Optimizing Nutritional Care with Machine Learning: Identifying Sarcopenia Risk Through Body Composition Parameters in Cancer Patients—Insights from the NUTritional and Sarcopenia RIsk SCREENing Project (NUTRISCREEN)
Abstract
:1. Introduction
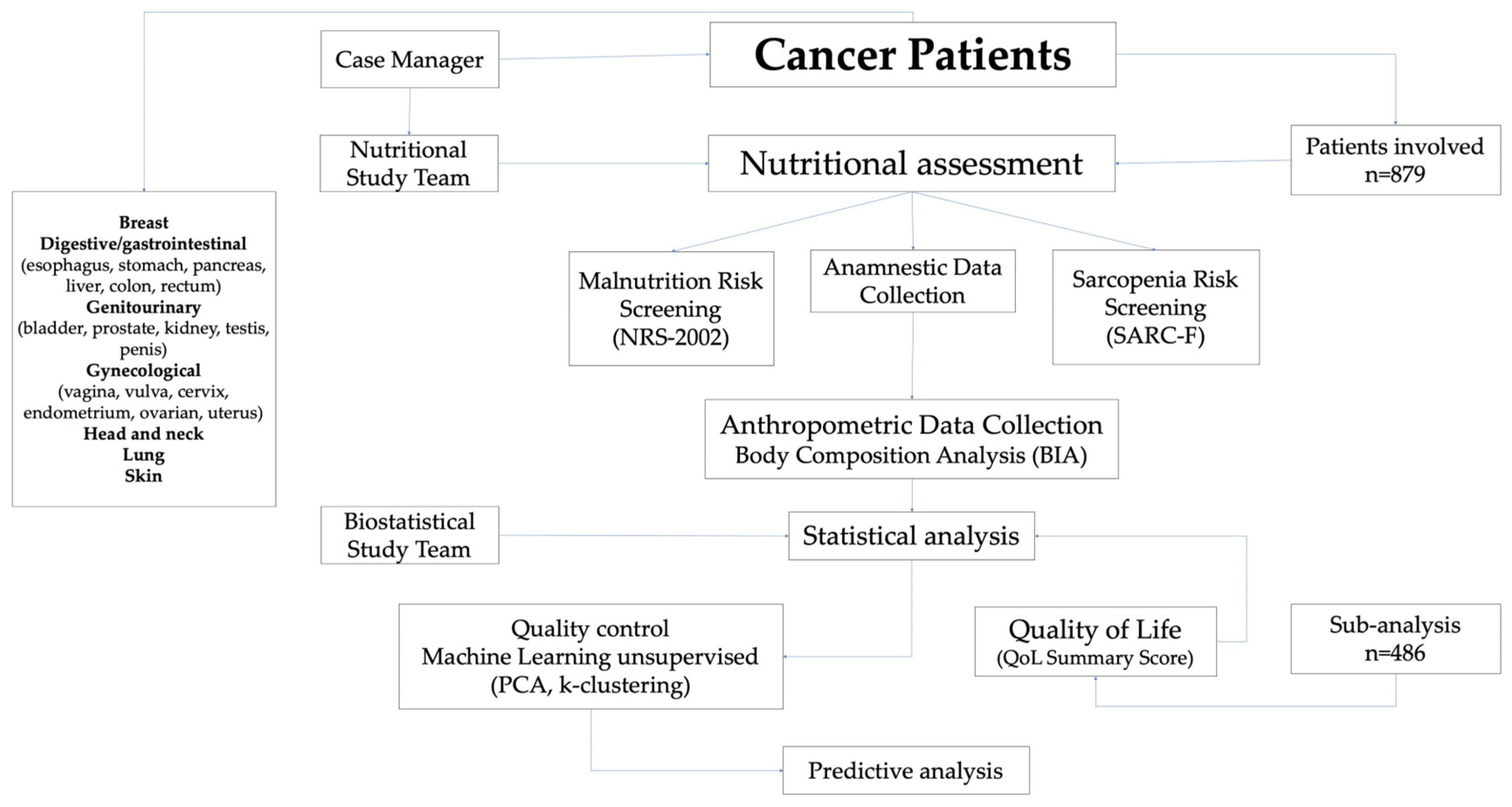
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Screening Tools: Malnutrition and Sarcopenia Risk
2.4. Anthropometric Measurements and BC Analysis
2.5. Evaluation of HRQoL
2.6. Statistical Analysis
3. Results
3.1. Descriptive Results
3.2. Univariable Analysis
3.3. Evaluation of BMI Across Age, Sex, and Cancer Types
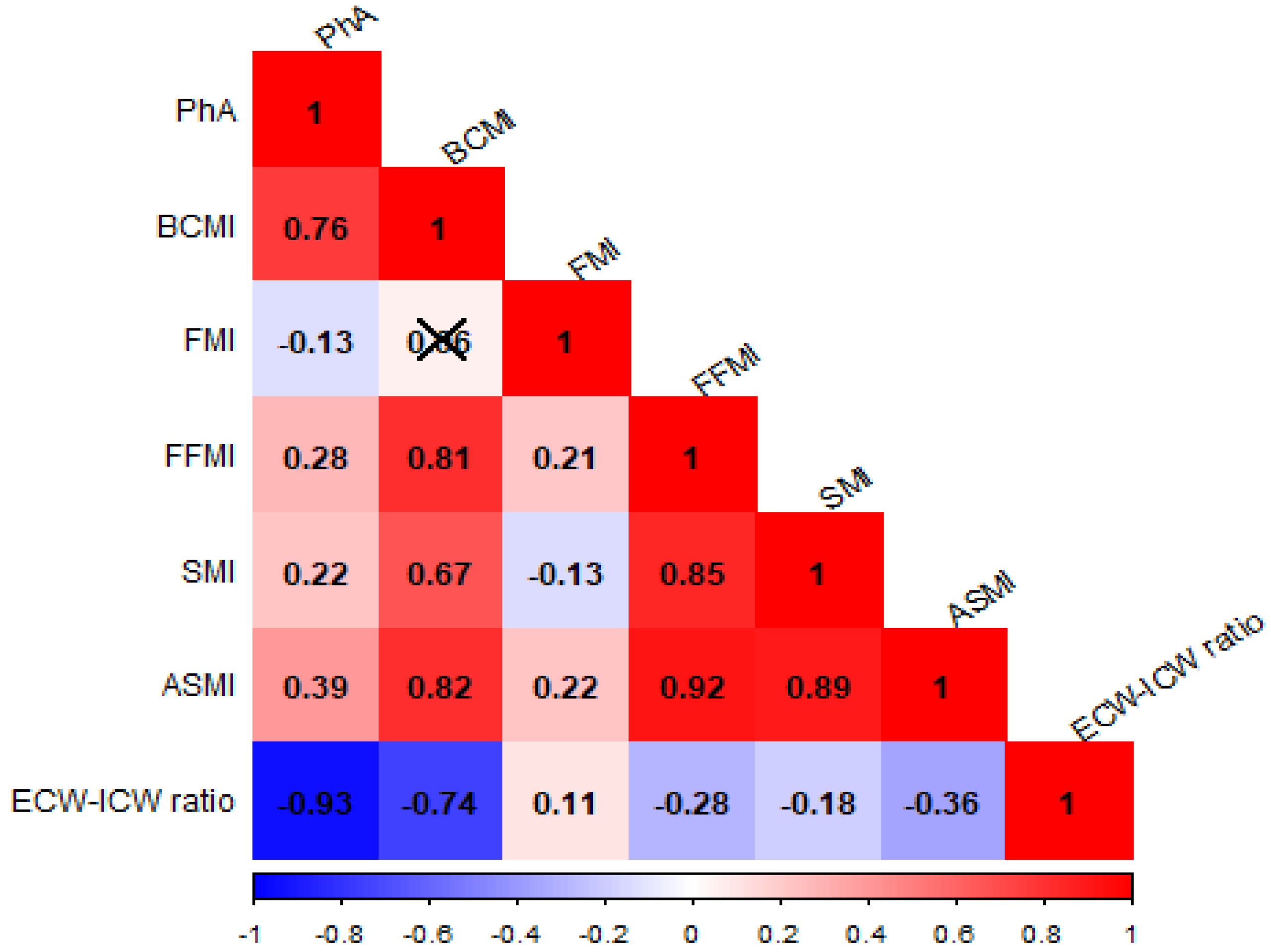
3.4. Correlation Analysis Between BIA-Derived Parameters
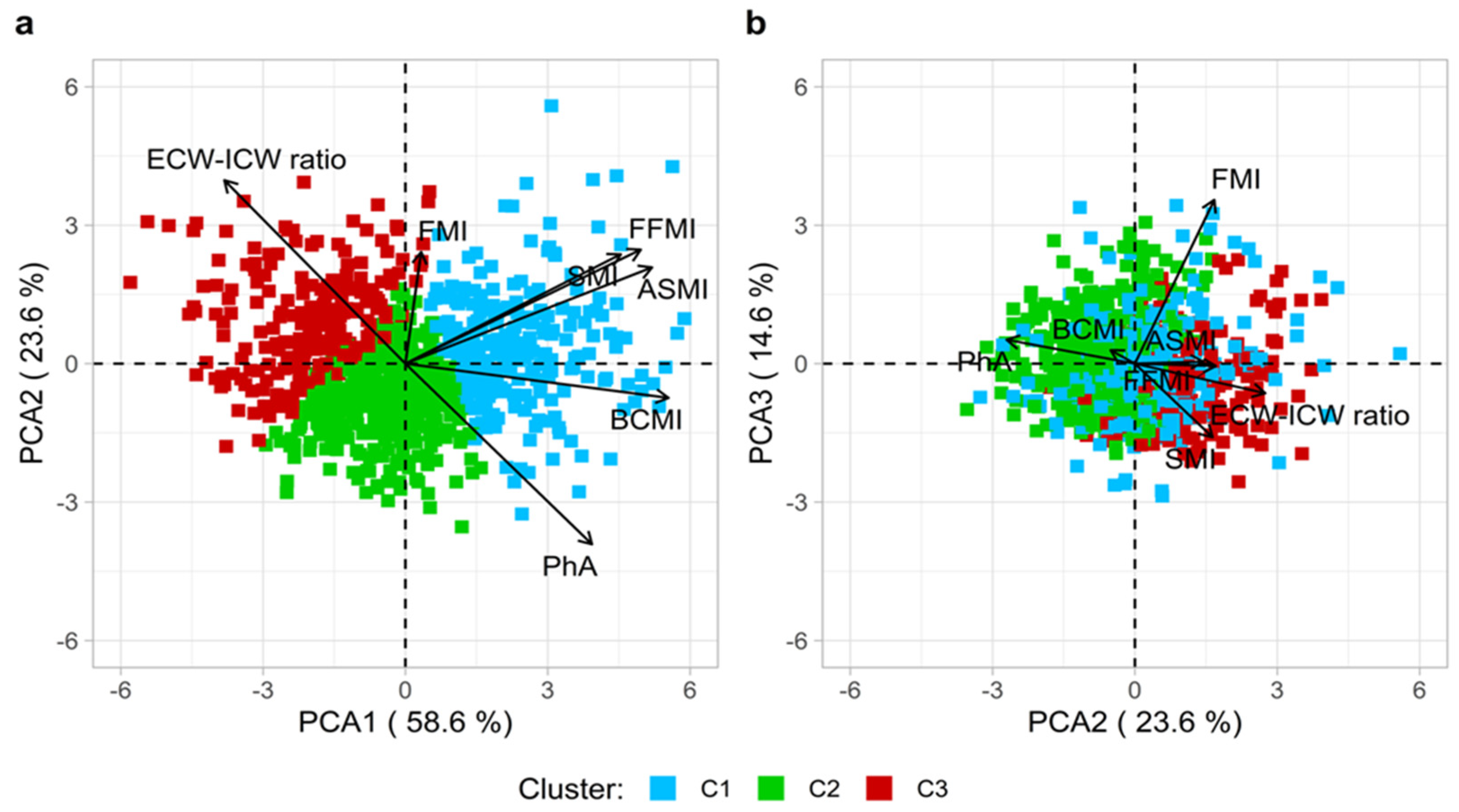
3.5. PCA and k-Means: Unsupervised ML Approaches
3.6. Multivariable Analysis
3.7. A Subgroup Analysis: SumSc and Clinical Associations in Cancer Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Body composition |
| MS | Muscle strength |
| QoL | Quality of Life |
| MM | Muscle mass |
| SMM | Skeletal muscle mass |
| FM | Fat mass |
| BIA | Bioelectrical Impedance Analysis |
| DXA | Dual-Energy X-Ray Absorptiometry |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| PhA | Phase Angle |
| TBW | Total Body Water |
| BCM | Body Cell Mass |
| ECW | Extracellular Water |
| ICW | Intracellular Water |
| ML | Machine learning |
| PCA | Principal Component Analysis |
| MOGs | Multidisciplinary Oncology Groups |
| NRS-2002 | Nutritional Risk Screening 2002 |
| BMI | Body Mass Index |
| SARC-F | Strength, Assistance with walking, Rising from a chair, Climbing stairs, and Falls |
| WC | Waist circumference |
| HC | Hip circumference |
| FFM | Fat-free Mass |
| SMM | Skeletal muscle mass |
| ASMM | Appendicular skeletal muscle mass |
| FMI | Fat Mass Index |
| BCMI | Body Cell Mass Index |
| FFMI | Fat-free Mass Index |
| SMI | Skeletal Muscle Index |
| ASMI | Appendicular Skeletal Muscle Index |
| SumSc | C30 Summary Score |
| PCs | Principal Components |
References
- World Health Organization. Global cancer burden growing, amidst mounting need for services. Saudi Med. J. 2024, 45, 326–327. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Bossi, P.; Delrio, P.; Mascheroni, A.; Zanetti, M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients 2021, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Modena, A.; Valerio, M.; Marchetti, P.; Magarotto, R.; Quadrini, S.; Narducci, F.; Tonini, G.; Grassani, T.; Cavanna, L.; et al. The Impact of NUTRItional Status at First Medical Oncology Visit on Clinical Outcomes: The NUTRIONCO Study. Cancers 2023, 15, 3206. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Bullock, A.F.; Patterson, M.J.; Paton, L.W.; Currow, D.C.; Johnson, M.J. Malnutrition, sarcopenia and cachexia: Exploring prevalence, overlap, and perceptions in older adults with cancer. Eur. J. Clin. Nutr. 2024, 78, 486–493. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Bar-Sela, G.; Battisti, N.M.L.; Belev, B.; Contreras-Martinez, J.; Cortesi, E.; de Brito-Ashurst, I.; Prado, C.M.; Ravasco, P.; Yalcin, S. Oncology-Led Early Identification of Nutritional Risk: A Pragmatic, Evidence-Based Protocol (PRONTO). Cancers 2023, 15, 380. [Google Scholar] [CrossRef]
