The Impact of Dietary Sugars and Saturated Fats on Body and Liver Fat in a Healthcare Worker Population
Abstract
1. Introduction
- Steatosis due to excess triglycerides: Accumulation of triglycerides in the liver.
- Persistent insulin resistance: Leading to decreased glycogen synthesis, increased hepatic fatty acid uptake, altered triglyceride transport, and inhibited beta-oxidation.
- Pro-inflammatory cytokine activity: Driven by lipotoxicity, apoptosis, inflammasome activation, and mitochondrial dysfunction.
- Oxidative stress: An imbalance between pro-inflammatory and anti-inflammatory mechanisms that exacerbates insulin resistance in the presence of genetic or environmental susceptibility.
- Impaired hepatocyte regeneration and apoptosis.
2. Methods
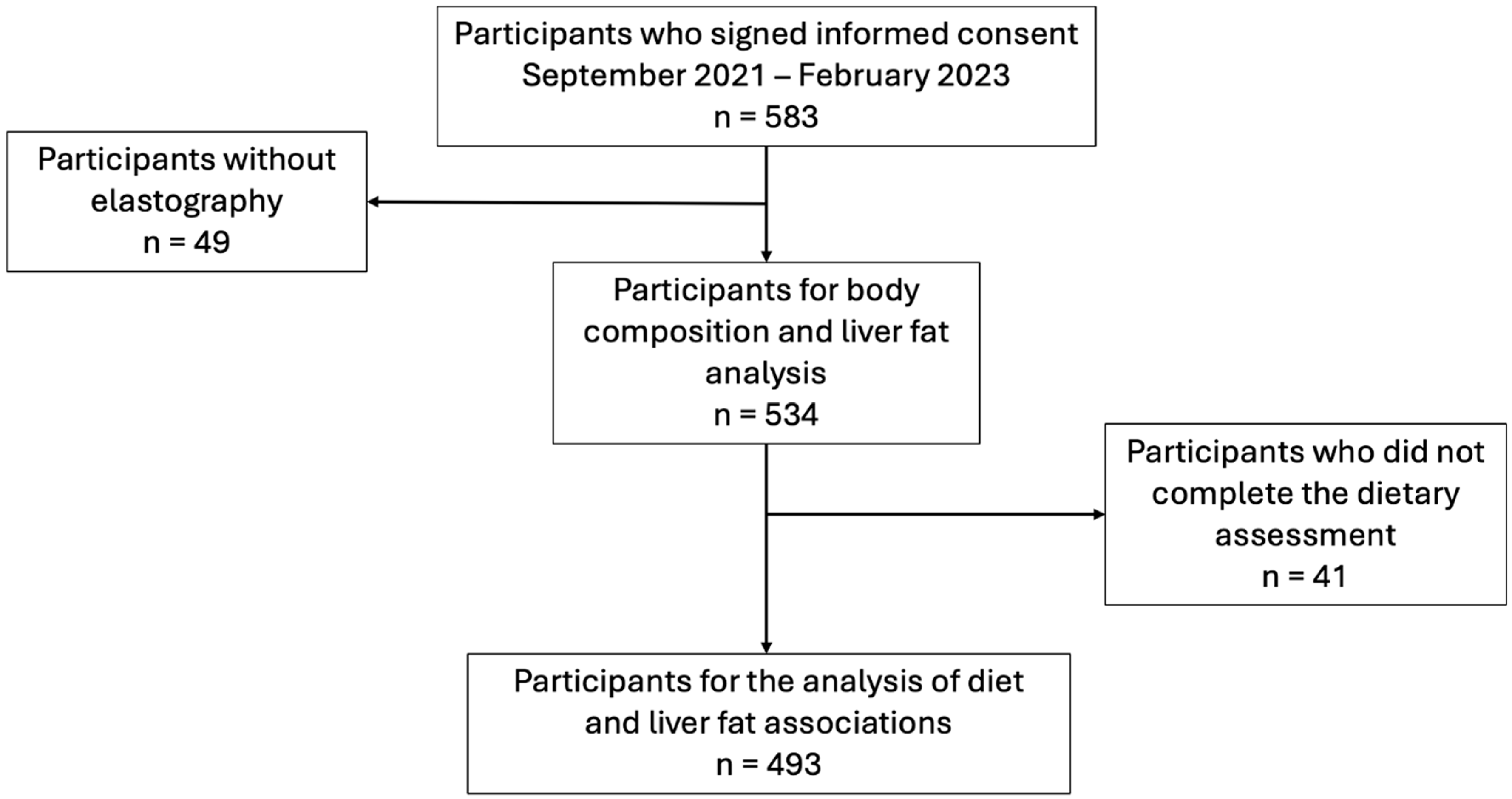
2.1. Population
2.2. Demographic, Clinical, and Biochemical Data
2.3. Body Composition Assessment
2.4. Liver Fat Assessment
2.5. Dietary Assessment
2.6. Sample Size
2.7. Statistical Analysis
- (1)
- Degree of association between nutrient intake (quantitative) and hepatic steatosis.
- (2)
- Nutrient intake distributed in quartiles, with quartile 1 as reference.
- (3)
- The association between the total sugar consumption > 10% (model 1) and saturated fat > 7% (model 2) with hepatic steatosis.
