Multiomics Analysis of a Micronutrient-Rich Dietary Pattern and the Aging Genotype 9p21 on the Plasma Proteome of Young Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Patterns
2.3. Genotyping
2.4. Proteomics Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strong, J.P.; Malcom, G.T.; Mcmahan, C.A.; Tracy, R.E.; Newman, W.P., III; Herderick, E.E.; Cornhill, J.F.; for the Pathobiological Determinants of Atherosclerosis in Youth Research Group. Prevalence and Extent of Atherosclerosis in Adolescents and Young Adults: Implications for Prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 1999, 281, 727–735. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Ashworth. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, H.; Rader, D.J. Management of lipids in the prevention of cardiovascular events. Annu. Rev. Med. 2008, 59, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ryu, S.; Tare, A.; Barzilai, N.; Atzmon, G.; Suh, Y. Targeted sequencing of the 9p21.3 region reveals association with reduced disease risks in Ashkenazi Jewish centenarians. Aging Cell 2023, 22, e13962. [Google Scholar] [CrossRef]
- Larson, M.G.; Atwood, L.D.; Benjamin, E.J.; Cupples, L.A.; D’AgostinoSr, R.B.; Fox, C.S.; Govindaraju, D.R.; Guo, C.-Y.; Heard-Costa, N.L.; Hwang, S.-J.; et al. Framingham Heart Study 100K project: Genome-wide associations for cardiovascular disease outcomes. BMC Med. Genet. 2007, 8 (Suppl. S1), S5. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Mahdavi, S.; Jenkins, D.J.A.; El-Sohemy, A. Genetic variation in 9p21 is associated with fasting insulin in women but not men. PLoS ONE 2018, 13, e0202365. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Balagopal, P.; De Ferranti, S.D.; Cook, S.; Daniels, S.R.; Gidding, S.S.; Hayman, L.L.; McCrindle, B.W.; Mietus-Snyder, M.L.; Steinberger, J. Nontraditional risk factors and biomarkers for cardiovascular disease: Mechanistic, research, and clinical considerations for youth: A scientific statement from the american heart association. Circulation 2011, 123, 2749–2769. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B., Sr.; Larson, M.G.; Massaro, J.M.; Vasan, R.S. Predicting the 30-Year Risk of Cardiovascular Disease. Circulation 2009, 119, 3078–3084. [Google Scholar] [CrossRef]
- Batsis, J.A.; Lopez-Jimenez, F. Cardiovascular risk assessment—From individual risk prediction to estimation of global risk and change in risk in the population. BMC Med. 2010, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, A.; Thorleifsson, G.; Manolescu, A.; Gretarsdottir, S.; Blondal, T.; Jonasdottir, A.; Jonasdottir, A.; Sigurdsson, A.; Baker, A.; Palsson, A.; et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science (1979) 2007, 316, 1491–1493. [Google Scholar] [CrossRef]
- Samani, N.J.; Erdmann, J.; Hall, A.S.; Hengstenberg, C.; Mangino, M.; Mayer, B.; Dixon, R.J.; Meitinger, T.; Braund, P.; Wichmann, H.-E.; et al. Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 2007, 357, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, K.G.; Li, L.; Shen, G.; Hu, Y.; Yang, Y.; MacKinlay, K.G.; Topol, E.J.; Wang, Q.K. Four SNPS on chromosome 9p21 confer risk to premature, familial CAD and MI in an American Caucasian population (GeneQuest). Ann. Hum. Genet. 2008, 72, 654–657. [Google Scholar] [CrossRef]
- Mahdavi, S.; Jenkins, D.J.; El-Sohemy, A. Genetic variation in 9p21, dietary patterns, and insulin sensitivity. Front. Genet. 2022, 13, 988873. [Google Scholar] [CrossRef]
