Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications
Abstract
:1. Introduction
2. Methods
3. Prevalence of Vitamin D Deficiency
4. Prevalence of Colorectal Cancer
5. The Biological Effects of Vitamin D
6. The Role of Vitamin D in Colorectal Cancer Prevention
- Inhibition of cancer cell growth: calcitriol induces G1 cell cycle arrest, reducing CRC cell proliferation, and restoring sensitivity to tumor suppressors like TGF-β [113].
- Regulation of the Wnt/β-Catenin pathway: the Wnt/β-catenin pathway is frequently hyperactivated in CRC. Calcitriol reduces β-catenin activity and increases E-cadherin expression, stabilizing cell–cell adhesion and reducing tumor invasiveness [114].
- Antiangiogenesis: calcitriol inhibits angiogenesis by downregulating VEGF and NF-κB signaling, limiting the tumor’s blood supply [6].
- Induction of apoptosis: calcitriol promotes pro-apoptotic proteins (BAX, BAK) while inhibiting anti-apoptotic proteins (BCL-2), driving CRC cell death [2].
- Anti-inflammatory effects: calcitriol reduces CRC-associated inflammation by inhibiting prostaglandin synthesis, stress-activated kinases, and pro-inflammatory cytokines [67].
7. The Role of Vitamin D in the Prevention of CRC: Immunological Mechanisms and Inflammatory Responses
8. The Role of Vitamin D in CRC Prevention: Mechanisms, Gut Microbiota Interaction, and Synergy with Chemotherapy and Healthy Diets
8.1. Vitamin D and Its Interaction with the Gut Microbiota
8.2. Vitamin D and Chemotherapeutic Synergy in Cancer
8.3. The Synergistic Role of Healthy Dietary Patterns
9. Association Between Serum Vitamin D Levels and CRC Outcomes
10. The Role of Dietary Vitamin D Intake in Colorectal Cancer Prevention and Prognosis
11. Ensuring Adequate Vitamin D Intake and Dietary Recommendations
12. Vitamin D Supplementation and Its Relationship with CRC
13. Recommended Intakes of Vitamin D
14. Vitamin D Supplementation and the Development of Adenomas and Polyps
15. Critical Remarks and Limitations
16. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, A.V.; Feldman, D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef]
- Muñoz, A.; Grant, W.B. Vitamin D and cancer: An historical overview of the epidemiology and mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.G.; Mehta, R.R. Vitamin D and cancer. J. Nutr. Biochem. 2002, 13, 252–264. [Google Scholar] [CrossRef]
- Kulda, V. Metabolizmus vitaminu D [Vitamin D metabolism]. Vnitrni Lekarstvi 2012, 58, 400–404. [Google Scholar] [PubMed]
- Kemeny, L.V.; Fisher, D.E. Hormones and Hormone Precursors of the Skin. In Hormonal Signaling in Biology and Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 531–556. [Google Scholar]
- Carlberg, C.; Raczyk, M.; Zawrotna, N. Vitamin D: A master example of nutrigenomics. Redox Biol. 2023, 62, 102695. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Singh, V.; Sen, A.; Yadav, D.; Agrawal, R.; Kishore, S.; Misra, S.; Sharma, P. Vitamin D in Disease Prevention and Cure-Part I: An Update on Molecular Mechanism and Significance on Human Health. Indian J. Clin. Biochem. 2024, 1–43. [Google Scholar] [CrossRef]
- Roseland, J.M.; Phillips, K.M.; Patterson, K.Y.; Pehrsson, P.R.; Taylor, C.L. Vitamin D in Foods: An Evolution of Knowledge. Vitamin D; Elsevier: Amsterdam, The Netherlands, 2018; pp. 41–77. [Google Scholar]
- Bilezikian, J.P.; Formenti, A.M.; Adler, R.A.; Binkley, N.; Bouillon, R.; Lazaretti-Castro, M.; Marcocci, C.; Napoli, N.; Rizzoli, R.; Giustina, A. Vitamin D: Dosing, levels, form, and route of administration: Does one approach fit all? Rev. Endocr. Metab. Disord. 2021, 22, 1201–1218. [Google Scholar] [CrossRef]
- Dusso, A.S. Kidney disease and vitamin D levels: 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and VDR activation. Kidney Int. Suppl. 2011, 1, 136–141. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Żmijewski, M.A. Nongenomic Activities of Vitamin D. Nutrients 2022, 14, 5104. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and chronic diseases. Aging Dis. 2017, 8, 346. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Oliveira, L.J.d.C.; Passarini, T.d.M.; Andrade, A.C.; Diniz, P.H.; Prolla, G.; Amorim, L.C.; Gil, M.; Lino, F.; Garicochea, B.; et al. Vitamin D and colorectal cancer–A practical review of the literature. Cancer Treat. Res. Commun. 2022, 32, 100616. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Althwanay, A.; Ahsan, F.; Oliveri, F.; Goud, H.K.; Mehkari, Z.; Mohammed, L.; Rutkofsky, I.H. Role of vitamin D in colorectal cancer: A holistic approach and review of the clinical utility. Cureus 2020, 12, e10734. [Google Scholar] [CrossRef] [PubMed]
- Giammanco, M.; Di Majo, D.; La Guardia, M.; Aiello, S.; Crescimannno, M.; Flandina, C.; Tumminello, F.M.; Leto, G. Vitamin D in cancer chemoprevention. Pharm. Biol. 2015, 53, 1399–1434. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Meshkin, A.; Badiee, F.; Salari, N.; Hassanabadi, M.; Khaleghi, A.A.; Mohammadi, M. The Global Prevalence of Vitamin D Deficiency in the Elderly: A Meta-analysis. Indian J. Orthop. 2024, 58, 223–230. [Google Scholar] [CrossRef]
- Tsiaras, W.G.; Weinstock, M.A. Factors influencing vitamin D status. Acta Derm. Venereol. 2011, 91, 115. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Rebecca, L.S.; Kimberly, D.; Ann, G.; Stacey, A.; Lynn, F.; Joseph, C. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar]
- Horváthné, K.Z. A Vastagbélszűrési Pilot Program Értékelése és a Vastagbéldaganatból Eredő Betegségteher Vizsgálata; University of Pécs: Pécs, Hungary, 2021. [Google Scholar]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Kenessey, I.; Nagy, P.; Polgár, C. A rosszindulatú daganatok hazai epidemiológiai helyzete a XXI. század második évtizedében. Magy. Onkológia 2022, 66, 175–184. [Google Scholar]
- Longobardi, S. Colorectal cancer: Local results and significance in Hungary. J. Gastrointest. Oncol. 2024, 15, 2552. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef]
- Zhang, Y.; Murata, S.; Schmidt-Mende, K.; Ebeling, M.; Modig, K. Do people reach 100 by surviving, delaying, or avoiding diseases? A life course comparison of centenarians and non-centenarians from the same birth cohorts. GeroScience 2024. [Google Scholar] [CrossRef] [PubMed]
- Cruces-Salguero, S.; Larranaga, I.; Mar, J.; Matheu, A. Centenarians of the Basque Country are resilient to cancer. GeroScience 2024, 47, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Andonian, B.J.; Hippensteel, J.A.; Abuabara, K.; Boyle, E.M.; Colbert, J.F.; Devinney, M.J.; Faye, A.S.; Kochar, B.; Lee, J.; Litke, R.; et al. Inflammation and aging-related disease: A transdisciplinary inflammaging framework. GeroScience 2024, 47, 1–28. [Google Scholar] [CrossRef]
- Ungvari, Z.; Ungvari, A.; Bianchini, G.; Gyorffy, B. Prognostic significance of a signature based on senescence-related genes in colorectal cancer. GeroScience 2024, 46, 4495–4504. [Google Scholar] [CrossRef]
- Cummings, S.R.; Lui, L.-Y.; Zaira, A.; Mau, T.; Fielding, R.A.; Atkinson, E.J.; Patel, S.; LeBrasseur, N. Biomarkers of cellular senescence and major health outcomes in older adults. GeroScience 2024. [Google Scholar] [CrossRef]
- Fekete, M.; Major, D.; Feher, A.; Fazekas-Pongor, V.; Lehoczki, A. Geroscience and pathology: A new frontier in understanding age-related diseases. Pathol. Oncol. Res. 2024, 30, 1611623. [Google Scholar] [CrossRef]
- Pandics, T.; Major, D.; Fazekas-Pongor, V.; Szarvas, Z.; Peterfi, A.; Mukli, P.; Gulej, R.; Ungvari, A.; Fekete, M.; Tompa, A.; et al. Exposome and unhealthy aging: Environmental drivers from air pollution to occupational exposures. Geroscience 2023, 45, 3381–3408. [Google Scholar] [CrossRef]
- Zhao, R.; Lu, H.; Yuan, H.; Chen, S.; Xu, K.; Zhang, T.; Liu, Z.; Jiang, Y.; Suo, C.; Chen, X. Plasma proteomics-based organ-specific aging for all-cause mortality and cause-specific mortality: A prospective cohort study. GeroScience 2024, 47, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Zhao, Y.; Huang, N.; Li, Y.; Wang, W.; Song, Z.; Dong, X.; Xiao, W.; Jia, J.; Liu, Z.; et al. Associations of healthy aging index and all-cause and cause-specific mortality: A prospective cohort study of UK Biobank participants. GeroScience 2024, 46, 1241–1257. [Google Scholar] [CrossRef]
- Zheng, H.T.; Li, D.L.; Lou, M.W.C.; Hodge, A.M.; Southey, M.C.; Giles, G.G.; Milne, R.L.; Lynch, B.M.; Dugué, P.-A. Physical activity and DNA methylation-based markers of ageing in 6208 middle-aged and older Australians: Cross-sectional and longitudinal analyses. GeroScience 2024, 47, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Magnano San Lio, R.; Li Destri, G.; Agodi, A.; Basile, G. Epigenetic Aging and Colorectal Cancer: State of the Art and Perspectives for Future Research. Int. J. Mol. Sci. 2020, 22, 200. [Google Scholar] [CrossRef]
- Bardelcikova, A.; Soltys, J.; Mojzis, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef]
- Kallai, A.; Ungvari, Z.; Fekete, M.; Maier, A.B.; Mikala, G.; Andrikovics, H.; Lehoczki, A. Genomic instability and genetic heterogeneity in aging: Insights from clonal hematopoiesis (CHIP), monoclonal gammopathy (MGUS), and monoclonal B-cell lymphocytosis (MBL). GeroScience 2024, 47, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T. Environmental Factors, Gut Microbiota, and Colorectal Cancer Prevention. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2019, 17, 275–289. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Lehoczki, A.; Laukkanen, J.A. Coffee consumption, cancer, and healthy aging: Epidemiological evidence and underlying mechanisms. GeroScience 2024, 47, 1517–1555. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Kaminsky, L.A.; Lehoczki, A.; Laukkanen, J.A. Unraveling the link between cardiorespiratory fitness and cancer: A state-of-the-art review. GeroScience 2024, 46, 5559–5585. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Jassal, D.S.; Ravandi, A.; Lehoczki, A. Dietary flaxseed: Cardiometabolic benefits and its role in promoting healthy aging. GeroScience 2025. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Woolbright, B.L.; Xuan, H.; Ahmed, I.; Rajendran, G.; Abbott, E.; Dennis, K.; Zhong, C.; Umar, S.; Taylor, J.A. Aging induces changes in cancer formation and microbial content in a murine model of bladder cancer. GeroScience 2024, 46, 3361–3375. [Google Scholar] [CrossRef]