2.8. Ethical Procedures
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.-Y.; Zheng, M.-H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Berná, G.; Romero-Gomez, M. The role of nutrition in non-alcoholic fatty liver disease: Pathophysiology and management. Liver Int. 2020, 40, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef]
- Maersk, M.; Belza, A.; Stødkilde-Jørgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef]
- Simons, N.; Veeraiah, P.; Simons, P.I.; Schaper, N.C.; Kooi, M.E.; Schrauwen-Hinderling, V.B.; Feskens, E.J.; Van Der Ploeg, E.; Van Den Eynde, M.D.; Schalkwijk, C.G.; et al. Effects of fructose restriction on liver steatosis (FRUITLESS); a double-blind randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 391–400. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Blendis, L.; Halpern, Z.; Oren, R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 2007, 47, 711–717. [Google Scholar] [CrossRef]
- Asgari-Taee, F.; Zerafati-Shoae, N.; Dehghani, M.; Sadeghi, M.; Baradaran, H.R.; Jazayeri, S. Association of sugar sweetened beverages consumption with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Nutr. 2019, 58, 1759–1769. [Google Scholar] [CrossRef]
- Ma, J.; Fox, C.S.; Jacques, P.F.; Speliotes, E.K.; Hoffmann, U.; Smith, C.E.; Saltzman, E.; McKeown, N.M. Sugar-sweetened beverage, diet soda, and fatty liver disease in the Framingham Heart Study cohorts. J. Hepatol. 2015, 63, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.-M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a High-Fructose Weight-Maintaining Diet on Lipogenesis and Liver Fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442. [Google Scholar] [CrossRef]
- Luukkonen, P.K.; Sädevirta, S.; Zhou, Y.; Kayser, B.; Ali, A.; Ahonen, L.; Lallukka, S.; Pelloux, V.; Gaggini, M.; Jian, C.; et al. Saturated Fat Is More Metabolically Harmful for the Human Liver Than Unsaturated Fat or Simple Sugars. Diabetes Care 2018, 41, 1732–1739. [Google Scholar] [CrossRef]
- Sobrecases, H.; Lê, K.-A.; Bortolotti, M.; Schneiter, P.; Ith, M.; Kreis, R.; Boesch, C.; Tappy, L. Effects of short-term overfeeding with fructose, fat and fructose plus fat on plasma and hepatic lipids in healthy men. Diabetes Metab. 2010, 36, 244–246. [Google Scholar] [CrossRef]
- Schneider, B.C.; Dumith, S.C.; Orlandi, S.P.; Assunção, M.C.F. Diet and body fat in adolescence and early adulthood: A systematic review of longitudinal studies. Ciênc. Saúde Coletiva 2017, 22, 1539–1552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perdomo, C.M.; Frühbeck, G.; Escalada, J. Impact of Nutritional Changes on Nonalcoholic Fatty Liver Disease. Nutrients 2019, 11, 677. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings. Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.P.; Bartholomew, J.; Bau, S.; Gilles, H.; Keller, A.; Moore, A.; Nader, K.; Richards, L.; Henry, L.; Younossi, Z.M. Focused Recommendations for the Management of Metabolic Dysfunction-Associated Steatohepatitis (MASH) by Advanced Practice Providers in the United States. J. Clin. Gastroenterol. 2025, 59, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Yki-Järvinen, H.; Luukkonen, P.K.; Hodson, L.; Moore, J.B. Dietary carbohydrates and fats in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 770–786. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wai-Sun Wong, V.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, Y.; Bian, S.; Lu, Z.; Zhang, Q.; Liu, L.; Meng, G.; Yao, Z.; Wu, H.; Wang, Y.; et al. Soft drink consumption and risk of nonalcoholic fatty liver disease: Results from the Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIH) cohort study. Am. J. Clin. Nutr. 2021, 113, 1265–1274. [Google Scholar] [CrossRef]
- Eslam, M.; Sarin, S.K.; Wong, V.W.-S.; Fan, J.-G.; Kawaguchi, T.; Ahn, S.H.; Zheng, M.-H.; Shiha, G.; Yilmaz, Y.; Gani, R.; et al. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol. Int. 2020, 14, 889–919. [Google Scholar] [CrossRef]
- Sato-Espinoza, K.; Chotiprasidhi, P.; Huaman, M.R.; Díaz-Ferrer, J. Update in lean metabolic dysfunction-associated steatotic liver disease. World J. Hepatol. 2024, 16, 452–464. [Google Scholar] [CrossRef]
- Tao, M.; Liu, J.; Chen, X.; Wang, Q.; He, M.; Chen, W.; Wang, C.; Zhang, L. Correlation between serum uric acid and body fat distribution in patients with MAFLD. BMC Endocr. Disord. 2023, 23, 204. [Google Scholar] [CrossRef]
- Iyengar, K.P.; Ish, P.; Upadhyaya, G.K.; Malhotra, N.; Vaishya, R.; Jain, V.K. COVID-19 and mortality in doctors. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1743–1746. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, U.J.; Lee, H.A.; Choi, E.J.; Park, H.J.; Ahn, H.S.; Park, H.; Policy Development Committee of NAMOK. Health and Mortality in Korean Healthcare Workers. J. Korean Med. Sci. 2022, 37, e22. [Google Scholar] [CrossRef] [PubMed]
- Kunyahamu, M.S.; Daud, A.; Jusoh, N. Obesity among Health-Care Workers: Which Occupations Are at Higher Risk of Being Obese? Int. J. Environ. Res. Public Health 2021, 18, 4381. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, V.D.S.; Jost, T.D.A.; Pasqual, H.M.; Becker, A.L.G.; Marques, L.M.; Manica, M.; Delani, B.L.L.; Langaro, J.P.; Afonso, D.T.; Hoppe, L.; et al. Non-alcoholic fatty liver disease and associated risk factors in health care professionals in a community hospital in Brazil. Rev. Bras. Med. Trab. 2020, 18, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Cevallos, P.; Ordóñez-Vázquez, A.L.; Procopio-Mosso, O.; Cardoso-Arias, R.; Uribe, M.; Chávez-Tapia, N.C. Cross-sectional pilot study to assess primary healthcare workers’ knowledge of nonalcoholic fatty liver disease in a marginalized community in Mexico. Sci. Rep. 2021, 11, 12100. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK541070/ (accessed on 28 March 2025).