- Prado, C.M.; Maia, Y.L.; Ormsbee, M.; Sawyer, M.B.; Baracos, V.E. Assessment of nutritional status in cancer--the relationship between body composition and pharmacokinetics. Anti Cancer Agents Med. Chem. 2013, 13, 1197–1203. [Google Scholar] [CrossRef]
- Brown, J.C.; Cespedes Feliciano, E.M.; Caan, B.J. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: Facts and numbers. J. Cachexia Sarcopenia Muscle 2018, 9, 1200–1208. [Google Scholar] [CrossRef]
- Yip, C.; Dinkel, C.; Mahajan, A.; Siddique, M.; Cook, G.J.; Goh, V. Imaging body composition in cancer patients: Visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging 2015, 6, 489–497. [Google Scholar] [CrossRef]
- Cereda, E.; Casirati, A.; Klersy, C.; Nardi, M.; Vandoni, G.; Agnello, E.; Crotti, S.; Masi, S.; Ferrari, A.; Pedrazzoli, P.; et al. Bioimpedance-derived body composition parameters predict mortality and dose-limiting toxicity: The multicenter ONCO-BIVA study. ESMO Open 2024, 9, 103666. [Google Scholar] [CrossRef]
- Branco, M.G.; Mateus, C.; Capelas, M.L.; Pimenta, N.; Santos, T.; Makitie, A.; Ganhao-Arranhado, S.; Trabulo, C.; Ravasco, P. Bioelectrical Impedance Analysis (BIA) for the Assessment of Body Composition in Oncology: A Scoping Review. Nutrients 2023, 15, 4792. [Google Scholar] [CrossRef]
- Lemos, T.; Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Prete, M.; Ballarin, G.; Porciello, G.; Arianna, A.; Luongo, A.; Belli, V.; Scalfi, L.; Celentano, E. Bioelectrical impedance analysis-derived phase angle (PhA) in lung cancer patients: A systematic review. BMC Cancer 2024, 24, 608. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, O.; Marra, M.; Di Gregorio, A.; Pasanisi, F.; Scalfi, L. Bioelectrical impedance analysis (BIA) -derived phase angle in sarcopenia: A systematic review. Clin. Nutr. 2021, 40, 3052–3061. [Google Scholar] [CrossRef]
- Bosy-Westphal, A.; Danielzik, S.; Dorhofer, R.P.; Later, W.; Wiese, S.; Muller, M.J. Phase angle from bioelectrical impedance analysis: Population reference values by age, sex, and body mass index. JPEN J. Parenter. Enter. Nutr. 2006, 30, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, M.C.; Barros, A.J.; Wang, J.; Heymsfield, S.B.; Pierson, R.N., Jr. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Kusakabe, T.; Arai, H.; Yamamoto, Y.; Nakao, K.; Ikeue, K.; Ishihara, Y.; Tagami, T.; Yasoda, A.; Ishii, K.; et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J. Cachexia Sarcopenia Muscle 2022, 13, 180–189. [Google Scholar] [CrossRef]
- Basile, C.; Della-Morte, D.; Cacciatore, F.; Gargiulo, G.; Galizia, G.; Roselli, M.; Curcio, F.; Bonaduce, D.; Abete, P. Phase angle as bioelectrical marker to identify elderly patients at risk of sarcopenia. Exp. Gerontol. 2014, 58, 43–46. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Li, J.; Wang, Y.; Xiao, Y.; Sha, T. The Diagnostic Accuracy and Cutoff Value of Phase Angle for Screening Sarcopenia: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2024, 25, 105283. [Google Scholar] [CrossRef]
- Kirk, D.; Kok, E.; Tufano, M.; Tekinerdogan, B.; Feskens, E.J.M.; Camps, G. Machine Learning in Nutrition Research. Adv. Nutr. 2022, 13, 2573–2589. [Google Scholar] [CrossRef] [PubMed]
- Mateussi, N.; Rogers, M.P.; Grimsley, E.A.; Read, M.; Parikh, R.; Pietrobon, R.; Kuo, P.C. Clinical Applications of Machine Learning. Ann. Surg. Open Perspect. Surg. Hist. Educ. Clin. Approaches 2024, 5, e423. [Google Scholar] [CrossRef]
