericium erinaceus (Yamabushitake): A unique resource for developing functional foods and medicines. Food Funct. 2014, 5, 3055–3064. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, W.; Shen, Y.; Wang, Y.; Dai, Y.C. Edible mushroom cultivation for food security and rural development in China: Bio-innovation, technological dissemination and marketing. Sustainability 2014, 6, 2961–2973. [Google Scholar] [CrossRef]
- Berovic, M. Cultivation of medicinal mushroom biomass by solid-state bioprocessing in bioreactors. In Solid State Fermentation: Research and Industrial Applications; Springer: Cham, Switzerland, 2019; pp. 3–25. [Google Scholar]
- Grace, J.; Mudge, K.W. Production of Hericium sp. (lion’s mane) mushrooms on totem logs in a forest farming system. Agrofor. Syst. 2015, 89, 549–556. [Google Scholar] [CrossRef]
- Chang, H.Y.; Roh, M.G. Effect of different cultivation methods on yield of Hericium erinaceus. Korean J. Mycol. 1999, 27, 249–251. [Google Scholar]
- Mizuno, T. Bioactive substances in Hericium erinaceus (Bull.: Fr.) Pers. (Yamabushitake, and its medicinal utilization. Int. J. Med. Mushrooms 1999, 1, 105–119. [Google Scholar] [CrossRef]
- Li, T.; Lo, Y.M.; Moon, B. Feasibility of using Hericium erinaceus as the substrate for vinegar fermentation. LWT-Food Sci. Technol. 2014, 55, 323–328. [Google Scholar] [CrossRef]
- Wong, K.H.; Sabaratnam, V.; Abdullah, N.; Kuppusamy, U.R.; Naidu, M. Effects of cultivation techniques and processing on antimicrobial and antioxidant activities of Hericium erinaceus (Bull.: Fr.) Pers. extracts. Food Technol. Biotechnol. 2009, 47, 47–55. [Google Scholar]
- Zhuang, H.; Dong, H.; Zhang, X.; Feng, T. Antioxidant activities and prebiotic activities of water-soluble, alkali-soluble polysaccharides extracted from the fruiting bodies of the fungus Hericium erinaceus. Polymers 2023, 15, 4165. [Google Scholar] [CrossRef]
- Ghosh, S.; Nandi, S.; Banerjee, A.; Sarkar, S.; Chakraborty, N.; Acharya, K. Prospecting medicinal properties of Lion’s mane mushroom. J. Food Biochem. 2021, 45, e13833. [Google Scholar] [CrossRef]
- Kushairi, N.; Phan, C.W.; Sabaratnam, V.; David, P.; Naidu, M. Lion’s mane mushroom, Hericium erinaceus (Bull.: Fr.) Pers. suppresses H2O2-induced oxidative damage and LPS-induced inflammation in HT22 hippocampal neurons and BV2 microglia. Antioxidants 2019, 8, 261. [Google Scholar] [CrossRef]
- Szydłowska-Tutaj, M.; Szymanowska, U.; Tutaj, K.; Domagała, D.; Złotek, U. The addition of reishi and lion’s mane mushroom powder to pasta influences the content of bioactive compounds and the antioxidant, potential anti-inflammatory, and anticancer properties of pasta. Antioxidants 2023, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecka, B.; Wessely-Szponder, J.; Świeca, M.; Espinal, P.; Fusté, E.; Fernández-De La Cruz, E. Bioactive peptides and other immunomodulators of mushroom origin. Biomedicines 2024, 12, 1483. [Google Scholar] [CrossRef]
- García-Carnero, L.C.; Martínez-Álvarez, J.A.; Salazar-García, L.M.; Lozoya-Pérez, N.E.; González-Hernández, S.E.; Tamez-Castrellón, A.K. Recognition of fungal components by the host immune system. Curr. Protein Pept. Sci. 2020, 21, 245–264. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Li, S.; Di, R.; Ho, C.T.; Yang, G. Ribosome-inactivating proteins (RIPs) and their important health promoting property. RSC Adv. 2016, 6, 46794–46805. [Google Scholar] [CrossRef]
- Piscitelli, A.; Cicatiello, P.; Gravagnuolo, A.M.; Sorrentino, I.; Pezzella, C.; Giardina, P. Applications of functional amyloids from fungi: Surface modification by class I hydrophobins. Biomolecules 2017, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible mushrooms and beta-glucans: Impact on human health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Kujawowicz, K.; Witkowska, A.M. Beta-glucans from fungi: Biological and health-promoting potential in the COVID-19 pandemic era. Nutrients 2021, 13, 3960. [Google Scholar] [CrossRef]
- Boulifa, A.; Raftery, M.J.; Franzén, A.S.; Radecke, C.; Stintzing, S.; Blohmer, J.U.; Pecher, G. Role of beta-(1 → 3)(1 → 6)-D-glucan derived from yeast on natural killer (NK) cells and breast cancer cell lines in 2D and 3D cultures. BMC Cancer 2024, 24, 339. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, D.D.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S96–S115. [Google Scholar] [CrossRef]
- Arunachalam, K.; Sreeja, P.S.; Yang, X. The antioxidant properties of mushroom polysaccharides can potentially mitigate oxidative stress, beta-cell dysfunction and insulin resistance. Front. Pharmacol. 2022, 13, 874474. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef]
- Szućko-Kociuba, I.; Trzeciak-Ryczek, A.; Kupnicka, P.; Chlubek, D. Neurotrophic and neuroprotective effects of Hericium erinaceus. Int. J. Mol. Sci. 2023, 24, 15960. [Google Scholar] [CrossRef]
- Kostanda, E.; Musa, S.; Pereman, I. Unveiling the chemical composition and biofunctionality of Hericium spp. fungi: A comprehensive overview. Int. J. Mol. Sci. 2024, 25, 5949. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, W.B.; Shehata, R.M.; Younis, A.M. In vitro assessment of multipotential therapeutic importance of Hericium erinaceus mushroom extracts using different solvents. Bioresour. Bioprocess. 2022, 9, 99. [Google Scholar] [CrossRef]
- Ma, B.J.; Shen, J.W.; Yu, H.Y.; Ruan, Y.; Wu, T.T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef]
- Tong, Z.; Chu, G.; Wan, C.; Wang, Q.; Yang, J.; Meng, Z.; Du, L.; Yang, J.; Ma, H. Multiple metabolites derived from mushrooms and their beneficial effect on Alzheimer’s diseases. Nutrients 2023, 15, 2758. [Google Scholar] [CrossRef]