- López Alvarenga, J.C.; Reyes Díaz, S.; Castillo Martínez, L.; Dávalos Ibañez, A. Reproducibilidad y sensibilidad de un cuestionario de actividad física en población mexicana. Salud Pública México 2001, 43, 306–312. [Google Scholar] [CrossRef]
- de los Ángeles Espinosa-Cuevas, M.; Rivas-Rodríguez, L.; González-Medina, E.; Atilano-Carsi, X. Vectores de impedancia bioeléctrica para la composición corporal en población mexicana. Rev. Investig. Clin. 2007, 59, 15–24. [Google Scholar]
- Jensen, B.; Moritoyo, T.; Kaufer-Horwitz, M.; Peine, S.; Norman, K.; Maisch, M.J.; Matsumoto, A.; Masui, Y.; Velázquez-González, A.; Domínguez-García, J.; et al. Ethnic differences in fat and muscle mass and their implication for interpretation of bioelectrical impedance vector analysis. Appl. Physiol. Nutr. Metab. 2019, 44, 619–626. [Google Scholar] [CrossRef]
- Durnin, J.V.G.A.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 Years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Salvador Castell, G.; Serra-Majem, L.; Ribas-Barba, L. What and how much do we eat? 24-h dietary recall method. Nutr. Hosp. 2015, 31 (Suppl. 3), 46–48. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Issues in analysis and presentation of dietary data. In Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2012; pp. 305–333. Available online: https://academic.oup.com/book/27443/chapter-abstract/197325993?redirectedFrom=fulltext (accessed on 28 March 2025).
- World Health Organization. Directriz: Ingesta de Azúcares para Adultos y Niños: Resumen. 2015. Available online: https://iris.who.int/handle/10665/154587 (accessed on 28 March 2025).
- Diaz Romero, P. Sobre el Reconocimiento de la Enfermedad Como Experiencia Subjetiva y su Impacto en la Salud Pública. RBD. 2022. Available online: https://revistes.ub.edu/index.php/RBD/article/view/32562 (accessed on 28 March 2025).
- Noroña-Salcedo, D.R. Estrés Laboral y Autopercepción del Personal de Salud. 2023. Available online: https://zenodo.org/doi/10.5281/zenodo.10048611 (accessed on 28 March 2025).
- Priego-Parra, B.A.; Reyes-Diaz, S.A.; Ordaz-Alvarez, H.R.; Bernal-Reyes, R.; Icaza-Chávez, M.E.; Martínez-Vázquez, S.E.; Amieva-Balmori, M.; Vivanco-Cid, H.; Velasco, J.A.V.-R.; Gracia-Sancho, J.; et al. Diagnostic performance of sixteen biomarkers for MASLD: A study in a Mexican cohort. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102400. [Google Scholar] [CrossRef]
- Pan, Z.; Derbala, M.; AlNaamani, K.; Ghazinian, H.; Fan, J.-G.; Eslam, M. MAFLD criteria are better than MASLD criteria at predicting the risk of chronic kidney disease. Ann. Hepatol. 2024, 29, 101512. [Google Scholar] [CrossRef] [PubMed]
- NCEP. National Cholesterol Education Program. Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). NCEP ATP III. Available online: https://www.nhlbi.nih.gov/files/docs/guidelines/atp3xsum.pdf (accessed on 22 October 2024).
- Ewers, B.; Vilsbøll, T.; Andersen, H.U.; Bruun, J.M. The dietary education trial in carbohydrate counting (DIET-CARB Study): Study protocol for a randomised, parallel, open-label, intervention study comparing different approaches to dietary self-management in patients with type 1 diabetes. BMJ Open 2019, 9, e029859. [Google Scholar] [CrossRef] [PubMed]
- Howes, E.M.; Parker, M.K.; Misyak, S.A.; DiFeliceantonio, A.G.; Davy, B.M.; Brown, L.E.C.; Hedrick, V.E. The Impact of Weight Bias and Stigma on the 24 h Dietary Recall Process in Adults with Overweight and Obesity: A Pilot Study. Nutrients 2024, 16, 191. [Google Scholar] [CrossRef]
