Ergonutrition Supplementation and Recovery in Water Polo: A Systematic Review
Abstract
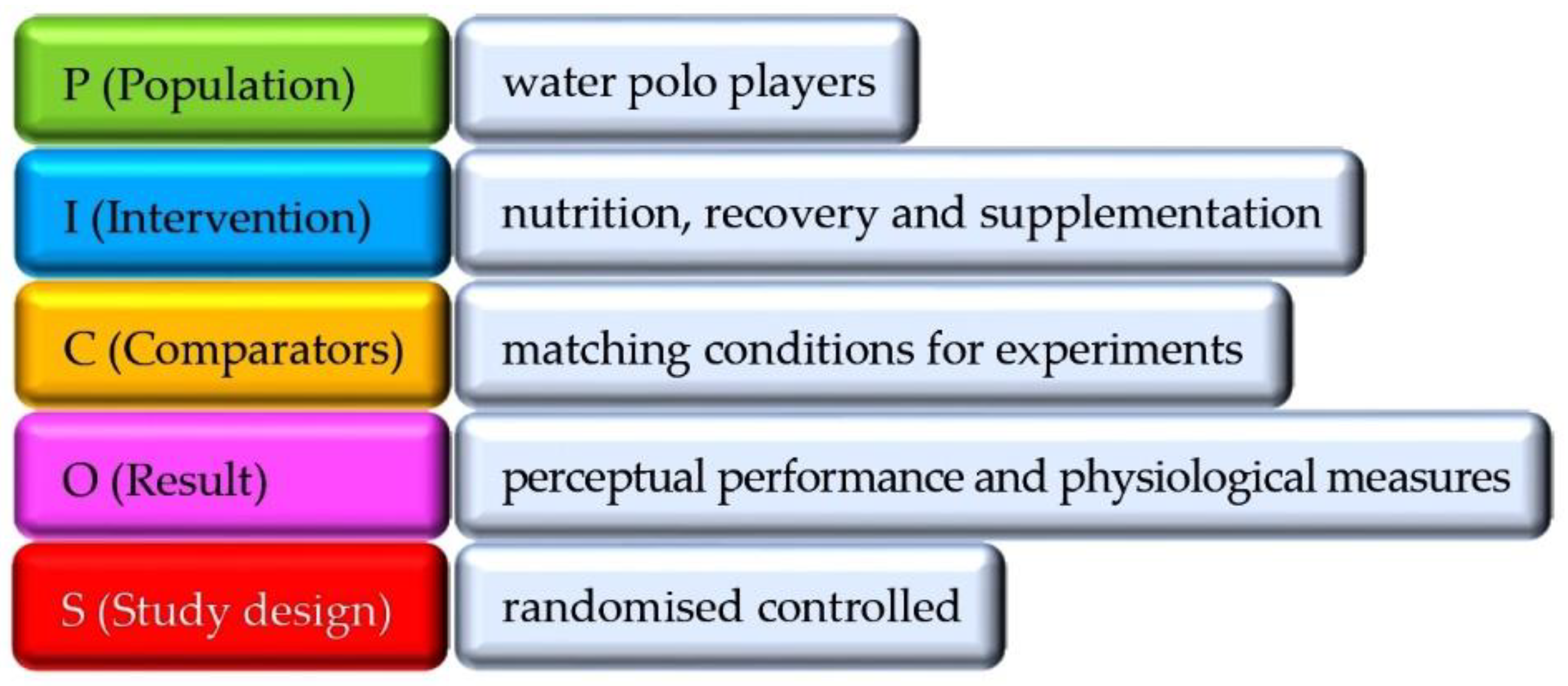
1. Introduction
2. Methods
2.1. Sources of Information
2.2. Study Selection
2.3. Data Extraction
2.4. Assessing the Quality of Experiments: Risk of Bias and Levels of Evidence
3. Results
3.1. Assessing the Quality of Experiments: Risk of Bias and Levels of Evidence
3.2. Nutritional Strategies
3.3. Recovery
3.4. Ergogenic Supplementation
3.5. Effect of Nitrates and β-Alanine: A Narrative Meta-Analysis
4. Discussion
4.1. Nutrition
4.2. Recovery
4.3. Ergogenic Supplements: Scientific Evidence
5. Strengths, Limitations, Future Research Lines, Practical Applications
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3AO | 3 min all-out |
| Ad | abdominal discomfort |
| TLA | three letter acronym |
| LD | linear dichroism |
| aFCr | average heart rate reserve |
| Antrop | anthropometry |
| ASPS | Athlete’s Subjective Performance Scale |
| Atc | anthocyanin |
| BC | body composition |
| BcM | body cell mass |
| Bf | bowel fullness |
| BfM | body fat mass |
| BL | blood lactate |
| BMC | bone mineral content |
| BMR | basal metabolic rate |
| Ca | calcium |
| CE | cycle ergometer |
| Ch | cholesterol |
| CHO | carbohydrate |
| CK | creatine kinase |
| CM | creatine monohydrate |
| CPK | creatine phosphokinase |
| Cr | creatinine |
| CRP | C-reactive protein |
| Cu | copper |
| DAt | dynamic apnoea test |
| DB | data-based |
| Di | daily intake |
| DI | dietary intake |
| DOMS | delayed-onset muscle soreness |
| EE | efficiency of exercise |
| eE | energetic efficiency |
| ee | energy expenditure |
| EW | extracellular water |
| EWPp | elite WP players |
| F | ferritin |
| F2-IsoP | F2-isoprostane |
| FDC | fatigue during competition |
| FFM | fat-free mass |
| FS | food science |
| G | glucose |
| Gd | glycolytic demands |
| GGT | gamma-glutamyl transferase |
| GOT | glutamate oxaloacetate transaminase |
| GPT | glutamate pyruvate transaminase |
| H | hydration |
| HDL | high-density lipoprotein |
| Hg | haemoglobin |
| HIs | high-intensity swimming |
| Hm | haematocrit |
| HMB | b-hydroxy-b-methyl butyrate |
| HR | heart rate |
| IGF1 | insulin-like growth factor |
| IL-6 | interleukin 6 |
| Ist | intermittent sprint test |
| IW | intracellular water |
| K | potassium |
| LDL | low-density lipoproteins |
| Lk | leucocyte |
| ll | low level |
| Me | medicine |
| M | minutes |
| maxFC | maximum heart rate |
| Mdtx | maltodextrin |
| Mg | magnesium |
| MPul | maximum power of the upper limbs |
| MSs | maximal-sprint swims |
| MsS | maximum swimming speed |
| MTS | match-simulation test |
| Mx | maximal 30 s tethered swimming in alternate eggbeater kick |
| N | nutrition |
| Na | sodium |
| NaHCO3 | sodium bicarbonate |
| Nh | nutritional habit |
| NS | nutritional strategy |
| opt | optimal time |
| P | phosphorus |
| PD | physiological demands |
| PDDC | physiological demands during competition |
| PER | perceived effort rating |
| PhCs | phytochemicals |
| pt | perceived tiredness |
| Pt | protein |
| RBC | red blood cell sink |
| RBCn | red blood cell number |
| RQ | respiratory quotient |
| RR | respiratory rate |
| RSA | repeated-sprint ability |
| RSAwft | specific WP repeated-sprint ability with free throw |
| S | strength |
| s | swimming |
| sCJ | sour cherry juice |