- Chen, Z.G.; Bishop, K.S.; Tanambell, H.; Buchanan, P.; Smith, C.; Quek, S.Y. Characterization of the bioactivities of an ethanol extract and some of its constituents from the New Zealand native mushroom Hericium novae-zealandiae. Food Funct. 2019, 10, 6633–6643. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; Priori, E.C.; Ratto, D.; De Luca, F.; Di Iorio, C.; Angelone, P.; Locatelli, C.A.; Desiderio, A.; Goppa, L.; Savino, E.; et al. Neuroprotective metabolites of Hericium erinaceus promote neuro-healthy aging. Int. J. Mol. Sci. 2021, 22, 6379. [Google Scholar] [CrossRef] [PubMed]
- Phan, C.W.; David, P.; Sabaratnam, V. Edible and medicinal mushrooms: Emerging brain food for the mitigation of neurodegenerative diseases. J. Med. Food 2017, 20, 1–10. [Google Scholar] [CrossRef]
- Chen, Z.G.; Bishop, K.S.; Zhang, J.; Quek, S.Y. Neuroprotective and anticarcinogenic properties of Hericium mushrooms and the active constituents associated with these effects: A review. Food Sci. Eng. 2022, 3, 69–90. [Google Scholar] [CrossRef]
- Kim, Y.O.; Lee, S.W.; Kim, J.S. A comprehensive review of the therapeutic effects of Hericium erinaceus in neurodegenerative disease. J. Mushroom 2014, 12, 77–81. [Google Scholar] [CrossRef]
- Bacha, S.A.S.; Ali, S.; Li, Y.; Rehman, H.U.; Farooq, S.; Mushtaq, A.; Wahocho, S.A.; Aslam, S.M. Lion’s mane mushroom; new addition to food and natural bounty for human wellness: A review. Int. J. Biosci. 2018, 13, 396–402. [Google Scholar]
- Li, I.C.; Lee, L.Y.; Tzeng, T.T.; Chen, W.P.; Chen, Y.P.; Shiao, Y.J.; Chen, C.C. Neurohealth properties of Hericium erinaceus mycelia enriched with erinacines. Behav. Neurol. 2018, 2018, 5802634. [Google Scholar] [CrossRef]
- Lee, L.Y.; Chou, W.; Chen, W.P.; Wang, M.F.; Chen, Y.J.; Chen, C.C.; Tung, K.C. Erinacine a-enriched Hericium erinaceus mycelium delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (samp8) mice. Nutrients 2021, 13, 3659. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Hsu, J.Y.; Chen, T.C.; Huang, C.C.; Wu, T.Y.; Chin, T.Y. Erinacine A prevents lipopolysaccharide-mediated glial cell activation to protect dopaminergic neurons against inflammatory factor-induced cell death in vitro and in vivo. Int. J. Mol. Sci. 2022, 23, 810. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, H.; Liu, Z.; Feng, G.; Shi, C.; Wu, Y. Unveiling the therapeutic potentials of mushroom bioactive compounds in Alzheimer’s disease. Foods 2023, 12, 2972. [Google Scholar] [CrossRef] [PubMed]
- Rascher, M.; Wittstein, K.; Winter, B.; Rupcic, Z.; Wolf-Asseburg, A.; Stadler, M.; Köster, R.W. Erinacine C activates transcription from a consensus ETS DNA binding site in astrocytic cells in addition to NGF induction. Biomolecules 2020, 10, 1440. [Google Scholar] [CrossRef]
- Lee, D.G.; Kang, H.W.; Park, C.G.; Ahn, Y.S.; Shin, Y. Isolation and identification of phytochemicals and biological activities of Hericium erinaceus and their contents in Hericium strains using HPLC/UV analysis. J. Ethnopharmacol. 2016, 184, 219–225. [Google Scholar] [CrossRef]
- Wei, J.; Li, J.Y.; Feng, X.L.; Zhang, Y.; Hu, X.; Hui, H.; Xue, X.; Qi, J. Unprecedented neoverrucosane and cyathane diterpenoids with anti-neuroinflammatory activity from cultures of the culinary-medicinal mushroom Hericium erinaceus. Molecules 2023, 28, 6380. [Google Scholar] [CrossRef]
- Ratto, D.; Corana, F.; Mannucci, B.; Priori, E.C.; Cobelli, F.; Roda, E.; Ferrari, B.; Occhinegro, A.; Di Iorio, C.; De Luca, F.; et al. Hericium erinaceus improves recognition memory and induces hippocampal and cerebellar neurogenesis in frail mice during aging. Nutrients 2019, 11, 715. [Google Scholar] [CrossRef]
- Arya, C. Potential uses of mushrooms as dietary supplement to enhance memory. In Biology, Cultivation and Applications of Mushrooms; Springer: Singapore, 2022; pp. 387–402. [Google Scholar]
- Shen, T.; Morlock, G.; Zorn, H. Production of cyathane type secondary metabolites by submerged cultures of Hericium erinaceus and evaluation of their antibacterial activity by direct bioautography. Fungal Biol. Biotechnol. 2015, 2, 8. [Google Scholar] [CrossRef]
- Sk, D.; Kr, S.; Mk, G. Hericium erinaceus—A rich source of diverse bioactive metabolites. Fungal Biotec 2021, 1, 10–38. [Google Scholar]
- Zhang, C.C.; Cao, C.Y.; Kubo, M.; Harada, K.; Yan, X.T.; Fukuyama, Y.; Gao, J.M. Chemical constituents from Hericium erinaceus promote neuronal survival and potentiate neurite outgrowth via the TrkA/Erk1/2 pathway. Int. J. Mol. Sci. 2017, 18, 1659. [Google Scholar] [CrossRef] [PubMed]
- Aramsirirujiwet, Y.; Leepasert, T.; Piamariya, D.; Thong-Asa, W. Benefits of erinacines from different cultivate formulas on cognitive deficits and anxiety-like behaviour in mice with trimethyltin-induced toxicity. Trop. Life Sci. Res. 2023, 34, 165. [Google Scholar] [CrossRef]
- Lee, K.F.; Hsieh, Y.Y.; Tung, S.Y.; Teng, C.C.; Cheng, K.C.; Hsieh, M.C.; Huang, C.Y.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; et al. The cerebral protective effect of novel erinacines from Hericium erinaceus mycelium on in vivo mild traumatic brain injury animal model and primary mixed glial cells via Nrf2-dependent pathways. Antioxidants 2024, 13, 371. [Google Scholar] [CrossRef]
- Rossi, P.; Cesaroni, V.; Brandalise, F.; Occhinegro, A.; Ratto, D.; Perrucci, F.; Lanaia, V.; Girometta, C.; Orrù, G.; Savino, E. Dietary supplementation of lion’s mane medicinal mushroom, Hericium erinaceus (Agaricomycetes, and spatial memory in wild-type mice. Int. J. Med. Mushrooms 2018, 20, 485–494. [Google Scholar] [CrossRef]
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.W.; Stadler, M.; Wittstein, K. Two new cyathane diterpenoids from mycelial cultures of the medicinal mushroom Hericium erinaceus and the rare species, Hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef]