- Rosqvist, F.; Kullberg, J.; Ståhlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.-E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating Saturated Fat Promotes Fatty Liver and Ceramides Compared With Polyunsaturated Fat: A Randomized Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef]
- Donghia, R.; Tatoli, R.; Campanella, A.; Losurdo, G.; Di Leo, A.; De Pergola, G.; Bonfiglio, C.; Giannelli, G. Extra Virgin Olive Oil Reduces the Risk of Non-Alcoholic Fatty Liver Disease in Females but Not in Males: Results from the NUTRIHEP Cohort. Nutrients 2024, 16, 3234. [Google Scholar] [CrossRef]
- Rezaei, S.; Akhlaghi, M.; Sasani, M.R.; Barati Boldaji, R. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Nutrition 2019, 57, 154–161. [Google Scholar] [CrossRef]
- Lee, D.; Chiavaroli, L.; Ayoub-Charette, S.; Khan, T.A.; Zurbau, A.; Au-Yeung, F.; Cheung, A.; Liu, Q.; Qi, X.; Ahmed, A.; et al. Important Food Sources of Fructose-Containing Sugars and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis of Controlled Trials. Nutrients 2022, 14, 2846. [Google Scholar] [CrossRef] [PubMed]
- Sepehrinia, M.; Khanmohammadi, S.; Rezaei, N.; Kuchay, M.S. Dietary inflammatory potential and metabolic (dysfunction)-associated steatotic liver disease and its complications: A comprehensive review. Clin. Nutr. ESPEN 2025, 65, 162–171. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Tian, F.; Qian, X.-L.; Ying, H.-M.; Zhou, Z.-F. Effect of 5:2 intermittent fasting diet versus daily calorie restriction eating on metabolic-associated fatty liver disease—A randomized controlled trial. Front. Nutr. 2024, 11, 1439473. [Google Scholar] [CrossRef]
- Dobbie, L.J.; Burgess, J.; Hamid, A.; Nevitt, S.J.; Hydes, T.J.; Alam, U.; Cuthbertson, D.J. Effect of a Low-Calorie Dietary Intervention on Liver Health and Body Weight in Adults with Metabolic-Dysfunction Associated Steatotic Liver Disease (MASLD) and Overweight/Obesity: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1030. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Rustichelli, A.; Dongiovanni, P. Nutrition and Genetics in NAFLD: The Perfect Binomium. Int. J. Mol. Sci. 2020, 21, 2986. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D.; Cogger, V.C.; Macia, L.; Solon-Biet, S.M.; Le Couteur, D.G.; George, J. The nutritional geometry of liver disease including non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 316–325. [Google Scholar] [CrossRef]
- Makhsous, S.; Bharadwaj, M.; Atkinson, B.E.; Novosselov, I.V.; Mamishev, A.V. DietSensor: Automatic Dietary Intake Measurement Using Mobile 3D Scanning Sensor for Diabetic Patients. Sensors 2020, 20, 3380. [Google Scholar] [CrossRef] [PubMed]
- Satija, A.; Yu, E.; Willett, W.C.; Hu, F.B. Understanding nutritional epidemiology and its role in policy. Adv. Nutr. 2015, 6, 5–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freedman, L.S.; Commins, J.M.; Moler, J.E.; Willett, W.; Tinker, L.F.; Subar, A.F.; Spiegelman, D.; Rhodes, D.; Potischman, N.; Neuhouser, M.L.; et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am. J. Epidemiol. 2015, 181, 473–487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Betancourt-Nuñez, A.; Márquez-Sandoval, F.; González-Zapata, L.I.; Babio, N.; Vizmanos, B. Unhealthy dietary patterns among healthcare professionals and students in Mexico. BMC Public Health 2018, 18, 1246. [Google Scholar] [CrossRef]
- Krag, A.; Torp, N.; Younossi, Z.M.; Israelsen, M. Reporting discrepancy of alcohol intake affecting estimated prevalence of MetALD and ALD. Lancet Gastroenterol. Hepatol. 2025, 10, 282–284. [Google Scholar] [CrossRef] [PubMed]

| Total Sample (n = 534) | No Steatosis (n = 307) | With Steatosis (n = 227) | p Value | |
|---|---|---|---|---|
| Age (years) | 41.5 (29.0–52.0) | 36.0 (27.0–51.0) | 45.0 (34.0–53.0) | <0.0001 |