| SM | sport medicine |
| SMM | skeletal muscle mass |
| Ss | shooting skill |
| SSFs | supplements and sports foods |
| SVO2pmax | strength associated with VO2pmax |
| TBW | total body water |
| Tg | triglyceride |
| TMA | time–motion analysis |
| TQR | total quality of recovery |
| tV | training volume |
| U | urea |
| Ua | uric acid |
| vD | vitamin D |
| VO2max | maximum oxygen consumption |
| VO2pmax | peak maximum oxygen uptake |
| WBPA | whole-body phase angle |
| Wt | whey protein |
| β-a | β-alanine |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Botonis, P.G.; Toubekis, A.G.; Platanou, T.I. Physiological and Tactical on-Court Demands of Water Polo. J. Strength Cond. Res. 2019, 33, 3188–3199. [Google Scholar] [CrossRef]
- Smith, H.K. Applied physiology of water polo. Sport. Med. 1998, 26, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, G.; Castagna, C.; Sorge, R.; Bonifazi, M. Game activity and blood lactate in men’s elite water-polo players. J. Strength Cond. Res. 2010, 24, 2647–2651. [Google Scholar] [CrossRef] [PubMed]
- Glaister, M. Multiple sprint work: Physiological responses, mechanisms of fatigue and the influence of aerobic fitness. Sports Med. 2005, 35, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports Nutr. 2012, 9, 33. [Google Scholar] [CrossRef]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef]
- Grgic, J.; Rodriguez, R.F.; Garofolini, A.; Saunders, B.; Bishop, D.J.; Schoenfeld, B.J.; Pedisic, Z. Effects of Sodium Bicarbonate Supplementation on Muscular Strength and Endurance: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 1361–1375. [Google Scholar] [CrossRef]
- Saunders, B.; de Oliveira, L.F.; Dolan, E.; Durkalec-Michalski, K.; McNaughton, L.; Artioli, G.G.; Swinton, P.A. Sodium bicarbonate supplementation and the female athlete: A brief commentary with small scale systematic review and meta-analysis. Eur. J. Sport Sci. 2022, 22, 745–754. [Google Scholar] [CrossRef]
- Heaton, L.E.; Davis, J.K.; Rawson, E.S.; Nuccio, R.P.; Witard, O.C.; Stein, K.W.; Baar, K.; Carter, J.M.; Baker, L.B. Selected In-Season Nutritional Strategies to Enhance Recovery for Team Sport Athletes: A Practical Overview. Sports Med. 2017, 47, 2201–2218. [Google Scholar] [CrossRef]
- Botonis, P.G.; Toubekis, A.G.; Platanou, T.I. Training Loads, Wellness and Performance before and during Tapering for a Water-Polo Tournament. J. Hum. Kinet. 2019, 66, 131–141. [Google Scholar] [CrossRef]
- Barrenetxea-Garcia, J.; Murua-Ruiz, A.; Mielgo-Ayuso, J.; Nuell, S.; Calleja-González, J.; de Villarreal, E.S. Recovery in water polo: How much do we have to know? A systematic review. J. Exerc. Rehabil. 2022, 18, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Balk, Y.A.; de Jonge, J. The “underrecovery trap”: When physical fatigue impairs the physical and mental recovery process. Sport Exerc. Perform. Psychol. 2021, 10, 88. [Google Scholar] [CrossRef]
- Jones, C.M.; Griffiths, P.C.; Mellalieu, S.D. Training Load and Fatigue Marker Associations with Injury and Illness: A Systematic Review of Longitudinal Studies. Sports Med. 2017, 47, 943–974. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Barrenetxea-Garcia, J.; Perazzetti, A.; Nuell, S.; Mielgo-Ayuso, J.; de Villarreal, E.S.; Calleja-González, J. Perceptions and use of recovery strategies in water polo players and coaches: A worldwide survey. J. Exerc. Rehabil. 2024, 20, 112–123. [Google Scholar] [CrossRef]