- Cugino, D.; Gianfagna, F.; Santimone, I.; de Gaetano, G.; Donati, M.; Iacoviello, L.; Di Castelnuovo, A. Type 2 diabetes and polymorphisms on chromosome 9p21: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 619–625. [Google Scholar] [CrossRef]
- Beckie, T.M.; Beckstead, J.W.; Groer, M.W. The Association Between Variants on Chromosome 9p21 and Inflammatory Biomarkers in Ethnically Diverse Women With Coronary Heart Disease: A Pilot Study. Biol. Res. Nurs. 2011, 13, 306–319. [Google Scholar] [CrossRef]
- Dandona, S.; Stewart, A.F.; Chen, L.; Williams, K.; So, D.; O’Brien, E.; Glover, C.; LeMay, M.; Assogba, O.; Vo, L.; et al. Gene Dosage of the Common Variant 9p21 Predicts Severity of Coronary Artery Disease. J. Am. Coll. Cardiol. 2010, 56, 479–486. [Google Scholar] [CrossRef]
- Harismendy, O.; Notani, D.; Song, X.; Rahim, N.G.; Tanasa, B.; Heintzman, N.; Ren, B.; Fu, X.-D.; Topol, E.J.; Rosenfeld, M.G.; et al. 9p21 DNA variants associated with coronary artery disease impair interferon-γ signalling response. Nature 2011, 470, 264–268. [Google Scholar] [CrossRef]
- Reaven, G.M.; Knowles, J.W.; Leonard, D.; Barlow, C.E.; Willis, B.L.; Haskell, W.L.; Maron, D.J. Relationship between simple markers of insulin resistance and coronary artery calcification. J. Clin. Lipidol. 2017, 11, 1007. [Google Scholar] [CrossRef]
- Cooper, J.A.; Miller, G.J.; Humphries, S.E. Humphries. A comparison of the PROCAM and Framingham point-scoring systems for estimation of individual risk of coronary heart disease in the Second Northwick Park Heart Study. Atherosclerosis 2005, 181, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Kuzyk, M.A.; Smith, D.; Yang, J.; Cross, T.J.; Jackson, A.M.; Hardie, D.B.; Anderson, N.L.; Borchers, C.H. Multiple reaction monitoring-based, multiplexed, absolute quantitation of 45 proteins in human plasma. Mol. Cell. Proteom. 2009, 8, 1860–1877. [Google Scholar] [CrossRef] [PubMed]
- García-Bailo, B.; Brenner, D.R.; Nielsen, D.; Lee, H.-J.; Domanski, D.; Kuzyk, M.; Borchers, C.H.; Badawi, A.; Karmali, M.A.; El-Sohemy, A. Dietary patterns and ethnicity are associated with distinct plasma proteomic groups. Am. J. Clin. Nutr. 2012, 95, 352–361. [Google Scholar] [CrossRef]
- Mahdavi, S.; Jenkins, D.J.A.; Borchers, C.H.; El-Sohemy, A. Genetic Variation in 9p21 and the Plasma Proteome. J. Proteome Res. 2018, 17, 2649–2656. [Google Scholar] [CrossRef]
- Anderson, L. Candidate-based proteomics in the search for biomarkers of cardiovascular disease. J. Physiol. 2005, 563, 23–60. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, Y.; Zhang, K.; Yuan, Y.; Jia, S.; Liu, J. The Complement System, Aging, and Aging-Related Diseases. Int. J. Mol. Sci. 2022, 23, 8689. [Google Scholar] [CrossRef]
- Walker, D.G.; McGeer, P.L. Complement gene expression in human brain: Comparison between normal and Alzheimer disease cases. Brain Res. Mol. 1992, 14, 109–116. [Google Scholar] [CrossRef]
- Badawi, A.; Costa, D.; Arora, P.; Bailo, G.; Karmali, M.; Sohemy, E. The association between obesity, cardiometabolic disease biomarkers, and innate immunity-related inflammation in Canadian adults. Diabetes Metab. Syndr. Obes. 2012, 5, 347–355. [Google Scholar] [CrossRef]
- El Ghoch, M.; El Shamieh, S. Nutrition, Genetics, and Cardiovascular Disease. MDPI: Basel, Switzerland. Available online: www.mdpi.com/journal/jcdd (accessed on 5 June 2024).
