Dynamic Changes in Antibodies and Proteome in Breast Milk of Mothers Infected with Wild-Type SARS-CoV-2 and Omicron: A Longitudinal Study
Highlights
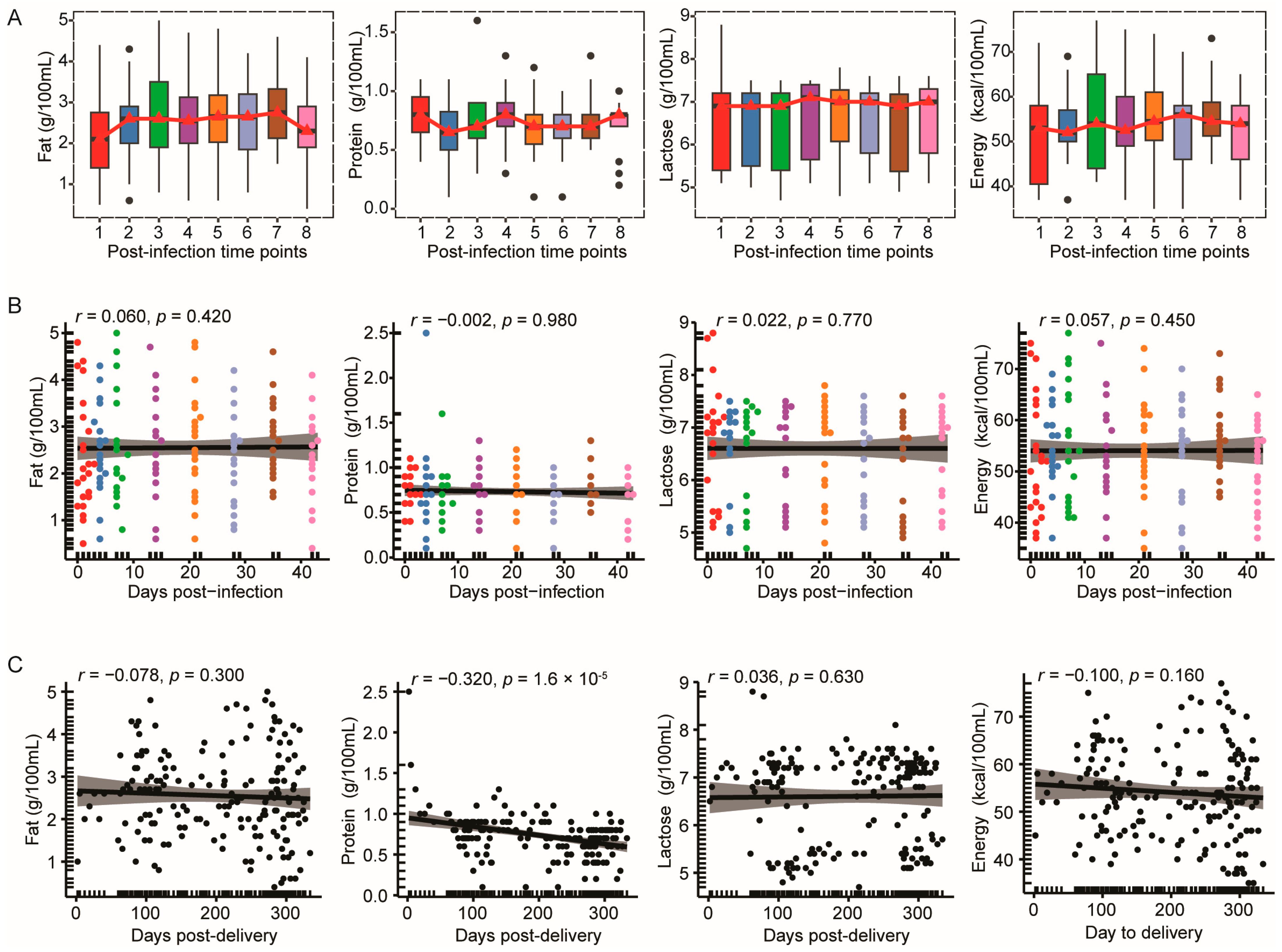
- Macronutrient composition in breast milk remained stable for 42 days after Omicron infection
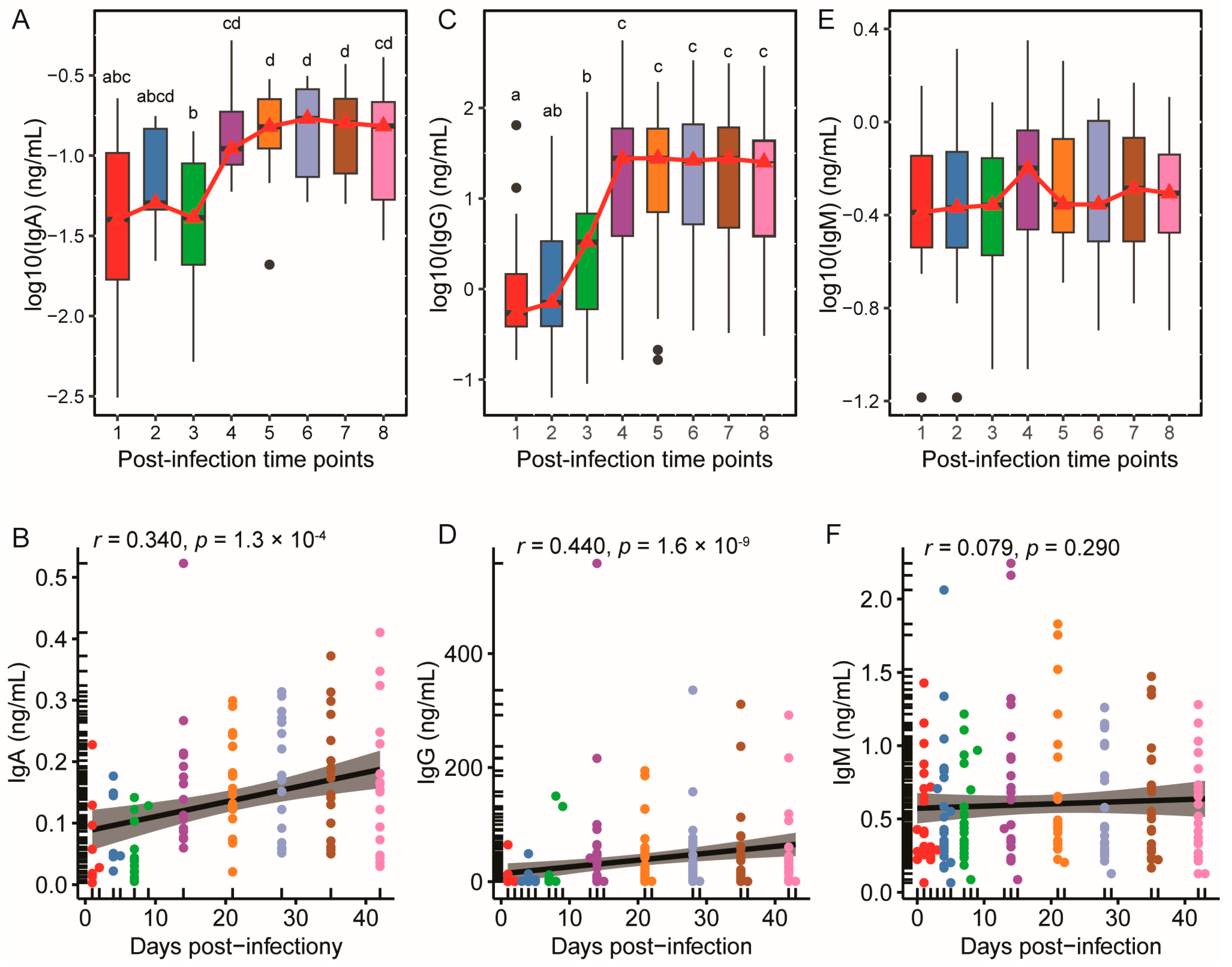
- SARS-CoV-2-specific IgA and IgG exhibited biphasic kinetics with prolonged plateau phases throughout the post-infection period
- Proteomic signatures illustrate the dynamic balance between innate antigen presentation and adaptive antibody-driven mechanisms
- Proteins with conserved expression trends across both wild-type and Omicron variants are predominantly acute-phase response proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Sample Collection
2.3. Laboratory Analysis
2.3.1. Nutritional Composition Analysis
2.3.2. SARS-CoV-2-Specific Antibodies Analysis
2.3.3. Proteomic Analysis
2.4. Statistical Analysis
3. Results
3.1. The Macronutrient Composition of Breast Milk in the Omicron Cohort Exhibited Temporal Stability Following Infection
3.2. SARS-CoV-2-Specific IgA and IgG Levels Increased and Plateaued Post-Infection, While IgM Remained Stable
3.3. Temporal Coordination of the Breast Milk Proteome with SARS-CoV-2-Specific Antibody Dynamics
3.4. Proteomic Analysis Revealed 24 Proteins with Consistent Change Trends Between Wild-Type and Omicron Variants
4. Discussion
4.1. Main Findings and Comparison with Previous Studies
4.2. Strengths and Limitations
4.3. Clinical Implications and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Victora, C.G.; Bahl, R.; Barros, A.J.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal breastfeeding practices and infant and child mortality: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 3–13. [Google Scholar] [CrossRef]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef]
- Matson, A.P.; Thrall, R.S.; Rafti, E.; Lingenheld, E.G.; Puddington, L. IgG transmitted from allergic mothers decreases allergic sensitization in breastfed offspring. Clin. Mol. Allergy 2010, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.P.; Ma, C.; Good, M.; et al. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Eker, F.; Akdaşçi, E.; Duman, H.; Yalçıntaş, Y.M.; Canbolat, A.A.; Kalkan, A.E.; Karav, S.; Šamec, D. Antimicrobial Properties of Colostrum and Milk. Antibiotics 2024, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Cheema, R.; Partridge, E.; Kair, L.R.; Kuhn-Riordon, K.M.; Silva, A.I.; Bettinelli, M.E.; Chantry, C.J.; Underwood, M.A.; Lakshminrusimha, S.; Blumberg, D. Protecting breastfeeding during the COVID-19 pandemic. Am. J. Perinatol. 2023, 40, 260–266. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Feketea, G.; Koumbi, L.; Mesiari, C.; Berghea, E.C.; Konstantinou, G.N. Breastfeeding and COVID-19: From nutrition to immunity. Front. Immunol. 2021, 12, 661806. [Google Scholar] [CrossRef]