- Smith-Ryan, A.E.; Hirsch, K.R.; Saylor, H.E.; Gould, L.M.; Blue, M.N.M. Nutritional Considerations and Strategies to Facilitate Injury Recovery and Rehabilitation. J. Athl. Train. 2020, 55, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Morton, J.P.; Burke, L.M. A Framework for Periodized Nutrition for Athletics. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 141–151. [Google Scholar] [CrossRef]
- Downer, S.; Berkowitz, S.A.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food is medicine: Actions to integrate food and nutrition into healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Drenowatz, C. Integrated role of nutrition and physical activity for lifelong health. Nutrients 2019, 11, 1437. [Google Scholar] [CrossRef]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and cognitive health: A life course approach. Front. Public Health 2023, 11, 1023907. [Google Scholar] [CrossRef]
- Muscaritoli, M. The Impact of Nutrients on Mental Health and Well-Being: Insights From the Literature. Front. Nutr. 2021, 8, 656290. [Google Scholar] [CrossRef] [PubMed]
- Amawi, A.; AlKasasbeh, W.; Jaradat, M.; Almasri, A.; Alobaidi, S.; Hammad, A.A.; Bishtawi, T.; Fataftah, B.; Turk, N.; Al Saoud, H.; et al. Athletes’ nutritional demands: A narrative review of nutritional requirements. Front. Nutr. 2024, 10, 1331854. [Google Scholar] [CrossRef]
- Baranauskas, M.; Kupčiūnaitė, I.; Stukas, R. Dietary Intake of Protein and Essential Amino Acids for Sustainable Muscle Development in Elite Male Athletes. Nutrients 2023, 15, 4003. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.T.; Kerksick, C.M.; Smith-Ryan, A.E.; Janse de Jonge, X.A.K.; Hirsch, K.R.; Arent, S.M.; Hewlings, S.J.; Kleiner, S.M.; Bustillo, E.; Tartar, J.L.; et al. International society of sports nutrition position stand: Nutritional concerns of the female athlete. J. Int. Soc. Sports Nutr. 2023, 20, 2204066. [Google Scholar] [CrossRef]
- Cox, G.R.; Mujika, I.; Van Den Hoogenband, C.R. Nutritional recommendations for water polo. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 382–391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Papadopoulou, S.K. Rehabilitation nutrition for injury recovery of athletes: The role of macronutrient intake. Nutrients 2020, 12, 2449. [Google Scholar] [CrossRef]
- Sygo, J.; Killer, S.C.; Glass, A.K.; Stellingwerff, T. Fueling for the Field: Nutrition for Jumps, Throws, and Combined Events. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 95–105. [Google Scholar] [CrossRef]
- Beck, K.; Thomson, J.S.; Swift, R.J.; von Hurst, P.R. Role of nutrition in performance enhancement and postexercise recovery. Open Access J. Sports Med. 2015, 6, 259. [Google Scholar] [CrossRef]
- Martín-Rodríguez, A.; Belinchón-deMiguel, P.; Rubio-Zarapuz, A.; Tornero-Aguilera, J.F.; Martínez-Guardado, I.; Villanueva-Tobaldo, C.V.; Clemente-Suárez, V.J. Advances in Understanding the Interplay between Dietary Practices, Body Composition, and Sports Performance in Athletes. Nutrients 2024, 16, 571. [Google Scholar] [CrossRef] [PubMed]
- Calleja-González, J.; Mielgo-Ayuso, J.; Sampaio, J.; Delextrat, A.; Ostojic, S.M.; Marques-Jiménez, D.; Arratibel, I.; Sánchez-Ureña, B.; Dupont, G.; Schelling, X.; et al. Brief ideas about evidence-based recovery in team sports. J. Exerc. Rehabil. 2018, 14, 545–550. [Google Scholar] [CrossRef]
