Intestinal Activation of LXRα Counteracts Metabolic-Associated Steatohepatitis Features in Mice
Highlights
- Specific expression of LXRα in the intestines alleviates liver damage and metabolism in vitro and in murine models of MASH.
- The intestinal activation of LXRα exerts a protective effect against hepatic steatosis and fibrosis by modulating the PPARγ and SMAD2/3 pathways on the HDL/SRB1 axis.
- Our findings support the hypothesis that the regulation of LXRα at the intestinal level represents a potential therapeutic option to reestablish cholesterol metabolism and reduce liver damage in MASH.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Real-Time Quantitative PCR (QRT-PCR) in Mice
2.3. Tissue Histology
2.4. ALT and AST Serum Levels Quantification
2.5. Hepatic Triglycerides Determination
2.6. Immunofluorescence in Liver Tissues
2.7. Lipopolysaccharide (LPS) Assay
2.8. Inflammatory Protein Array
2.9. RNA Extraction and Analysis of Inflammatory Genes
2.10. Determination of Serum and Hepatic Total Cholesterol and Lipoproteins
2.11. Cell Culture Experiments
2.12. Statistics
3. Results
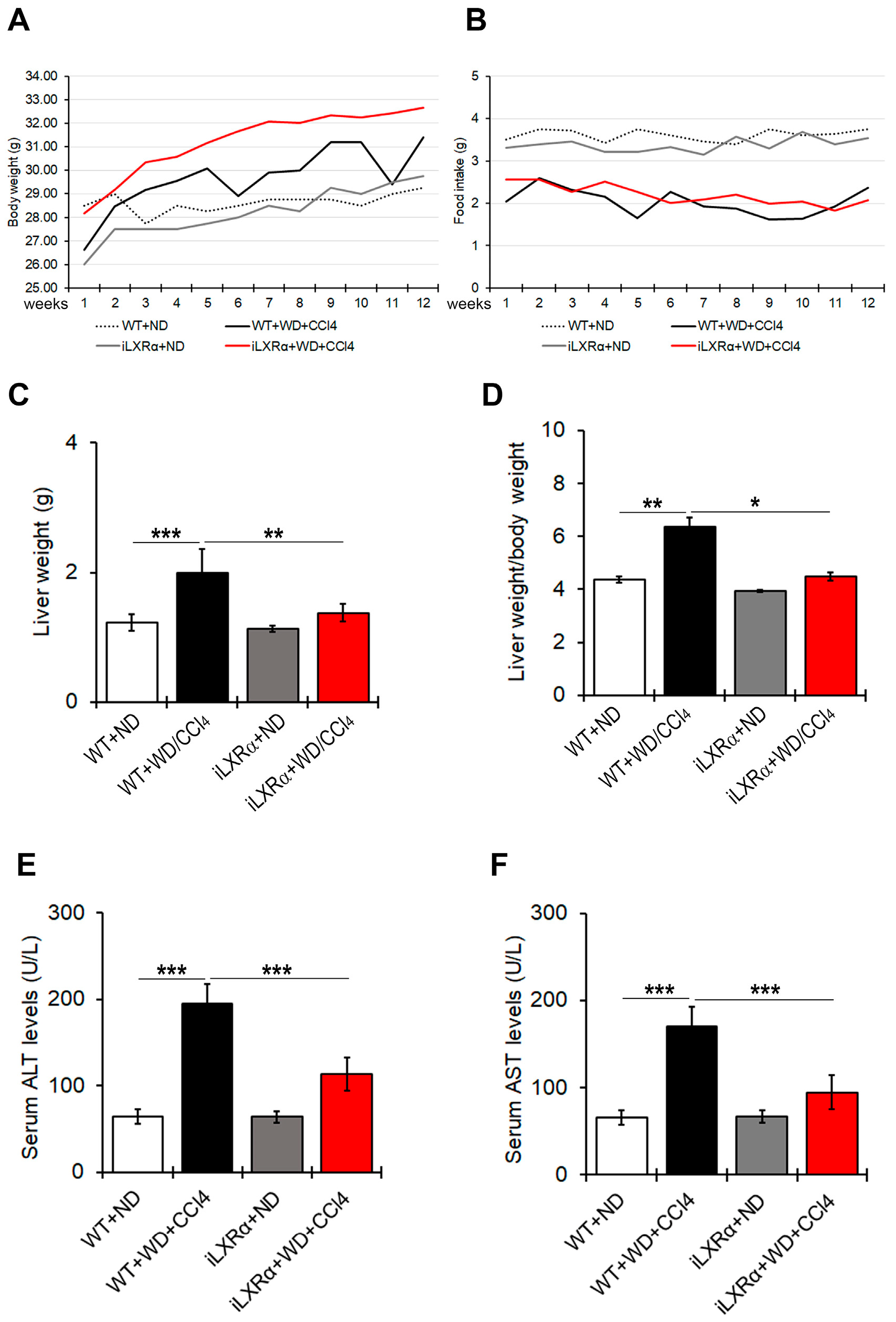
3.1. Constitutive Intestinal Activation of LXRα Reduces WD/CCl4-Dependent Liver Weight and Liver Function
3.2. Constitutive Intestinal Activation of LXRα Reduces WD/CCl4-Dependent Liver Weight and Steatosis
3.3. Constitutive Intestinal Activation of LXRα Reduces WD/CCl4-Dependent Inflammation in Mice
3.4. Constitutive Intestinal Activation of LXRα Reduces WD/CCl4-Dependent Fibrosis in Mice
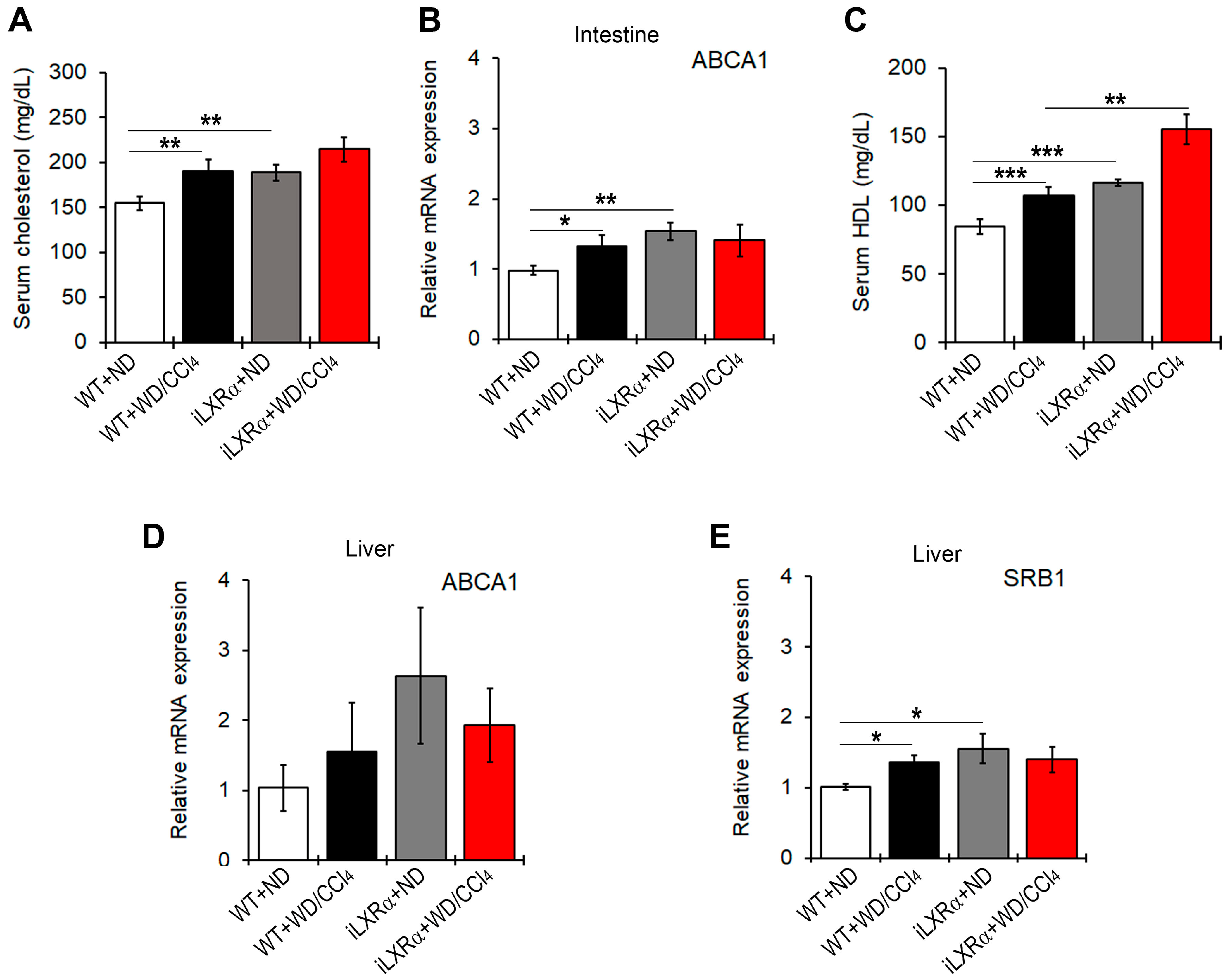
3.5. Constitutive Intestinal Activation of LXRα Decreases WD/CCl4-Dependent Cholesterol Absorption and Induces Reverse Cholesterol Transport (RCT)
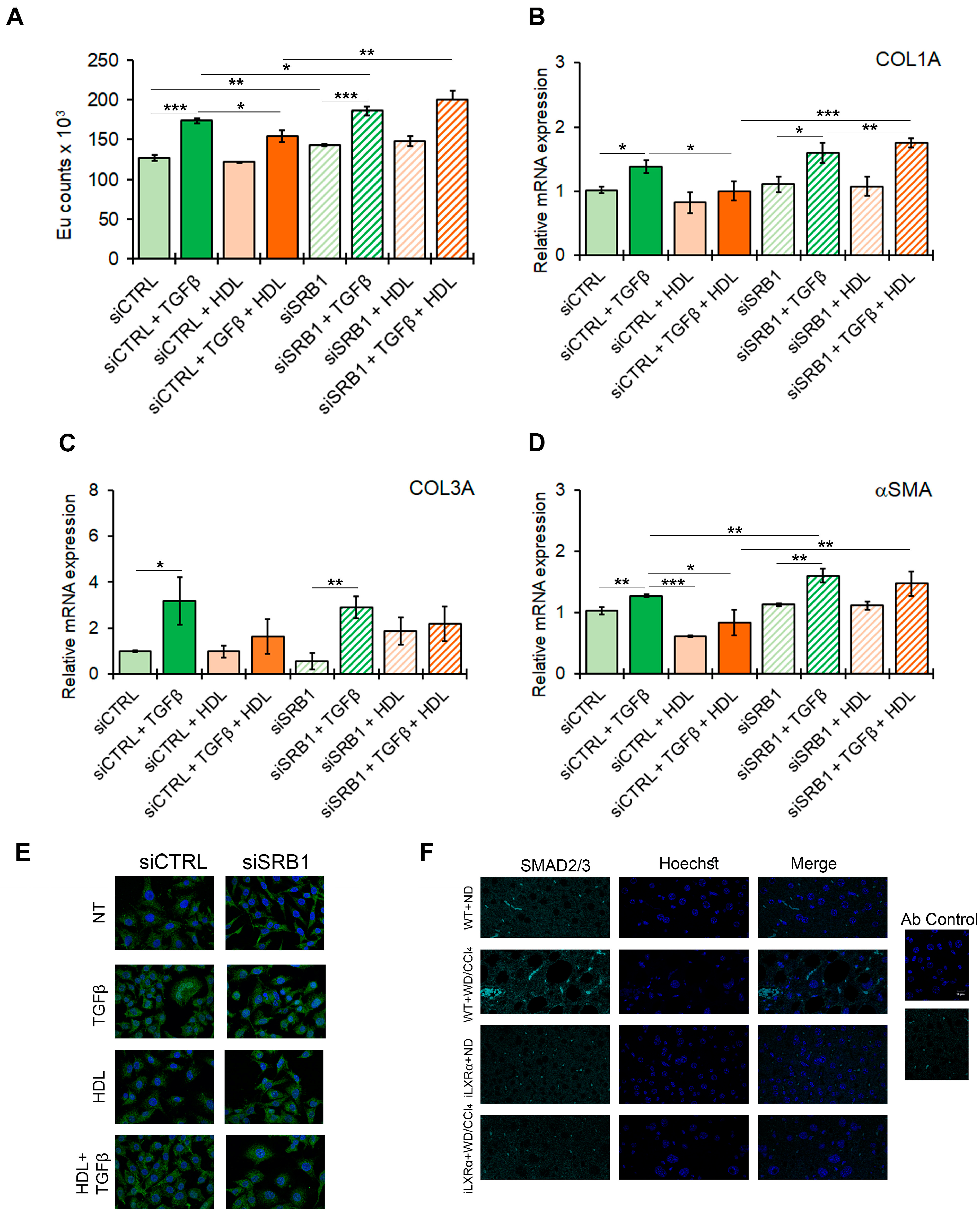
3.6. SRB1 Mediates the HDL-Dependent Anti-Steatogenic Effects In Vitro