- Mente, A.; Dehghan, M.; Rangarajan, S.; O’donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.A.; Wei, L.; Diaz, R.; et al. Diet, cardiovascular disease, and mortality in 80 countries. Eur. Heart J. 2023, 44, 2560. [Google Scholar] [CrossRef]
- Brenner, D.R.; Boucher, B.A.; Kreiger, N.; Jenkins, D.; El-Sohemy, A. Dietary Patterns in an Ethnoculturally Diverse Population: Of Young Canadian Adults. Can. J. Diet. Pract. Res. 2011, 72, e161–e168. [Google Scholar] [CrossRef]
- Nielsen, D.E.; Boucher, B.A.; Da Costa, L.A.; Jenkins, D.J.A.; El-Sohemy, A. Reproducibility and validity of the Toronto-modified Harvard food frequency questionnaire in a multi-ethnic sample of young adults. Eur. J. Clin. Nutr. 2023, 77, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Fontaine-Bisson, B.; Wolever, T.M.; Connelly, P.W.; Corey, P.N.; El-Sohemy, A. NF-kappaB-94Ins/Del ATTG polymorphism modifies the association between dietary polyunsaturated fatty acids and HDL-cholesterol in two distinct populations. Atherosclerosis 2009, 204, 465–470. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.; Pertsemlidis, A.; Kavaslar, N.; Stewart, A.; Roberts, R.; Cox, D.R.; Hinds, D.A.; Pennacchio, L.A.; Tybjaerg-Hansen, A.; Folsom, A.R.; et al. A common allele on chromosome 9 associated with coronary heart disease. Science 2007, 316, 1488–1491. [Google Scholar] [CrossRef] [PubMed]
- Addona, T.A.; Abbatiello, S.E.; Schilling, B.; Skates, S.J.; Mani, D.R.; Bunk, D.M.; Spiegelman, C.H.; Zimmerman, L.J.; Ham, A.-J.L.; Keshishian, H.; et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat. Biotechnol. 2009, 27, 633–641. [Google Scholar] [CrossRef]
- Hortin, G.L.; Sviridov, D.; Anderson, N.L. Anderson. High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin. Chem. 2008, 54, 1608–1616. [Google Scholar] [CrossRef]
- Josse, A.R.; Garcia-Bailo, B.; Fischer, K.; El-Sohemy, A. Novel effects of hormonal contraceptive use on the plasma proteome. PLoS ONE 2012, 7, e45162. [Google Scholar] [CrossRef]
- Markiewski, M.M.; Lambris, J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef]
- Carroll, M.C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004, 5, 981–986. [Google Scholar] [CrossRef]
- Donkin, R.; Fung, Y.L.; Singh, I. Fibrinogen, Coagulation, and Ageing. In Biochemistry and Cell Biology of Ageing: Part III Biomedical Science; Springer: Cham, Switzerland, 2023; Volume 102, pp. 313–342. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, X.; Fang, L.; Tang, J.; Zhang, J. Association between lipoprotein(a), fibrinogen and their combination with all-cause, cardiovascular disease and cancer-related mortality: Findings from the NHANES. BMC Public Health 2024, 24, 1927. [Google Scholar] [CrossRef]