- Diaz-Lara, J.; Nieto-Acevedo, R.; Abian-Vicen, J.; Del Coso, J. Can Caffeine Change the Game? Effects of Acute Caffeine Intake on Specific Performance in Intermittent Sports During Competition: A Systematic Review and Meta-Analysis. Int. J. Sports Physiol. Perform. 2024, 19, 1180–1196. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Eghbali, E.; Suzuki, K. Creatine Supplementation, Physical Exercise and Oxidative Stress Markers: A Review of the Mechanisms and Effectiveness. Nutrients 2021, 13, 869. [Google Scholar] [CrossRef]
- Senefeld, J.W.; Wiggins, C.C.; Regimbal, R.J.; Dominelli, P.B.; Baker, S.E.; Joyner, M.J. Ergogenic Effect of Nitrate Supplementation: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2020, 52, 2250–2261. [Google Scholar] [CrossRef]
- Ojeda, Á.H.; Cerda, C.T.; Salvatierra, M.F.P.; Barahona-Fuentes, G.; Aguilera, C.J. Effects of beta-alanine supplementation on physical performance in aerobic–anaerobic transition zones: A systematic review and meta-analysis. Nutrients 2020, 12, 2490. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Rezaei, M.J. The benefits of ashwagandha (Withania somnifera) supplements on brain function and sports performance. Front. Nutr. 2024, 11, 1439294. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Ott, I.; Calleja-González, J.; Mielgo-Ayuso, J. Ergo-Nutritional Intervention in Basketball: A Systematic Review. Nutrients 2022, 14, 638. [Google Scholar] [CrossRef]
- Miguel-Ortega, Á.; Calleja-Gonzalez, J.; Mielgo-Ayuso, J. Nutrition-Related considerations for health and performance in female Volleyball: A narrative review. J. Hum. Sport Exerc. 2023, 18, 462–477. [Google Scholar] [CrossRef]
- López-Samanes, Á.; Moreno-Pérez, V.; Kovacs, M.S.; Pallarés, J.G.; Mora-Rodríguez, R.; Ortega Fonseca, J.F. Use of nutritional supplements and ergogenic aids in professional tennis players. Nutr. Hosp. 2017, 34, 163–1468. [Google Scholar] [CrossRef]
- Miguel-Ortega, Á.; Calleja-González, J.; Mielgo-Ayuso, J. Endurance in Long-Distance Swimming and the Use of Nutritional Aids. Nutrients 2024, 16, 3949. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; Clark, J.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- O’Connor, D.; Green, S.; Higgins, J.P. Defining the Review Question and Developing Criteria for Including Studies. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008; pp. 81–94. [Google Scholar] [CrossRef]
- Horsley, T.; Dingwall, O.; Sampson, M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database Syst. Rev. 2011, 2011, MR000026. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008; Volume 2010, pp. 1–649. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.R.; Sackett, D.L.; Guyatt, G.H.; Tugwell, P. Clinical Epidemiology: How to Do Clinical Practice Research, 3rd ed.; Lippincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2012. [Google Scholar]
- Olivo, S.A.; Macedo, L.G.; Gadotti, I.C.; Fuentes, J.; Stanton, T.; Magee, D.J. Scales to assess the quality of randomized controlled trials: A systematic review. Phys. Ther. 2008, 88, 156–175. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, N.; Shaheen, A.; Ramadan, A.; Hefnawy, M.T.; Ramadan, A.; Ibrahim, I.A.; Hassanein, M.E.; Ashour, M.E.; Flouty, O. Appraising systematic reviews: A comprehensive guide to ensuring validity and reliability. Front. Res. Metr. Anal. 2023, 8, 1268045. [Google Scholar] [CrossRef]