- Coppola, E.; De Santo, I.; Luongo, A.; Rocco, P.; Ciardiello, F.; Pignata, S.; Crispo, A.; Celentano, E.; Grimaldi, A.M.; Schiavone, F.; et al. Establishment of the Campania Oncology Network (Rete Oncologica Campana, ROC): A paradigm shift in oncological care and real world research in the Campania region. ESMO Real World Data Digit. Oncol. 2025, 8, 100126. [Google Scholar] [CrossRef]
- Trollebo, M.A.; Skeie, E.; Revheim, I.; Stangeland, H.; Erstein, M.H.; Gronning, M.K.; Tangvik, R.J.; Morken, M.H.; Nygard, O.; Eagan, T.M.L.; et al. Comparison of nutritional risk screening with NRS2002 and the GLIM diagnostic criteria for malnutrition in hospitalized patients. Sci. Rep. 2022, 12, 19743. [Google Scholar] [CrossRef]
- Malmstrom, T.K.; Miller, D.K.; Simonsick, E.M.; Ferrucci, L.; Morley, J.E. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J. Cachexia Sarcopenia Muscle 2016, 7, 28–36. [Google Scholar] [CrossRef]
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut Off Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Cocks, K.; Wells, J.R.; Johnson, C.; Schmidt, H.; Koller, M.; Oerlemans, S.; Velikova, G.; Pinto, M.; Tomaszewski, K.A.; Aaronson, N.K.; et al. Content validity of the EORTC quality of life questionnaire QLQ-C30 for use in cancer. Eur. J. Cancer 2023, 178, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; Oerlemans, S.; Mols, F.; Aaronson, N.K.; van der Graaf, W.T.A.; van de Poll-Franse, L.V. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the "Real-World": Results from the Population-Based PROFILES Registry. Oncologist 2020, 25, e722–e732. [Google Scholar] [CrossRef]
- Giesinger, J.M.; Kieffer, J.M.; Fayers, P.M.; Groenvold, M.; Petersen, M.A.; Scott, N.W.; Sprangers, M.A.; Velikova, G.; Aaronson, N.K.; EORTC Quality of Life Group. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 2016, 69, 79–88. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, Statistics and Computing, 4th illustrated ed.; Springer Science & Business Media: New York, NY, USA, 2003; 498p. [Google Scholar]
- Forgy, E.W. Cluster Analysis of Multivariate Data: Efficiency vs Interpretability of Classifications. Biometrics 1965, 21, 768–780. [Google Scholar]
- Fox, J.; Monette, G. Generalized Collinearity Diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Bartlett, M.S. The effect of standardization on a Chi-square approximation in factor analysis. Biometrika 1951, 38, 337–344. [Google Scholar]
- Kaiser, H.F. An Index of Factorial Simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Cross, G.R.; Jain, A.K. Measurement of clustering tendency. In Theory and Application of Digital Control; Pergamon: Bergama, Turkey, 1982; pp. 315–320. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Plummer, M. Improved estimates of floating absolute risk. Stat. Med. 2004, 23, 93–104. [Google Scholar] [CrossRef]
- van Vugt, J.L.; van Putten, Y.; van der Kall, I.M.; Buettner, S.; D’Ancona, F.C.; Dekker, H.M.; Kimenai, H.J.; de Bruin, R.W.; Warlé, M.C.; IJzermans, J.N. Estimated skeletal muscle mass and density values measured on computed tomography examinations in over 1000 living kidney donors. Eur. J. Clin. Nutr. 2019, 73, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Moroni, A.; Perna, S.; Azzolino, D.; Gasparri, C.; Zupo, R.; Micheletti Cremasco, M.; Rondanelli, M. Discovering the Individualized Factors Associated with Sarcopenia and Sarcopenic Obesity Phenotypes-A Machine Learning Approach. Nutrients 2023, 15, 4536. [Google Scholar] [CrossRef]