- Sapey, E. Neutrophil Modulation in Alpha-1 Antitrypsin Deficiency. Chronic Obstr. Pulm. Dis. 2020, 7, 247–259. [Google Scholar] [CrossRef]
- Walport, M.J. Complement. First of two parts. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Crossley, D.; Stockley, R.; Sapey, E. Alpha-1 Antitrypsin Deficiency and Accelerated Aging: A New Model for an Old Disease? Drugs Aging 2019, 36, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Maillet, F.; Goetz, J.; Hauptmann, G.; Clauvel, J.P.; Mery, J.P.; Kahn, M.F.; Kazatchkine, M. Components of the classical complement pathway in systemic lupus erythematosus. Presse Med. 1987, 16, 378–382. [Google Scholar] [PubMed]
- Vinchi, F.; De Franceschi, L.; Ghigo, A.; Townes, T.; Cimino, J.; Silengo, L.; Hirsch, E.; Altruda, F.; Tolosano, E. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation 2013, 127, 1317–1329. [Google Scholar] [CrossRef]
- Koenig, W. Serum amyloid P component and cardiovascular disease: Is there a sensible link? Arterioscler. Thromb. Vasc. Biol. 2007, 27, 698–700. [Google Scholar] [CrossRef]
- Jenny, N.S.; Arnold, A.M.; Kuller, L.H.; Tracy, R.P.; Psaty, B.M. Serum amyloid P and cardiovascular disease in older men and women: Results from the Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 352–358. [Google Scholar] [CrossRef]
- Engström, G.; Hedblad, B.; Janzon, L.; Lindgärde, F. Complement C3 and C4 in plasma and incidence of myocardial infarction and stroke: A population-based cohort study. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 392–397. [Google Scholar] [CrossRef]
- Fortney, K.; Dobriban, E.; Garagnani, P.; Pirazzini, C.; Monti, D.; Mari, D.; Atzmon, G.; Barzilai, N.; Franceschi, C.; Owen, A.B.; et al. Genome-Wide Scan Informed by Age-Related Disease Identifies Loci for Exceptional Human Longevity. PLoS Genet. 2015, 11, e1005728. [Google Scholar] [CrossRef]
- Pinós, T.; Fuku, N.; Cámara, Y.; Arai, Y.; Abe, Y.; Rodríguez-Romo, G.; Garatachea, N.; Santos-Lozano, A.; Miro-Casas, E.; Ruiz-Meana, M.; et al. The rs1333049 polymorphism on locus 9p21.3 and extreme longevity in Spanish and Japanese cohorts. AGE 2014, 36, 933–943. [Google Scholar] [CrossRef]