3.7. SRB1 Mediates the HDL-Dependent Anti-Fibrotic Effects In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| αSMA | alpha smooth muscle actin |
| ABCA1 | ATP-Binding Cassette Transporter 1 |
| ABCG | ATP-binding cassette transporters G |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| CCl4 | Carbon tetrachloride |
| CD206 | Mannose receptor C-type 1 |
| CD36 | Thrombospondin receptor |
| CD68 | Type I transmembrane glycoprotein |
| COL | Collagen |
| CPT1A | Carnitine Palmitoyltransferase 1A |
| FABP4 | Fatty Acid Binding Protein 4 |
| FAS | Fatty acid synthase |
| GAPDH | Glyceraldehyde 3 Phosphate Dehydrogenase |
| H&E | Haematoxylin-eosin |
| HDL | High-density lipoprotein |
| IL | Interleukin |
| iLXRα | Intestinal Liver X receptors alpha |
| LDL | Low-density lipoprotein; |
| LPS | Lipopolysaccharide |
| LXRs | Liver X receptors |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| miRNAs | Small noncoding microRNAs |
| NAFLD | Non-alcoholic fatty liver disease |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| ND | Normal diet |
| PPAR | Peroxisome proliferator-activated receptors gamma SMAD2/3 |
| RCT | Reverse cholesterol transport |
| SMAD2/3 | Small mothers against decapentaplegic homolog protein proteins 2/3 |
| SRB1 | Scavenger receptor class B type 1 |
| T2D | Type 2 diabetes |
| TGFβ | Transforming Growth Factor-Beta |
| TNFα | Tumor necrosis factor-α |
| VLDL | Very low-density lipoprotein |
| WD | Western diet |
| WT | Wild-type |
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Friedman, S.L. Found in translation-Fibrosis in metabolic dysfunction-associated steatohepatitis (MASH). Sci. Transl. Med. 2023, 15, eadi0759. [Google Scholar] [CrossRef]
- Pirola, C.J.; Sookoian, S. Metabolic dysfunction-associated fatty liver disease: Advances in genetic and epigenetic implications. Curr. Opin. Lipidol. 2022, 33, 95–102. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Dixon, L.J.; Feldstein, A.E. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol. 2009, 3, 445–451. [Google Scholar] [CrossRef]