- Bozzetti, F. Age-related and cancer-related sarcopenia: Is there a difference? Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 410–418. [Google Scholar] [CrossRef]
- Ligibel, J.A.; Schmitz, K.H.; Berger, N.A. Sarcopenia in aging, obesity, and cancer. Transl. Cancer Res. 2020, 9, 5760–5771. [Google Scholar] [CrossRef]
- Hwang, J.; Park, S. Gender-Specific Risk Factors and Prevalence for Sarcopenia among Community-Dwelling Young-Old Adults. Int. J. Environ. Res. Public Health 2022, 19, 7232. [Google Scholar] [CrossRef]
- Dam, T.T.; Peters, K.W.; Fragala, M.; Cawthon, P.M.; Harris, T.B.; McLean, R.; Shardell, M.; Alley, D.E.; Kenny, A.; Ferrucci, L.; et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 584–590. [Google Scholar] [CrossRef]
- Tay, L.; Ding, Y.Y.; Leung, B.P.; Ismail, N.H.; Yeo, A.; Yew, S.; Tay, K.S.; Tan, C.H.; Chong, M.S. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age 2015, 37, 121. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Chen, Y.F.; Wu, W.T.; Han, D.S.; Tsai, I.C.; Chang, K.V.; Ozcakar, L. Impact of sarcopenia on the prognosis and treatment of lung cancer: An umbrella review. Discov. Oncol. 2022, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, S.; Eckert, I.D.C.; Burgel, C.F.; Teixeira, P.J.Z.; Silva, F.M. Increased energy and/or protein intake improves anthropometry and muscle strength in chronic obstructive pulmonary disease patients: A systematic review with meta-analysis on randomised controlled clinical trials. Br. J. Nutr. 2022, 129, 1332–1349. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Chen, Q.; Yu, D.; Zhou, Q.; Tang, C.; Qiao, C. Prevalence and Prognostic Significance of Sarcopenia in Gynecologic Oncology: A Systematic Review and Meta-Analysis. J. Cachexia Sarcopenia Muscle 2025, 16, e13699. [Google Scholar] [CrossRef]
- Anjanappa, M.; Corden, M.; Green, A.; Roberts, D.; Hoskin, P.; McWilliam, A.; Choudhury, A. Sarcopenia in cancer: Risking more than muscle loss. Tech. Innov. Patient Support Radiat. Oncol. 2020, 16, 50–57. [Google Scholar] [CrossRef]
- de Bree, R.; Meerkerk, C.D.A.; Halmos, G.B.; Makitie, A.A.; Homma, A.; Rodrigo, J.P.; Lopez, F.; Takes, R.P.; Vermorken, J.B.; Ferlito, A. Measurement of Sarcopenia in Head and Neck Cancer Patients and Its Association With Frailty. Front. Oncol. 2022, 12, 884988. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Dong, M.; Wen, S.; Zhou, L.; Yuan, X. The Association Between Sarcopenia and Diabetes: From Pathophysiology Mechanism to Therapeutic Strategy. Diabetes Metab. Syndr. Obes. 2023, 16, 1541–1554. [Google Scholar] [CrossRef]
- Williams, G.R.; Al-Obaidi, M.; Dai, C.; Mir, N.; Challa, S.A.; Daniel, M.; Patel, H.; Barlow, B.; Young-Smith, C.; Gbolahan, O.; et al. Association of malnutrition with geriatric assessment impairments and health-related quality of life among older adults with gastrointestinal malignancies. Cancer 2020, 126, 5147–5155. [Google Scholar] [CrossRef]