- Mourtzi, N.; Hatzimanolis, A.; Xiromerisiou, G.; Ntanasi, E.; Georgakis, M.K.; Ramirez, A.; Heilmann-Heimbach, S.; Grenier-Boley, B.; Lambert, J.C.; Yannakoulia, M.; et al. Association between 9p21–23 Locus and Frailty in a Community-Dwelling Greek Population: Results from the Hellenic Longitudinal Investigation of Ageing and Diet. J. Prev. Alzheimer’s Dis. 2022, 9, 77–85. [Google Scholar] [CrossRef]
- Kim, W.Y.; Sharpless, N.E. The Regulation of INK4/ARF in Cancer and Aging. Cell 2006, 127, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Notani, D. Transcriptional regulation of INK4/ARF locus by cis and trans mechanisms. Front. Cell Dev. Biol. 2022, 10, 948351. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.J.P. Highlight the major clinical outcomes of vitamins and minerals in healthy aging: A systematic review. Int. J. Nutrol. 2023, 16. [Google Scholar] [CrossRef]
- Castiglione, D.; Platania, A.; Conti, A.; Falla, M.; D’urso, M.; Marranzano, M. Dietary micronutrient and mineral intake in the mediterranean healthy eating, ageing, and lifestyle (Meal) study. Antioxidants 2018, 7, 79. [Google Scholar] [CrossRef]
- Copenhaver, M.M.; Yu, C.-Y.; Zhou, D.; Hoffman, R.P. Relationships of complement components C3 and C4 and their genetics to cardiometabolic risk in healthy, non-Hispanic white adolescents. Pediatr. Res. 2020, 87, 88–94. [Google Scholar] [CrossRef]
- Xing, Z.; Wang, Y.; Gong, K.; Chen, Y. Plasma C4 level was associated with mortality, cardiovascular and cerebrovascular complications in hemodialysis patients. BMC Nephrol. 2022, 23, 232. [Google Scholar] [CrossRef]
- Nilsson, B.; Hamad, O.A.; Ahlström, H.; Kullberg, J.; Johansson, L.S.O.; Lindhagen, L.; Haenni, A.; Ekdahl, K.N.; Lind, L. C3 and C4 are strongly related to adipose tissue variables and cardiovascular risk factors. Eur. J. Clin. Investig. 2014, 44, 587–596. [Google Scholar] [CrossRef]
- Iltumur, K.; Karabulut, A.; Toprak, G.; Toprak, N. Complement activation in acute coronary syndromes. APMIS 2005, 113, 167–174. [Google Scholar] [CrossRef]
- Heurich, M.; Föcking, M.; Cotter, D. Complement C4, C4A and C4a—What they do and how they differ. Brain Behav. Immun. Health 2024, 39, 100809. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M. Complement C4, Infections, and Autoimmune Diseases. Front. Immunol. 2021, 12, 694928. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Do, R.; Xie, C.; Zhang, X.; Männistö, S.; Harald, K.; Islam, S.; Bailey, S.D.; Rangarajan, S.; McQueen, M.J.; Diaz, R.; et al. The effect of chromosome 9p21 variants on cardiovascular disease may be modified by dietary intake: Evidence from a case/control and a prospective study. PLoS Med. 2011, 8, e1001106. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Ordovas, J.M. Nutrigenomics in cardiovascular medicine. Circ. Cardiovasc. Genet. 2009, 2, 637–651. [Google Scholar] [CrossRef]