- Pang, Z.; Hu, R.; Tian, L.; Lou, F.; Chen, Y.; Wang, S.; He, S.; Zhu, S.; An, X.; Song, L.; et al. Overview of Breastfeeding Under COVID-19 Pandemic. Front. Immunol. 2022, 13, 896068. [Google Scholar] [CrossRef]
- Atyeo, C.; Alter, G. The multifaceted roles of breast milk antibodies. Cell 2021, 184, 1486–1499. [Google Scholar] [CrossRef]
- Andreas, N.J.; Kampmann, B.; Mehring Le-Doare, K. Human breast milk: A review on its composition and bioactivity. Early Hum. Dev. 2015, 91, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Halsey, J.F.; Mitchell, C.S.; McKenzie, S.J. The origins of secretory IgA in milk: A shift during lactation from a serum origin to local synthesis in the mammary gland. Ann. N. Y. Acad. Sci. 1983, 409, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Moor, K.; Diard, M.; Sellin, M.E.; Felmy, B.; Wotzka, S.Y.; Toska, A.; Bakkeren, E.; Arnoldini, M.; Bansept, F.; Co, A.D.; et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 2017, 544, 498–502. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; An, M.; Yang, W.; Wen, Y.; Cai, Z.; Wang, L.; Zhou, Q. Recommendations for breastfeeding during Coronavirus Disease 2019 (COVID-19) pandemic. Int. Breastfeed. J. 2022, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, H. Should COVID-19 Mother Breastfeed her Newborn Child? A Literature Review on the Safety of Breastfeeding for Pregnant Women with COVID-19. Curr. Nutr. Rep. 2021, 10, 71–75. [Google Scholar] [CrossRef]
- Ismail, S.; Unger, S.; Budylowski, P.; Poutanen, S.; Yau, Y.; Jenkins, C.; Anwer, S.; Christie-Holmes, N.; Kiss, A.; Mazzulli, T.; et al. SARS-CoV-2 antibodies and their neutralizing capacity against live virus in human milk after COVID-19 infection and vaccination: Prospective cohort studies. Am. J. Clin. Nutr. 2024, 119, 485–495. [Google Scholar] [CrossRef]
- Pace, R.M.; Williams, J.E.; Järvinen, K.M.; Belfort, M.B.; Pace, C.D.; Lackey, K.A.; Gogel, A.C.; Nguyen-Contant, P.; Kanagaiah, P.; Fitzgerald, T.; et al. COVID-19 and human milk: SARS-CoV-2, antibodies, and neutralizing capacity. medRxiv 2020. [Google Scholar] [CrossRef]