| Sex, n (%) | ||||
| Women | 328 (61.4) | 199 (64.8) | 129 (56.8) | 0.061 |
| Smoking, n (%) | 41 (7.68) | 18 (5.86) | 23 (10.13) | 0.062 |
| Comorbidities, n (%) | ||||
| Diabetes | 9 (1.69) | 3 (0.98) | 6 (2.64) | 0.178 * |
| Hypertension | 21 (3.93) | 9 (2.93) | 12 (5.29) | 0.166 |
| Dyslipidemia | 3 (0.56) | 0 (0.00) | 3 (1.32) | 0.077 |
| Hypothyroidism | 9 (1.69) | 5 (1.63) | 4 (1.76) | 0.999 |
| Insulin resistance | 4 (0.75) | 1 (0.33) | 3 (1.32) | 0.316 |
| Job categories | ||||
| Medical staff | 31 (5.8) | 20 (6.5) | 11 (4.8) | 0.857 |
| Nursing staff | 46 (8.6) | 23 (7.5) | 23 (10.1) | |
| Non-medical clinical staff | 43 (8.1) | 26 (8.5) | 17 (7.5) | |
| Administrative staff | 126 (23.8) | 74 (24.1) | 52 (22.9) | |
| Not disclosed | 288 (53.9) | 164 (53.4) | 124 (54.6) | |
| Body composition | ||||
| Weight (kg) | 70.5 (60.6–80.7) | 64.2 (56.7–74.5) | 77.8 (69.4–86.4) | <0.0001 |
| BMI | 27.1 (23.8–30.3) | 25.1 (22.3–27.8) | 29.4 (27.1–32.8) | <0.0001 |
| <18.5 | 5 (0.9) | 5 (1.6) | 0 (0.0) | <0.0001 |
| 18–24.9 | 164 (30.7) | 146 (47.6) | 18 (7.9) | |
| 25–29.9 | 224 (41.9) | 117 (38.1) | 107 (47.1) | |
| 30–34.9 | 110 (20.6) | 34 (11.1) | 76 (33.5) | |
| 35–39.9 | 26 (4.9) | 3 (1.0) | 23 (10.1) | |
| ≥40 | 5 (0.09) | 2 (0.7) | 3 (1.3) | |
| Fat mass (kg) | 25.1 (18.7–31.5) | 20.8 (15.5–27.4) | 29.6 (25.2–35.8) | <0.0001 |
| Fat mass (%) | 35.6 (29.0–42.2) | 32.9 (26.2–38.7) | 39.7 (32.6–44.7) | <0.0001 |
| Visceral fat (L) | 2.50 (1.80–3.50) | 2.00 (1.50–2.70) | 3.20 (2.48–4.20) | <0.0001 |
| Waist circumference (m) | 0.89 (0.80–0.99) | 0.84 (0.76–0.92) | 0.96 (0.89–1.04) | <0.0001 |
| Bicipital skinfold (mm) | 10.0 (7.0–14.0) | 8.0 (6.0–12.0) | 12.0 (9.0–17.0) | <0.0001 |
| Triceps skinfold (mm) | 17.0 (12.0–22.0) | 15.0 (12.0–19.0) | 20.0 (14.0–25.0) | <0.0001 |
| Subscapular skinfold (mm) | 23.0 (17.0–30.0) | 19.0 (15.0–25.0) | 28.0 (23.0–33.0) | <0.0001 |
| Suprailiac skinfold (mm) | 24.0 (17.0–30.0) | 20.0 (15.0–27.0) | 28.0 (24.0–35.0) | <0.0001 |
| Physical activity | ||||
| Kcals from PAQ * | 737.7 (636.5–852.2) | 699.4 (600.9–814.2) | 781.3 (697.7–928.2) | <0.0001 |
| Z Score PAQ * | 0.00 (1.00) | −0.27 (0.89) | 0.36 (−0.13) | <0.0001 |
| <−1 SD | 75 (14.0) | 65 (22.0) | 10 (4.5) | <0.0001 |
| −1 to 1 SD | 368 (68.9) | 203 (68.6) | 165 (74.3) | |
| >1 SD | 75 (14.0) | 28 (9.5) | 47 (21.2) | |
| Energy balance | 529.5 (203.1–920.9) | 557.9 (249.8–980.9) | 463.00 (153.1–829.4) | 0.013 |
| Z Score Energy balance | 0.00 (1.00) | 0.09 (1.01) | −0.13 (0.97) | 0.01 |
| <−1 SD | 84 (15.7) | 14 (13.0) | 44 (19.4) | 0.04 |
| −1 to 1 SD | 370 (69.3) | 216 (70.4) | 154 (67.8) | |
| >1 SD | 80 (15.0) | 51 (16.6) | 29 (12.8) | |
| Biochemical data | ||||
| HOMA IR | 1.58 (1.04–2.62) | 1.25 (0.81–1.78) | 2.45 (1.55–3.85) | <0.0001 |
| Glucose (mg/dL) | 89.0 (84.0–96.0) | 87.0 (82.0–92.0) | 93.0 (88.0–101.0) | <0.0001 |
| Creatinine (mg/dL) | 0.76 (0.66–0.89) | 0.74 (0.66–0.87) | 0.77 (0.66–0.92) | 0.152 |
| Cholesterol (mg/dL) | 180.0 (155.0–206.0) | 177.0 (155.0–202.0) | 185.0 (156.0–217.0) | 0.009 |
| Low-density cholesterol (LDL-c; mg/dL) | 110.0 (89.0–129.0) | 105.0 (86.0–124.0) | 116.0 (92.5–134.5) | 0.001 |
| LDL-c, Martin’s method (mg/dL) | 107.0 (86.0–126.0) | 104.0 (83.0–122.0) | 112.0 (88.0–133.0) | 0.003 |
| High-density cholesterol (HDL-c; mg/dL) | 47.0 (40.0–56.0) | 50.0 (43.0–59.0) | 43.0 (37.0–51.0) | <0.0001 |
| Triglycerides (mg/dL) | 124.0 (90.0–174.0) | 106.0 (79.0–142.0) | 163.0 (114.5–233.0) | <0.0001 |
| Total bilirubin (mg/dL) | 0.64 (0.49–0.85) | 0.64 (0.49–0.85) | 0.63 (0.51–0.83) | 0.762 |
| Alanine aminotransferase (ALT; U/L) | 21.6 (15.4–32.2) | 18.1 (13.9–26.0) | 26.5 (18.0–40.7) | <0.0001 |