- Bruins, M.J.; Van Dael, P.; Eggersdorfer, M. The role of nutrients in reducing the risk for noncommunicable diseases during aging. Nutrients 2019, 11, 85. [Google Scholar] [CrossRef]
- Tsirimiagkou, C.; Karatzi, K.; Basdeki, E.D.; Argyris, A.; Papaioannou, T.G.; Yannakoulia, M.; Protogerou, A.D. Dietary sodium estimation methods: Accuracy and limitations of old and new methods in individuals at high cardiovascular risk. Public Health Nutr. 2022, 25, 866–878. [Google Scholar] [CrossRef]
- Corella, D.; Coltell, O.; Portolés, O.; Sotos-Prieto, M.; Fernández-Carrión, R.; Ramirez-Sabio, J.B.; Zanón-Moreno, V.; Mattei, J.; Sorlí, J.V.; Ordovas, J.M. A guide to applying the sex-gender perspective to nutritional genomics. Nutrients 2019, 11, 4. [Google Scholar] [CrossRef]
- Brennan, L.; Hu, F.B. Metabolomics-Based Dietary Biomarkers in Nutritional Epidemiology—Current Status and Future Opportunities. Mol. Nutr. Food Res. 2019, 63, e1701064. [Google Scholar] [CrossRef]
| Characteristic | AA and AG (n = 958) | GG (n = 322) | p-Value |
|---|---|---|---|
| Age (Years) | 22.7 ± (2.5) | 22.5 ± (2.5) | 0.32 |
| Body Mass Index (kg/m2) * | 23.0 ± (3.7) | 23.1 ± (3.7) | 0.56 |
| Waist Circumference (cm) | 74.8 ± (9.5) | 75.7 ± (10.3) | 0.12 |
| Systolic Blood Pressure (mm Hg) | 114.3 ± (12.2) | 115.5 ± (12.0) | 0.13 |
| Diastolic Blood Pressure (mm Hg) | 69.3 ± (7.9) | 69.6 ± (9.0) | 0.56 |
| Glucose (mmol/L) | 4.8 ± (0.4) | 4.8 ± (0.4) | 0.20 |
| Insulin (pmol/L) * | 45.5 ± (38.3) | 49.9 ± (38.4) | 0.03 |
| CRP (mmol/L) | 0.9 ± (2.3) | 1.0 ± (2.0) | 0.50 |
| TG (mmol/L) * | 0.9 ± (0.6) | 0.9 ± (0.6) | 0.61 |
| HDL (mmol/L) | 1.5 ± (0.4) | 1.5 ± (0.4) | 0.11 |
| LDL (mmol/L) | 2.3 ± (0.6) | 2.2 ± (0.6) | 0.05 |
| Prudent Diet Score | 2.9 ± (1.6) | 2.8 ± (1.6) | 0.21 |
| Eastern Diet Score | 1.2 ± (1.0) | 1.1 ± (0.8) | 0.03 |
| Western Diet Score | 1.3 ± (1.0) | 1.3 ± (1.0) | 0.39 |
| Females, N (%) | 593 (76.9) | 178 (23.1) | |
| Males, N (%) | 365 (71.7) | 144 (28.3) | |
| Caucasian, N (%) | 391 (72.1) | 151 (27.9) | |
| East Asian, N (%) | 396 (79.7) | 101 (20.3) | |