- Roda, E.; De Luca, F.; Ratto, D.; Priori, E.C.; Savino, E.; Bottone, M.G.; Rossi, P. Cognitive healthy aging in mice: Boosting memory by an ergothioneine-rich Hericium erinaceus primordium extract. Biology 2023, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; Ratto, D.; De Luca, F.; Desiderio, A.; Ramieri, M.; Goppa, L.; Savino, E.; Bottone, M.G.; Locatelli, C.A.; Rossi, P. Searching for a longevity food, we bump into Hericium erinaceus primordium rich in ergothioneine: The “longevity vitamin” improves locomotor performances during aging. Nutrients 2022, 14, 1177. [Google Scholar] [CrossRef]
- Corana, F.; Cesaroni, V.; Mannucci, B.; Baiguera, R.M.; Picco, A.M.; Savino, E.; Ratto, D.; Perini, C.; Kawagishi, H.; Girometta, C.E.; et al. Array of metabolites in Italian Hericium erinaceus mycelium, primordium, and sporophore. Molecules 2019, 24, 3511. [Google Scholar] [CrossRef] [PubMed]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: Promising bioactive compounds and market value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
- Gao, X.; Homayoonfal, M. Exploring the anti-cancer potential of Ganoderma lucidum polysaccharides (GLPs) and their versatile role in enhancing drug delivery systems: A multifaceted approach to combat cancer. Cancer Cell Int. 2023, 23, 324. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Zhao, Z.; Zhang, N. Structural and pharmacological insights into cordycepin for neoplasms and metabolic disorders. Front. Pharmacol. 2024, 15, 1367820. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Arif, M.; Xu, M.; Zhang, J.; Ding, Y.; Lyu, F. Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review. Trends Food Sci. Technol. 2023, 134, 123–135. [Google Scholar] [CrossRef]
- Gusev, E.; Zhuravleva, Y. Inflammation: A new look at an old problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef]
- Soliman, A.M.; Barreda, D.R. Acute inflammation in tissue healing. Int. J. Mol. Sci. 2023, 24, 641. [Google Scholar] [CrossRef] [PubMed]
- Nasef, N.A.; Mehta, S.; Ferguson, L.R. Susceptibility to chronic inflammation: An update. Arch. Toxicol. 2017, 91, 1131–1141. [Google Scholar] [CrossRef]
- Ren, Y.; Geng, Y.; Du, Y.; Li, W.; Lu, Z.M.; Xu, H.Y.; Xu, G.H.; Shi, J.S.; Xu, Z.H. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J. Nutr. Biochem. 2018, 57, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Tangen, J.M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjønnfjord, G.E.; Johnson, E. Antitumor, anti-inflammatory and antiallergic effects of Agaricus blazei mushroom extract and the related medicinal basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A review of preclinical and clinical studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef]
- Bhatt, D.; Ghosh, S. Regulation of the NF-κB-mediated transcription of inflammatory genes. Front. Immunol. 2014, 5, 71. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, Z.; Guo, M. Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef]
- Wang, L.Y.; Huang, C.S.; Chen, Y.H.; Chen, C.C.; Chen, C.C.; Chuang, C.H. Anti-inflammatory effect of erinacine C on NO production through down-regulation of NF-κB and activation of Nrf2-mediated HO-1 in BV2 microglial cells treated with LPS. Molecules 2019, 24, 3317. [Google Scholar] [CrossRef]
- Long, T.; Liu, Z.; Shang, J.; Zhou, X.; Yu, S.; Tian, H.; Bao, Y. Polygonatum sibiricum polysaccharides play anti-cancer effect through TLR4-MAPK/NF-κB signaling pathways. Int. J. Biol. Macromol. 2018, 111, 813–821. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.J.; Kim, J.U.; Yook, T.H.; Kim, K.H.; Lee, J.Y.; Yang, G. A novel treatment strategy by natural products in NLRP3 inflammasome-mediated neuroinflammation in Alzheimer’s and Parkinson’s disease. Int. J. Mol. Sci. 2021, 22, 1324. [Google Scholar] [CrossRef]
- Williams, L.M.; Berthon, B.S.; Stoodley, I.L.; Williams, E.J.; Wood, L.G. Medicinal mushroom extracts from Hericium coralloides and Trametes versicolor exert differential immunomodulatory effects on immune cells from older adults in vitro. Nutrients 2023, 15, 2227. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Nigro, M.; Travagli, A.; Gessi, S. Microglia and Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 12990. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, H.K.; Kim, S.E.; Kim, M.J.; Kim, D.H.; Lee, H.S. Chondroprotective effects of a standardized extract (KBH-JP-040) from Kalopanax pictus, Hericium erinaceus, and Astragalus membranaceus in experimentally induced in vitro and in vivo osteoarthritis models. Nutrients 2018, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Iova, O.M.; Marin, G.E.; Lazar, I.; Stanescu, I.; Dogaru, G.; Nicula, C.A.; Bulboacă, A.E. Nitric oxide/nitric oxide synthase system in the pathogenesis of neurodegenerative disorders—An overview. Antioxidants 2023, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Fontes, A.; Alemany-Pagès, M.; Oliveira, P.J.; Ramalho-Santos, J.; Zischka, H.; Azul, A.M. Antioxidant versus pro-apoptotic effects of mushroom-enriched diets on mitochondria in liver disease. Int. J. Mol. Sci. 2019, 20, 3987. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. Role of oxidative stress in blood–brain barrier disruption and neurodegenerative diseases. Antioxidants 2024, 13, 1462. [Google Scholar] [CrossRef]
- Wang, D.; Xu, D.; Zhao, D.; Wang, M. Screening and comparison of anti-intestinal inflammatory activities of three polysaccharides from the mycelium of lion’s mane culinary-medicinal mushroom, Hericium erinaceus (Agaricomycetes). Int. J. Med. Mushrooms 2021, 23, 63–71. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, M.; Wu, D.; Li, W.; Chen, W.; Liu, P.; Zhang, H.; Yang, Y. Resveratrol-loaded sulfated Hericium erinaceus β-glucan-chitosan nanoparticles: Preparation, characterization and synergistic anti-inflammatory effects. Carbohydr. Polym. 2024, 332, 121916. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Hong, Q.; Zhang, X.; Liu, Z.; Zhang, T. Polysaccharides from Hericium erinaceus fruiting bodies: Structural characterization, immunomodulatory activity and mechanism. Nutrients 2022, 14, 3721. [Google Scholar] [CrossRef] [PubMed]