- Tzemah-Shahar, R.; Turjeman, S.; Sharon, E.; Gamliel, G.; Hochner, H.; Koren, O.; Agmon, M. Signs of aging in midlife: Physical function and sex differences in microbiota. GeroScience 2024, 46, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Sun, H.; Wang, Y.; Li, Y.; Piao, R.; Bu, L.; Xu, H. Characterizing the oral and gastrointestinal microbiome associated with healthy aging: Insights from long-lived populations in Northeastern China. GeroScience 2024, 47, 2275–2292. [Google Scholar] [CrossRef]
- Thomas, R.M. Role of Bacteria in the Development of Colorectal Cancer. Clin. Colon. Rectal Surg. 2023, 36, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mikó, E.; Sipos, A.; Tóth, E.; Lehoczki, A.; Fekete, M.; Sebő, É.; Kardos, G.; Bai, P. Guideline for designing microbiome studies in neoplastic diseases. GeroScience 2024, 46, 4037–4057. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Varga, P.; Lehoczki, A.; Fekete, J.T.; Ungvari, A.; Győrffy, B. Overweight and obesity significantly increase colorectal cancer risk: A meta-analysis of 66 studies revealing a 25-57% elevation in risk. GeroScience 2024. [Google Scholar] [CrossRef]
- Lega, I.C.; Lipscombe, L.L. Review: Diabetes, Obesity, and Cancer-Pathophysiology and Clinical Implications. Endocr. Rev. 2020, 41, 33–52. [Google Scholar] [CrossRef]
- Socol, C.T.; Chira, A.; Martinez-Sanchez, M.A.; Nuñez-Sanchez, M.A.; Maerescu, C.M.; Mierlita, D.; Rusu, A.V.; Ruiz-Alcaraz, A.J.; Trif, M.; Ramos-Molina, B. Leptin Signaling in Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 4713. [Google Scholar] [CrossRef]
- Mak, J.K.L.; Kuja-Halkola, R.; Wang, Y.; Hagg, S.; Jylhava, J. Can frailty scores predict the incidence of cancer? Results from two large population-based studies. GeroScience 2023, 45, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Melia, F.; Udomjarumanee, P.; Zinovkin, D.; Arghiani, N.; Pranjol, M.Z.I. Pro-tumorigenic role of type 2 diabetes-induced cellular senescence in colorectal cancer. Front. Oncol. 2022, 12, 975644. [Google Scholar] [CrossRef] [PubMed]
- Takács, I.; Dank, M.; Majnik, J.; Nagy, G.; Szabó, A.; Szabó, B.; Szekanecz, Z.; Sziller, I.; Toldy, E.; Tislér, A.; et al. Hungarian consensus recommendation on the role of vitamin D in disease prevention and treatment. Orvosi Hetil. 2022, 163, 575–584. [Google Scholar] [CrossRef]
- Bhattoa, H.P. A Csontanyagcsere és a D-Vitaminháztartás Biokémiai Markerei; Analitikai kihívások, klinikai alkalmazások; DE ÁOK: Debrecen, Hungary, 2023. [Google Scholar]
- Li, Y.C.; Chen, Y.; Du, J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J. Steroid Biochem. Mol. Biol. 2015, 148, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D: Newer concepts of its metabolism and function at the basic and clinical level. J. Endocr. Soc. 2020, 4, bvz038. [Google Scholar] [CrossRef]
- Beauchet, O.; Launay, C.P.; Fantino, B.; Annweiler, C.; Allali, G. Motor imagery of gait in non-demented older community-dwellers: Performance depends on serum 25-hydroxyvitamin D concentrations. AGE 2015, 37, 18. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Physiology of vitamin D—Focusing on disease prevention. Nutrients 2024, 16, 1666. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef]
- Galvão, L.O.; Galvão, M.F.; Reis, C.M.S.; Batista, C.d.Á.; Casulari, L.A. Considerações atuais sobre a vitamina D. Brasília Med. 2013, 50, 324–332. [Google Scholar]
- Sîrbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An update on the effects of vitamin D on the immune system and autoimmune diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Samuel, S.; Sitrin, M.D. Vitamin D’s role in cell proliferation and differentiation. Nutr. Rev. 2008, 66 (Suppl. 2), S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The anti-inflammatory effects of vitamin D in tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef]
- Dou, R.; Ng, K.; Giovannucci, E.L.; Manson, J.E.; Qian, Z.R.; Ogino, S. Vitamin D and colorectal cancer: Molecular, epidemiological and clinical evidence. Br. J. Nutr. 2016, 115, 1643–1660. [Google Scholar] [CrossRef] [PubMed]
- McGee, K.C.; Sullivan, J.; Hazeldine, J.; Schmunk, L.J.; Martin-Herranz, D.E.; Jackson, T.; Lord, J.M. A combination nutritional supplement reduces DNA methylation age only in older adults with a raised epigenetic age. GeroScience 2024, 46, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Huggins, B.; Farris, M. Vitamin D(3) promotes longevity in Caenorhabditis elegans. GeroScience 2023, 45, 345–358. [Google Scholar] [CrossRef]
- Vetter, V.M.; Sommerer, Y.; Kalies, C.H.; Spira, D.; Bertram, L.; Demuth, I. Vitamin D supplementation is associated with slower epigenetic aging. GeroScience 2022, 44, 1847–1859. [Google Scholar] [CrossRef]
- Díaz, L.; Díaz-Muñoz, M.; García-Gaytán, A.C.; Méndez, I. Mechanistic Effects of Calcitriol in Cancer Biology. Nutrients 2015, 7, 5020–5050. [Google Scholar] [CrossRef]
- Bover, J.; Egido, J.; Ferández-Giráldez, E.; Fernández-Giráldez, E.; Praga, M.; Solozábal-Campos, C.; Torregrosa, J.V.; Torregrosa, J.V.; Martínez-Castelao, A. Vitamin D, vitamin D receptor and the importance of its activation in patients with chronic kidney disease. Nefrología 2015, 35, 28–41. [Google Scholar]
- Misiorowski, W. Vitamin D, infections and immunity. Wiedza Med. 2020, 2, 6–15. [Google Scholar] [CrossRef]
- Alswailmi, F.K.; Shah, S.I.A.; Nawaz, H. Immunomodulatory role of vitamin D: Clinical implications in infections and autoimmune disorders. Gomal J. Med. Sci. 2020, 18, 132–138. [Google Scholar] [CrossRef]
- Bray, N.W.; Pieruccini-Faria, F.; Witt, S.T.; Bartha, R.; Doherty, T.J.; Nagamatsu, L.S.; Almeida, Q.J.; Liu-Ambrose, T.; Middleton, L.E.; Bherer, L.; et al. Combining exercise with cognitive training and vitamin D3 to improve functional brain connectivity (FBC) in older adults with mild cognitive impairment (MCI). Results from the SYNERGIC trial. GeroScience 2023, 45, 1967–1985. [Google Scholar] [CrossRef]
- Banerjee, A.; Khemka, V.K.; Ganguly, A.; Roy, D.; Ganguly, U.; Chakrabarti, S. Vitamin D and Alzheimer’s disease: Neurocognition to therapeutics. Int. J. Alzheimer’s Dis. 2015, 2015, 192747. [Google Scholar] [CrossRef] [PubMed]
- Casseb, G.A.; Kaster, M.P.; Rodrigues, A.L.S. Potential role of vitamin D for the management of depression and anxiety. CNS Drugs 2019, 33, 619–637. [Google Scholar] [CrossRef]
- Vanga, S.R.; Good, M.; Howard, P.A.; Vacek, J.L. Role of vitamin D in cardiovascular health. Am. J. Cardiol. 2010, 106, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Marini, F.; Parri, S.; Bandinelli, S.; Iantomasi, T.; Giusti, F.; Talluri, E.; Sini, G.; Nannipieri, F.; Battaglia, S.; et al. Association of vitamin D and bisphenol A levels with cardiovascular risk in an elderly Italian population: Results from the InCHIANTI study. GeroScience 2024, 46, 6141–6156. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kumawat, S.; Labieb, F.; Kaur, P. Vitamin D Deficiency and Heart Health: A Narrative Review. J. Nutr. Res. 2023, 11, 47–52. [Google Scholar]
- Dibaba, D.T. Effect of vitamin D supplementation on serum lipid profiles: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 890–902. [Google Scholar] [CrossRef]
- Bryson, K.; Nash, A.; Norval, M. Does vitamin D protect against respiratory viral infections? Epidemiol. Infect. 2014, 142, 1789–1801. [Google Scholar] [CrossRef]
- Fekete, M.; Horvath, A.; Santa, B.; Tomisa, G.; Szollosi, G.; Ungvari, Z.; Fazekas-Pongor, V.; Major, D.; Tarantini, S.; Varga, J.T. COVID-19 vaccination coverage in patients with chronic obstructive pulmonary disease—A cross-sectional study in Hungary. Vaccine 2023, 41, 193–200. [Google Scholar] [CrossRef]
- Percze, A.R.; Nagy, A.; Polivka, L.; Barczi, E.; Czaller, I.; Kovats, Z.; Varga, J.T.; Ballai, J.H.; Muller, V.; Horvath, G. Fatigue, sleepiness and sleep quality are SARS-CoV-2 variant independent in patients with long COVID symptoms. Inflammopharmacology 2023, 31, 2819–2825. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Dosa, N.; Lehoczki, A.; Tarantini, S.; Varga, J.T. COVID-19 infection in patients with chronic obstructive pulmonary disease: From pathophysiology to therapy. Mini-Rev. Physiol. Int. 2022, 109, 9–19. [Google Scholar] [CrossRef]
- Lương Kvq Nguyễn, L.T.H. Beneficial role of vitamin D3 in the prevention of certain respiratory diseases. Ther. Adv. Respir. Dis. 2013, 7, 327–350. [Google Scholar] [CrossRef]
- Banerjee, A.; Panettieri, R.A. Vitamin D modulates airway smooth muscle function. Vitamin D Lung Mech. Dis. Assoc. 2012, 127–150. [Google Scholar] [CrossRef]
- Bossé, Y.; Maghni, K.; Hudson, T.J. 1α, 25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol. Genom. 2007, 29, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Szewczuk, M.R. Age and immunity to respiratory tract infections. AGE 1989, 12, 25–35. [Google Scholar] [CrossRef]