- Yang, X.; Fox, A.; DeCarlo, C.; Pineda, N.; Powell, R.L.R. The Secretory IgA Response in Human Milk Against the SARS-CoV-2 Spike Is Highly Durable and Neutralizing for at Least 1 Year of Lactation Postinfection. Breastfeed. Med. 2023, 18, 602–611. [Google Scholar] [CrossRef]
- Duncombe, C.J.; McCulloch, D.J.; Shuey, K.D.; Logue, J.K.; Franko, N.M.; Wolf, C.R.; Frivold, C.J.; Chu, H.Y. Dynamics of breast milk antibody titer in the six months following SARS-CoV-2 infection. J. Clin. Virol. 2021, 142, 104916. [Google Scholar] [CrossRef]
- Pace, R.M.; Williams, J.E.; Järvinen, K.M.; Meehan, C.L.; Martin, M.A.; Ley, S.H.; Barbosa-Leiker, C.; Andres, A.; Yeruva, L.; Belfort, M.B.; et al. Milk from Women Diagnosed with COVID-19 Does Not Contain SARS-CoV-2 RNA but Has Persistent Levels of SARS-CoV-2-Specific IgA Antibodies. Front. Immunol. 2021, 12, 801797. [Google Scholar] [CrossRef]
- Fox, A.; Marino, J.; Amanat, F.; Krammer, F.; Hahn-Holbrook, J.; Zolla-Pazner, S.; Powell, R.L. Robust and Specific Secretory IgA Against SARS-CoV-2 Detected in Human Milk. iScience 2020, 23, 101735. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Katzourakis, A.; Stilianakis, N.I. Antigenic evolution will lead to new SARS-CoV-2 variants with unpredictable severity. Nat. Rev. Microbiol. 2022, 20, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; COVID-19 Genomics UK Consortium; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Kirca, F.; Aydoğan, S.; Gözalan, A.; Kayipmaz, A.E.; Özdemir, F.A.E.; Tekçe, Y.T.; Beşer, İ.O.; Gün, P.; Ökten, R.S.; Dinç, B. Comparison of clinical characteristics of wild-type SARS-CoV-2 and Omicron. Rev. Assoc. Med. Bras. 2022, 68, 1476–1480. [Google Scholar] [CrossRef]
- Uthman, O.A.; Lyngse, F.P.; Anjorin, S.; Hauer, B.; Hakki, S.; Martinez, D.A.; Ge, Y.; Jonnerby, J.; Julin, C.H.; Lin, G.; et al. Susceptibility and infectiousness of SARS-CoV-2 in children versus adults, by variant (wild-type, alpha, delta): A systematic review and meta-analysis of household contact studies. PLoS ONE 2024, 19, e0306740. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- van Keulen, B.J.; Romijn, M.; Bondt, A.; Dingess, K.A.; Kontopodi, E.; van der Straten, K.; den Boer, M.A.; Burger, J.A.; Poniman, M.; Bosch, B.J.; et al. Human Milk from Previously COVID-19-Infected Mothers: The Effect of Pasteurization on Specific Antibodies and Neutralization Capacity. Nutrients 2021, 13, 1645. [Google Scholar] [CrossRef] [PubMed]
- Akkız, H. The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Corcern. Front. Med. 2022, 9, 849217. [Google Scholar] [CrossRef] [PubMed]