- Chau, S.C.; Chong, P.S.; Jin, H.; Tsui, K.C.; Khairuddin, S.; Tse, A.C.K.; Lew, S.Y.; Tipoe, G.L.; Lee, C.W.; Fung, M.L.; et al. Hericium erinaceus promotes anti-inflammatory effects and regulation of metabolites in an animal model of cerebellar ataxia. Int. J. Mol. Sci. 2023, 24, 6089. [Google Scholar] [CrossRef]
- Li, H.; Feng, J.; Liu, C.; Hou, S.; Meng, J.; Liu, J.Y.; Zilong, S.; Chang, M.C. Polysaccharides from an edible mushroom, Hericium erinaceus, alleviate ulcerative colitis in mice by inhibiting the NLRP3 inflammasomes and reestablish intestinal homeostasis. Int. J. Biol. Macromol. 2024, 267, 131251. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Sun, Q.; Gao, R.; Sheng, Y.; Guan, T.; Li, W.; Zhou, L.; Liu, C.; Li, H.; Lu, Z.; et al. Low weight polysaccharide of Hericium erinaceus ameliorates colitis via inhibiting the NLRP3 inflammasome activation in association with gut microbiota modulation. Nutrients 2023, 15, 739. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.T.; Wu, K.W.; Chen, C.C.; Nguyen, A.T.; Huang, W.J.; Lee, L.Y.; Chem, W.P.; Huang, C.Y.; Shiao, Y.J. Exploring the synergistic effects of erinacines on microglial regulation and Alzheimer’s pathology under metabolic stress. CNS Neurosci. Ther. 2024, 30, e70137. [Google Scholar] [CrossRef]
- Lu, H.; Yang, S.; Li, W.; Zheng, B.; Zeng, S.; Chen, H. Hericium erinaceus protein alleviates high-fat diet-induced hepatic lipid accumulation and oxidative stress in vivo. Foods 2025, 14, 459. [Google Scholar] [CrossRef]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef]
- Li, I.C.; Chang, H.H.; Lin, C.H.; Chen, W.P.; Lu, T.H.; Lee, L.Y.; Che, Y.W.; Chen, Y.P.; Chen, C.C.; Lin, D.P.C. Prevention of early Alzheimer’s disease by erinacine A-enriched Hericium erinaceus mycelia pilot double-blind placebo-controlled study. Front. Aging Neurosci. 2020, 12, 155. [Google Scholar] [CrossRef]
- Tursi, A.; D’Avino, A.; Brandimarte, G.; Mocci, G.; Pellegrino, R.; Savarino, E.V.; Gravina, A.G.; Hericium-Uc Study Group. Enhancing oral 5-ASA effectiveness in mild-to-moderate ulcerative colitis through an H. erinaceus-based nutraceutical add-on multi-compound: The “HERICIUM-UC” two-arm multicentre retrospective study. Pharmaceutics 2024, 16, 1133. [Google Scholar]
- Gravina, A.G.; Pellegrino, R.; Palladino, G.; Coppola, A.; Brandimarte, G.; Tuccillo, C.; Ciadierllo, F.; Romano, F.; Federico, A. Hericium erinaceus, in combination with natural flavonoid/alkaloid and B3/B8 vitamins, can improve inflammatory burden in Inflammatory bowel diseases tissue: An ex vivo study. Front. Immunol. 2023, 14, 1215329. [Google Scholar] [CrossRef]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef]
- Tsai, P.C.; Wu, Y.K.; Hu, J.H.; Li, I.C.; Lin, T.W.; Chen, C.C.; Kuo, C.F. Preclinical bioavailability, tissue distribution, and protein binding studies of erinacine A, a bioactive compound from Hericium erinaceus mycelia using validated LC-MS/MS method. Molecules 2021, 26, 4510. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Wen, Y.T.; Hsu, T.C.; Chen, C.C.; Lee, L.Y.; Chen, W.P.; Tsai, R.K. Neuroprotective effects of erinacine A on an experimental model of traumatic optic neuropathy. Int. J. Mol. Sci. 2023, 24, 1504. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuan, X.; Buchanan, P.; Quek, S.Y. Isolation and determination of lipophilic mycochemicals from a New Zealand edible native mushroom Hericium novae-zealandiae. J. Food Compos. Anal. 2020, 88, 103456. [Google Scholar] [CrossRef]
- Moukham, H.; Lambiase, A.; Barone, G.D.; Tripodi, F.; Coccetti, P. Exploiting natural niches with neuroprotective properties: A comprehensive review. Nutrients 2024, 16, 1298. [Google Scholar] [CrossRef]
- Altemimi, A.B.; Farag, H.A.M.; Salih, T.H.; Awlqadr, F.H.; Al-Manhel, A.J.A.; Vieira, I.R.S.; Conte-Junior, C.A. Application of nanoparticles in human nutrition: A review. Nutrients 2024, 16, 636. [Google Scholar] [CrossRef]
- Shazwani, S.S.; Marlina, A.; Misran, M. Development of nanostructured lipid carrier-loaded flavonoid-enriched Zingiber officinale. ACS Omega 2024, 9, 17379–17388. [Google Scholar] [CrossRef]
- Lelis, C.A.; de Carvalho, A.P.A.; Conte Junior, C.A. A systematic review on nanoencapsulation natural antimicrobials in foods: In vitro versus in situ evaluation, mechanisms of action and implications on physical-chemical quality. Int. J. Mol. Sci. 2021, 22, 12055. [Google Scholar] [CrossRef]
- Pinar, O.; Rodríguez-Couto, S. Biologically active secondary metabolites from white-rot fungi. Front. Chem. 2024, 12, 1363354. [Google Scholar] [CrossRef]
- Tu, J.Q.; Liu, H.P.; Wen, Y.H.; Chen, P.; Liu, Z.T. A novel polysaccharide from Hericium erinaceus: Preparation, structural characteristics, thermal stabilities, and antioxidant activities in vitro. J. Food Biochem. 2021, 45, e13871. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.H.; Jang, H.D.; Ma, J.Y.; Kim, Y.H. Antioxidant and anti-osteoporotic activities of aromatic compounds and sterols from Hericium erinaceum. Molecules 2017, 22, 108. [Google Scholar] [CrossRef]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective effects of Hericium erinaceus (Bull.: Fr.) Pers. against high-dose corticosterone-induced oxidative stress in PC-12 cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, G.M.; Petraglia, T.; Latronico, T.; Crescenzi, A.; Rossano, R. Antioxidant compounds from edible mushrooms as potential candidates for treating age-related neurodegenerative diseases. Nutrients 2023, 15, 1913. [Google Scholar] [CrossRef]