- Szarvas, Z.; Fekete, M.; Szollosi, G.J.; Kup, K.; Horvath, R.; Schimizu, M.; Tsuchiya, F.; Choi, H.E.; Wu, H.-T.; Pongor-Fazekas, V.; et al. Optimizing Cardiopulmonary Rehabilitation Duration for Long COVID Patients: An Exercise Physiology Monitoring Approach. Eur. Respir. J. 2025, 64 (Suppl. 68), PA704. [Google Scholar]
- Abidi, Y.; Kovats, Z.; Bohacs, A.; Fekete, M.; Naas, S.; Madurka, I.; Torok, K.; Bogyo, L.; Varga, J.T. Lung Transplant Rehabilitation—A Review. Life 2023, 13, 506. [Google Scholar] [CrossRef]
- Akimbekov, N.S.; Digel, I.; Sherelkhan, D.K.; Lutfor, A.B.; Razzaque, M.S. Vitamin D and the host-gut microbiome: A brief overview. Acta Histochem. Cytochem. 2020, 53, 33–42. [Google Scholar] [CrossRef]
- Marfil-Sánchez, A.; Seelbinder, B.; Ni, Y.; Varga, J.; Berta, J.; Hollosi, V.; Dome, B.; Megyesfalvi, Z.; Dulka, E.; Galffy, G.; et al. Gut microbiome functionality might be associated with exercise tolerance and recurrence of resected early-stage lung cancer patients. PLoS ONE 2021, 16, e0259898. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ananthakrishnan, A.N. vitamin D and inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2014, 39, 125–136. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Vitamin D: Metabolism and Function; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Tai, K.; Need, A.G.; Horowitz, M.; Chapman, I.M. Vitamin D, glucose, insulin, and insulin sensitivity. Nutrition 2008, 24, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xia, H.; Li, L.; Lee, R.J.; Xie, J.; Liu, Z.; Qiu, Z.; Teng, L. Liposomal vitamin D3 as an anti-aging agent for the skin. Pharmaceutics 2019, 11, 311. [Google Scholar] [CrossRef]
- Kocyigit, B.F.; Kocyigit, E.; Ozturk, G.Y. Anti-aging nutrition therapy. Anti-Aging East. Eur. 2024, 3, 59–65. [Google Scholar] [CrossRef]
- Gy, B.N.; Balikó, Z.; Kovács, G. Egészségügyi szakmai irányelv a krónikus obstruktív tüdőbetegség (COPD) diagnosztikájáról és kezeléséről, az alap, a szak és a sürgősségi ellátás területén. Med. Thor. 2014, 67, 76112. [Google Scholar]
- Fekete, M.; Pákó, J.; Szőllősi, G.; Tóth, K.; Szabó, M.; Horváth, D.; Varga, J.T. [Significance of nutritional status in chronic obstructive pulmonary disease: A survey]. Orv. Hetil. 2020, 161, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Boughanem, H.; Canudas, S.; Becerra-Tomás, N.; Fernández de la Puente, M.; Babio, N.; Macias-Gonzalez, M.; Salas-Salvadó, J. Circulating vitamin D levels and colorectal cancer risk: A meta-analysis and systematic review of case-control and prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1–17. [Google Scholar] [CrossRef]
- Garland, C.; Garland, F.; Shaw, E.; Comstock, G.; Helsing, K.; Gorham, E. Serum 25-hydroxyvitamin D and colon cancer: Eight-year prospective study. Lancet 1989, 334, 1176–1178. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Cross, H.S.; Bises, G.; Lechner, D.; Manhardt, T.; Kállay, E. The vitamin D endocrine system of the gut—Its possible role in colorectal cancer prevention. J. Steroid Biochem. Mol. Biol. 2005, 97, 121–128. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Liu, Z.; Qin, H. Association between vitamin D and risk of colorectal cancer: A systematic review of prospective studies. J. Clin. Oncol. 2011, 29, 3775–3782. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Mayorga, G.; Larriba, M.J.; Crespo, P.; Muñoz, A. Mechanisms of action of vitamin D in colon cancer. J. Steroid Biochem. Mol. Biol. 2019, 185, 1–6. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, C.; Zhong, Y.N.; Zhao, F.; Hao, Z.; Xu, Y.; Lai, R.; Shen, G.; Yin, X. Effect and mechanism of vitamin D on the development of colorectal cancer based on intestinal flora disorder. J. Gastroenterol. Hepatol. 2020, 35, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Bellerba, F.; Muzio, V.; Gnagnarella, P.; Facciotti, F.; Chiocca, S.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Serrano, D.; Raimondi, S.; et al. The Association between Vitamin D and Gut Microbiota: A Systematic Review of Human Studies. Nutrients 2021, 13, 3378. [Google Scholar] [CrossRef]
- Meeker, S.; Seamons, A.; Paik, J.; Treuting, P.M.; Brabb, T.; Grady, W.M.; Maggio-Prince, L. Increased dietary vitamin D suppresses MAPK signaling, colitis, and colon cancer. Cancer Res. 2014, 74, 4398–4408. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Fehér, Á.; Varga, J.T. Az emberi szervezetben élő baktériumok klinikai jelentősége a gyakorlatban. Egészségfejlesztés 2021, 62, 31–43. [Google Scholar] [CrossRef]
- Chen, A.; Davis, B.H.; Sitrin, M.D.; Brasitus, T.A.; Bissonnette, M. Transforming growth factor-beta 1 signaling contributes to Caco-2 cell growth inhibition induced by 1,25(OH)(2)D(3). Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G864–G874. [Google Scholar] [CrossRef] [PubMed]
- Kósa, J.P.; Horváth, P.; Wölfling, J.; Kovács, D.; Balla, B.; Mátyus, P.; Horváth, E.; Speer, G.; Takács, I.; Nagy, Z.; et al. CYP24A1 inhibition facilitates the anti-tumor effect of vitamin D3 on colorectal cancer cells. World J. Gastroenterol. 2013, 19, 2621–2628. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260.e16. [Google Scholar] [CrossRef]
- Na, S.-Y.; Kim, K.B.; Lim, Y.J.; Song, H.J. Vitamin D and Colorectal Cancer: Current Perspectives and Future Directions. J. Cancer Prev. 2022, 27, 147. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- El-Sharkawy, A.; Malki, A. Vitamin D Signaling in Inflammation and Cancer: Molecular Mechanisms and Therapeutic Implications. Molecules 2020, 25, 3219. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, M.; Nicoletti, F.; Malaguarnera, L. Vitamin D3: A helpful immuno-modulator. Immunology 2011, 134, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Muthusami, S.; Ramachandran, I.K.; Babu, K.N.; Krishnamoorthy, S.; Guruswamy, A.; Queimado, L.; Chaudhuri, G.; Ramachandran, I. Role of inflammation in the development of colorectal cancer. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2021, 21, 77–90. [Google Scholar]
- Pereira, F.; Fernández-Barral, A.; Larriba, M.J.; Barbáchano, A.; González-Sancho, J.M. From molecular basis to clinical insights: A challenging future for the vitamin D endocrine system in colorectal cancer. FEBS J. 2024, 291, 2485–2518. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and immune function: Understanding common pathways. Curr. Osteoporos. Rep. 2009, 7, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, W. The roles of vitamin D and its analogs in inflammatory diseases. Curr. Top. Med. Chem. 2016, 16, 1242–1261. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Molina, A.M.; Téllez Santana, T.; Redondo, M.; Bravo Romero, M.J. The Crucial Role of Inflammation and the Immune System in Colorectal Cancer Carcinogenesis: A Comprehensive Perspective. Int. J. Mol. Sci. 2024, 25, 6188. [Google Scholar] [CrossRef]
- Piemonti, L.; Monti, P.; Sironi, M.; Fraticelli, P.; Leone, B.E.; Dal Cin, E.; Allavena, P.; Di Carlo, V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 2000, 164, 4443–4451. [Google Scholar] [CrossRef]
- Bscheider, M.; Butcher, E.C. Vitamin D immunoregulation through dendritic cells. Immunology 2016, 148, 227–236. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Di Rosa, M.; Malaguarnera, G.; De Gregorio, C.; Palumbo, M.; Nunnari, G.; Malaguarnera, L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell. Immunol. 2012, 280, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory role of vitamin D: A review. In Current Trends in Immunity and Respiratory Infections; Springer: Cham, Switzerland, 2018; pp. 13–23. [Google Scholar]
- Meeker, S.; Seamons, A.; Maggio-Price, L.; Paik, J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J. Gastroenterol. 2016, 22, 933. [Google Scholar] [CrossRef]
- Raman, M.; Milestone, A.N.; Walters, J.R.; Hart, A.L.; Ghosh, S. Vitamin D and gastrointestinal diseases: Inflammatory bowel disease and colorectal cancer. Ther. Adv. Gastroenterol. 2011, 4, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.-C.C.; Chen, T. The anti-cancer actions of vitamin D. Anti-Cancer Agents Med. Chem.-Anti-Cancer Agents 2013, 13, 126–139. [Google Scholar] [CrossRef]
- Starska-Kowarska, K. Role of vitamin D in head and neck cancer—Immune function, anti-tumour effect, and its impact on patient prognosis. Nutrients 2023, 15, 2592. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhao, W.; Zhang, W.; Li, S.; Teng, G.; Liu, L. Vitamin D promotes ferroptosis in colorectal cancer stem cells via SLC7A11 downregulation. Oxidative Med. Cell. Longev. 2023, 2023, 4772134. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, W.; Zhang, T.; Li, S.; Guo, J.; Liu, L. Identification of a ferroptosis-related gene signature for prognosis prediction in colorectal cancer patients and relationship with vitamin D. J. Steroid Biochem. Mol. Biol. 2023, 227, 106234. [Google Scholar] [CrossRef]
- Nemeth, Z.; Patonai, A.; Simon-Szabó, L.; Takács, I. Interplay of vitamin D and SIRT1 in tissue-specific metabolism—Potential roles in prevention and treatment of non-communicable diseases including cancer. Int. J. Mol. Sci. 2023, 24, 6154. [Google Scholar] [CrossRef]
- Kabra, N.; Li, Z.; Chen, L.; Li, B.; Zhang, X.; Wang, C.; Yeatman, T.; Coppola, D.; Chen, J. SirT1 is an inhibitor of proliferation and tumor formation in colon cancer. J. Biol. Chem. 2009, 284, 18210–18217. [Google Scholar] [CrossRef]
- Singh, P.K.; Campbell, M.J. 2 Vitamin D Receptor: Genomic and Epigenomic Effects. In Vitamin D: Oxidative Stress, Immunity, and Aging; CRC Press: Boca Raton, FL, USA, 2012; p. 37. [Google Scholar]