- Barrera, A.; Martínez-Valdebenito, C.; Angulo, J.; Palma, C.; Hormazábal, J.; Vial, C.; Aguilera, X.; Castillo-Torres, P.; Pardo-Roa, C.; Balcells, M.E.; et al. SARS-CoV-2 infectivity and antigenic evasion: Spotlight on isolated Omicron sub-lineages. Front. Med. 2024, 11, 1414331. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Dapporto, F.; Marchi, S.; Leonardi, M.; Piu, P.; Lovreglio, P.; Decaro, N.; Buonvino, N.; Stufano, A.; Lorusso, E.; Bombardieri, E.; et al. Antibody Avidity and Neutralizing Response against SARS-CoV-2 Omicron Variant after Infection or Vaccination. J. Immunol. Res. 2022, 2022, 4813199. [Google Scholar] [CrossRef]
- Chen, Z.; Deng, X.; Fang, L.; Sun, K.; Wu, Y.; Che, T.; Zou, J.; Cai, J.; Liu, H.; Wang, Y.; et al. Epidemiological characteristics and transmission dynamics of the outbreak caused by the SARS-CoV-2 Omicron variant in Shanghai, China: A descriptive study. Lancet Reg. Health West. Pac. 2022, 29, 100592. [Google Scholar] [CrossRef]
- Zuo, F.; Marcotte, H.; Hammarström, L.; Pan-Hammarström, Q. Mucosal IgA against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 387, e55. [Google Scholar]
- Verheul, M.K.; Kaczorowska, J.; Hofstee, M.I.; Schepp, R.M.; Smits, G.P.; Wessels Beljaars, D.; Kuijer, M.; Schuin, W.; Middelhof, I.; Wong, D.; et al. Protective mucosal SARS-CoV-2 antibodies in the majority of the general population in The Netherlands. Mucosal Immunol. 2024, 17, 554–564. [Google Scholar] [CrossRef]
- Singh, P.; Dhakal, J.; Kumar, P. Antiviral properties of milk proteins and peptides against SARS-CoV-2: A review. J. Funct. Foods 2024, 117, 106237. [Google Scholar] [CrossRef]
- Quitadamo, P.A.; Comegna, L.; Cristalli, P. Anti-Infective, Anti-Inflammatory, and Immunomodulatory Properties of Breast Milk Factors for the Protection of Infants in the Pandemic from COVID-19. Front. Public Health 2021, 8, 589736. [Google Scholar] [CrossRef]
- Romero Ramírez, D.S.; Lara Pérez, M.M.; Carretero Pérez, M.; Suárez Hernández, M.I.; Martín Pulido, S.; Pera Villacampa, L.; Fernández Vilar, A.M.; Rivero Falero, M.; González Carretero, P.; Reyes Millán, B.; et al. SARS-CoV-2 Antibodies in Breast Milk After Vaccination. Pediatrics 2021, 148, e2021052286. [Google Scholar] [CrossRef] [PubMed]