| Aspartate aminotransferase (AST; U/L) | 19.6 (17.0–24.8) | 19.1 (16.9–23.7) | 21.4 (17.2–26.7) | 0.002 |
| Gammaglutamyl transferase (U/L) | 21.4 (15.1–35.3) | 17.9 (13.1–26.7) | 27.9 (19.1–42.6) | <0.0001 |
| Alkaline phosphatase (U/L) | 74.0 (61.5–88.0) | 71.0 (58.0–85.0) | 78.0 (66.0–92.0) | <0.0001 |
| Albumin (g/dL) | 4.42 (4.23–4.62) | 4.46 (4.25–4.64) | 4.38 (4.22–4.59) | 0.066 |
| Ultra-sensitive C-reactive protein (mg/dL) | 0.15 (0.08–0.31) | 0.13 (0.06–0.24) | 0.20 (0.11–0.41) | <0.0001 |
| Insulin (μIU/mL) | 7.19 (4.95–11.38) | 5.72 (3.96–8.15) | 10.43 (6.91–16.02) | <0.0001 |
| Platelets (103/μL) | 249.0 (213.0–289.5) | 251.0 (214.0–290.0) | 242.5 (211.0–287.2) | 0.496 |
| Total Sample (n = 493) | No Steatosis (n = 282) | With Steatosis (n = 211) | p Value | |
|---|---|---|---|---|
| Kilocalories | 1278.6 (1021.5–1533.9) | 1326.2 (1050.7–1557.7) | 1188.7 (991.8–1499.0) | 0.009 |
| Nutrients | ||||
| Carbohydrates (g) | 143.6 (67.5–211.7) | 145.1 (53.6–216.96) | 142.9 (78.0–208.5) | 0.959 |
| Protein (g) | 55.2 (44.7–69.0) | 56.4 (46.5–70.5) | 53.1 (40.8–65.8) | 0.019 |
| Fat (g) | 43.1 (30.6–75.2) | 45.2 (32.5–86.6) | 41.3 (28.8–62.3) | 0.008 |
| Kilocalories per carbohydrate | 574.5 (270.1–846.7) | 580.2 (214.4–867.8) | 571.7 (312.2–834.1) | 0.959 |
| Carbohydrates (%) | 53.7 (28.2–60.0) | 51.8 (13.6–60.0) | 55.4 (41.2–60.0) | 0.253 |
| Kilocalories from protein | 221.0 (179.0–276.2) | 225.9 (186.2–281.9) | 212.3 (163.5–263.4) | 0.019 |
| Protein (%) | 16.6 (15.2–18.9) | 16.9 (15.4–19.4) | 16.5 (15.0–18.6) | 0.241 |
| Kilocalories from fat | 387.7 (275.7–676.8) | 407.3 (293.1–780.1) | 372.0 (259.2–560.9) | 0.008 |
| Fats (%) | 26.8 (23.2–49.6) | 27.8 (23.2–70.5) | 26.8 (23.1–39.4) | 0.435 |
| Saturated fat (g) | 16.8 (11.6–28.6) | 17.7 (12.1–32.1) | 15.8 (10.9–24.9) | 0.022 |
| Kilocalories per saturated fat | 151.4 (104.3–257.9) | 159.4 (109.1–288.7) | 142.4 (98.4–224.9) | 0.022 |
| Saturated Fat (%) | 10.6 (9.05–18.35) | 10.6 (9.11–25.98) | 10.5 (9.05–14.82) | 0.587 |
| Monounsaturated fat (g) | 3.91 (1.56–7.47) | 3.98 (1.62–7.56) | 3.50 (1.41–7.24) | 0.516 |
| Polyunsaturated fat (g) | 1.76 (0.81–3.36) | 2.02 (0.90–3.39) | 1.60 (0.78–3.34) | 0.232 |
| Trans fats (g) | 0.00 (0.00–0.12) | 0.00 (0.00–0.08) | 0.00 (0.00–0.13) | 0.250 |
| Types of carbohydrates | ||||
| Available CH (g) | 134.2 (57.8–197.5) | 134.3 (47.5–202.4) | 134.2 (68.4–190.0) | 0.886 |
| Total sugars (g) | 28.0 (17.98–55.39) | 28.4 (18.61–52.03) | 28.0 (16.68–55.99) | 0.352 |
| Added sugar (g) | 7.53 (3.39–20.14) | 7.53 (3.52–21.04) | 7.67 (3.28–19.36) | 0.649 |
| Total sugars (%) | 8.57 (6.43–14.73) | 8.57 (6.43–14.64) | 8.57 (6.43–16.14) | 0.728 |
| Added sugars (%) | 2.44 (1.30–5.65) | 2.43 (1.30–5.71) | 2.49 (1.25–5.63) | 0.845 |
| Monosaccharides (g) | 7.80 (3.69–12.40) | 8.44 (4.40–12.72) | 6.76 (2.33–12.00) | 0.037 |
| Galactose (g) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.794 |
| Glucose (g) | 2.91 (1.52–4.71) | 3.06 (1.99–4.67) | 2.51 (0.99–4.72) | 0.049 |
| Fructose (g) | 4.86 (1.97–8.78) | 5.42 (2.28–8.78) | 4.35 (0.96–7.69) | 0.017 |
| Fructose (%) | 1.56 (0.75–2.45) | 1.65 (0.77–2.46) | 1.50 (0.42–2.45) | 0.266 |
| Disaccharides (g) | 2.28 (1.18–3.75) | 2.37 (1.41–3.75) | 2.11 (0.71–3.62) | 0.127 |
| Lactose (g) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.834 |
| Maltose (g) | 0.00 (0.00–0.01) | 0.00 (0.00–0.01) | 0.00 (0.00–0.01) | 0.404 |
| Other HC (g) | 75.8 (15.3–142.7) | 68.7 (15.1–143.3) | 78.9 (15.6–138.6) | 0.871 |
| Net HC (g) | 134.4 (63.1–197.5) | 134.4 (47.9–202.0) | 134.4 (68.4–190.0) | 0.938 |