- García-Martínez, J.M.; Chocarro-Calvo, A.; Martínez-Useros, J.; Fernández-Aceñero, M.J.; Fiuza, M.C.; Cáceres-Rentero, J.; De la Vieja, A.; Barbáchano, A.; Muñoz, A.; Larriba, M.J.; et al. Vitamin D induces SIRT1 activation through K610 deacetylation in colon cancer. eLife 2023, 12, RP86913. [Google Scholar] [CrossRef] [PubMed]
- Strycharz, J.; Rygielska, Z.; Swiderska, E.; Drzewoski, J.; Szemraj, J.; Szmigiero, L.; Sliwinska, A. SIRT1 as a therapeutic target in diabetic complications. Curr. Med. Chem. 2018, 25, 1002–1035. [Google Scholar] [CrossRef] [PubMed]
- Firestein, R.; Blander, G.; Michan, S.; Oberdoerffer, P.; Ogino, S.; Campbell, J.; Bhimavarapu, A.; Luikenhuis, S.; de Cabo, R.; Fuchs, C.; et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE 2008, 3, e2020. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.S.; Khan, Z.; Hu, C.; Galligan, M.A.; Dussik, C.M.; Mallick, S.; Stone, A.D.; Batie, S.F.; Jacobs, E.T.; Whitfield, G.K.; et al. SIRT1 enzymatically potentiates 1, 25-dihydroxyvitamin D3 signaling via vitamin D receptor deacetylation. J. Steroid Biochem. Mol. Biol. 2017, 172, 117–129. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, R.; Sun, M.; Guo, X.; Yang, J.; Bian, W.; Xie, C.; Miao, D.; Mao, L. Sirt1 Mediates Vitamin D Deficiency-Driven Gluconeogenesis in the Liver via mTorc2/Akt Signaling. J. Diabetes Res. 2022, 2022, 1755563. [Google Scholar] [CrossRef]
- Borojević, A.; Jauković, A.; Kukolj, T.; Mojsilović, S.; Obradović, H.; Trivanović, D.; Živanović, M.; Zečević, Ž.; Simić, M.; Gobeljić, B.; et al. Vitamin D3 stimulates proliferation capacity, expression of pluripotency markers, and osteogenesis of human bone marrow mesenchymal stromal/stem cells, partly through SIRT1 signaling. Biomolecules 2022, 12, 323. [Google Scholar] [CrossRef]
- Carafa, V.; Altucci, L.; Nebbioso, A. Dual tumor suppressor and tumor promoter action of sirtuins in determining malignant phenotype. Front. Pharmacol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Ren, N.S.; Ji, M.; Tokar, E.J.; Busch, E.L.; Xu, X.; Lewis, D.; Li, X.; Jin, A.; Zhang, Y.; Wu, W.K.; et al. Haploinsufficiency of SIRT1 enhances glutamine metabolism and promotes cancer development. Curr. Biol. 2017, 27, 483–494. [Google Scholar] [CrossRef]
- Assa, A.; Vong, L.; Pinnell, L.J.; Avitzur, N.; Johnson-Henry, K.C.; Sherman, P.M. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J. Infect. Dis. 2014, 210, 1296–1305. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Lu, R.; Wu, S.; Chatterjee, I.; Zhou, D.; Xia, Y.; Sun, J. Vitamin D receptor protects against dysbiosis and tumorigenesis via the JAK/STAT pathway in intestine. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 729–746. [Google Scholar] [CrossRef]
- Naderpoor, N.; Mousa, A.; Fernanda Gomez Arango, L.; Barrett, H.L.; Dekker Nitert, M.; de Courten, B. Effect of Vitamin D Supplementation on Faecal Microbiota: A Randomised Clinical Trial. Nutrients 2019, 11, 2888. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.; Choudhury, A.; Von Dohlen, G.; Heileson, J.L.; Forsse, J.S.; Rajakaruna, S.; Zec, M.; Tfaily, M.M.; Greathouse, L. Randomized control trial of moderate dose vitamin D alters microbiota stability and metabolite networks in healthy adults. Microbiol. Spectr. 2024, 12, e00083-24. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Q.; Huang, G.; Cong, J.; Wang, T.; Zhai, X.; Zhang, J.; Qi, G.; Zhou, L.; Jin, J. Combined effects of vitamin D and neferine on the progression and metastasis of colorectal cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 6203–6210. [Google Scholar] [CrossRef]
- Dasari, S.; Bakthavachalam, V.; Chinnapaka, S.; Venkatesan, R.; Samy, A.L.; Munirathinam, G. Neferine, an alkaloid from lotus seed embryo targets HELA and SIHA cervical cancer cells via pro-oxidant anticancer mechanism. Phytother. Res. 2020, 34, 2366–2384. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Fekete, M.; Varga, P.; Fekete, J.T.; Buda, A.; Szappanos, Á.; Lehoczki, A.; Mózes, N.; Grosso, G.; Menyhart, O.; et al. Impact of adherence to the Mediterranean diet on stroke risk. GeroScience 2025. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Varga, P.; Lehoczki, A.; Munkácsy, G.; Fekete, J.T.; Bianchini, G.; Ocana, A.; Buda, A.; Ungvari, A.; et al. Association between red and processed meat consumption and colorectal cancer risk: A comprehensive meta-analysis of prospective studies. GeroScience 2025. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Q.; Zhang, Q.; Li, S.; Zhao, J.; Chen, W.; Yang, J.; Xia, M.; Liu, Y. Association of adherence to the EAT-Lancet diet with risk of dementia according to social economic status: A prospective cohort in UK Biobank. GeroScience 2024. [Google Scholar] [CrossRef]
- Trabado-Fernández, A.; García-Colomo, A.; Cuadrado-Soto, E.; Peral-Suárez, Á.; Salas-González, M.D.; Lorenzo-Mora, A.M.; Aparicio, A.; Delgado-Losada, M.L.; Maestú-Unturbe, F.; López-Sobaler, A.M. Association of a DASH diet and magnetoencephalography in dementia-free adults with different risk levels of Alzheimer’s disease. GeroScience 2024, 47, 1747–1759. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Lehoczki, A.; Munkácsy, G.; Fekete, J.T.; Zábó, V.; Purebl, G.; Varga, P.; Ungvari, A.; Győrffy, B. Sleep disorders increase the risk of dementia, Alzheimer’s disease, and cognitive decline: A meta-analysis. GeroScience 2025. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Fekete, J.T.; Grosso, G.; Ungvari, A.; Győrffy, B. Adherence to the Mediterranean diet and its protective effects against colorectal cancer: A meta-analysis of 26 studies with 2,217,404 participants. GeroScience 2024, 47, 1105–1121. [Google Scholar] [CrossRef]
- Fekete, M.; Csípő, T.; Fazekas-Pongor, V.; Bálint, M.; Csizmadia, Z.; Tarantini, S.; Varga, J.T. The Possible Role of Food and Diet in the Quality of Life in Patients with COPD-A State-of-the-Art Review. Nutrients 2023, 15, 3902. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Varga, P.; Ungvari, Z.; Fekete, J.T.; Buda, A.; Szappanos, Á.; Lehoczki, A.; Mózes, N.; Grosso, G.; Godos, J.; et al. The role of the Mediterranean diet in reducing the risk of cognitive impairement, dementia, and Alzheimer’s disease: A meta-analysis. GeroSscience 2025, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Lee, S.Y.; Sharma, S.; Ullah, S.; Tan, E.C.; Brodaty, H.; Schutte, A.E.; Sachdev, P.S. A systematic review of diet and medication use among centenarians and near-centenarians worldwide. GeroScience 2024, 46, 6625–6639. [Google Scholar] [CrossRef]
- Fekete, M.; Liotta, E.M.; Molnar, T.; Fülöp, G.A.; Lehoczki, A. The role of atrial fibrillation in vascular cognitive impairment and dementia: Epidemiology, pathophysiology, and preventive strategies. GeroScience 2025, 47, 287–300. [Google Scholar] [CrossRef]
- Dobreva, I.; Marston, L.; Mukadam, N. Which components of the Mediterranean diet are associated with dementia? A UK Biobank cohort study. Geroscience 2022, 44, 2541–2554. [Google Scholar] [CrossRef]
- Madarász, B.; Fazekas-Pongor, V.; Szarvas, Z.; Fekete, M.; Varga, J.T.; Tarantini, S.; Csiszar, A.; Lionetti, V.; Tabák, A.G.; Ungvari, Z.; et al. Survival and longevity of European rulers: Geographical influences and exploring potential factors, including the Mediterranean diet—A historical analysis from 1354 to the twentieth century. GeroScience 2024, 46, 3801–3818. [Google Scholar] [CrossRef]
- Bizzozero-Peroni, B.; Díaz-Goñi, V.; Beneit, N.; Oliveira, A.; Jiménez-López, E.; Martínez-Vizcaíno, V.; Mesas, A.E. Nut consumption is associated with a lower risk of all-cause dementia in adults: A community-based cohort study from the UK Biobank. Geroscience 2024, 47, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Micek, A.; Currenti, W.; Franchi, C.; Poli, A.; Battino, M.; Dolci, A.; Ricci, C.; Ungvari, Z.; Grosso, G. Fish consumption, cognitive impairment and dementia: An updated dose-response meta-analysis of observational studies. Aging Clin. Exp. Res. 2024, 36, 171. [Google Scholar] [CrossRef]
- Gensous, N.; Garagnani, P.; Santoro, A.; Giuliani, C.; Ostan, R.; Fabbri, C.; Milazzo, M.; Gentilini, D.; di Blasio, A.M.; Pietruszka, B.; et al. One-year Mediterranean diet promotes epigenetic rejuvenation with country-and sex-specific effects: A pilot study from the NU-AGE project. Geroscience 2020, 42, 687–701. [Google Scholar] [CrossRef]
- Selb, J.; Cvetko, F.; Deutsch, L.; Bedrac, L.; Kuscer, E.; Maier, A.B. Personalization matters: The effect of sex in multivitamin-multimineral-based cancer prevention. Geroscience 2024, 46, 1351–1356. [Google Scholar] [CrossRef]
- Shang, X.; Liu, J.; Zhu, Z.; Zhang, X.; Huang, Y.; Liu, S.; Wang, W.; Zhang, X.; Tang, S.; Hu, Y.; et al. Healthy dietary patterns and the risk of individual chronic diseases in community-dwelling adults. Nat. Commun. 2023, 14, 6704. [Google Scholar] [CrossRef] [PubMed]
- Romanos-Nanclares, A.; Guasch-Ferré, M.; Willett, W.C.; Chen, W.Y.; Holmes, M.D.; Rosner, B.A.; Martinez-Gonzalez, M.A.; Eliassen, A.H. Consumption of olive oil and risk of breast cancer in U.S. women: Results from the Nurses’ Health Studies. Br. J. Cancer 2023, 129, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Szőllősi, G.; Németh, A.N.; Varga, J.T. Az ómega-3 zsírsavak pótlásának klinikai értéke krónikus obstruktív tüdőbetegségben. Orvosi Hetil. 2021, 162, 23–30. [Google Scholar] [CrossRef]
- GGu, Y.; Honig, L.S.; Schupf, N.; Lee, J.H.; Luchsinger, J.A.; Stern, Y.; Scarmeas, N. Mediterranean diet and leukocyte telomere length in a multi-ethnic elderly population. Age 2015, 37, 24. [Google Scholar] [CrossRef]
- Marin, C.; Delgado-Lista, J.; Ramirez, R.; Carracedo, J.; Caballero, J.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Garcia-Rios, A.; Delgado-Casado, N.; Cruz-Teno, C.; et al. Mediterranean diet reduces senescence-associated stress in endothelial cells. Age 2012, 34, 1309–1316. [Google Scholar] [CrossRef]