- Tomita, K.; Teratani, T.; Suzuki, T.; Shimizu, M.; Sato, H.; Narimatsu, K.; Okada, Y.; Kurihara, C.; Irie, R.; Yokoyama, H.; et al. Free cholesterol accumulation in hepatic stellate cells: Mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology 2014, 59, 154–169. [Google Scholar] [CrossRef]
- Ho, C.M.; Ho, S.L.; Jeng, Y.M.; Lai, Y.S.; Chen, Y.H.; Lu, S.C.; Chen, H.L.; Chang, P.Y.; Hu, R.H.; Lee, P.H. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. J. Inflamm. 2019, 16, 7. [Google Scholar] [CrossRef]
- Zhang, Y.; Breevoort, S.R.; Angdisen, J.; Fu, M.; Schmidt, D.R.; Holmstrom, S.R.; Holmstrom, S.R.; Kliewer, S.A.; Mangelsdorf, D.J.; Schulman, I.G. Liver LXRα expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J. Clin. Investig. 2012, 122, 1688–1699. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Lioci, G.; Gurrado, F.; Giordano, D.M.; Rychlicki, C.; Bocca, C.; Trozzi, L.; Novo, E.; Panera, N.; De Stefanis, C.; et al. HDL cholesterol protects from liver injury in mice with intestinal specific LXRα activation. Liver Int. 2020, 40, 3127–3139. [Google Scholar] [CrossRef]
- Lo Sasso, G.; Murzilli, S.; Salvatore, L.; D’Errico, I.; Petruzzelli, M.; Conca, P.; Conca, P.; Jiang, Z.Y.; Calabresi, L.; Parini, P.; et al. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010, 12, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.I.; Hoshida, Y.; et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Skat-Rørdam, J.; Højland Ipsen, D.; Lykkesfeldt, J.; Tveden-Nyborg, P. A role of peroxisome proliferator-activated receptor γ in non-alcoholic fatty liver disease. Basic Clin. Pharmacol. Toxicol. 2019, 124, 528–537. [Google Scholar] [CrossRef]