- Malhotra, A.; Fransen, H.P.; Quaresma, M.; Raijmakers, N.; Versluis, M.A.J.; Rachet, B.; van Maaren, M.C.; Leyrat, C. Associations between treatments, comorbidities and multidimensional aspects of quality of life among patients with advanced cancer in the Netherlands-a 2017-2020 multicentre cross-sectional study. Qual. Life Res. 2023, 32, 3123–3133. [Google Scholar] [CrossRef]
- Vissers, P.A.; Thong, M.S.; Pouwer, F.; Zanders, M.M.; Coebergh, J.W.; van de Poll-Franse, L.V. The impact of comorbidity on Health-Related Quality of Life among cancer survivors: Analyses of data from the PROFILES registry. J. Cancer Surviv. 2013, 7, 602–613. [Google Scholar] [CrossRef]
- Wang, J.W.; Sun, L.; Ding, N.; Li, J.; Gong, X.H.; Chen, X.F.; Yu, D.H.; Luo, Z.N.; Yuan, Z.P.; Yu, J.M. The association between comorbidities and the quality of life among colorectal cancer survivors in the People’s Republic of China. Patient Prefer. Adherence 2016, 10, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Guedon, A.F.; Ricard, L.; Laurent, C.; De Moreuil, C.; Urbanski, G.; Deriaz, S.; Gerotziafas, G.; Elalamy, I.; Audemard, A.; Chasset, F.; et al. Identifying high-risk profile in primary antiphospholipid syndrome through cluster analysis: French multicentric cohort study. RMD Open 2023, 9, e002881. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Shi, J.; Zheng, X.; Chen, Y.; Liu, X.; Bu, Z.; Zhao, H.; Xu, C.; Yin, B.; et al. The association of metabolic disorders and prognosis in cancer patients. BMC Cancer 2025, 25, 278. [Google Scholar] [CrossRef] [PubMed]
- Grune, E.; Nattenmuller, J.; Kiefer, L.S.; Machann, J.; Peters, A.; Bamberg, F.; Schlett, C.L.; Rospleszcz, S. Subphenotypes of body composition and their association with cardiometabolic risk—Magnetic resonance imaging in a population-based sample. Metabolism 2025, 164, 156130. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Boutin, R.D.; Lenchik, L.; Kim, J.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L., 3rd; Guthrie, K.A.; Chiorean, E.G.; Ahmad, S.A.; et al. Body composition measurements and clinical outcomes in patients with resectable pancreatic adenocarcinoma—analysis from SWOG S1505. J. Gastrointest. Surg. 2024, 28, 232–235. [Google Scholar] [CrossRef]
- Kang, H.; Kim, I.; Park, H.; Ahn, W.; Kim, S.K.; Lee, S. Prognostic value of body composition measures in breast cancer patients treated with chemotherapy. Sci. Rep. 2024, 14, 23309. [Google Scholar] [CrossRef]
- Huang, Y.; Cun, H.; Mou, Z.; Yu, Z.; Du, C.; Luo, L.; Jiang, Y.; Zhu, Y.; Zhang, Z.; Chen, X.; et al. Multiparameter body composition analysis on chest CT predicts clinical outcomes in resectable non-small cell lung cancer. Insights Imaging 2025, 16, 32. [Google Scholar] [CrossRef]
| (a) | ||||
| Sample Characteristics | Univariable Analysis | |||
| Variable | Overall (n = 879) | SARC-F < 4, n = 751 (85%) | SARC-F ≥ 4, n = 128 (15%) | p-Value * |
| Gender, n (%) | 0.890 1 | |||
| Female | 388 (44.1%) | 330 (43.9%) | 58 (45.3%) | |
| Male | 491 (55.9%) | 421 (56.1%) | 70 (54.7%) | |
| Age (ys) | <0.001 2 | |||
| Mean (SD) | 63.1 (12.5) | 61.9 (12.3) | 70.2 (11.0) | |
| Median (IQR) | 64.7 (54.7, 72.9) | 63.3 (53.5, 71.7) | 72.3 (64.7, 77.6) | |
| Neoplasm, n (%) | <0.001 1 | |||
| Head and neck | 43 (4.9%) | 40 (5.3%) | 3 (2.3%) | |
| Breast | 91 (10.4%) | 85 (11.3%) | 6 (4.7%) | |