- Ahn, E.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef]
- Kolaski, K.; Logan, L.R.; Ioannidis, J.P.A. Guidance to best tools and practices for systematic reviews. Syst. Rev. 2023, 12, 241–273. [Google Scholar] [CrossRef]
- Viswanathan, M.; Ansari, M.T.; Berkman, N.D.; Chang, S.; Hartling, L.; McPheeters, M.; Santaguida, P.L.; Shamliyan, T.; Singh, K.; Tsertsvadze, A.; et al. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. In Methods Guide for Effectiveness and Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012. [Google Scholar]
- Tan, F.; Polglaze, T.; Cox, G.; Dawson, B.; Mujika, I.; Clark, S. Effects of Induced Alkalosis on Simulated Match Performance in Elite Female Water Polo Players. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 198–205. [Google Scholar] [CrossRef]
- McCormick, R.; Peeling, P.; Binnie, M.; Dawson, B.; Sim, M. Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players. J. Int. Soc. Sports Nutr. 2016, 13, 41. [Google Scholar] [CrossRef]
- Brisola, G.M.P.; Artioli, G.G.; Papoti, M.; Zagatto, A.M. Effects of four weeks of β-alanine supplementation on repeated sprint ability in water polo players. PLoS ONE 2016, 11, e0167968. [Google Scholar] [CrossRef]
- Claus, G.M.; Redkva, P.E.; Brisola, G.M.P.; Malta, E.S.; De Araujo Bonetti De Poli, R.; Miyagi, W.E.; Zagatto, A.M. Beta-alanine supplementation improves throwing velocities in repeated sprint ability and 200-m swimming performance in young water polo players. Pediatr. Exerc. Sci. 2017, 29, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Van Dijk, J.W.; Senden, J.M.G.; Van Loon, L.J.C.; Verdijk, L.B. The effect of beetroot juice supplementation on dynamic apnea and intermittent sprint performance in elite female water polo players. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 468–473. [Google Scholar] [CrossRef]
- Pinheiro Brisola, G.M.; Redkva, P.E.; Pessoa Filho, D.M.; Papoti, M.; Zagatto, A.M. Effects of 4 weeks of β-alanine supplementation on aerobic fitness in water polo players. PLoS ONE 2018, 13, e0205129. [Google Scholar] [CrossRef]
- Elhusseini, R.; Fares, E.J.; Obeid, O. Phosphorus supplementation raised the heart rate of male water polo players during a randomised graded dryland exercise test. BMJ Open Sport Exerc. Med. 2020, 6, e000714. [Google Scholar] [CrossRef]
- Gambardella, J.; Fiordelisi, A.; Spigno, L.; Boldrini, L.; Lungonelli, G.; Di Vaia, E.; Santulli, G.; Sorriento, D.; Cerasuolo, F.A.; Trimarco, V.; et al. Effects of Chronic Supplementation of L-Arginine on Physical Fitness in Water Polo Players. Oxid. Med. Cell. Longev. 2021, 2021, 6684568. [Google Scholar] [CrossRef] [PubMed]
- Fritz, P.; Fritz, R.; Mayer, L.; Németh, B.; Ressinka, J.; Ács, P.; Oláh, C. Hungarian male water polo players’ body composition can predict specific playing positions and highlight different nutritional needs for optimal sports performance. BMC Sports Sci. Med. Rehabil. 2022, 14, 165. [Google Scholar] [CrossRef]
- La Mantia, I.; Maniaci, A.; Scibilia, G.; Scollo, P. Effects of a Dietary Microalgae (Arthrospira platensis) Supplement on Stress, Well-Being, and Performance in Water Polo Players: A Clinical Case Series. Nutrients 2024, 16, 2421. [Google Scholar] [CrossRef]