- Yao, F.; Gao, H.; Yin, C.M.; Shi, D.F.; Lu, Q.; Fan, X.Z. Evaluation of in vitro antioxidant and antihyperglycemic activities of extracts from the lion’s mane medicinal mushroom, Hericium erinaceus (Agaricomycetes). Int. J. Med. Mushrooms 2021, 23, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Liu, S.; Qin, M. Effects of extraction conditions on crude polysaccharides and antioxidant activities of the lion’s mane medicinal mushroom, Hericium erinaceus (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 1007–1018. [Google Scholar] [CrossRef]
- Ghosh, S.; Chakraborty, N.; Banerjee, A.; Chatterjee, T.; Acharya, K. Mycochemical profiling and antioxidant activity of two different tea preparations from Lion’s Mane medicinal mushroom, Hericium erinaceus (Agaricomycetes). Int. J. Med. Mushrooms 2021, 23, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Jalani, S.A.M. Effects of Different Cooking Methods on the Antioxidant Activities of Hericium erinaceus (Bull.: Fr.) Pers. Master’s Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2017. [Google Scholar]
- Wang, G.; Zhang, X.; Maier, S.E.; Zhang, L.; Maier, R.J. In vitro and in vivo inhibition of Helicobacter pylori by ethanolic extracts of lion’s mane medicinal mushroom, Hericium erinaceus (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 1–11. [Google Scholar] [CrossRef]
- Kim, S.P.; Kang, M.Y.; Choi, Y.H.; Kim, J.H.; Nam, S.H.; Friedman, M. Mechanism of Hericium erinaceus (Yamabushitake) mushroom-induced apoptosis of U937 human monocytic leukemia cells. Food Funct. 2011, 2, 348–356. [Google Scholar] [CrossRef]
- Song, X.; Gaascht, F.; Schmidt-Dannert, C.; Salomon, C.E. Discovery of antifungal and biofilm preventative compounds from mycelial cultures of a unique North American Hericium sp. fungus. Molecules 2020, 25, 963. [Google Scholar] [CrossRef]
- Glover, R.L.; Nyanganyura, D.; Mufamadi, M.S.; Mulaudzi, R.B. (Eds.) Green Synthesis in Nanomedicine and Human Health; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Moussa, A.Y.; Fayez, S.; Xiao, H.; Xu, B. New insights into antimicrobial and antibiofilm effects of edible mushrooms. Food Res. Int. 2022, 162, 111982. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, H.; Wu, D.; Zhang, Z.; Zhang, J.; Bao, D.; Yang, Y. Key metabolism pathways and regulatory mechanisms of high polysaccharide yielding in Hericium erinaceus. BMC Genom. 2021, 22, 160. [Google Scholar] [CrossRef]
- Lu, C.C.; Huang, W.S.; Lee, K.F.; Lee, K.C.; Hsieh, M.C.; Huang, C.Y.; Lee, L.Y.; Lee, B.O.; Teng, C.C.; Shen, C.H.; et al. Inhibitory effect of Erinacines A on the growth of DLD-1 colorectal cancer cells is induced by generation of reactive oxygen species and activation of p70S6K and p21. J. Funct. Foods 2016, 21, 474–484. [Google Scholar] [CrossRef]
- Khalaf, M.S.; Shawkat, M.S. Antibaterial activity of (Hericium erinaceus) extract on some clinical pathogenic isolates. Iraqi J. Agric. Sci. 2023, 54, 691–699. [Google Scholar] [CrossRef]
- Lomberg, M.; Krupodorova, T.; Krasinko, V.; Mykchaylova, O. The antibacterial activity of culture filtrates and mycelia of selected strains of macromycetes from the genus Hericium. Bot. Serbica 2023, 47, 241–249. [Google Scholar] [CrossRef]
- Shrivastava, A.; Jain, S. Hericium erinaceus (Bull.) and Hericium coralloides. In Edible and Medicinal Mushrooms of the Himalayas; CRC Press: Boca Raton, FL, USA, 2023; pp. 111–127. [Google Scholar]
- Liu, J.H.; Li, L.; Shang, X.D.; Zhang, J.L.; Tan, Q. Anti-Helicobacter pylori activity of bioactive components isolated from Hericium erinaceus. J. Ethnopharmacol. 2016, 183, 54–58. [Google Scholar] [CrossRef]
- Darmasiwi, S.; Aramsirirujiwet, Y.; Kimkong, I. Antibiofilm activity and bioactive phenolic compounds of ethanol extract from the Hericium erinaceus basidiome. J. Adv. Pharm. Technol. Res. 2022, 13, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Darmasiwi, S.; Aramsirirujiwet, Y.; Kimkong, I. Biological activities and chemical profile of Hericium erinaceus mycelium cultivated on mixed red and white jasmine rice. Food Sci. Technol. 2022, 42, e08022. [Google Scholar] [CrossRef]
- Shang, X.; Tan, Q.; Liu, R.; Yu, K.; Li, P.; Zhao, G.P. In vitro anti-Helicobacter pylori effects of medicinal mushroom extracts, with special emphasis on the Lion’s Mane mushroom, Hericium erinaceus (higher Basidiomycetes). Int. J. Med. Mushrooms 2013, 15, 165–174. [Google Scholar] [CrossRef]
- Dörr, T. Understanding tolerance to cell wall–active antibiotics. Ann. N. Y. Acad. Sci. 2021, 1496, 35–58. [Google Scholar] [CrossRef]
- Kim, M.U.; Lee, E.H.; Jung, H.Y.; Lee, S.Y.; Cho, Y.J. Inhibitory activity against biological activities and antimicrobial activity against pathogenic bacteria of extracts from Hericium erinaceus. J. Appl. Biol. Chem. 2019, 62, 173–179. [Google Scholar] [CrossRef]
- Chopra, H.; Mishra, A.K.; Baig, A.A.; Mohanta, T.K.; Mohanta, Y.K.; Baek, K.H. Narrative review: Bioactive potential of various mushrooms as the treasure of versatile therapeutic natural product. J. Fungi 2021, 7, 728. [Google Scholar] [CrossRef] [PubMed]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef]
- Altenburg, J.; De Graaff, C.S.; Van der Werf, T.S.; Boersma, W.G. Immunomodulatory effects of macrolide antibiotics–part 2: Advantages and disadvantages of long-term, low-dose macrolide therapy. Respiration 2010, 81, 75–87. [Google Scholar] [CrossRef]
- Pham, T.D.; Ziora, Z.M.; Blaskovich, M.A. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Panda, G.; Dash, S.; Sahu, S.K. Harnessing the role of bacterial plasma membrane modifications for the development of sustainable membranotropic phytotherapeutics. Membranes 2022, 12, 914. [Google Scholar] [CrossRef]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, K.; Kapoor, N.; Kaur, H.; Abu-Seer, E.A.; Tariq, M.; Siddiqui, S.; Yadav, V.K.; Niazi, P.; Kumar, P.; Alghamdi, S. A comprehensive review of the diversity of fungal secondary metabolites and their emerging applications in healthcare and environment. Mycobiology 2024, 52, 335. [Google Scholar] [CrossRef]