| Non-digestible HC (g) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.833 |
| Dietary fiber (g) | 8.57 (4.96–13.36) | 8.56 (4.97–13.72) | 8.57 (4.95–13.16) | 0.807 |
| Starch (g) | 0.00 (0.00–1.84) | 0.00 (0.00–1.84) | 0.00 (0.00–1.83) | 0.418 |
| Micronutrients | ||||
| Cholesterol (mg) | 132.5 (83.4–217.4) | 141.7 (94.1–221.1) | 130.2 (78.8–205.9) | 0.063 |
| Folate (μg) | 106.9 (58.7–157.1) | 108.4 (59.4–159.3) | 104.9 (54.9–156.9) | 0.458 |
| Folic acid (μg) | 13.9 (0.00–62.3) | 7.90 (0.00–66.1) | 14.28 (0.00–60.1) | 0.478 |
| Vitamin B1 (mg) | 0.48 (0.25–0.71) | 0.49 (0.27–0.77) | 0.45 (0.24–0.70) | 0.158 |
| Vitamin B2 (mg) | 0.88 (0.58–1.28) | 0.92 (0.58–1.33) | 0.84 (0.57–1.26) | 0.519 |
| Vitamin B3 (mg) | 12.0 (8.26–19.04) | 12.4 (8.46–19.43) | 11.6 (8.17–18.67) | 0.468 |
| Pantothenic acid (mg) | 0.57 (0.25–1.02) | 0.57 (0.19–1.02) | 0.57 (0.27–1.05) | 0.505 |
| Vitamin B6 (mg) | 0.85 (0.50–1.34) | 0.92 (0.50–1.40) | 0.79 (0.50–1.27) | 0.158 |
| Vitamin B12 (μg) | 1.12 (0.41–2.37) | 1.12 (0.37–2.45) | 1.07 (0.41–2.35) | 0.746 |
| Vitamin C (mg) | 42.3 (13.7–96.4) | 40.1 (14.6–96.9) | 42.9 (11.6–96.4) | 0.952 |
| Vitamin D (μg) | 0.18 (0.00–0.79) | 0.19 (0.00–0.74) | 0.18 (0.00–0.82) | 0.490 |
| Vitamin E (mg) | 0.48 (0.23–1.02) | 0.50 (0.23–1.11) | 0.47 (0.22–0.95) | 0.521 |
| Vitamin K (μg) | 14.7 (5.03–51.57) | 14.6 (5.19–52.50) | 15.0 (4.62–49.98) | 0.483 |
| Biotin (μg) | 3.59 (0.50–7.27) | 3.68 (1.33–7.27) | 3.49 (0.36–7.27) | 0.401 |
| Vitamin A (IU) | 426.9 (135.4–856.3) | 413.5 (124.9–863.7) | 440.1 (155.9–849.0) | 0.691 |
| Calcium (mg) | 916.6 (517.7–1311.1) | 875.1 (453.7–1299.9) | 958.4 (572.5–1320.7) | 0.152 |
| Copper (mg) | 0.34 (0.21–0.53) | 0.35 (0.21–0.54) | 0.32 (0.21–0.51) | 0.517 |
| Iron (mg) | 12.68 (7.52–18.20) | 12.89 (7.35–18.56) | 12.56 (8.04–17.71) | 0.929 |
| Magnesium (mg) | 109.5 (62.3–163.3) | 114.2 (62.6–173.3) | 104.2 (61.6–157.5) | 0.574 |
| Boron (μg) | 155.1 (0.00–529.19) | 248.4 (0.00–529.19) | 122.1 (0.00–529.19) | 0.102 |
| Chlorine (mg) | 62.0 (0.0–150.0) | 84.7 (0.0–150.0) | 46.8 (0.0–150.0) | 0.193 |
| Chromium (μg) | 1.39 (0.00–2.32) | 1.39 (0.00–2.54) | 1.05 (0.00–2.32) | 0.380 |
| Fluoride (mg) | 0.00 (0.00–0.01) | 0.00 (0.00–0.01) | 0.00 (0.00–0.01) | 0.841 |
| Iodine (μg) | 1.13 (0.15–33.51) | 1.42 (0.15–36.74) | 0.88 (0.12–19.79) | 0.147 |
| Manganese (mg) | 0.32 (0.10–0.58) | 0.32 (0.09–0.58) | 0.32 (0.10–0.58) | 0.657 |
| Molybdenum (μg) | 0.00 (0.00–1.26) | 0.00 (0.00–1.29) | 0.00 (0.00–1.22) | 0.833 |
| Phosphorus (mg) | 490.8 (305.7–724.6) | 499.5 (300.4–772.5) | 486.1 (309.1–684.1) | 0.669 |
| Potassium (mg) | 1404.2 (845.2–1957.7) | 1424.5 (807.9–1986.6) | 1373.7 (888.0–1953.6) | 0.833 |
| Selenium (μg) | 45.58 (30.59–70.16) | 46.53 (30.36–72.71) | 44.96 (30.59–68.29) | 0.562 |
| Sodium (mg) | 2332.6 (1597.9–3033.6) | 2335.7 (1589.2–3146.7) | 2289.8 (1610.1–2968.2) | 0.578 |
| Zinc (mg) | 3.73 (1.76–8.89) | 4.33 (1.66–9.27) | 3.47 (1.87–8.22) | 0.496 |
| CAP Model | Fat Model | Model Waist | Visceral Fat Model | |||||
|---|---|---|---|---|---|---|---|---|
| Nutrient | B (95% CI) | p-Value | B (95% CI) | p-Value | B (95% CI) | p-Value | B (95% CI) | p-Value |
| Kilocalories ‡ | −0.006 (−0.016 to 0.004) | 0.204 | 0.001 (0.044–0.000) | 0.002 | −0.001 (−0.002–0.000) | 0.042 | 0.000 (0.000–0.000) | 0.567 |
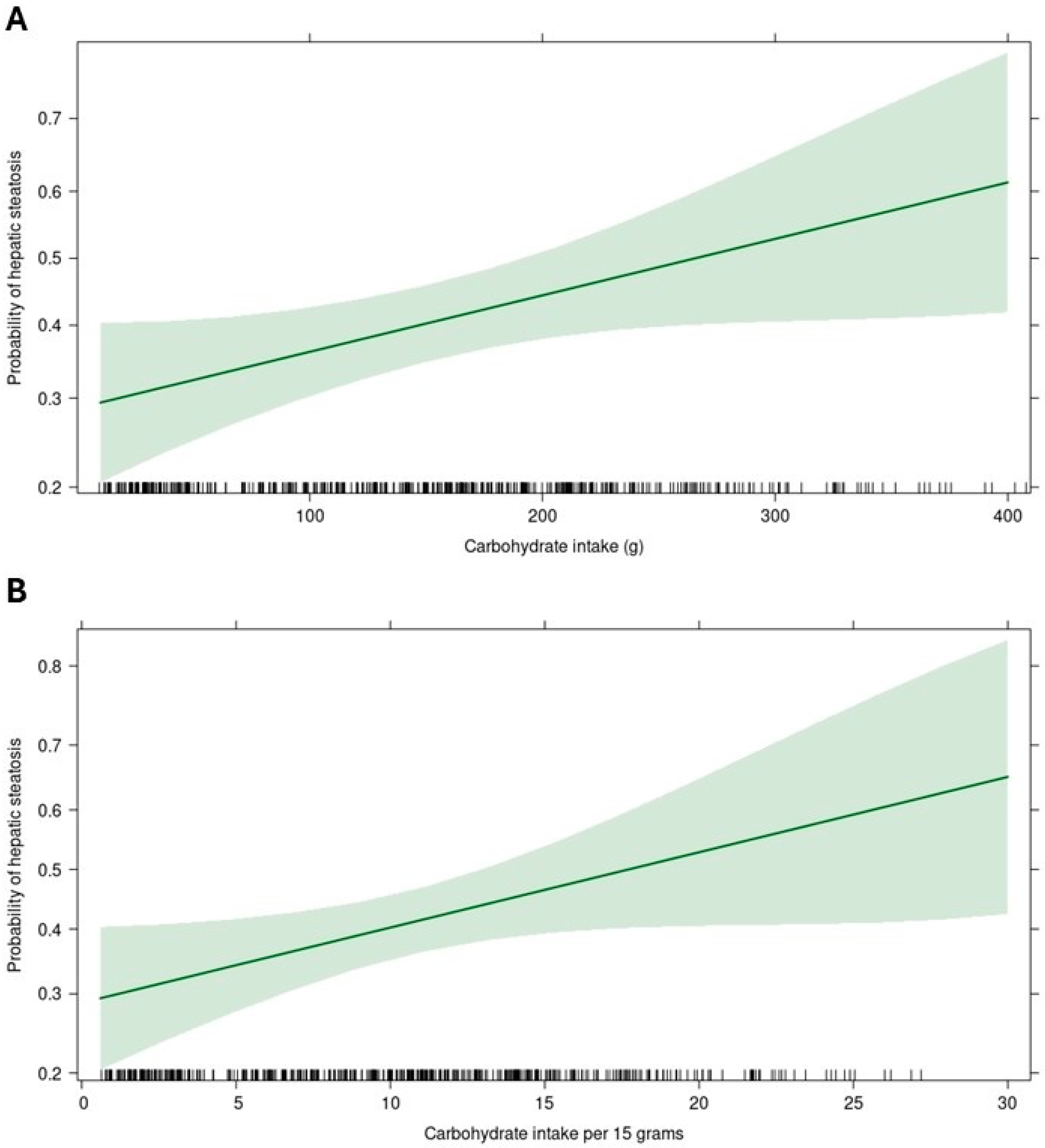
| Carbohydrates (g) * | 0.060 (−0.001 to 0.121) | 0.053 | −0.002 (0.372–−0.007) | 0.003 | 0.001 (−0.006–0.007) | 0.862 | 0.000 (0.000–0.001) | 0.589 |
| Protein (g) * | −0.082 (−0.434 to 0.271) | 0.649 | −0.009 (0.541–−0.039) | 0.020 | 0.002 (−0.034–0.038) | 0.900 | −0.001 (−0.005–0.003) | 0.682 |