- Close, G.L.; Kasper, A.M.; Morton, J.P. From Paper to Podium: Quantifying the Translational Potential of Performance Nutrition Research. Sport. Med. 2019, 49, 25–37. [Google Scholar] [CrossRef]
- Solly, H.; Badenhorst, C.E.; McCauley, M.; Slater, G.J.; Gifford, J.A.; Erueti, B.; Beck, K.L. Athlete Preferences for Nutrition Education: Development of and Findings from a Quantitative Survey. Nutrients 2023, 15, 2519. [Google Scholar] [CrossRef]
- Bellinger, P.M. β-alanine supplementation for athletic performance: An update. J. Strength Cond. Res. 2014, 28, 1751–1770. [Google Scholar] [CrossRef] [PubMed]
- Viribay, A.; Burgos, J.; Fernández-Landa, J.; Seco-Calvo, J.; Mielgo-Ayuso, J. Effects of arginine supplementation on athletic performance based on energy metabolism: A systematic review and meta-analysis. Nutrients 2020, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Burgos, J.; Viribay, A.; Calleja-González, J.; Fernández-lázaro, D.; Olasagasti-Ibargoien, J.; Seco-Calvo, J.; Mielgo-Ayuso, J. Long-Term Combined Effects of Citrulline and Nitrate-Rich Beetroot Extract Supplementation on Recovery Status in Trained Male Triathletes: A Randomized, Double-Blind, Placebo-Controlled Trial. Biology 2022, 11, 75. [Google Scholar] [CrossRef]
- Fernández-Landa, J.; Todorovic, N.; Santibañez-Gutierrez, A.; Ostojic, S.M.; Calleja-González, J.; Sekulic, D.; Mielgo-Ayuso, J. Effects of HMB on Endurance Performance in a Healthy Population: A Systematic Review and Meta-Analysis. J. Strength Cond. Res. 2024, 38, E202–E210. [Google Scholar] [CrossRef]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, S.; Ramaglia, M.; Bellistri, G.; Pavei, G.; Pugliese, L.; Montorsi, M.; Rasica, L.; Marzorati, M. Aerobic fitness affects the exercise performance responses to nitrate supplementation. Med. Sci. Sports Exerc. 2015, 47, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Macuh, M.; Knap, B. Effects of nitrate supplementation on exercise performance in humans: A narrative review. Nutrients 2021, 13, 3183. [Google Scholar] [CrossRef]
- Bishop, D.; Claudius, B. Effects of induced metabolic alkalosis on prolonged intermittent-sprint performance. Med. Sci. Sports Exerc. 2005, 37, 759–767. [Google Scholar] [CrossRef]
- Price, M.; Moss, P.; Rance, S. Effects of sodium bicarbonate ingestion on prolonged intermittent exercise. Med. Sci. Sports Exerc. 2003, 35, 1303–1308. [Google Scholar] [CrossRef]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; da Silva, S.F.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J. Int. Soc. Sports Nutr. 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Hobson, R.M.; Saunders, B.; Ball, G.; Harris, R.C.; Sale, C. Effects of β-alanine supplementation on exercise performance: A meta-analysis. Amino Acids 2012, 43, 25–37. [Google Scholar] [CrossRef]