- Hu, T.; Hui, G.; Li, H.; Guo, Y. Selenium biofortification in Hericium erinaceus (Lion’s Mane mushroom) and its in vitro bioaccessibility. Food Chem. 2020, 331, 127287. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewska, A.; Wójciak, M.; Mroziak-Lal, K.; Zagórska-Dziok, M.; Bujak, T.; Nizioł-Łukaszewska, Z.; Szczepanek, D.; Sowa, I. Assessment of cosmetic properties and safety of use of model washing gels with Reishi, Maitake and Lion’s Mane extracts. Molecules 2022, 27, 5090. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y.; Li, S.; Li, H.; Li, M.; Gao, Z. Edible fungus-derived bioactive components as innovative and sustainable options in health promotion. Food Biosci. 2024, 59, 104215. [Google Scholar] [CrossRef]
- Silva, M.; Vida, M.; Ramos, A.C.; Lidon, F.J.; Reboredo, F.H.; Gonçalves, E.M. Storage temperature effect on quality and shelf-life of Hericium erinaceus mushroom. Horticulturae 2025, 11, 158. [Google Scholar] [CrossRef]
- You, H.; Abraham, E.J.; Mulligan, J.; Zhou, Y.; Montoya, M.; Willig, J.; Chen, B.K.; Wang, C.K.; Wang, L.S.; Dong, A.; et al. Label compliance for ingredient verification: Regulations, approaches, and trends for testing botanical products marketed for “immune health” in the United States. Crit. Rev. Food Sci. Nutr. 2024, 64, 2441–2460. [Google Scholar] [CrossRef]
- Gu, H.; Liang, L.; Kang, Y.; Yu, R.; Wang, J.; Fan, D. Preparation, characterization, and property evaluation of Hericium erinaceus peptide–calcium chelate. Front. Nutr. 2024, 10, 1337407. [Google Scholar] [CrossRef]
- Pikor, D.; Hurła, M.; Słowikowski, B.; Szymanowicz, O.; Poszwa, J.; Banaszek, N.; Drelichowska, A.; Jagodziński, P.P.; Kozubski, W.; Dorszewska, J. Calcium ions in the physiology and pathology of the central nervous system. Int. J. Mol. Sci. 2024, 25, 13133. [Google Scholar] [CrossRef]
- Sukumaran, P.; da Conceicao, V.N.; Sun, Y.; Ahamad, N.; Saraiva, L.R.; Selvaraj, S.; Singh, B.B. Calcium signaling regulates autophagy and apoptosis. Cells 2021, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Bading, H. Nuclear calcium signalling in the regulation of brain function. Nat. Rev. Neurosci. 2013, 14, 593–608. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.E.; Frailey, D.; Nohe, A.; Duncan, R.; Czymmek, K.J.; Kang, S. Roles of three Fusarium oxysporum calcium ion (Ca2+) channels in generating Ca2+ signatures and controlling growth. Fungal Genet. Biol. 2015, 82, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Involvement of oxidative stress in protective cardiac functions of calprotectin. Cells 2022, 11, 1226. [Google Scholar] [CrossRef]
- Camins, A.; Verdaguer, E.; Folch, J.; Pallàs, M. Involvement of calpain activation in neurodegenerative processes. CNS Drug Rev. 2006, 12, 135–148. [Google Scholar] [CrossRef]
- Sangtitanu, T.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Peptides obtained from edible mushrooms: Hericium erinaceus offers the ability to scavenge free radicals and induce apoptosis in lung cancer cells in humans. Food Funct. 2020, 11, 4927–4939. [Google Scholar] [CrossRef]
- Krzywoszyńska, K.; Witkowska, D.; Świątek-Kozłowska, J.; Szebesczyk, A.; Kozłowski, H. General aspects of metal ions as signaling agents in health and disease. Biomolecules 2020, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Hor, S.L.; Teoh, S.L.; Lim, W.L. Plant polyphenols as neuroprotective agents in Parkinson’s disease targeting oxidative stress. Curr. Drug Targets 2020, 21, 458–476. [Google Scholar] [CrossRef]
- Wang, X.; An, P.; Gu, Z.; Luo, Y.; Luo, J. Mitochondrial metal ion transport in cell metabolism and disease. Int. J. Mol. Sci. 2021, 22, 7525. [Google Scholar] [CrossRef] [PubMed]
- Akinola, S.A.; Adeyemo, R.O.; Binuyo, M.O.; Adegboyega, T.T.; Buhari, M.O.; Adebayo, I.A. Edible mushroom bioactive compounds and neurogenic diseases. In Bioactive Compounds in Edible Mushrooms: Sustainability and Health Applications; Springer Nature: Cham, Switzerland, 2025; pp. 1–37. [Google Scholar]
- Docherty, S.; Doughty, F.L.; Smith, E.F. The acute and chronic effects of lion’s mane mushroom supplementation on cognitive function, stress and mood in young adults: A double-blind, parallel groups, pilot study. Nutrients 2023, 15, 4842. [Google Scholar] [CrossRef]
- Barcan, A.S.; Barcan, R.A.; Vamanu, E. Therapeutic potential of fungal polysaccharides in gut microbiota regulation: Implications for diabetes, neurodegeneration, and oncology. J. Fungi 2024, 10, 394. [Google Scholar] [CrossRef]
- Picone, P.; Girgenti, A.; Buttacavoli, M.; Nuzzo, D. Enriching the Mediterranean diet could nourish the brain more effectively. Front. Nutr. 2024, 11, 1489489. [Google Scholar] [CrossRef] [PubMed]
- Uffelman, C.N.; Doenges, K.A.; Armstrong, M.L.; Quinn, K.; Reisdorph, R.M.; Tang, M.; Krebs, N.F.; Reisdorph, N.A.; Campbell, W.W. Metabolomics profiling of white button, crimini, portabella, lion’s mane, maitake, oyster, and shiitake mushrooms using untargeted metabolomics and targeted amino acid analysis. Foods 2023, 12, 2985. [Google Scholar] [CrossRef]
- Li, H.; Zhao, H.; Liu, W.; Feng, Y.; Zhang, Y.; Yuan, F.; Jia, L. Liver and brain protective effect of sulfated polysaccharides from residue of lion’s mane medicinal mushroom, Hericium erinaceus (Agaricomycetes, on D-galactose—Induced aging mice. Int. J. Med. Mushrooms 2021, 23, 55–65. [Google Scholar] [CrossRef]
- Kumar, D.; Kashyap, S.; Gupta, S. Mushrooms as Functional Foods: Trends and Innovations. In Mushroom Magic; CRC Press: Boca Raton, FL, USA, 2024; pp. 307–322. [Google Scholar]
- Nieman, K.M.; Zhu, Y.; Tucker, M.; Koecher, K. The role of dietary ingredients in mental energy–a scoping review of randomized controlled trials. J. Am. Nutr. Assoc. 2024, 43, 167–182. [Google Scholar] [CrossRef]