- Tognon, G.; Rothenberg, E.; Eiben, G.; Sundh, V.; Winkvist, A.; Lissner, L. Does the Mediterranean diet predict longevity in the elderly? A Swedish perspective. Age 2011, 33, 439–450. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Detopoulou, P.; Voulgaridou, G.; Tsoumana, D.; Spanoudaki, M.; Sadikou, F.; Papadopoulou, V.G.; Zidrou, C.; Chatziprodromidou, I.P.; Giaginis, C.; et al. Mediterranean Diet and Sarcopenia Features in Apparently Healthy Adults over 65 Years: A Systematic Review. Nutrients 2023, 15, 1104. [Google Scholar] [CrossRef] [PubMed]
- Maggi, S.; Ticinesi, A.; Limongi, F.; Noale, M.; Ecarnot, F. The role of nutrition and the Mediterranean diet on the trajectories of cognitive decline. Exp. Gerontol. 2023, 173, 112110. [Google Scholar] [CrossRef]
- Hoffmann, A.; Meir, A.Y.; Hagemann, T.; Czechowski, P.; Müller, L.; Engelmann, B.; Haange, S.-B.; Rolle-Kampczyk, U.; Tsaban, G.; Zelicha, H.; et al. A polyphenol-rich green Mediterranean diet enhances epigenetic regulatory potential: The DIRECT PLUS randomized controlled trial. Metabolism 2023, 145, 155594. [Google Scholar] [CrossRef]
- Zábó, V.; Lehoczki, A.; Fekete, M.; Szappanos, Á.; Varga, P.; Moizs, M.; Giovannetti, G.; Loscalzo, Y.; Giannini, M.; Polidori, M.C.; et al. The role of purpose in life in healthy aging: Implications for the Semmelweis Study and the Semmelweis-EUniWell Workplace Health Promotion Model Program. GeroScience, 2025; Advance online publication. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G.; Ferri, R.; Caraci, F.; Lanza, G.; Al-Qahtani, W.H.; Caruso, G.; Castellano, S. Mediterranean diet, mental health, cognitive status, quality of life, and successful aging in southern Italian older adults. Exp. Gerontol. 2023, 175, 112143. [Google Scholar] [CrossRef]
- Chen, H.; Dhana, K.; Huang, Y.; Huang, L.; Tao, Y.; Liu, X.; van Lent, D.M.; Zheng, Y.; Ascherio, A.; Willett, W.; et al. Association of the Mediterranean Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) Diet with the Risk of Dementia. JAMA Psychiatry 2023, 80, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.S.; Simpson, B.S.; Murphy, K.J. The role of a Mediterranean diet and physical activity in decreasing age-related inflammation through modulation of the gut microbiota composition. Br. J. Nutr. 2022, 128, 1299–1314. [Google Scholar] [CrossRef]
- Shannon, O.M.; Ashor, A.W.; Scialo, F.; Saretzki, G.; Martin-Ruiz, C.; Lara, J.; Matu, J.; Griffiths, A.; Robinson, N.; Lillà, L.; et al. Mediterranean diet and the hallmarks of ageing. Eur. J. Clin. Nutr. 2021, 75, 1176–1192. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Peng, H.; Hu, Z.; Xu, C.; Ning, M.; Zhou, M.; Mi, Y.; Yu, P.; Fazekas-Pongor, V.; Major, D.; et al. Exploring the global impact of obesity and diet on dementia burden: The role of national policies and sex differences. GeroScience 2025, 47, 1345–1360. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition strategies promoting healthy aging: From improvement of cardiovascular and brain health to prevention of age-associated diseases. Nutrients 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Lehoczki, A.; Tarantini, S.; Fazekas-Pongor, V.; Csípő, T.; Csizmadia, Z.; Varga, J.T. Improving Cognitive Function with Nutritional Supplements in Aging: A Comprehensive Narrative Review of Clinical Studies Investigating the Effects of Vitamins, Minerals, Antioxidants, and Other Dietary Supplements. Nutrients 2023, 15, 5116. [Google Scholar] [CrossRef]
- Saketkoo, L.A.; Escorpizo, R.; Varga, J.; Keen, K.J.; Fligelstone, K.; Birring, S.S.; Alexanderson, H.; Pettersson, H.; Chaudhry, H.A.; Poole, J.L.; et al. World Health Organization (WHO) international classification of functioning, disability and health (ICF) core set development for interstitial lung disease. Front. Pharmacol. 2022, 13, 979788. [Google Scholar] [CrossRef]
- Patai, R.; Patel, K.; Csik, B.; Gulej, R.; Nagaraja, R.Y.; Nagy, D.; Chandragiri, S.S.; Shanmugarama, S.; Kordestan, K.V.; Nagykaldi, M.; et al. Aging, mitochondrial dysfunction, and cerebral microhemorrhages: A preclinical evaluation of SS-31 (elamipretide) and development of a high-throughput machine learning-driven imaging pipeline for cerebromicrovascular protection therapeutic screening. GeroScience 2025. [Google Scholar] [CrossRef]
- Talavera-Rodríguez, I.; Banegas, J.R.; de la Cruz, J.J.; Martínez-Gómez, D.; Ruiz-Canela, M.; Ortolá, R.; Hershey, M.S.; Artalejo, F.R.; Sotos-Prieto, M. Mediterranean lifestyle index and 24-h systolic blood pressure and heart rate in community-dwelling older adults. GeroScience 2024, 46, 1357–1369. [Google Scholar] [CrossRef]
- Fekete, M.; Csípő, T.; Fazekas-Pongor, V.; Fehér, Á.; Szarvas, Z.; Kaposvári, C.; Horváth, K.; Lehoczki, A.; Tarantini, S.; Varga, J.T. The Effectiveness of Supplementation with Key Vitamins, Minerals, Antioxidants and Specific Nutritional Supplements in COPD-A Review. Nutrients 2023, 15, 2741. [Google Scholar] [CrossRef]
- Zupo, R.; Donghia, R.; Castellana, F.; Bortone, I.; De Nucci, S.; Sila, A.; Tatoli, R.; Lampignano, L.; Sborgia, G.; Panza, F.; et al. Ultra-processed food consumption and nutritional frailty in older age. GeroScience 2023, 45, 2229–2243. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Rodriguez, J.; Delgado-Velandia, M.; Ortolá, R.; Carballo-Casla, A.; García-Esquinas, E.; Rodríguez-Artalejo, F.; Sotos-Prieto, M. Plant-based diets and risk of frailty in community-dwelling older adults: The Seniors-ENRICA-1 cohort. GeroScience 2023, 45, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, H.; Zhao, M.; Peng, P. Prognostic value of circulating vitamin D binding protein, total, free and bioavailable 25-hydroxy vitamin D in patients with colorectal cancer. Oncotarget 2017, 8, 40214–40221. [Google Scholar] [CrossRef]
- Facciorusso, A.; Del Prete, V.; Muscatiello, N.; Crucinio, N.; Barone, M. Prognostic role of 25-hydroxyvitamin D in patients with liver metastases from colorectal cancer treated with radiofrequency ablation. J. Gastroenterol. Hepatol. 2016, 31, 1483–1488. [Google Scholar] [CrossRef]
- Maalmi, H.; Walter, V.; Jansen, L.; Chang-Claude, J.; Owen, R.W.; Ulrich, A.; Schöttker, B.; Hoffmeister, M.; Brenner, H. Relationship of very low serum 25-hydroxyvitamin D 3 levels with long-term survival in a large cohort of colorectal cancer patients from Germany. Eur. J. Epidemiol. 2017, 32, 961–971. [Google Scholar] [CrossRef]
- Tretli, S.; Schwartz, G.G.; Torjesen, P.A.; Robsahm, T.E. Serum levels of 25-hydroxyvitamin D and survival in Norwegian patients with cancer of breast, colon, lung, and lymphoma: A population-based study. Cancer Causes Control 2012, 23, 363–370. [Google Scholar] [CrossRef]
- Zgaga, L.; Theodoratou, E.; Farrington, S.M.; Din, F.V.N.; Ooi, L.Y.; Glodzik, D.; Johnston, S.; Tenesa, A.; Campbell, H.; Dunlop, M.G. Plasma vitamin D concentration influences survival outcome after a diagnosis of colorectal cancer. J. Clin. Oncol. 2014, 32, 2430–2439. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Sargent, D.J.; Goldberg, R.M.; Meyerhardt, J.A.; Green, E.M.; Pitot, H.C.; Hollis, B.W.; Pollak, M.N.; Fuchs, C.S. Vitamin D status in patients with stage IV colorectal cancer: Findings from Intergroup trial N9741. J. Clin. Oncol. 2011, 29, 1599–1606. [Google Scholar] [CrossRef]
- Mezawa, H.; Sugiura, T.; Watanabe, M.; Norizoe, C.; Takahashi, D.; Shimojima, A.; Tamez, S.; Tsutsumi, Y.; Yanaga, K.; Urashima, M. Serum vitamin D levels and survival of patients with colorectal cancer: Post-hoc analysis of a prospective cohort study. BMC Cancer 2010, 10, 347. [Google Scholar] [CrossRef]
- Fedirko, V.; Riboli, E.; Tjønneland, A.; Ferrari, P.; Olsen, A.; Bueno-De-Mesquita, H.B.; van Duijnhoven, F.J.; Norat, T.; Jansen, E.H.; Dahm, C.C.; et al. Prediagnostic 25-hydroxyvitamin D, VDR and CASR polymorphisms, and survival in patients with colorectal cancer in western European populations. Cancer Epidemiol. Biomark. Prev. 2012, 21, 582–593. [Google Scholar] [CrossRef]
- Yuan, C.; Sato, K.; Hollis, B.W.; Zhang, S.; Niedzwiecki, D.; Ou, F.-S.; Chang, I.-W.; O’Neil, B.H.; Innocenti, F.; Lenz, H.-J.; et al. Plasma 25-hydroxyvitamin D levels and survival in patients with advanced or metastatic colorectal cancer: Findings from CALGB/SWOG 80405 (Alliance). Clin. Cancer Res. 2019, 25, 7497–7505. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.A.; Yuan, C.; Sato, K.; Niedzwiecki, D.; Ye, X.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; et al. Predicted vitamin D status and colon cancer recurrence and mortality in CALGB 89803 (Alliance). Ann. Oncol. 2017, 28, 1359–1367. [Google Scholar] [CrossRef]
- Zhu, K.; Knuiman, M.; Divitini, M.; Hung, J.; Lim, E.M.; Cooke, B.R.; Walsh, J.P. Lower serum 25-hydroxyvitamin D is associated with colorectal and breast cancer, but not overall cancer risk: A 20-year cohort study. Nutr. Res. 2019, 67, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.K.; Hodge, A.M.; Ebeling, P.R.; Kvaskoff, D.; Eyles, D.W.; Giles, G.G.; English, D.R.; Williamson, E.J. Circulating 25-hydroxyvitamin D concentration and cause-specific mortality in the Melbourne Collaborative Cohort Study. J. Steroid Biochem. Mol. Biol. 2020, 198, 105612. [Google Scholar] [CrossRef] [PubMed]
- Vojdeman, F.J.; Madsen, C.M.; Frederiksen, K.; Durup, D.; Olsen, A.; Hansen, L.; Heegaard, A.; Lind, B.; Tjønneland, A.; Jørgensen, H.L.; et al. Vitamin D levels and cancer incidence in 217,244 individuals from primary health care in Denmark. Int. J. Cancer 2019, 145, 338–346. [Google Scholar] [CrossRef]