- Burke, L.M.; Cox, G.R.; Cummings, N.K.; Desbrow, B. Guidelines for daily carbohydrate intake: Do athletes achieve them? Sports Med. 2001, 31, 267–299. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.; Kreider, R.B.; Ziegenfuss, T.; La Bounty, P.; Roberts, M.; Burke, D.; Landis, J.; Lopez, H.; Antonio, J. International Society of Sports Nutrition position stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2007, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Sánchez-Oliver, A.J.; Cuenca, E.; Jodra, P.; da Silva, S.F.; Mata-Ordóñez, F. Nutritional needs in the professional practice of swimming: A review. J. Exerc. Nutr. Biochem. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Samanipour, M.H.; Mohammadian, S.; Del Coso, J.; Salehian, O.; Jeddi, F.K.; Khosravi, M.; González-Ravé, J.M.; Ceylan, H.İ.; Liu, H.; Abou Sawan, S.; et al. Body Composition and Dietary Intake Profiles of Elite Iranian Swimmers and Water Polo Athletes. Nutrients 2024, 16, 2393. [Google Scholar] [CrossRef]
- Fritz, P.; Katona, S.; Ignits, D. The nutritional and physiological effect of carbohydrates, their influences on sports nutrition. Recreation 2018, 8, 10–14. [Google Scholar] [CrossRef]
- Kanter, M. High-Quality Carbohydrates and Physical Performance. Nutr. Today 2018, 53, 35–39. [Google Scholar] [CrossRef]
- Mountjoy, M.; Fina, B.; Maughan, R.; Burke, L.; Benardot, D.; Costill, D.; Cox, G.; Derave, W.; Koivsto, A.; Melin, A.; et al. Nutrition for Aquatic Athletes a Practical Guide to Eating for Health and Performance Nutrition for Aquatic Athletes. 2013. 50p. Available online: https://resources.fina.org/fina/document/2021/02/04/5c14b311-7eba-4d2b-9114-acf13d300683/nutrition_for_aquatic_athletes_booklet_v5_final.pdf (accessed on 31 January 2025).
- Tzelatis, D.; Mougios, V. Nutritional Knowledge of Water Polo Players. Proceedings 2019, 25, 39. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Periodized Nutrition for Athletes. Sports Med. 2017, 47, 51–63. [Google Scholar] [CrossRef]
- Halson, S.L. Monitoring Training Load to Understand Fatigue in Athletes. Sports Med. 2014, 44, 139. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Mujika, I. Nutrition for recovery in aquatic sports. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Meng, Q.; Su, C.H. From Food Supplements to Functional Foods: Emerging Perspectives on Post-Exercise Recovery Nutrition. Nutrients 2024, 16, 4081. [Google Scholar] [CrossRef] [PubMed]
- Kellmann, M.; Bertollo, M.; Bosquet, L.; Brink, M.; Coutts, A.J.; Duffield, R.; Erlacher, D.; Halson, S.L.; Hecksteden, A.; Heidari, J.; et al. Recovery and performance in sport: Consensus statement. Int. J. Sports Physiol. Perform. 2018, 13, 240–245. [Google Scholar] [CrossRef] [PubMed]



| Study | Level of Evidence |
|---|---|
| Tan et el. (2010) [53] | 1B |
| Cox et al. (2014) [25] | 1A |
| McCormick et al. (2016) [54] | 1B |
| Brisola et al. (2016) [55] | 1B |
| Claus et al. (2017) [56] | 1B |
| Jonvik et al. (2018) [57] | 1B |
| Brisola et al. (2018) [58] | 1B |
| Elhusseini et al. (2020) [59] | 1B |
| Gambardella et al. (2021) [60] | 1B |
| Fritz et al. (2022) [61] | 1B |
| La Mantia et al. (2024) [62] | 1B |
| Scope of Knowledge | Journal | Q | Authors | Population | Age Ranges (Years) | Method | Intervention | Variables | Outcomes Analysed | Main Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| SM | Int J Sport Nutr Exerc Metab | 1 | Tan et el. (2010) [53] | 12 ♀ EWPp | 23.7 ± 3.0 | DB | 1 week | NaHCO3 | Antrop; MTS; 4 × 10 MSs; BL; PER; Bf; Ad; HR | Average sprint performance |