- Juncker, H.G.; Mulleners, S.J.; Ruhe, E.J.; Coenen, E.R.; Bakker, S.; van Doesburg, M.; Harinck, J.E.; Rood, R.D.; Bouhuijs, J.H.; Oomen, M. Comparing the human milk antibody response after vaccination with four COVID-19 vaccines: A prospective, longitudinal cohort study in the Netherlands. eClinicalMedicine 2022, 47, 101393. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.S.; Valcarce, V.; Henry, M.; Neu, J.; Parker, L.; Mueller, M.; Vicuna, V.; Gowen, T.; Cato, E.; Kosik, I. Detection of SARS-CoV-2 IgA and IgG in human milk and breastfeeding infant stool 6 months after maternal COVID-19 vaccination. J. Perinatol. 2023, 43, 775–781. [Google Scholar] [CrossRef]
- Perl, S.H.; Uzan-Yulzari, A.; Klainer, H.; Asiskovich, L.; Youngster, M.; Rinott, E.; Youngster, I. SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA 2021, 325, 2013–2014. [Google Scholar] [CrossRef]
- Zhao, S.; Lok, K.Y.; Sin, Z.Y.; Peng, Y.; Fan, H.S.; Nagesh, N.; Choi, M.S.; Kwok, J.Y.; Choi, E.P.; Zhang, X. COVID-19 mRNA vaccine-mediated antibodies in human breast milk and their association with breast milk microbiota composition. npj Vaccines 2023, 8, 151. [Google Scholar] [CrossRef]
- Guo, J.; Tan, M.; Zhu, J.; Tian, Y.; Liu, H.; Luo, F.; Wang, J.; Huang, Y.; Zhang, Y.; Yang, Y.; et al. Proteomic Analysis of Human Milk Reveals Nutritional and Immune Benefits in the Colostrum from Mothers with COVID-19. Nutrients 2022, 14, 2513. [Google Scholar] [CrossRef]
- van den Heever, W.; de Wet, G.; Hattingh, M. Possible association between the stage of HIV disease, antiretroviral treatment and the nutrient composition of breast milk in the Mangaung area, South Africa. J. Int. AIDS Soc. 2014, 17, 19702. [Google Scholar] [CrossRef] [PubMed]
- Fouché, C.; van Niekerk, E.; du Plessis, L.M. Differences in Breast Milk Composition of HIV-Infected and HIV-Uninfected Mothers of Premature Infants: Effects of Antiretroviral Therapy. Breastfeed. Med. 2016, 11, 455–460. [Google Scholar] [CrossRef]
- Zhang, P.; Jian, H.; Li, C.; Yao, L.; He, T.; Tan, Z.; Zhou, J.; Yin, Y. Effect of hepatitis B virus infection on the nutrient composition of human breast milk: A prospective cohort study. Food Chem. 2025, 465, 141947. [Google Scholar] [CrossRef]
- Flores-Fong, I.; Pérez-Montaño, J.A.; Amézcua-López, J.A. Presence of Lactic Acid Bacteria and Nutritional Content in Human Milk in Vaccinated and COVID-19 Infected Mothers. Microbiol. Infect. Dis. 2025, 9, 1–7. [Google Scholar]
- Bäuerl, C.; Randazzo, W.; Sánchez, G.; Selma-Royo, M.; García Verdevio, E.; Martínez, L.; Parra-Llorca, A.; Lerin, C.; Fumadó, V.; Crovetto, F.; et al. SARS-CoV-2 RNA and antibody detection in breast milk from a prospective multicentre study in Spain. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 216–221. [Google Scholar] [CrossRef]