- Szydłowska-Tutaj, M.; Złotek, U.; Wójtowicz, A.; Combrzyński, M. The effect of the addition of various species of mushrooms on the physicochemical and sensory properties of semolina pasta. Food Funct. 2022, 13, 8425–8435. [Google Scholar] [CrossRef]
- Meyer, F.; Hutmacher, A.; Lu, B.; Steiger, N.; Nyström, L.; Narciso, J.O. Vegan shrimp alternative made with pink oyster and lion’s mane mushrooms: Nutritional profiles, presence of conjugated phenolic acids, and prototyping. Curr. Res. Food Sci. 2023, 7, 100572. [Google Scholar] [CrossRef] [PubMed]
- Atila, F.; Tuzel, Y.; Fernández, J.A.; Cano, A.F.; Sen, F. The effect of some agro–industrial wastes on yield, nutritional characteristics and antioxidant activities of Hericium erinaceus isolates. Sci. Hortic. 2018, 238, 246–254. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, Y.J.; Hsu, C.H.; Lin, Y.H.; Chen, P.T.; Kuo, T.H.; Ho, C.T.; Chen, H.H.; Huang, S.J.; Chiu, H.C.; et al. Erinacine S from Hericium erinaceus mycelium promotes neuronal regeneration by inducing neurosteroids accumulation. J. Food Drug Anal. 2023, 31, 32. [Google Scholar] [CrossRef]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Polzella, M.; Fabrizi, C.; Fornai, F. Potential antidepressant effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants 2020, 9, 234. [Google Scholar] [CrossRef]
- Zang, P.; Chen, P.; Chen, J.; Sun, J.; Lan, H.; Dong, H.; Liu, W.; Xu, N.; Wang, W.; Hou, L.; et al. Alteration of gastrointestinal function and the ameliorative effects of Hericium erinaceus polysaccharides in tail suspension rats. Nutrients 2025, 17, 724. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, F.; Liu, L.; Feng, J.; Zhang, J.; Yang, Y.; Wu, D.; Guo, Q.; Liu, Y. Physicochemical properties of polysaccharides from Hericium erinaceus by steam explosion pretreatment and its effects on human gut microbiota. Food Hydrocoll. 2024, 156, 110365. [Google Scholar] [CrossRef]
- Cui, M.; Ma, Q.; Zhang, Z.; Li, W.; Chen, W.; Liu, P.; Wu, D.; Yang, Y. Semi-solid enzymolysis enhanced the protective effects of fruiting body powders and polysaccharides of Hericium erinaceus on gastric mucosal injury. Int. J. Biol. Macromol. 2023, 251, 126388. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, H.; Raman, J.; David, P.; Wong, K.H.; Naidu, M.; Sabaratnam, V. Haematological, biochemical and histopathological aspects of Hericium erinaceus ingestion in a rodent model: A sub-chronic toxicological assessment. J. Ethnopharmacol. 2016, 194, 1051–1059. [Google Scholar] [CrossRef]
- Naumoska, K.; Gregori, A.; Albreht, A. Two-dimensional chromatographic isolation of high purity erinacine A from Hericium erinaceus. J. Fungi 2025, 11, 150. [Google Scholar] [CrossRef]
- Dudekula, U.T.; Doriya, K.; Devarai, S.K. A critical review on submerged production of mushroom and their bioactive metabolites. 3 Biotech 2020, 10, 337. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Jiang, Q.; Roubík, H.; Xu, Q.; Gharsallaoui, A.; Cai, M.; Yang, K.; Sun, P. Fungal solid-state fermentation of crops and their by-products to obtain protein resources: The next frontier of food industry. Trends Food Sci. Technol. 2023, 138, 628–644. [Google Scholar] [CrossRef]
- Arshadi, N.; Nouri, H.; Moghimi, H. Increasing the production of the bioactive compounds in medicinal mushrooms: An omics perspective. Microb. Cell Factories 2023, 22, 11. [Google Scholar] [CrossRef]
- Wang, D.; Jin, S.; Lu, Q.; Chen, Y. Advances and challenges in CRISPR/Cas-based fungal genome engineering for secondary metabolite production: A review. J. Fungi 2023, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Negi, T.; Rawat, N.; Sagar, N.A.; Sindhu, R.; Tarafdar, A. Emerging technologies for the extraction of bioactives from mushroom waste. J. Food Sci. Technol. 2024, 61, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, F.B.; Aranha, A.C.R.; Nardino, D.A.; Defendi, R.O.; Suzuki, R.M. Extraction and encapsulation of bioactive compounds: A review. J. Food Process Eng. 2022, 45, e14167. [Google Scholar] [CrossRef]
- Macit, C.; Eyupoglu, O.E.; Macit, M.; Zengin, G. Chemical characterization and biological properties of oyster and shiitake mushrooms extracts and their liposomal formulations. Food Biosci. 2024, 61, 104737. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Sukdee, S.; Prajuabjinda, O.; Thepsilvisut, O.; Panthong, S.; Athinuwat, D.; Chuaboon, W.; Poomipan, P.; Vachirayagorn, V. The effects of soybean meal on growth, bioactive compounds, and antioxidant activity of Hericium erinaceus. Horticulturae 2023, 9, 693. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Stathopoulos, P.; Kaliora, A.C.; Zervakis, G.I. Bioactive compounds and antioxidant activity exhibit high intraspecific variability in Pleurotus ostreatus mushrooms and correlate well with cultivation performance parameters. World J. Microbiol. Biotechnol. 2017, 33, 98. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, J.; Li, S. Quantitative estimation of enzymatic released specific oligosaccharides from Hericium erinaceus polysaccharides using CE-LIF. J. Pharm. Anal. 2023, 13, 201–208. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, T.W.; Lin, J.Y.; Chen, Y.W.; Li, T.J.; Chen, C.C. Identification of common liver metabolites of the natural bioactive compound erinacine A, purified from Hericium erinaceus mycelium. Appl. Sci. 2022, 12, 1201. [Google Scholar] [CrossRef]
- Gong, W.; Song, X.; Xie, C.; Zhou, Y.; Zhu, Z.; Xu, C.; Peng, Y. Detection of quantitative trait loci underlying fruiting body and yield-related traits in Hericium erinaceus. Sci. Hortic. 2022, 293, 110729. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Available online: https://eur-lex.europa.eu (accessed on 3 March 2025).