| Digestive/gastrointestinal | 311 (35.4%) | 254 (33.8%) | 57 (44.5%) | |
| Genitourinary | 195 (22.2%) | 186 (24.8%) | 9 (7.0%) | |
| Gynecological | 75 (8.5%) | 66 (8.8%) | 9 (7.0%) | |
| Lung | 117 (13.3%) | 73 (9.7%) | 44 (34.4%) | |
| Skin | 47 (5.3%) | 47 (6.3%) | 0 (0.0%) | |
| Civil status, n (%) | 0.002 1 | |||
| Bachelor | 86 (9.8%) | 83 (11.1%) | 3 (2.3%) | |
| Married/cohabiting | 663 (75.4%) | 565 (75.2%) | 98 (76.6%) | |
| Divorced | 57 (6.5%) | 49 (6.5%) | 8 (6.3%) | |
| Widow | 73 (8.3%) | 54 (7.2%) | 19 (14.8%) | |
| Education, n (%) | <0.001 1 | |||
| Less than 1st grade | 136 (15.5%) | 93 (12.4%) | 43 (33.6%) | |
| Middle school | 272 (30.9%) | 228 (30.4%) | 44 (34.4%) | |
| High school | 329 (37.4%) | 297 (39.5%) | 32 (25.0%) | |
| Graduated | 142 (16.2%) | 133 (17.7%) | 9 (7.0%) | |
| Smoking status, n (%) | 0.180 1 | |||
| No smoking | 276 (31.4%) | 243 (32.4%) | 33 (25.8%) | |
| Former smoker | 360 (41.0%) | 297 (39.5%) | 63 (49.2%) | |
| Smoker | 243 (27.6%) | 211 (28.1%) | 32 (25.0%) | |
| Hypertension, n (%) a | 393 (44.7%) | 320 (42.6%) | 73 (57.0%) | 0.006 1 |
| Diabetes, n (%) a | 141 (16.0%) | 105 (14.0%) | 36 (28.1%) | <0.001 1 |
| Cardiovascular disease, n (%) a | 83 (9.4%) | 66 (8.8%) | 17 (13.3%) | 0.210 1 |
| Dyslipidemia, n (%) a | 42 (4.8%) | 34 (4.5%) | 8 (6.3%) | 0.660 1 |
| Hypercholesterolemia, n (%) a | 187 (21.3%) | 157 (20.9%) | 30 (23.4%) | 0.670 1 |
| Cancer surgery, n (%) a | 135 (15.4%) | 115 (15.3%) | 20 (15.6%) | >0.990 1 |
| Other comorbidities, n (%) a | <0.001 1 | |||
| One | 175 (19.9%) | 132 (17.6%) | 43 (33.6%) | |
| More | 33 (3.8%) | 24 (3.2%) | 9 (7.0%) | |
| (b) | ||||
| Sample Characteristics | Univariable Analysis | |||
| Variable | Overall (n = 879) | SARC-F < 4, n = 751 (85%) | SARC-F ≥ 4, n = 128 (15%) | p-Value * |
| NRS-2002, n (%) | <0.001 1 | |||
| <3 | 762 (86.7%) | 682 (90.8%) | 80 (62.5%) | |
| ≥3 | 117 (13.3%) | 69 (9.2%) | 48 (37.5%) | |
| PhA | <0.001 2 | |||
| Mean (SD) | 5.6 (1.1) | 5.7 (1.1) | 5.1 (1.2) | |
| Median (IQR) | 5.5 (4.8, 6.2) | 5.6 (5.0, 6.3) | 5.0 (4.3, 5.8) | |
| BCMI | <0.001 2 | |||
| Mean (SD) | 10.0 (2.0) | 10.1 (1.9) | 9.4 (2.1) | |
| Median (IQR) | 9.9 (8.6, 11.1) | 9.9 (8.7, 11.2) | 9.4 (8.1, 10.5) | |
| FMI | 0.670 2 | |||
| Mean (SD) | 7.7 (3.7) | 7.7 (3.6) | 8.0 (4.3) | |
| Median (IQR) | 7.3 (5.1, 9.9) | 7.2 (5.2, 9.7) | 7.4 (4.7, 10.5) | |
| FFMI | 0.470 2 | |||
| Mean (SD) | 19.6 (2.5) | 19.6 (2.4) | 19.4 (2.5) | |
| Median (IQR) | 19.3 (17.9, 21.1) | 19.3 (17.9, 21.2) | 19.2 (17.7, 20.7) | |
| SMI | 0.210 2 | |||
| Mean (SD) | 9.2 (1.7) | 9.2 (1.8) | 8.9 (1.6) | |
| Median (IQR) | 9.0 (7.9, 10.2) | 9.1 (7.9, 10.3) | 8.9 (7.8, 9.7) | |
| ASMI | 0.013 2 | |||
| Mean (SD) | 7.4 (1.2) | 7.4 (1.2) | 7.2 (1.2) | |
| Median (IQR) | 7.3 (6.6, 8.1) | 7.3 (6.6, 8.1) | 7.1 (6.5, 7.7) | |
| ECW-ICW ratio | <0.001 2 | |||
| Mean (SD) | 1.0 (0.2) | 0.9 (0.2) | 1.1 (0.3) | |
| Median (IQR) | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.0) | 1.0 (0.9, 1.2) | |
| (a) | ||||
| Characteristic | HMP, n = 253 | MMP, n = 410 | LMP, n = 216 | p-Value * |
| Gender, n (%) | <0.001 2 | |||
| Female | 33 (13.0%) | 244 (59.5%) | 111 (51.4%) | |
| Male | 220 (87.0%) | 166 (40.5%) | 105 (48.6%) | |
| Age (ys) | <0.001 2 | |||