- Agustanti, N.; Soetedjo, N.N.M.; Damara, F.A.; Iryaningrum, M.R.; Permana, H.; Bestari, M.B.; Supriyadi, R. The association between metabolic dysfunction-associated fatty liver disease and chronic kidney disease: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2023, 17, 102780. [Google Scholar] [CrossRef]
- Kim, H.; Park, C.; Kim, T.H. Targeting Liver X Receptors for the Treatment of Non-Alcoholic Fatty Liver Disease. Cells 2023, 12, 1292. [Google Scholar] [CrossRef]
- Laurencikiene, J.; Rydén, M. Liver X receptors and fat cell metabolism. Int. J. Obes. 2012, 36, 1494–1502. [Google Scholar] [CrossRef]
- Komati, R.; Spadoni, D.; Zheng, S.; Sridhar, J.; Riley, K.E.; Wang, G. Ligands of Therapeutic Utility for the Liver X Receptors. Molecules 2017, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Griffett, K.; Solt, L.A.; El-Gendy, B.-D.; Kamenecka, T.M.; Burris, T.P. A liver-selective LXR inverse agonist that suppresses hepatic steatosis. ACS Chem. Biol. 2013, 8, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Kirchgessner, T.G.; Sleph, P.; Ostrowski, J.; Lupisella, J.; Ryan, C.S.; Liu, X.; Fernando, G.; Grimm, D.; Shipkova, P.; Zhang, R. Beneficial and Adverse Effects of an LXR Agonist on Human Lipid and Lipoprotein Metabolism and Circulating Neutrophils. Cell Metab. 2016, 24, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Wei, X. Liver X receptors: From pharmacology to nanoparticle-based drug delivery. Eur. J. Pharmacol. 2023, 956, 175953. [Google Scholar] [CrossRef]
- Malerød, L.; Juvet, L.K.; Hanssen-Bauer, A.; Eskild, W.; Berg, T. Oxysterol-activated LXRalpha/RXR induces hSR-BI-promoter activity in hepatoma cells and preadipocytes. Biochem. Biophys. Res. Commun. 2002, 299, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, E.; Arconzo, M.; Pasculli, E.; Tricase, A.F.; Cultrera, S.; Bertrand-Michel, J.; Loiseau, N.; Villani, G.; Guillou, H.; Moschetta, A. Pivotal role of intestinal cholesterol and nuclear receptor LXR in metabolic liver steatohepatitis and hepatocarcinoma. Cell Biosci. 2024, 14, 69. [Google Scholar] [CrossRef]