| SM | Int J Sport Nutr Exerc Metab | 1 | Cox et al. (2014) [25] | ♂ and ♀ EWPp | - | DB | - | PD; DI; BC; N; SSFs | TMA; PDDC; FDC; DI; BC; NS; H | Uses all metabolic pathways in ♂ HIs is + important for ♀ in the game area In central ♀ players are + important in the fight maxFC above 80% in ♂ aFCr in ♀ is 80% Gd high due to sustained intensity Ss deteriorates with fatigue Di Pt higher in ♂ tan in ♀ ♂ balanced mesomorphs ♀ endomorphs. HMB does not influence S and FFM use of NaHCO3 is beneficial in en ♀ CM no clear benefit in ♂ |
| FS | J Int Soc Sports Nutr | 2 | McCormick et al. (2016) [54] | 9 ♂ EWPp | 18.6 ± 1.4 | DB | 7 days | sCJ with [high] of PhCs and Atc | IL-6; CRP; Ua; F2-IsoP; TQR; DOMS | Recovery of performance |
| M | PLoS ONE | 1 | Brisola et al. (2016) [55] | 22 ♂ EWPp | 18 ± 4 | DB | 4 weeks | RSA; PER; BL; 30 m s | β-a | Slight improvement for RSA |
| SM | Pediatr Exerc Sci | 1 | Claus et al. (2017) [56] | 15 ♂ EWPp | 16 ± 2 | DB | 6 weeks | Wt; Mdtx; β-a | RSAwft; Mx; 200-m s; BL | Performance |
| SM | Int J Sport Nutr Exerc Metab | 1 | Jonvik et al. (2018) [57] | 14 ♀ EWPp | 22 ± 4 | DB | 4 weeks | DAt; Ist | Nitrate | Performance in Ist |
| DAt | ||||||||||
| Me | PLoS ONE | 1 | Brisola et al. (2018) [58] | 22 ♂ EWPp | 18 ± 4 | DB | 4 weeks | β-a | VO2pmax; SVO2pmax; 3AO | SVO2pmax |
| VO2pmax | ||||||||||
| SM | BMJ Open Sport Exerc Med | 2 | Elhusseini et al. (2020) [59] | 12 ♂ EWPp | 18–22 | DB | 2 measurements on a CE | P G | BC; HR; ee; EE; RQ; RR; pt | P does not affect ee P does not affect EE Significant increase in the HR. No significant differences in the eE. |
| Me | Oxid Med Cell Longev | 1 | Gambardella et al. (2021) [60] | 17 ♂ EWPp | 29.3 ± 1.66 | DB | 4 weeks | MsS; BL; IGF1; CPK; MPul: | L-Arginine | Performance |
| SM | BMC Sports Sci Med Rehabil | 3 | Fritz et al. (2022) [61] | 19 ♂ EWPp | 18–34 | DB | 4 meses | BC Antrop Nh | BMC; EW; SMM; BcM IW; FFM; TBW; BMR; BfM; WBPA; RBC; Hm; RBCn; G; Cr; GGT; GPT; GOT; F; Lk; U; CK; Ua; Hg; Ch; HDL; LDL; Tg; Na; K; Mg; Ca; vD | Di Pt and CHO are much lower than the recommendations opt DI Pt is before and after training, and regularly 3 to 5 times a day High [K] for big tV U ll vD ll associated with overload |
| FS | Nutrients | 1 | La Mantia et al. (2024) [62] | 20 ♂ EWPp | 18–35 | DB | 8 weeks | Spirulina + Cu | ASPS CPK | ASPS Performance Muscle tension |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguel-Ortega, Á.; Barrenetxea-Garcia, J.; Rodríguez-Rodrigo, M.-A.; García-Ordóñez, E.; Mielgo-Ayuso, J.; Calleja-González, J. Ergonutrition Supplementation and Recovery in Water Polo: A Systematic Review. Nutrients 2025, 17, 1319. https://doi.org/10.3390/nu17081319
Miguel-Ortega Á, Barrenetxea-Garcia J, Rodríguez-Rodrigo M-A, García-Ordóñez E, Mielgo-Ayuso J, Calleja-González J. Ergonutrition Supplementation and Recovery in Water Polo: A Systematic Review. Nutrients. 2025; 17(8):1319. https://doi.org/10.3390/nu17081319
Chicago/Turabian StyleMiguel-Ortega, Álvaro, Josu Barrenetxea-Garcia, María-Azucena Rodríguez-Rodrigo, Enrique García-Ordóñez, Juan Mielgo-Ayuso, and Julio Calleja-González. 2025. "Ergonutrition Supplementation and Recovery in Water Polo: A Systematic Review" Nutrients 17, no. 8: 1319. https://doi.org/10.3390/nu17081319
APA StyleMiguel-Ortega, Á., Barrenetxea-Garcia, J., Rodríguez-Rodrigo, M.-A., García-Ordóñez, E., Mielgo-Ayuso, J., & Calleja-González, J. (2025). Ergonutrition Supplementation and Recovery in Water Polo: A Systematic Review. Nutrients, 17(8), 1319. https://doi.org/10.3390/nu17081319