- Young, B.E.; Seppo, A.E.; Diaz, N.; Rosen-Carole, C.; Nowak-Wegrzyn, A.; Vasquez, J.M.C.; Ferri-Huerta, R.; Nguyen-Contant, P.; Fitzgerald, T.; Sangster, M.Y. Association of human milk antibody induction, persistence, and neutralizing capacity with SARS-CoV-2 infection vs. mRNA vaccination. JAMA Pediatr. 2022, 176, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; DeRiso, E.A.; Davis, C.; Bordt, E.A.; De Guzman, R.M.; Shook, L.L.; Yonker, L.M.; Fasano, A.; Akinwunmi, B.; Lauffenburger, D.A. COVID-19 mRNA vaccines drive differential antibody Fc-functional profiles in pregnant, lactating, and nonpregnant women. Sci. Transl. Med. 2021, 13, eabi8631. [Google Scholar] [CrossRef]
- Shook, L.L.; Atyeo, C.G.; Yonker, L.M.; Fasano, A.; Gray, K.J.; Alter, G.; Edlow, A.G. Durability of anti-spike antibodies in infants after maternal COVID-19 vaccination or natural infection. JAMA 2022, 327, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Juncker, H.G.; Mulleners, S.J.; Coenen, E.R.; van Goudoever, J.B.; van Gils, M.J.; van Keulen, B.J. Comparing human milk antibody response after 4 different vaccines for COVID-19. JAMA Pediatr. 2022, 176, 611–612. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.C.; Carter, E.B.; Raghuraman, N.; Nolan, L.S.; Gong, Q.; Lewis, A.N.; Good, M. Anti–severe acute respiratory syndrome coronavirus 2 antibodies induced in breast milk after Pfizer-BioNTech/BNT162b2 vaccination. Am. J. Obstet. Gynecol. 2021, 225, 101–103. [Google Scholar] [CrossRef]
- Rosenberg-Friedman, M.; Kigel, A.; Bahar, Y.; Werbner, M.; Alter, J.; Yogev, Y.; Dror, Y.; Lubetzky, R.; Dessau, M.; Gal-Tanamy, M. BNT162b2 mRNA vaccine elicited antibody response in blood and milk of breastfeeding women. Nat. Commun. 2021, 12, 6222. [Google Scholar] [CrossRef]
- Chen, M.; Qin, R.; Jiang, M.; Yang, Z.; Wen, W.; Li, J. Clinical applications of detecting IgG, IgM or IgA antibody for the diagnosis of COVID-19: A meta-analysis and systematic review. Int. J. Infect. Dis. 2021, 104, 415–422. [Google Scholar] [CrossRef]
- Sun, J.; Tang, X.; Bai, R.; Liang, C.; Zeng, L.; Lin, H.; Yuan, R.; Zhou, P.; Huang, X.; Xiong, Q.; et al. The kinetics of viral load and antibodies to SARS-CoV-2. Clin. Microbiol. Infect. 2020, 26, 1690.e1–1690.e4. [Google Scholar] [CrossRef]
- Pinto, A.L.; Rai, R.K.; Brown, J.C.; Griffin, P.; Edgar, J.R.; Shah, A.; Singanayagam, A.; Hogg, C.; Barclay, W.S.; Futter, C.E.; et al. Ultrastructural insight into SARS-CoV-2 entry and budding in human airway epithelium. Nat. Commun. 2022, 13, 1609. [Google Scholar] [CrossRef] [PubMed]