- Wouters, K.; van Bilsen, M.; van Gorp, P.J.; Bieghs, V.; Lütjohann, D.; Kerksiek, A.; Staels, B.; Hofker, M.H.; Shiri-Sverdlov, R. Intrahepatic cholesterol influences progression, inhibition and reversal of non-alcoholic steatohepatitis in hyperlipidemic mice. FEBS Lett. 2010, 584, 1001–1005. [Google Scholar] [CrossRef]
- Yasuda, T.; Grillot, D.; Billheimer, J.T.; Briand, F.; Delerive, P.; Huet, S.; Rader, D.J. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 2010, 3, 781–786. [Google Scholar] [CrossRef]
- Jain, M.R.; Giri, S.R.; Bhoi, B.; Trivedi, C.; Rath, A.; Rathod, R.; Ranvir, R.; Kadam, S.; Patel, H.; Swain, P. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018, 38, 1084–1094. [Google Scholar] [CrossRef]
- Rivera, K.; Quiñones, V.; Amigo, L.; Santander, N.; Salas-Pérez, F.; Xavierm, A.; Fernández-Galilea, M.; Carrasco, G.; Cabrera, D.; Arrese, M.; et al. Lipoprotein receptor SR-B1 deficiency enhances adipose tissue inflammation and reduces susceptibility to hepatic steatosis during diet-induced obesity in mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158909. [Google Scholar] [CrossRef]
- Lin, F.Y.; Lin, Y.W.; Shih, C.M.; Lin, S.J.; Tung, Y.T.; Li, C.Y.; Chen, Y.H.; Lin, C.Y.; Tsai, Y.T.; Huang, C.Y. A Novel Relative High-Density Lipoprotein Index to Predict the Structural Changes in High-Density Lipoprotein and Its Ability to Inhibit Endothelial-Mesenchymal Transition. Int. J. Mol. Sci. 2021, 22, 5210. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Poulsen, K.L.; Wu, L.; Liu, S.; Miyata, T.; Song, Q.; Wei, Q.; Zhao, C.; Lin, C.; Yang, J. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH). Signal Transduct. Target Ther. 2022, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Griffett, K.; Burris, T.P. Development of LXR inverse agonists to treat MAFLD, NASH, and other metabolic diseases. Front. Med. 2023, 10, 1102469. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Casirati, E.; Tomasi, M.; Valenti, L. PNPLA3 as a therapeutic target for fatty liver disease: The evidence to date. Expert Opin. Ther. Targets 2021, 25, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Panera, N.; Braghini, M.R.; Crudele, A.; Smeriglio, A.; Bianchi, M.; Condorelli, A.G.; Nobili, R.; Conti, L.A.; Lioci, G.; De Stefanis, C.; et al. Combination Treatment with Hydroxytyrosol and Vitamin E Improves NAFLD-Related Fibrosis. Nutrients 2022, 14, 3791. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lioci, G.; Gurrado, F.; Panera, N.; Bianchi, M.; De Stefanis, C.; D’Oria, V.; Cicolani, N.; Santini, S.J.; Schiadà, L.; Alisi, A.; et al. Intestinal Activation of LXRα Counteracts Metabolic-Associated Steatohepatitis Features in Mice. Nutrients 2025, 17, 1349. https://doi.org/10.3390/nu17081349
Lioci G, Gurrado F, Panera N, Bianchi M, De Stefanis C, D’Oria V, Cicolani N, Santini SJ, Schiadà L, Alisi A, et al. Intestinal Activation of LXRα Counteracts Metabolic-Associated Steatohepatitis Features in Mice. Nutrients. 2025; 17(8):1349. https://doi.org/10.3390/nu17081349
Chicago/Turabian StyleLioci, Gessica, Fabio Gurrado, Nadia Panera, Marzia Bianchi, Cristiano De Stefanis, Valentina D’Oria, Nicolò Cicolani, Silvano Junior Santini, Laura Schiadà, Anna Alisi, and et al. 2025. "Intestinal Activation of LXRα Counteracts Metabolic-Associated Steatohepatitis Features in Mice" Nutrients 17, no. 8: 1349. https://doi.org/10.3390/nu17081349
APA StyleLioci, G., Gurrado, F., Panera, N., Bianchi, M., De Stefanis, C., D’Oria, V., Cicolani, N., Santini, S. J., Schiadà, L., Alisi, A., & Svegliati-Baroni, G. (2025). Intestinal Activation of LXRα Counteracts Metabolic-Associated Steatohepatitis Features in Mice. Nutrients, 17(8), 1349. https://doi.org/10.3390/nu17081349