- Ordóñez-Mena, J.M.; Schöttker, B.; Fedirko, V.; Jenab, M.; Olsen, A.; Halkjær, J.; Kampman, E.; de Groot, L.; Jansen, E.; Bueno-De-Mesquita, H.B.; et al. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: An analysis of cohorts participating in the CHANCES consortium. Eur. J. Epidemiol. 2016, 31, 311–323. [Google Scholar] [CrossRef]
- Ordóñez-Mena, J.M.; Schöttker, B.; Haug, U.; Müller, H.; Köhrle, J.; Schomburg, L.; Holleczek, B.; Brenner, H. Serum 25-hydroxyvitamin d and cancer risk in older adults: Results from a large German prospective cohort study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 905–916. [Google Scholar] [CrossRef]
- Skaaby, T.; Husemoen, L.L.N.; Thuesen, B.H.; Pisinger, C.; Jørgensen, T.; Roswall, N.; Larsen, S.C.; Linneberg, A. Prospective population-based study of the association between serum 25-hydroxyvitamin-D levels and the incidence of specific types of cancer. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1220–1229. [Google Scholar] [CrossRef]
- Wong, Y.Y.E.; Hyde, Z.; McCaul, K.A.; Yeap, B.B.; Golledge, J.; Hankey, G.J.; Flicker, L. In older men, lower plasma 25-hydroxyvitamin D is associated with reduced incidence of prostate, but not colorectal or lung cancer. PLoS ONE 2014, 9, e99954. [Google Scholar] [CrossRef]
- Cooney, R.V.; Chai, W.; Franke, A.A.; Wilkens, L.R.; Kolonel, L.N.; Le Marchand, L. C-reactive protein, lipid-soluble micronutrients, and survival in colorectal cancer patients. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1278–1288. [Google Scholar] [CrossRef]
- Ng, K.; Meyerhardt, J.A.; Wu, K.; Feskanich, D.; Hollis, B.W.; Giovannucci, E.L.; Fuchs, C.S. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J. Clin. Oncol. 2008, 26, 2984–2991. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cheng, S.; Cai, T.; Cagan, A.; Gainer, V.S.; Szolovits, P.; Shaw, S.Y.; Churchill, S.; Karlson, E.W.; Murphy, S.N.; et al. Association between reduced plasma 25-hydroxy vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2014, 12, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Cheney, C.P.; Thorand, B.; Huth, C.; Berger, K.; Peters, A.; Seifert-Klauss, V.; Kiechle, M.; Strauch, K.; Quante, A.S. The Association between Serum 25-Hydroxyvitamin D and Cancer Risk: Results from the Prospective KORA F4 Study. Oncol. Res. Treat. 2018, 41, 117–121. [Google Scholar] [CrossRef]
- Boughanem, H.; Canudas, S.; Hernandez-Alonso, P.; Becerra-Tomás, N.; Babio, N.; Salas-Salvadó, J.; Macias-Gonzalez, M. Vitamin D intake and the risk of colorectal cancer: An updated meta-analysis and systematic review of case-control and prospective cohort studies. Cancers 2021, 13, 2814. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Robertson, A.S.; Rodriguez, C.; Jacobs, E.J.; Chao, A.; Jonas, C.; Calle, E.E.; Willett, W.C.; Thun, M.J. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States). Cancer Causes Control 2003, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- MMartínez, M.E.; Giovannucci, E.L.; Colditz, G.A.; Stampfer, M.J.; Hunter, D.J.; Speizer, F.E.; Wing, A.; Willett, W.C. Calcium, vitamin D, and the occurrence of colorectal cancer among women. J. Natl. Cancer Inst. 1996, 88, 1375–1382. [Google Scholar] [CrossRef]
- Bostick, R.M.; Potter, J.D.; Sellers, T.A.; McKenzie, D.R.; Kushi, L.H.; Folsom, A.R. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am. J. Epidemiol. 1993, 137, 1302–1317. [Google Scholar] [CrossRef]
- Keamey, J.; Giovannucci, E.; Rimm, E.B.; Ascherio, A.; Stampfer, M.J.; Colditz, G.A.; Wing, A.; Kampman, E.; Willett, W.C. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am. J. Epidemiol. 1996, 143, 907–917. [Google Scholar] [CrossRef]
- Zheng, W.; Anderson, K.E.; Kushi, L.H.; Sellers, T.A.; Greenstein, J.; Hong, C.P.; Cerhan, J.R.; Bostick, R.M.; Folsom, A.R. A prospective cohort study of intake of calcium, vitamin D, and other micronutrients in relation to incidence of rectal cancer among postmenopausal women. Cancer Epidemiol. Biomark. Prev. 1998, 7, 221–225. [Google Scholar]
- Hernández-Alonso, P.; Canudas, S.; Boughanem, H.; Toledo, E.; Sorlí, J.V.; Estruch, R.; Castañer, O.; Lapetra, J.; Alonso-Gómez, A.M.; Gutiérrez-Bedmar, M.; et al. Dietary vitamin D intake and colorectal cancer risk: A longitudinal approach within the PREDIMED study. Eur. J. Nutr. 2021, 60, 4367–4378. [Google Scholar] [CrossRef]
- Kopp, T.I.; Vogel, U.; Andersen, V. Associations between common polymorphisms in CYP2R1 and GC, Vitamin D intake and risk of colorectal cancer in a prospective case-cohort study in Danes. PLoS ONE 2020, 15, e0228635. [Google Scholar] [CrossRef] [PubMed]
- Kesse, E.; Boutron-Ruault, M.C.; Norat, T.; Riboli, E.; Clavel-Chapelon, F. Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int. J. Cancer 2005, 117, 137–144. [Google Scholar] [CrossRef]
- Nakano, S.; Yamaji, T.; Hidaka, A.; Shimazu, T.; Shiraishi, K.; Kuchiba, A.; Saito, M.; Kunishima, F.; Nakaza, R.; Kohno, T.; et al. Dietary vitamin D intake and risk of colorectal cancer according to vitamin D receptor expression in tumors and their surrounding stroma. J. Gastroenterol. 2024, 59, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.; Barrett-Connor, E.; Rossof, A.; Shekelle, R.; Criqui, M.; Paul, O. Dietary vitamin D and calcium and risk of colorectal cancer: A 19-year prospective study in men. Lancet 1985, 325, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, J.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Tsugane, S. Dietary calcium, vitamin D, and the risk of colorectal cancer. Am. J. Clin. Nutr. 2008, 88, 1576–1583. [Google Scholar] [CrossRef]
- Järvinen, R.; Knekt, P.; Hakulinen, T.; Aromaa, A. Prospective study on milk products, calcium and cancers of the colon and rectum. Eur. J. Clin. Nutr. 2001, 55, 1000–1007. [Google Scholar] [CrossRef]
- Terry, P.; Baron, J.A.; Bergkvist, L.; Holmberg, L.; Wolk, A. Dietary calcium and vitamin D intake and risk of colorectal cancer: A prospective cohort study in women. Nutr. Cancer 2002, 43, 39–46. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Erem, S.; Razzaque, M.S. Benefits of safe sunlight exposure: Vitamin D and beyond. J. Steroid Biochem. Mol. Biol. 2021, 27, 105957. [Google Scholar]
- Vallis, J.; Wang, P.P. The Role of Diet and Lifestyle in Colorectal Cancer Incidence and Survival. In Gastrointestinal Cancers; Morgado-Diaz, J.A., Ed.; Exon Publications: Brisbane, Australia, 2022. [Google Scholar]
- Cowbrough, K. Identifying vitamin D deficiency and recommendations for at-risk groups. J. Health Visit. 2014, 2, 304–310. [Google Scholar] [CrossRef]
- Misra, M.; Pacaud, D.; Petryk, A.; Collett-Solberg, P.F.; Kappy, M.; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: Review of current knowledge and recommendations. Pediatrics 2008, 122, 398–417. [Google Scholar] [CrossRef]
- Lopez-Caleya, J.F.; Ortega-Valín, L.; Fernández-Villa, T.; Delgado-Rodríguez, M.; Martín-Sánchez, V.; Molina, A.J. The role of calcium and vitamin D dietary intake on risk of colorectal cancer: Systematic review and meta-analysis of case-control studies. Cancer Causes Control 2022, 33, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qian, M.; Hong, J.; Ng, D.M.; Yang, T.; Xu, L.; Ye, X. The effect of vitamin D on the occurrence and development of colorectal cancer: A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.; Nimeiri, H.S.; McCleary, N.J.; Abrams, T.A.; Yurgelun, M.B.; Cleary, J.M.; Rubinson, D.A.; Schrag, D.; Miksad, R.; Bullock, A.J.; et al. Effect of high-dose vs standard-dose vitamin D3 supplementation on progression-free survival among patients with advanced or metastatic colorectal cancer: The SUNSHINE randomized clinical trial. Jama 2019, 321, 1370–1379. [Google Scholar] [CrossRef]
- Um, C.Y.; Prizment, A.; Hong, C.P.; Lazovich, D.; Bostick, R.M. Associations of Calcium, Vitamin D, and Dairy Product Intakes with Colorectal Cancer Risk among Older Women: The Iowa Women’s Health Study. Nutr. Cancer 2019, 71, 739–748. [Google Scholar] [CrossRef]
- Park, S.Y.; Murphy, S.P.; Wilkens, L.R.; Nomura, A.M.; Henderson, B.E.; Kolonel, L.N. Calcium and vitamin D intake and risk of colorectal cancer: The Multiethnic Cohort Study. Am. J. Epidemiol. 2007, 165, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Wactawski-Wende, J.; Kotchen, J.M.; Anderson, G.L.; Assaf, A.R.; Brunner, R.L.; O’Sullivan, M.J.; Margolis, K.L.; Ockene, J.K.; Phillips, L.; Pottern, L.; et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N. Engl. J. Med. 2006, 354, 684–696. [Google Scholar] [CrossRef]
- Urashima, M.; Ohdaira, H.; Akutsu, T.; Okada, S.; Yoshida, M.; Kitajima, M.; Suzuki, Y. Effect of vitamin D supplementation on relapse-free survival among patients with digestive tract cancers: The AMATERASU randomized clinical trial. JAMA 2019, 321, 1361–1369. [Google Scholar] [CrossRef]
- Antunac Golubić, Z.; Baršić, I.; Librenjak, N.; Pleština, S. Vitamin D supplementation and survival in metastatic colorectal cancer. Nutr. Cancer 2018, 70, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, S.M.; Cook, N.R.; Manson, J.E.; Lee, I.M.; Buring, J.E. Intakes of calcium and vitamin D and risk of colorectal cancer in women. Am. J. Epidemiol. 2005, 161, 755–764. [Google Scholar] [CrossRef]
- Serrano, D.; Bellerba, F.; Johansson, H.; Macis, D.; Aristarco, V.; Accornero, C.A.; Guerrieri-Gonzaga, A.; Trovato, C.M.; Zampino, M.G.; Salè, E.O.; et al. Vitamin D Supplementation and Adherence to World Cancer Research Fund (WCRF) Diet Recommendations for Colorectal Cancer Prevention: A Nested Prospective Cohort Study of a Phase II Randomized Trial. Biomedicines 2023, 11, 1766. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, E.M.; Rylander, C.; Brustad, M.; Jensen, T.E. Pre-diagnostic intake of vitamin D and incidence of colorectal cancer by anatomical subsites: The Norwegian Women and Cancer Cohort Study (NOWAC). Br. J. Nutr. 2023, 130, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Kováts, Z.; Müller, V.; Varga, J.T. Ambuláns rehabilitációs programok COVID–19-betegek számára. Orvosi Hetil. 2021, 162, 1671–1677. [Google Scholar] [CrossRef]