| South Asian, N (%) | 102 (66.7) | 51 (33.3) | |
| Other, N (%) | 69 (78.4) | 19 (21.6) |
| Prudent Dietary Score | p-Value | |||||
|---|---|---|---|---|---|---|
| Protein and Genotype | Low | Medium | High | Diet | Gene | Interaction |
| α1 Antichymotrypsin | 0.20 | <0.01 | 0.02 | |||
| AA and GA | 3.2 ± 0.7 | 3.4 ± 0.8 | 3.4 ± 0.8 | |||
| GG | 3.6 ± 0.8 | 3.5 ± 0.9 | 3.6 ± 0.7 | |||
| Complement C4 β chain | 0.45 | <0.01 | 0.05 | |||
| AA and GA | 1.4 ± 0.5 | 1.4 ± 0.5 | 1.4 ± 0.5 | |||
| GG | 1.6 ± 0.6 | 1.4 ± 0.6 | 1.4 ± 0.6 | |||
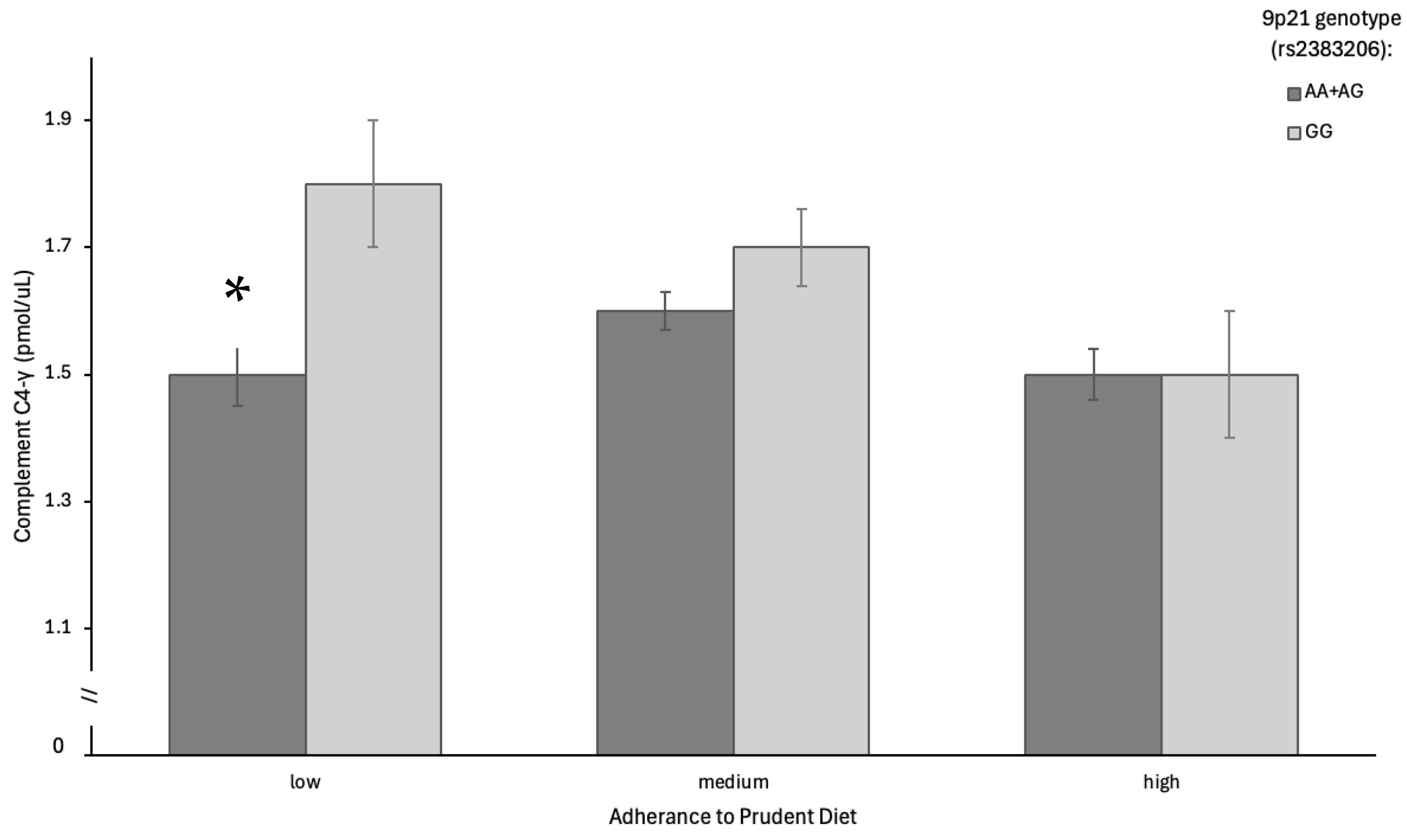
| Complement C4 γ chain | 0.59 | <0.01 | 0.02 | |||
| AA and GA | 1.5 ± 0.6 | 1.6 ± 0.6 | 1.5 ± 0.5 | |||
| GG | 1.8 ± 0.7 | 1.7 ± 0.7 | 1.5 ± 0.7 | |||
| Complement C9 | 0.02 | 0.09 | 0.03 | |||
| AA and GA | 2.5 ± 0.9 | 2.7 ± 0.8 | 2.8 ± 0.9 | |||
| GG | 2.8 ± 0.7 | 2.8 ± 1.0 | 2.8 ± 0.7 | |||
| Fibrinogen α chain | 0.08 | 0.91 | 0.03 | |||
| AA and GA | 10.7 ± 4.5 | 12.2 ± 7.0 | 11.9 ± 5.8 | |||
| GG | 11.6 ± 3.5 | 11.9 ± 4.4 | 11.8 ± 3.5 | |||
| Fibrinopeptide A | 0.09 | 0.55 | 0.02 | |||
| AA and GA | 6.4 ± 2.2 | 7.1 ± 3.5 | 7.0 ± 2.7 | |||
| GG | 7.1 ± 2.2 | 7.1 ± 2.5 | 7.0 ± 1.9 | |||
| Hemopexin | 0.04 | 0.01 | 0.01 | |||
| AA and GA | 9.5 ± 1.8 | 9.7 ± 2.0 | 10.0 ± 2.3 | |||
| GG | 10.2 ± 2.0 | 9.9 ± 2.6 | 10.6 ± 2.1 | |||
| Serum amyloid P-component | 0.18 | <0.01 | <0.01 | |||
| AA and GA | 0.4 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.1 | |||
| GG | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.4 ± 0.1 | |||
| Protein | Primary Function | Direction of Association | Interaction Interpretation | Selected References |
|---|---|---|---|---|
| α1 Antichymotrypsin | Serine protease inhibitor; modulates inflammation | * ↑ in GG across diet adherence levels | May reflect persistent low-grade inflammation among GG carriers regardless of dietary pattern | [36,40] |
| Complement C4 β chain | Classical complement pathway protein | ↑ in GG at low diet adherence → at higher adherence | Suggests increased innate immune activity in GG carriers mitigated by Prudent diet | [41,42] |
| Complement C4 γ chain | Immune-related isoform of C4 | ↑ in GG at low adherence ↓ at high adherence | Strong diet–genotype interaction; Prudent diet buffers genotype-related inflammatory activation | [5,6,43] |
| Complement C9 | Forms membrane attack complex (MAC) | ↑ in GG at low adherence → at medium/high adherence | May reflect subclinical complement activation modifiable by dietary antioxidants | [43,44,45] |