| Mean (SD) | 61.6 (11.7) | 61.1 (12.6) | 68.8 (11.6) | |
| Median (IQR) | 63.3 (54.6, 69.9) | 61.8 (52.2, 71.0) | 71.4 (63.4, 76.7) | |
| Neoplasm, n (%) | <0.001 1 | |||
| Head and neck | 18 (7.1%) | 13 (3.2%) | 12 (5.6%) | |
| Breast | 9 (3.6%) | 59 (14.4%) | 23 (10.6%) | |
| Digestive/gastrointestinal | 99 (39.1%) | 142 (34.6%) | 70 (32.4%) | |
| Genitourinary | 75 (29.6%) | 70 (17.1%) | 50 (23.1%) | |
| Gynecological | 10 (4.0%) | 50 (12.2%) | 15 (6.9%) | |
| Lung | 23 (9.1%) | 55 (13.4%) | 39 (18.1%) | |
| Skin | 19 (7.5%) | 21 (5.1%) | 7 (3.2%) | |
| Civil status, n (%) | <0.001 2 | |||
| Bachelor | 20 (7.9%) | 43 (10.5%) | 23 (10.6%) | |
| Married/cohabiting | 205 (81.0%) | 308 (75.1%) | 150 (69.4%) | |
| Divorced | 18 (7.1%) | 29 (7.1%) | 10 (4.6%) | |
| Widow | 10 (4.0%) | 30 (7.3%) | 33 (15.3%) | |
| Education, n (%) | <0.001 1 | |||
| Less than 1st grade | 31 (12.3%) | 52 (12.7%) | 53 (24.5%) | |
| Middle school | 97 (38.3%) | 107 (26.1%) | 68 (31.5%) | |
| High school | 89 (35.2%) | 176 (42.9%) | 64 (29.6%) | |
| Graduated | 36 (14.2%) | 75 (18.3%) | 31 (14.4%) | |
| Smoking status, n (%) | 0.337 1 | |||
| No smoking | 72 (28.5%) | 143 (34.9%) | 61 (28.2%) | |
| Former smoker | 107 (42.3%) | 158 (38.5%) | 95 (44.0%) | |
| Smoker | 74 (29.2%) | 109 (26.6%) | 60 (27.8%) | |
| Hypertension, n (%) | 112 (44.3%) | 166 (40.5%) | 115 (53.2%) | 0.015 1 |
| Diabetes, n (%) | 44 (17.4%) | 53 (12.9%) | 44 (20.4%) | 0.059 1 |
| Cardiovascular disease, n (%) | 28 (11.1%) | 29 (7.1%) | 26 (12.0%) | 0.097 1 |
| Dyslipidemia, n (%) | 17 (6.7%) | 17 (4.1%) | 8 (3.7%) | 0.245 1 |
| Hypercholesterolemia, n (%) | 48 (19.0%) | 85 (20.7%) | 54 (25.0%) | 0.277 1 |
| Cancer surgery, n (%) | 30 (11.9%) | 67 (16.3%) | 38 (17.6%) | 0.199 1 |
| Other comorbidities, n (%) | 0.015 1 | |||
| None | 208 (82.2%) | 312 (76.1%) | 151 (69.9%) | |
| One | 42 (16.6%) | 81 (19.8%) | 52 (24.1%) | |
| More | 3 (1.2%) | 17 (4.1%) | 13 (6.0%) | |
| (b) | ||||
| Characteristic | HMP, n = 253 | MMP, n = 410 | LMP, n = 216 | p-Value * |
| NRS-2002, n (%) | <0.001 1 | |||
| <3 | 229 (90.5%) | 366 (89.3%) | 167 (77.3%) | |
| ≥3 | 24 (9.5%) | 44 (10.7%) | 49 (22.7%) | |
| SARC-F, n (%) | <0.001 1 | |||
| <4 | 227 (89.7%) | 361 (88.0%) | 163 (75.5%) | |
| ≥4 | 26 (10.3%) | 49 (12.0%) | 53 (24.5%) | |
| PhA | <0.001 1 | |||
| Mean (SD) | 6.3 (1.0) | 5.8 (0.7) | 4.2 (0.5) | |
| Median (IQR) | 6.1 (5.6, 6.9) | 5.7 (5.3, 6.2) | 4.3 (3.9, 4.6) | |
| BCMI | <0.001 1 | |||
| Mean (SD) | 12.2 (1.4) | 9.7 (0.9) | 7.8 (1.0) | |
| Median (IQR) | 12.0 (11.3, 13.0) | 9.8 (9.1, 10.4) | 8.0 (7.2, 8.6) | |
| FMI | 0.165 1 | |||
| Mean (SD) | 8.1 (4.1) | 7.5 (3.5) | 7.8 (3.5) | |
| Median (IQR) | 7.3 (5.3, 10.5) | 7.1 (4.9, 9.6) | 7.4 (5.6, 10.0) | |
| FFMI | <0.001 1 | |||
| Mean (SD) | 22.5 (1.8) | 18.5 (1.5) | 18.2 (1.7) | |
| Median (IQR) | 22.1 (21.2, 23.5) | 18.6 (17.5, 19.6) | 18.4 (16.9, 19.6) | |
| SMI | <0.001 1 | |||
| Mean (SD) | 11.0 (1.5) | 8.4 (1.0) | 8.5 (1.4) | |
| Median (IQR) | 10.8 (10.1, 11.6) | 8.4 (7.5, 9.2) | 8.3 (7.3, 9.5) | |
| ASMI | <0.001 1 | |||
| Mean (SD) | 8.8 (0.9) | 7.0 (0.6) | 6.6 (0.9) | |
| Median (IQR) | 8.6 (8.2, 9.2) | 7.0 (6.5, 7.4) | 6.6 (6.0, 7.2) | |
| ECW-ICW ratio | <0.001 1 | |||
| Mean (SD) | 0.8 (0.1) | 0.9 (0.1) | 1.3 (0.2) | |