- Cazzolla, A.P.; Lovero, R.; Spirito, F.; Di Cosola, M.; Santacroce, L.; Lo Muzio, E.; Ciavarella, D.; Dioguardi, M.; Crincoli, V.; Pepe, M.; et al. Evaluation of qualitative and quantitative taste alterations in COVID-19. Biomol. Biomed. 2023, 23, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Barham, H.P.; Taha, M.A.; Broyles, S.T.; Stevenson, M.M.; Zito, B.A.; Hall, C.A. Association Between Bitter Taste Receptor Phenotype and Clinical Outcomes Among Patients with COVID-19. JAMA Netw. Open 2021, 4, e2111410. [Google Scholar] [CrossRef]
- Perez, L. Acute phase protein response to viral infection and vaccination. Arch. Biochem. Biophys. 2019, 671, 196–202. [Google Scholar] [CrossRef]
- Mosaad, Y.M. Clinical Role of Human Leukocyte Antigen in Health and Disease. Scand. J. Immunol. 2015, 82, 283–306. [Google Scholar] [CrossRef]
- Alsoussi, W.B.; Malladi, S.K.; Zhou, J.Q.; Liu, Z.; Ying, B.; Kim, W.; Schmitz, A.J.; Lei, T.; Horvath, S.C.; Sturtz, A.J.; et al. SARS-CoV-2 Omicron boosting induces de novo B cell response in humans. Nature 2023, 617, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Utrata, A.; Schmidtner, N.; Mester, P.; Schmid, S.; Müller, M.; Pavel, V.; Buechler, C. Plasma Lipopolysaccharide-Binding Protein (LBP) Is Induced in Critically Ill Females with Gram-Negative Infections-Preliminary Study. Infect. Dis. Rep. 2025, 17, 10. [Google Scholar] [CrossRef]
- Hoel, H.; Heggelund, L.; Reikvam, D.H.; Stiksrud, B.; Ueland, T.; Michelsen, A.E.; Otterdal, K.; Muller, K.E.; Lind, A.; Muller, F.; et al. Elevated markers of gut leakage and inflammasome activation in COVID-19 patients with cardiac involvement. J. Intern. Med. 2021, 289, 523–531. [Google Scholar] [CrossRef]
- Mestriner, F.; Francisco, D.F.; Campos, L.C.B.; Couto, A.E.S.; Fraga-Silva, T.F.C.; Flora Dugaich, V.; Avila-Mesquita, C.D.; Zukowski Kovacs, H.; Vasconcelos, J.L.; Milani, E.R.; et al. Alpha 1-acid glycoprotein is upregulated in severe COVID-19 patients and decreases neutrophil NETs in SARS-CoV-2 infection. Cytokine 2024, 176, 156503. [Google Scholar] [CrossRef]
- Mendes-Frias, A.; Gallo, V.; Iacobelli, V.; Gentile, R.; Antonini, G.; Silvestre, R.; Iacobelli, S. Galectin-3 binding protein stimulated IL-6 expression is impeded by antibody intervention in SARS-CoV-2 susceptible cell lines. Sci. Rep. 2022, 12, 17047. [Google Scholar] [CrossRef]
- Olarte-Castillo, X.A.; Dos Remédios, J.F.; Heeger, F.; Hofer, H.; Karl, S.; Greenwood, A.D.; East, M.L. The virus-host interface: Molecular interactions of Alphacoronavirus-1 variants from wild and domestic hosts with mammalian aminopeptidase N. Mol. Ecol. 2021, 30, 2607–2625. [Google Scholar] [CrossRef] [PubMed]