- Pludowski, P.; Grant, W.B.; Karras, S.N.; Zittermann, A.; Pilz, S. Vitamin D supplementation: A review of the evidence arguing for a daily dose of 2000 international units (50 µg) of vitamin D for adults in the general population. Nutrients 2024, 16, 391. [Google Scholar] [CrossRef]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr. 2003, 77, 204–210. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Rapidly Increasing Serum 25 (OH) D Boosts the Immune System, against Infections—Sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- He, X.; Wu, K.; Ogino, S.; Giovannucci, E.L.; Chan, A.T.; Song, M. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology 2018, 155, 355–373.e18. [Google Scholar] [CrossRef]
- Sutherland, R.L.; Ormsbee, J.; Pader, J.; Forbes, N.; Town, S.; Hilsden, R.J.; Brenner, D.R. Vitamin D supplementation reduces the occurrence of colorectal polyps in high-latitude locations. Prev. Med. 2020, 135, 106072. [Google Scholar] [CrossRef]
- Ahearn, T.U.; Shaukat, A.; Flanders, W.D.; Rutherford, R.E.; Bostick, R.M. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on the APC/β-catenin pathway in the normal mucosa of colorectal adenoma patients. Cancer Prev. Res. 2012, 5, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.K.; Um, C.Y.; Rutherford, R.E.; Seabrook, M.E.; Barry, E.L.; Fedirko, V.; Baron, J.A.; Bostick, R.M. Effects of vitamin D and calcium on expression of MSH2 and transforming growth factors in normal-appearing colorectal mucosa of sporadic colorectal adenoma patients: A randomized clinical trial. Mol. Carcinog. 2019, 58, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Crockett, S.D.; Barry, E.L.; Mott, L.A.; Ahnen, D.J.; Robertson, D.J.; Anderson, J.C.; Wallace, K.; Burke, C.A.; Bresalier, R.S.; Figueiredo, J.C.; et al. Calcium and vitamin D supplementation and increased risk of serrated polyps: Results from a randomised clinical trial. Gut 2019, 68, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Barry, E.L.; Mott, L.A.; Rees, J.R.; Snover, D.C.; Bostick, R.M.; Ivanova, A.; Cole, B.F.; Ahnen, D.J.; Beck, G.J.; et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N. Engl. J. Med. 2015, 373, 1519–1530. [Google Scholar] [CrossRef]
- Song, M.; Lee, I.-M.; Manson, J.E.; Buring, J.E.; Dushkes, R.; Gordon, D.; Walter, J.; Wu, K.; Chan, A.T.; Ogino, S.; et al. No association between vitamin D supplementation and risk of colorectal adenomas or serrated polyps in a randomized trial. Clin. Gastroenterol. Hepatol. 2021, 19, 128–135.e6. [Google Scholar] [CrossRef]
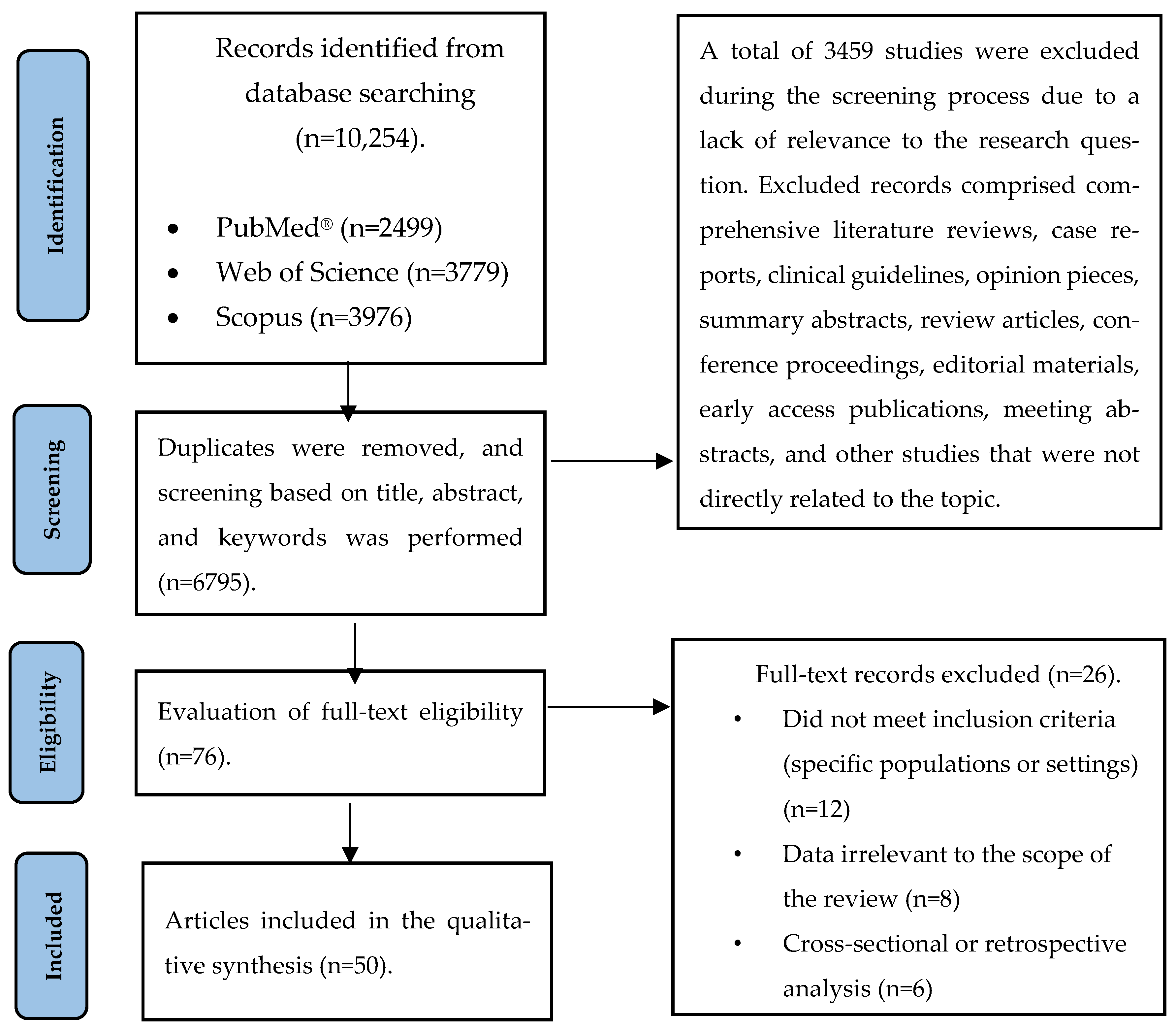
| PICO | Criteria |
|---|---|
| Population | Adult patients who are either healthy, have documented vitamin D deficiency, or have been diagnosed with CRC, as well as early-stage lesions such as adenomas or polyps. |
| Intervention | Vitamin D intake or supplementation and its effects on CRC development, immune response, and inflammatory processes. |
| Comparison | Individuals with vitamin D deficiency or those not receiving vitamin D supplementation. |
| Outcome | Incidence of colorectal cancer/adenomas/polyps, levels of immunological markers, concentrations of inflammatory factors, tumor progression, and overall disease course. |
| Study | Design | Mean Follow-Up | Country | Sample Size | Average Age (Year) | Sex Male/ Female (%) | CRC Stage | Main Results (HR, 95% CI) |
|---|---|---|---|---|---|---|---|---|
| Yang L et al. [192] | Prospective cohort | 45 months | China | 206 | 63 | 63.5/36.5 | Stage I–III CRC | Higher free 25(OH)D levels (≥0.01–0.02 pg/mL) were identified as an independent factor for improved overall survival (HR = 0.442, 95% CI = 0.238–0.819, p < 0.010) |
| Facciorusso A et al. [193] | Prospective cohort | 72 months | Italy | 143 | 68 | 71.3/28.7 | CRC with liver metastases | HR based on 25(OH)D levels (≥20 ng/mL) HR: 0.35 (95% CI: 0.21–0.59), p < 0.001 |
| Maalmi H et al. [194] | Prospective cohort | 4.8 years | Germany | 2910 | 69 | 60/40 | Stage I–IV CRC | All-cause mortality: HR = 1.78 (95% CI: 1.39–2.27); CRC-specific mortality: HR = 1.65 (95% CI: 1.24–2.21); 25(OH)D < 30 nmol/L |
| Tretli S et al. [195] | Prospective cohort | 30 years | Norway | 658 (CRC: 52) | 59.1 | 61.5/38.5 | Stage I–IV CRC (with and without metastases) | HR = 0.36 (95% CI: 0.27–0.51); 25(OH)D < 46 nmol/L |
| Zgaga L et al. [196] | Prospective cohort | 12,323 person-years of follow-up | Ireland | 1598 | 62.5 | 58/42 | Stage I-III CRC | CRC-specific mortality: HR = 0.68 (95% CI: 0.50–0.90); all-cause mortality: HR = 0.70 (95% CI: 0.55–0.89); 25(OH)D ≥ 13.25 ng/mL |
| Ng K et al. [197] | Prospective cohort | 5.1 | Ireland | 515 | 61 | 59/41 | Unresectable metastatic colorectal cancer | No significant association between plasma 25(OH)D levels and overall survival (HR = 0.94, p trend = 0.55); 25(OH)D ≥ 33 ng/mL |
| Mezawa H et al. [198] | Prospective cohort | 32.4 months | Japan | 257 | 65 ± 12 | 65/35 | Stage I–IV CRC | Higher 25(OH)D levels (≥30 ng/mL) are associated with better overall survival (HR, 0.91; 95% CI, 0.84–0.99, p = 0.027) |
| Fedirko V et al. [199] | Prospective cohort | 73 months | Europe (EPIC Study) | 1202 | 62.1 (7.6) | 49.5/50.5 | Stage I–IV CRC | Higher prediagnostic 25(OH)D levels (≥76.9 nmol/L) are associated with lower CRC-specific mortality (HR 0.69, 95% CI: 0.50–0.93) and overall mortality (HR 0.67, 95% CI: 0.50–0.88) |
| Yuan C et al. [200] | Prospective cohort | 5.6 years | USA | 1041 | 59 (12) | 58/42 | Advanced or metastatic CRC | OS: HR = 0.66 (95% CI: 0.53–0.83); PFS: HR = 0.81 (95% CI: 0.66–1.00); 25(OH)D ≥ 24.1 ng/mL |
| Fuchs MA et al. [201] | Prospective observational study | 3.5 (0.2–9.9) months | USA | 1016 | 60.4 | 56/44 | Stage III CRC | DFS: HR = 0.62 (95% CI: 0.44–0.86), Ptrend = 0.05. OS: HR = 0.55 (95% CI: 0.38–0.80), Ptrend = 0.0004; 25(OH)D: 30.1–36.4 ng/mL |
| Zhu K et al. [202] | Prospective cohort | 20 years | Australia | 3818 | 25–84 | 43/57 | Colorectal cancer | CRC risk: Low 25(OH)D < 50 nmol/L associated with higher CRC risk (HR 1.62, 95% CI 1.04–2.53) |
| Heath AK et al. [203] | Case–cohort study | 14 years | Australia | 2923 | 61.3 | 55.2/44.8 | Colorectal cancer | Colorectal cancer: HR = 0.75 (95% CI 0.57–0.99), women (25(OH)D: 53.1–121.3 nmol/L) HR = 0.63 (95% CI 0.40–1.01), men (25(OH)D: 68.9–201.8 nmol/L) HR = 0.82 (95% CI 0.58–1.14) |
| Vojdeman FJ et al. [204] | Observational cohort study | 10 years | Denmark | 1108 | 48.8 | 65.3/4.7 | Colorectal cancer (rectosigmoid cancer) | HR: 0.98 (95% CI: 0.96–1.00), p = 0.1; 25(OH)D < 30 nmol/L |
| Ordóñez-Mena JM et al. [205] | Cohort ESTHER/TROMSØ/EPIC-Elderly | 12 years | Germany/Norway/Greece, Denmark, Netherlands, Spain, Sweden | 616 | 63 | 42.9/57.1 | Colorectal cancer | ESTHER: HR 0.99 (0.60–1.65); TROMSØ: HR 1.33 (0.73–2.44); EPIC-Elderly: OR 1.24 (0.64–2.42); meta-analysis: RR 1.15 (0.82–1.61), p = 0.74; 25(OH)D < 50 nmol/L |
| Ordóñez-Mena JM et al. [206] | Prospective cohort | 8 years | Germany | 9949 | 50–74 | 42/58 | Stage I–IV CRC | HR for Q1 (lowest 25(OH)D quartile): 1.33 (1.06–1.68) in men, 0.95 (0.75–1.20) in women. Protective effect for obese individuals: HR: 0.65 (0.48–0.90) in the lowest quartile of 25(OH)D < 30 nmol/L |
| Skaaby T et al. [207] | Prospective cohort | 11.3 years | Denmark | 12,204 | ≥55 | 49.9/50.1 | Colorectal cancer | HR = 0.95 (95% CI, 0.88–1.02); 25(OH)D < 50 nmol/L |
| Wong YY et al. [208] | Prospective cohort | 6.7 ± 1.8 years | Australia | 4208 | 70–88 | 100% Male | Colorectal cancer | HR = 0.88 (95% CI, 0.55–1.40); 25(OH)D < 50 nmol/L |
| Cooney RV et al. [209] | Prospective cohort | 8.03 | USA | 368 | <85 | 58.7/41.3 | Stage I–IV CRC | HR = 0.98 (95% CI: 0.57–1.67); p-value for trend = 0.92, indicating no significant association between 25(OH)D levels and CRC-specific mortality; 25(OH)D > 30.8 ng/mL |
| Ng K et al. [210] | Prospective cohort study | Up to 14 years (1991–2005) | USA | 304 | 68.4 | 47/53 | Stage I–IV CRC | Higher prediagnosis 25(OH)D ≥ 40.0 ng/mL levels are associated with lower overall mortality (HR = 0.52, 95% CI: 0.29–0.94, p trend = 0.02) and a trend toward lower CRC-specific mortality (HR = 0.61, 95% CI: 0.31–1.19) |
| Ananthakrishnan AN et al. [211] | Observational cohort study | 11 years | USA | 2809 | 46 (IQR 32–60) | 39/61 | Stage I–IV CRC (with and without metastases) | Each 1 ng/mL increase in 25(OH)D reduced CRC risk by 8% (OR = 0.92, 95% CI: 0.88–0.96); median 25(OH)D: 26 ng/mL |
| Cheney CP et al. [212] | Population-based prospective cohort | 7 years | Germany | 2003 | 59.7 (SD 11.8) | 62.3/37.7 | Colorectal cancer | HR 0.97 (95% CI: 0.88–1.07) for CRC risk per 1 ng/mL increase in 25(OH)D (<20 ng/mL) |
| Study | Design | Mean Follow-Up | Country | Sample Size | Average Age (Year) | Sex Male/ Female (%) | CRC Stage | Main Results (HR, 95% CI) |
|---|---|---|---|---|---|---|---|---|
| McCullough ML et al. [214] | Prospective cohort | 5 years | USA | 127,749 | 62.8 | 48/52 | Incident CRC cases (421 men, 262 women) | Vitamin D intake (>240 IU/day vs. <90 IU/day): RR = 0.71 (95% CI: 0.51–0.98) in men, p trend = 0.02. |
| Martínez ME et al. [215] | Prospective cohort | 12 years | USA | 89,448 | 30–55 | 100% Female | Colorectal adenocarcinoma (colon and rectal cancer) | After excluding milk intake changers: total vitamin D (<76 IU/day vs. >477 IU/day): 0.42 (0.19–0.91); consistent high total vitamin D: 0.33 (0.16–0.70). |