- Li, C.; Xu, S. Edible mushroom industry in China: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.Z.; Zhang, X.Y.; Wang, Y.Y.; Zhao, Y.M.; Wang, J. Polysaccharides from Hericium erinaceus and its immunomodulatory effects on RAW 264.7 macrophages. Int. J. Biol. Macromol. 2024, 278, 134947. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Krakowska, A.; Sułkowska-Ziaja, K. Medicinal mushrooms as a source of therapeutic biopolymers. In Fungal Biotechnology; CRC Press: Boca Raton, FL, USA, 2022; pp. 54–83. [Google Scholar]


| Cultivation Method | Growth Time | Yield | Cost | Difficulty Level | Characteristics | Substrate | Inoculation | Harvest | Application | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Log cultivation | 6–12 months | Low | Low | Moderate | Mimics natural habitat; slow but high-quality mushrooms | Hardwood logs (oak, beech, maple) aged for a few weeks | Plug spawn inserted into drilled holes and sealed with wax | Annual harvest for up to 5 years | Traditional, gourmet markets | [52,53,54] |
| Sawdust Blocks (Indoor) | 6–8 weeks | High | Medium | High | Fast, controlled conditions, high predictability | Sterilized sawdust with wheat/rice bran and gypsum | Grain spawn mixed into the substrate and incubated for 2–4 weeks | Multiple harvests over a few weeks | Commercial mushroom production | [37,42,51] |
| Liquid fermentation | 5–10 days | Very high | High | Advanced | Produces high mycelial biomass and bioactive compounds | Liquid nutrient medium (glucose, yeast extract, peptone) | Inoculated with mycelium and incubated under controlled aeration | Mycelium harvested, dried, and processed | Pharmaceutical, nutraceutical industries | [55,56,57] |
| Terpenoid | Chemical Class | Source | Biological Activity | Potential Applications | References |
|---|---|---|---|---|---|
| Hericenones | |||||
| Hericenone A | Phenolic terpenoid | Fruiting body | Stimulates NGF synthesis, neuroprotective, anti-inflammatory | Neurodegenerative disease prevention, cognitive enhancement | [34,75] |
| Hericenone B | Phenolic terpenoid | Fruiting body | Promotes NGF synthesis, enhances cognitive function, memory improvement | Alzheimer’s treatment, cognitive health | [75,76] |
| Hericenone C | Phenolic terpenoid | Fruiting body | NGF synthesis promotion, neuroprotective, anti-inflammatory | Cognitive disorders, memory loss | [75,77] |
| Hericenone D | Phenolic terpenoid | Fruiting body | Enhances NGF production, antioxidant, neuroprotective | Neurodegeneration prevention, oxidative stress reduction | [75,78] |
| Hericenone E | Phenolic terpenoid | Fruiting body | Neurogenic effects, promotes NGF synthesis | Cognitive decline treatment, neurogenesis | [72,75] |
| Hericenone F | Phenolic terpenoid | Fruiting body | Stimulates NGF synthesis, neuroprotective, enhances brain function | Neuroprotective drugs, cognitive health | [75,79] |
| Hericenone G | Phenolic terpenoid | Fruiting body | Neuroprotective, cognitive enhancement | Nootropic supplements, memory improvement | [80,81] |
| Hericenone H | Phenolic terpenoid | Fruiting body | Anti-inflammatory, promotes nerve regeneration | Nerve damage repair | [34,81] |
| Hericenone I | Phenolic terpenoid | Fruiting body | No protective effect on estrogen receptor stress-dependent cell death | Cardiovascular health, anti-aging formulations | [29] |
| Hericenone J | Phenolic terpenoid | Fruiting body | Enhances NGF expression, neuroprotection | Neurodegenerative disease therapy, brain health | [72,75] |
| Hericenone L | Phenolic terpenoid | Fruiting body | Modulates inflammatory pathways, antioxidant | Chronic inflammation management, metabolic disease therapy | [73,82] |
| Erinacines | |||||
| Erinacine A | Sesquiterpenoid | Mycelium | Potent stimulator of NGF synthesis, enhances neurogenesis, promotes neuronal growth | Alzheimer’s, Parkinson’s disease, cognitive decline | [78,83,84,85] |
| Erinacine B | Sesquiterpenoid | Mycelium | Neuroprotective, enhances NGF synthesis, supports brain health | Cognitive function improvement, neurodegenerative disease | [72,86] |
| Erinacine C | Sesquiterpenoid | Mycelium | Stimulates NGF production, improves cognitive abilities, neurogenesis | Alzheimer’s prevention, brain health | [78,82,87] |
| Erinacine D | Sesquiterpenoid | Mycelium | NGF stimulation, neuroprotective, anti-inflammatory | Neurodegeneration, brain function recovery | [75,78,88] |
| Erinacine E | Sesquiterpenoid | Mycelium | Stimulates NGF synthesis, reduces neuroinflammation | Neuroprotective therapies, cognitive health | [75,89] |
| Erinacine F | Sesquiterpenoid | Mycelium | Promotes NGF production, neurogenesis stimulation | Cognitive disorders, neurodegenerative disease prevention | [75,90] |
| Erinacine G | Sesquiterpenoid | Mycelium | Stimulates NGF synthesis, neuroprotective effects | Neurodegenerative disease prevention, cognitive enhancement | [75,90] |
| Erinacine H | Sesquiterpenoid | Mycelium | Neuroprotective | Brain function recovery | [90] |
| Erinacine I | Sesquiterpenoid | Mycelium | Enhances cognitive function | Memory improvement, Alzheimer’s therapy | [91] |
| Erinacine K | Sesquiterpenoid | Mycelium | Promotes NGF production | Brain health | [75] |
| Erinacine P | Sesquiterpenoid | Mycelium | Potential antimicrobial and anti-inflammatory activity | Antimicrobial applications, immune modulation | [92,93] |
| Erinacine Q | Sesquiterpenoid | Mycelium | Neurotrophic effects, supports neuronal health | Nerve growth support, neurodegeneration prevention | [72,94] |
| Erinacine R | Sesquiterpenoid | Mycelium | Cognitive enhancement | Alzheimer’s treatment | [95] |
| Erinacine S | Sesquiterpenoid | Mycelium | Neuroprotective, improves memory function | Memory preservation, learning enhancement | [72,96] |
| Erinacine V | Sesquiterpenoid | Mycelium | Antioxidant, potential cognitive enhancer | Antioxidant therapy, cognitive support | [73,97] |
| Erinacine Z1 | Sesquiterpenoid | Mycelium | Increase the expression of this neurotrophin, regulating inflammatory processes | Inflammation control, neuroprotective drug development | [72] |
| Erinacine Z2 | Sesquiterpenoid | Mycelium | Potential application in neurodegenerative disease therapy | Potential Alzheimer’s and Parkinson’s therapy | [93,98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contato, A.G.; Conte-Junior, C.A. Lion’s Mane Mushroom (Hericium erinaceus): A Neuroprotective Fungus with Antioxidant, Anti-Inflammatory, and Antimicrobial Potential—A Narrative Review. Nutrients 2025, 17, 1307. https://doi.org/10.3390/nu17081307
Contato AG, Conte-Junior CA. Lion’s Mane Mushroom (Hericium erinaceus): A Neuroprotective Fungus with Antioxidant, Anti-Inflammatory, and Antimicrobial Potential—A Narrative Review. Nutrients. 2025; 17(8):1307. https://doi.org/10.3390/nu17081307
Chicago/Turabian StyleContato, Alex Graça, and Carlos Adam Conte-Junior. 2025. "Lion’s Mane Mushroom (Hericium erinaceus): A Neuroprotective Fungus with Antioxidant, Anti-Inflammatory, and Antimicrobial Potential—A Narrative Review" Nutrients 17, no. 8: 1307. https://doi.org/10.3390/nu17081307
APA StyleContato, A. G., & Conte-Junior, C. A. (2025). Lion’s Mane Mushroom (Hericium erinaceus): A Neuroprotective Fungus with Antioxidant, Anti-Inflammatory, and Antimicrobial Potential—A Narrative Review. Nutrients, 17(8), 1307. https://doi.org/10.3390/nu17081307