| Fat (g) * | −0.129 (−0.266 to 0.007) | 0.063 | 0.006 (0.318–−0.006) | 0.017 | −0.001 (−0.015–0.013) | 0.845 | 0.000 (−0.002–0.001) | 0.639 |
| Saturated fat (g) * | −0.325 (−0.706–0.056) | 0.094 | 0.015 (0.347–−0.017) | 0.047 | −0.006 (−0.045–0.033) | 0.747 | −0.001 (−0.005–0.003) | 0.701 |
| Monounsaturated Fats (g) | −0.037 (−0.952–0.878) | 0.937 | 0.017 (−0.058–0.092) | 0.658 | 0.047 (−0.045–0.138) | 0.316 | −0.007 (−0.017–0.003) | 0.149 |
| Polyunsaturated Fats (g) | 0.358 (−1.339–2.055) | 0.678 | 0.002 (−0.137–0.142) | 0.975 | 0.083 (−0.087–0.253) | 0.337 | −0.006 (−0.024–0.012) | 0.502 |
| Total sugars (g) * | 0.054 (−0.087–0.195) | 0.453 | 0.005 (0.395–−0.007) | 0.017 | −0.009 (−0.023–0.006) | 0.237 | 0.000 (−0.001–0.002) | 0.812 |
| Added sugar (g) * | 0.098 (−0.190–0.386) | 0.506 | 0.018 (0.148–−0.006) | 0.042 | −0.012 (−0.041–0.017) | 0.425 | 0.000 (−0.003–0.003) | 0.834 |
| Fructose (g) * | −0.129 (−0.920–0.661) | 0.748 | 0.026 (0.448–−0.041) | 0.092 | −0.052 (−0.132–0.029) | 0.210 | −0.001 (−0.009–0.008) | 0.884 |
| Protein (%) * | −0.285 (−1.336–0.766) | 0.594 | −0.019 (0.679–−0.106) | 0.069 | 0.006 (−0.101–0.113) | 0.912 | −0.001 (−0.012–0.010) | 0.862 |
| Carbohydrates (%) * | 0.234 (0.019–0.449) | 0.033 | −0.013 (0.155–−0.031) | 0.005 | 0.000 (−0.022–0.022) | 0.977 | 0.001 (−0.001–0.004) | 0.247 |
| Fat (%) * | −0.220 (−0.435–0.006) | 0.044 | 0.014 (0.133–−0.004) | 0.032 | 0.000 (−0.022–0.022) | 0.995 | −0.001 (−0.04–0.001) | 0.263 |
| Saturated Fat (%) * | −0.557 (−1.145–0.031) | 0.063 | 0.037 (0.145–−0.013) | 0.086 | −0.003 (−0.064–0.057) | 0.910 | −0.003 (−0.010–0.003) | 0.329 |
| Monounsaturated Fats (%) | 0.163 (−1.113–1.438) | 0.802 | 0.012 (−0.093–0.117) | 0.818 | 0.085 (−0.043–0.212) | 0.193 | −0.009 (−0.023–0.005) | 0.201 |
| Polyunsaturated Fats (%) | 0.819 (−1.593–3.232) | 0.505 | −0.020 (−0.219–0.178) | 0.841 | 0.127 (−0.115–0.369) | 0.302 | −0.007 (−0.033–0.019) | 0.582 |
| Total sugars (%) * | 0.165 (−0.293–0.622) | 0.480 | 0.009 (0.651–−0.029) | 0.047 | −0.028 (−0.075–0.018) | 0.236 | 0.000 (−0.005–0.005) | 0.954 |
| Added sugar (%) * | 0.211 (−0.649–1.072) | 0.630 | 0.034 (0.355–−0.038) | 0.106 | −0.030 (−0.118–0.058) | 0.503 | −0.001 (−0.011–0.008) | 0.791 |
| Fructose (%) * | −0.719 (−3.298–1.859) | 0.584 | 0.049 (0.654–−0.167) | 0.266 | −0.158 (−0.422–0.106) | 0.240 | −0.005 (−0.033–0.023) | 0.725 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Vazquez, S.E.; Kammar-García, A.; Moctezuma-Velázquez, C.; Mancilla-Galindo, J.; García-Juárez, I.; Uscanga-Domínguez, L.F. The Impact of Dietary Sugars and Saturated Fats on Body and Liver Fat in a Healthcare Worker Population. Nutrients 2025, 17, 1328. https://doi.org/10.3390/nu17081328
Martinez-Vazquez SE, Kammar-García A, Moctezuma-Velázquez C, Mancilla-Galindo J, García-Juárez I, Uscanga-Domínguez LF. The Impact of Dietary Sugars and Saturated Fats on Body and Liver Fat in a Healthcare Worker Population. Nutrients. 2025; 17(8):1328. https://doi.org/10.3390/nu17081328
Chicago/Turabian StyleMartinez-Vazquez, Sophia Eugenia, Ashuin Kammar-García, Carlos Moctezuma-Velázquez, Javier Mancilla-Galindo, Ignacio García-Juárez, and Luis Federico Uscanga-Domínguez. 2025. "The Impact of Dietary Sugars and Saturated Fats on Body and Liver Fat in a Healthcare Worker Population" Nutrients 17, no. 8: 1328. https://doi.org/10.3390/nu17081328
APA StyleMartinez-Vazquez, S. E., Kammar-García, A., Moctezuma-Velázquez, C., Mancilla-Galindo, J., García-Juárez, I., & Uscanga-Domínguez, L. F. (2025). The Impact of Dietary Sugars and Saturated Fats on Body and Liver Fat in a Healthcare Worker Population. Nutrients, 17(8), 1328. https://doi.org/10.3390/nu17081328