| Bostick RM et al. [216] | Prospective cohort | 5 years | USA | 35,216 | 55–69 | 100% Female | Colorectal cancer | Vitamin D (<159 IU/day vs. >618 IU/day): 0.54 (0.35–0.84) (age-adjusted), 0.73 (0.45–1.18) (multivariate-adjusted). |
| Kearney J et al. [217] | Prospective cohort | 6 years | USA | 47,935 | 40–75 | 100% Male | Colon cancer | Total vitamin D (810 IU/day): RR = 0.66 (95% CI: 0.42–1.05). Dietary vitamin D: RR = 0.88 (95% CI: 0.54–1.42). |
| Zheng W et al. [218] | Cohort Study | 9 years | USA | 34,702 | 55–69 | 100% Female | Colorectal cancer | Vitamin D intake: RR 1.00, 0.71, 0.76 (p = 0.20); highest intake of both calcium and vitamin D (Ca > 1278.7 mg/day + vitamin D > 337 IU/day): RR 0.55, 95% CI 0.32–0.93 (45% reduced risk). |
| Hernández-Alonso P et al. [219] | Cohort study, observational | 6 years | Spain | 7216 | 67 | 57/43 | Incident CRC and colon cancer | Colon cancer: 0.44 (0.22–0.90), p for trend = 0.032 (significant). The highest vitamin D intake was 618 IU/day. |
| Kopp TI et al. [220] | Nested case–cohort | 15 years | Denmark | 920 cases/1743 controls | 58 | 56/44 | Colorectal cancer | IRR: 1.01 (0.87–1.18) (vitamin D: 2.3 μg/day vs. 10.2 μg/day). Not significant. |
| Kesse E et al. [221] | Prospective cohort | 3.7 years | France | 67,484 | 52.7 (6.6) | 100% Female | Colorectal cancer | No significant association with vitamin D (<1.72 µg/day vs. >3.23 µg/day). |
| Nakano S et al. [222] | Prospective study | 15 years | Japan | 22,743 | 61.3 (6.2) | 46.7/53.3 | Various (82.3% high VDR in tumors, 12.1% high VDR in stroma) | HR 0.46 (0.23–0.94) (534.6 IU/day vs. 154.1 IU/day). |
| Garland C et al. [223] | Prospective cohort study | 19 years | USA | 1954 | 50 (4) | 100% Male | Colorectal cancer | The risk of CRC in the highest quartile of Vitamin D and calcium intake (75–208 vs. 2–30 IU/1000 kcal/day) was 14.3/1000, compared to 38.9/1000 in the lowest quartile. |
| Ishihara J et al. [224] | Prospective Cohort Study | 9.5 years | Japan | 74,639 | 50.8± 7.5 | 47/53 | All stages | No significant association (the highest D-vitamin intake was 21.0 ± 7.4 μg/day). |
| Järvinen R et al. [225] | Prospective cohort study | 24 years | Sweden | 9959 | 53,7 | 60/40 | Colon and rectal cancer | No significant association with vitamin D: 3.8 µg/day. |
| Terry P et al. [226] | Cohort study, observational | 11.3 years | Sweden | 61,463 | 53 | 100% Female | Colon and rectal CRC | Rate ratio (4th vs. 1st quartile): 1.05 (95% CI = 0.83–1.33; vitamin D intake: 2.9 µg/day (lowest quartile) to 3.7 µg/day (highest quartile). |
| Food | Vitamin D Content (IU) |
|---|---|
| Cow’s milk | 3–40/L |
| Fortified milk/infant formulas | 400/L |
| Fortified orange juice/soy milk/rice milk | 400/L |
| Butter | 35/100 g |
| Margarine, fortified | 60/tablespoon |
| Yogurt (normal, low fat, or nonfat) | 89/100 g |
| Cheddar cheese | 12/100 g |
| Parmesan cheese | 28/100 g |
| Swiss cheese | 44/100 g |
| Cereal fortified | 40/serving |
| Tofu fortified (1⁄5 block) | 120 |
| Fresh shiitake mushrooms | 100/100 g |
| Dried shiitake mushrooms (non-radiated) | 1660/100 g |
| Egg yolk | 20–25 per yolk |
| Shrimp | 152/100 g |
| Calf liver | 15–50/100 g |
| Canned tuna/sardines/salmon/mackerel in oil | 224–332/100 g |
| Canned pink salmon with bones in oil | 624/100 g |
| Cooked salmon/mackerel | 345–360/100 g |
| Atlantic mackerel (raw) | 360/100 g |
| Atlantic herring (raw) | 1628/100 g |
| Smoked herring | 120/100 g |
| Pickled herring | 680/100 g |
| Codfish (raw) | 44/100 g |
| Cod liver oil | 175/g; 1360/tablespoon |
| Study | Design | Mean Follow-Up | Country | Sample Size | Average Age (Year) | Sex Male/ Female (%) | CRC Stage | Main Results (HR, 95% CI) |
|---|---|---|---|---|---|---|---|---|
| Ng K et al. [235] | RCT | 22.9 months | USA | 139 | 56 | 57/43 | Metastatic | HR for PFS: 0.64 (95% CI, 0–0.90, p = 0.02). High-dose: 8000 IU initial, 4000 IU/day; standard-dose: 400 IU/day. |
| Um CY et al. [236] | Prospective cohort | 26 years | USA | 35,221 | 55–69 | 100% Female | Overall and distal CRC | HR = 0.85; 95% CI, 0.75–0.97. Total vitamin D: 656 IU/day; total calcium: 1957 mg/day. |
| Park SY et al. [237] | Cohort Study | 8 years | Los Angeles, California | 191,011 | 58.1 | 45/55 | Invasive CRC | Total vitamin D intake: Men: RR = 0.72, 95% CI: 0.51–1.00; p = 0.03. No significant association in women. (Men: 335 IU/day; women: 340 IU/day). |
| Manson JE et al. [238] | RCT | 5.3 years | USA | 25,871 | 67.1 | 49/51 | All cancer types | No significant reduction in invasive cancer HR: 0.96 (95% CI: 0.88–1.06; 2000 IU/day). |
| Wactawski-Wende J et al. [239] | RCT | 7 years | USA | 36,282 | 50–79 | 100% Female | Invasive CRC | HR = 1.08 (95% CI: 0.86–1.34), p = 0.51; (500 mg of calcium and 200 IU of D3 vitamin). |
| Urashima M et al. [240] | RCT | 3.5 years | Japan | 417 | 66 | 66/34 | Stage I-III CRC | Relapse-free survival: HR = 0.76 (95% CI, 0.50–1.14; p = 0.18). Overall survival: HR = 0.95 (95% CI, 0.57–1.57; p = 0.83); Vit D: 2000 IU/day. |
| Antunac Golubić Z et al. [241] | RCT | 46 months | Croatia | 71 | 63 (56–71) | 61.8/40.5 | Metastatic CRC | No significant difference in OS or PFS (HR = 1.0064, 95% CI = 0.3882–2.609, p = 0.9895); Vit D: 2000 IU/day. |
| Lin J et al. [242] | Prospective Cohort Study | 10 years | USA | 36,976 | 54 | 100% Female | Colorectal cancer | Total vitamin D: 1.34 (95% CI: 0.84, 2.13; p for trend = 0.08). No significant association with CRC risk. The median daily intake was 882 mg of calcium and 271 IU of vitamin D. |
| Serrano D et al. [243] | Randomized Phase II Trial | 2.6 years | Italy | 74 | 62 | 47/53 | Stage II-III CRC | WCRF adherence significantly decreased the risk of events (HR = 0.41, 95% CI: 0.18–0.92, p = 0.03). No significant difference with vitamin D supplementation alone (2000 IU/day). |
| Paulsen EM et al. [244] | Prospective cohort study | 6 years | Norway | 95,416 | 56 | Female 100% | Proximal colon cancer, distal colon cancer, rectal cancer | CRC: 5 μg increase in vitamin D intake: HR = 0.97 (95% CI: 0.93, 1.01). Proximal colon cancer: 10–19 μg intake: HR = 0.73 (95% CI: 0.57, 0.94; high intake vit D ≥ 20 µg/day). |
| Age Group | Recommended Daily Dose | Upper Safe Daily Intake Limit |
|---|---|---|
| Under 1.5 years | 400–500 IU | 1000 IU |
| Children (1.5–6 years) | 400–500 IU | 1000 IU |
| High-risk children (1.5–6 years) | 1000 IU | 2000 IU |
| Children (above 6 years) | 1000 IU | 2000 IU |
| Adults | 2000 IU | 4000 IU |
| Study | Design | Mean Follow-Up | Country | Sample Size | Average Age (Year) | Sex Male/ Female (%) | Stage | Main Results (HR, 95% CI) |
|---|---|---|---|---|---|---|---|---|
| He X et al. [249] | Prospective cohort | 20 years | USA | 141,143 | 60.2 | 20/80 | SPs, conventional adenomas | Higher intake of vitamin D (415 ± 214 IU/day) and marine omega-3 fatty acid (0.25 ± 0.20 g) were associated with lower risk. |
| Sutherland RL et al. [250] | Observational study | 14.7 years | Canada | 1409 | 60 ± 6 | 54.7/45.3 | Early-stage CRC (colorectal polyps) | ORadj = 0.67 (95% CI: 0.51–0.88); reduced odds of HRAPs: ORadj = 0.57 (95% CI: 0.33–0.96; vit D: 600 IU/day). |
| Ahearn TU et al. [251] | RCT | 6 months | USA | 92 | 61 | 70/30 | Colorectal adenoma | Calcium intake: 2000 mg/day Vitamin D intake: 800 IU/day Combination: β-catenin −11% (p = 0.20), E-cadherin +51% (p = 0.08). |
| Kwan AK et al. [252] | RCT | 1 year | USA | 104 | 59 | 46/54 | Colorectal adenoma (early-stage) | 1000 IU/day vitamin D and 1200 mg/day calcium supplements vs. calcium RR: 0.56 (0.26, 1.20). |
| Crockett SD et al. [253] | RCT | 5 years | USA | 2813 | 58.1 | 63/37 | Polyps, SSA/Ps (sessile serrated adenoma), and traditional serrated adenoma | During the treatment phase, neither calcium (1200 mg/day) nor vitamin D (1000 IU/day) had an effect on the incidence of SSA/Ps. |
| Baron JA et al. [254] | RCT | 5 years | USA | 2259 | 58.2 ± 7.0 | 85.5/14.5 | SPs, Conventional adenomas | HR (95% CI): 0.99 (0.89–1.09) for vitamin D, 0.93 (0.80–1.08) for D + calcium (1000 IU/day + 1200 mg/day). |
| Song M et al. [255] | RCT | 5.3 years | USA | 25,871 | 67.1 ± 7.1 | 49.4/50.6 | Adenoma, serrated polyps | D-vitamin supplementation (2000 IU/day) not associated with colorectal adenomas or serrated polyps risk. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete, M.; Lehoczki, A.; Szappanos, Á.; Zábó, V.; Kaposvári, C.; Horváth, A.; Farkas, Á.; Fazekas-Pongor, V.; Major, D.; Lipécz, Á.; et al. Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications. Nutrients 2025, 17, 1351. https://doi.org/10.3390/nu17081351
Fekete M, Lehoczki A, Szappanos Á, Zábó V, Kaposvári C, Horváth A, Farkas Á, Fazekas-Pongor V, Major D, Lipécz Á, et al. Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications. Nutrients. 2025; 17(8):1351. https://doi.org/10.3390/nu17081351
Chicago/Turabian StyleFekete, Mónika, Andrea Lehoczki, Ágnes Szappanos, Virág Zábó, Csilla Kaposvári, Alpár Horváth, Árpád Farkas, Vince Fazekas-Pongor, Dávid Major, Ágnes Lipécz, and et al. 2025. "Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications" Nutrients 17, no. 8: 1351. https://doi.org/10.3390/nu17081351
APA StyleFekete, M., Lehoczki, A., Szappanos, Á., Zábó, V., Kaposvári, C., Horváth, A., Farkas, Á., Fazekas-Pongor, V., Major, D., Lipécz, Á., Csípő, T., & Varga, J. T. (2025). Vitamin D and Colorectal Cancer Prevention: Immunological Mechanisms, Inflammatory Pathways, and Nutritional Implications. Nutrients, 17(8), 1351. https://doi.org/10.3390/nu17081351