| Median (IQR) | 0.8 (0.7, 0.9) | 0.9 (0.8, 1.0) | 1.2 (1.1, 1.4) | |
| Characteristic | n | OR 1 | 95% CI 1,2 | p-Value |
|---|---|---|---|---|
| Age (for each 5-years increase) | 832 | 1.17 | 1.10, 1.25 | <0.001 |
| Neoplasm | <0.001 | |||
| Head and neck | 43 | 1.00 | 0.52 to 1.93 | |
| Breast | 91 | 2.59 | 1.63 to 4.12 | |
| Digestive/gastrointestinal | 311 | 2.71 | 2.12 to 3.46 | |
| Genitourinary | 195 | 1.17 | 0.83 to 1.65 | |
| Gynecological | 75 | 4.97 | 3.16 to 7.82 | |
| Lung | 117 | 11.0 | 8.31 to 14.51 | |
| Education | <0.001 | |||
| Less than 1st grade | 136 | 1.00 | 0.75 to 1.33 | |
| Middle school | 269 | 0.53 | 0.42 to 0.66 | |
| High school | 299 | 0.38 | 0.31 to 0.48 | |
| Graduated | 128 | 0.32 | 0.22 to 0.47 | |
| Diabetes | 0.001 | |||
| No | 696 | — | — | |
| Yes | 136 | 1.70 | 1.23, 2.36 | |
| Dyslipidemia | 0.041 | |||
| No | 791 | — | — | |
| Yes | 41 | 1.76 | 1.02, 3.04 | |
| Cancer surgery | 0.012 | |||
| No | 698 | — | — | |
| Yes | 134 | 0.63 | 0.44, 0.90 | |
| Other comorbidities | 0.002 | |||
| None | 626 | 1.00 | 0.85 to 1.18 | |
| One | 173 | 1.60 | 1.25 to 2.06 | |
| More | 33 | 1.84 | 1.06 to 3.19 | |
| NRS-2002 | <0.001 | |||
| <3 | 715 | — | — | |
| ≥3 | 117 | 4.81 | 3.38, 6.93 | |
| Cluster | 0.006 | |||
| HMP | 234 | 1.00 | 0.77 to 1.3 | |
| MMP | 389 | 1.01 | 0.83 to 1.22 | |
| LMP | 209 | 1.62 | 1.26 to 2.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porciello, G.; Di Lauro, T.; Luongo, A.; Coluccia, S.; Prete, M.; Abbadessa, L.; Coppola, E.; Di Martino, A.; Mozzillo, A.L.; Racca, E.; et al. Optimizing Nutritional Care with Machine Learning: Identifying Sarcopenia Risk Through Body Composition Parameters in Cancer Patients—Insights from the NUTritional and Sarcopenia RIsk SCREENing Project (NUTRISCREEN). Nutrients 2025, 17, 1376. https://doi.org/10.3390/nu17081376
Porciello G, Di Lauro T, Luongo A, Coluccia S, Prete M, Abbadessa L, Coppola E, Di Martino A, Mozzillo AL, Racca E, et al. Optimizing Nutritional Care with Machine Learning: Identifying Sarcopenia Risk Through Body Composition Parameters in Cancer Patients—Insights from the NUTritional and Sarcopenia RIsk SCREENing Project (NUTRISCREEN). Nutrients. 2025; 17(8):1376. https://doi.org/10.3390/nu17081376
Chicago/Turabian StylePorciello, Giuseppe, Teresa Di Lauro, Assunta Luongo, Sergio Coluccia, Melania Prete, Ludovica Abbadessa, Elisabetta Coppola, Annabella Di Martino, Anna Licia Mozzillo, Emanuela Racca, and et al. 2025. "Optimizing Nutritional Care with Machine Learning: Identifying Sarcopenia Risk Through Body Composition Parameters in Cancer Patients—Insights from the NUTritional and Sarcopenia RIsk SCREENing Project (NUTRISCREEN)" Nutrients 17, no. 8: 1376. https://doi.org/10.3390/nu17081376
APA StylePorciello, G., Di Lauro, T., Luongo, A., Coluccia, S., Prete, M., Abbadessa, L., Coppola, E., Di Martino, A., Mozzillo, A. L., Racca, E., Piccirillo, A., Di Giacomo, V., Fontana, M., D’Amico, M., Palumbo, E., Vitale, S., D’Errico, D., Turrà, V., Parascandolo, I., ... Pignata, S. (2025). Optimizing Nutritional Care with Machine Learning: Identifying Sarcopenia Risk Through Body Composition Parameters in Cancer Patients—Insights from the NUTritional and Sarcopenia RIsk SCREENing Project (NUTRISCREEN). Nutrients, 17(8), 1376. https://doi.org/10.3390/nu17081376