- Scarcella, M.; d’Angelo, D.; Ciampa, M.; Tafuri, S.; Avallone, L.; Pavone, L.M.; De Pasquale, V. The Key Role of Lysosomal Protease Cathepsins in Viral Infections. Int. J. Mol. Sci. 2022, 23, 9089. [Google Scholar] [CrossRef] [PubMed]
- Polasky, D.A.; Geiszler, D.J.; Yu, F.; Li, K.; Teo, G.C.; Nesvizhskii, A.I. MSFragger-Labile: A Flexible Method to Improve Labile PTM Analysis in Proteomics. Mol. Cell. Proteom. 2023, 22, 100538. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An integrated proteome resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef]



| Characteristics | Mothers | Infants |
|---|---|---|
| Age (years) | 32.50 ± 3.59 | 2~334 (days) |
| Weight (kg) | 62.41 ± 11.32 | NA |
| BMI | 24.02 ± 3.87 | NA |
| Gestational Week, n (%) | ||
| 37–40 weeks | 17 (77.27) | NA |
| ≤37 weeks | 1 (4.55) | NA |
| ≥40 weeks | 4 (18.18) | NA |
| Gravidity, n (%) | ||
| First | 17 (77.27) | NA |
| Second | 5 (22.73) | NA |
| Symptom, n (%) | ||
| Fever | 13 (59.09) | 14 (63.64) |
| Headache or muscle aches | 16 (72.73) | 7 (31.82) |
| Sore throat | 16 (72.73) | 7 (31.82) |
| Runny nose | 18 (81.82) | 8 (36.36) |
| Cough | 21 (95.45) | 10 (45.45) |
| Loss of appetite | 8 (36.36) | 10 (45.45) |
| Diarrhea | 6 (27.27) | 2 (9.09) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Li, C.; Tan, M.; Chen, Y.; Zhu, S.; Zhi, C.; Zhu, J. Dynamic Changes in Antibodies and Proteome in Breast Milk of Mothers Infected with Wild-Type SARS-CoV-2 and Omicron: A Longitudinal Study. Nutrients 2025, 17, 1396. https://doi.org/10.3390/nu17081396
Guo Y, Li C, Tan M, Chen Y, Zhu S, Zhi C, Zhu J. Dynamic Changes in Antibodies and Proteome in Breast Milk of Mothers Infected with Wild-Type SARS-CoV-2 and Omicron: A Longitudinal Study. Nutrients. 2025; 17(8):1396. https://doi.org/10.3390/nu17081396
Chicago/Turabian StyleGuo, Yaqiong, Cheng Li, Minjie Tan, Yuexiao Chen, Shuai Zhu, Cheng Zhi, and Jing Zhu. 2025. "Dynamic Changes in Antibodies and Proteome in Breast Milk of Mothers Infected with Wild-Type SARS-CoV-2 and Omicron: A Longitudinal Study" Nutrients 17, no. 8: 1396. https://doi.org/10.3390/nu17081396
APA StyleGuo, Y., Li, C., Tan, M., Chen, Y., Zhu, S., Zhi, C., & Zhu, J. (2025). Dynamic Changes in Antibodies and Proteome in Breast Milk of Mothers Infected with Wild-Type SARS-CoV-2 and Omicron: A Longitudinal Study. Nutrients, 17(8), 1396. https://doi.org/10.3390/nu17081396