| Fibrinogen α chain | Precursor to fibrin in clot formation | ↑ in GG at low adherence → at higher adherence | Suggests vascular activation in GG carriers; diet may attenuate thrombotic risk | [38,39] |
| Fibrinopeptide A | Thrombin-cleaved peptide; associated with coagulation | ↑ in GG at low adherence → at medium/high adherence | Indicates diet-modifiable pro-thrombotic signaling in high-risk genotype | [9] |
| Hemopexin | Heme-binding antioxidant protein | ↑ in GG across diet; slight ↑ with diet in both genotypes | Reflects enhanced antioxidant demand in GG carriers, with additive benefits from Prudent diet | [10,46] |
| Serum amyloid P-component | Pentraxin protein; linked to tissue remodeling and amyloid stability | ↑ in GG across all diet levels → no modulation by diet | Suggests genotype-driven inflammatory profile unaffected by dietary adherence | [46,47,48] |
| Micronutrient Intake Derived from FFQ | r | p-Value |
|---|---|---|
| Vitamin A | 0.57 | 2.7 × 10−45 |
| Vitamin C | 0.31 | 2.3 × 10−13 |
| Vitamin D | 0.07 | 0.12 |
| Vitamin E | 0.46 | 1.6 × 10−28 |
| Folate | 0.31 | 5.7 × 10−13 |
| Sodium | 0.13 | 0.004 |
| Potassium | 0.55 | 6.8 × 10−43 |
| Magnesium | 0.62 | 3.3 × 10−58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahdavi, S.; Rosychuk, K.; Jenkins, D.J.A.; Percy, A.J.; Borchers, C.H.; El-Sohemy, A. Multiomics Analysis of a Micronutrient-Rich Dietary Pattern and the Aging Genotype 9p21 on the Plasma Proteome of Young Adults. Nutrients 2025, 17, 1398. https://doi.org/10.3390/nu17081398
Mahdavi S, Rosychuk K, Jenkins DJA, Percy AJ, Borchers CH, El-Sohemy A. Multiomics Analysis of a Micronutrient-Rich Dietary Pattern and the Aging Genotype 9p21 on the Plasma Proteome of Young Adults. Nutrients. 2025; 17(8):1398. https://doi.org/10.3390/nu17081398
Chicago/Turabian StyleMahdavi, Sara, Katie Rosychuk, David J. A. Jenkins, Andrew J. Percy, Christoph H. Borchers, and Ahmed El-Sohemy. 2025. "Multiomics Analysis of a Micronutrient-Rich Dietary Pattern and the Aging Genotype 9p21 on the Plasma Proteome of Young Adults" Nutrients 17, no. 8: 1398. https://doi.org/10.3390/nu17081398
APA StyleMahdavi, S., Rosychuk, K., Jenkins, D. J. A., Percy, A. J., Borchers, C. H., & El-Sohemy, A. (2025). Multiomics Analysis of a Micronutrient-Rich Dietary Pattern and the Aging Genotype 9p21 on the Plasma Proteome of Young Adults. Nutrients, 17(8), 1398. https://doi.org/10.3390/nu17081398








