The Effect of Increased Plant Protein Intake on the Lipid Profile of Chronic Kidney Disease Patients: A Meta-Analysis of Controlled Clinical Trials
Highlights
- Improved lipid regulation may aid in reducing cardiovascular risk, a major cause of death in CKD patients.
- Most current evidence pertains to soy-based proteins; further research is needed to assess the effects of non-soy plant protein sources.
- The lack of statistically significant changes in HDL and Apolipoprotein A levels warrants further investigation.
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
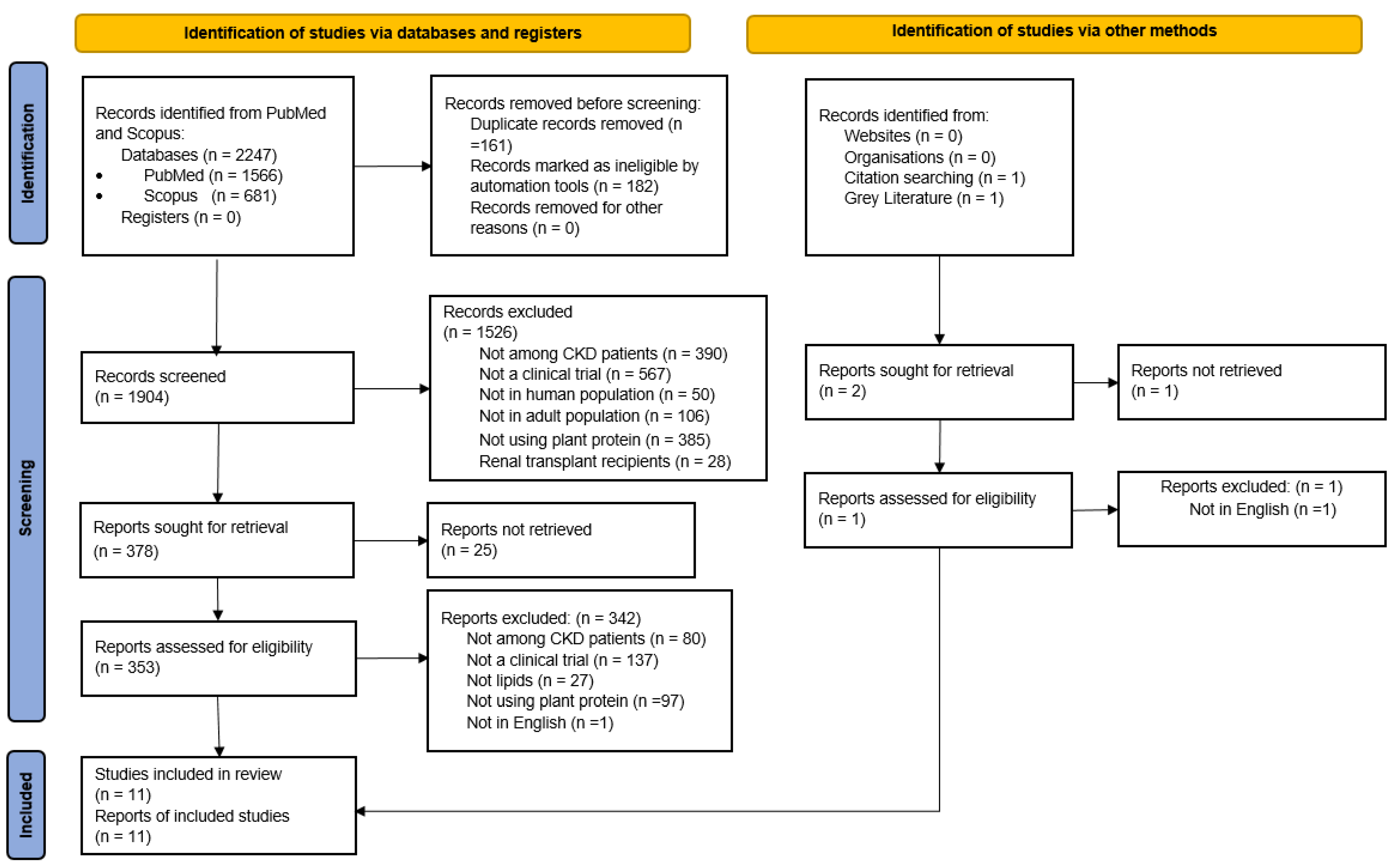
2.2. Eligibility Criteria and Study Selection
2.3. Data Extraction and Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
| Author, Year | Country | Study Type | Number of Study Participants | RRT | Intervention | Control | Duration (Weeks) |
|---|---|---|---|---|---|---|---|
| Ahmed, 2011 [32] | Brazil | RP | 18 | None | 100% vegetable protein | 100% animal protein | 8 |
| Anderson, 1998 [28] | USA | RC | 8 | None | 50% soy protein | 50% animal protein | 8 |
| Azadbakht, 2003 [29] | Iran | RC | 14 | None | 35% soy + 30% vegetable protein | 70% animal protein | 7 |
| Azadbakht, 2008 [33] | Iran | RP | 41 | None | 35% soy + 30% vegetable protein | 70% animal protein | 192 |
| Chen, 2005 [22] | Taiwan | RP | 37 | Hemodialysis | 30 g soy protein/d | 30 g cow milk protein/d | 12 |
| Chen, 2006 [23] | Taiwan | RP | 26 | Hemodialysis | 30 g soy protein/d | 30 g milk protein/d | 12 |
| D’Amico, 1992 [31] | Italy | NRC | 20 | None | 100% vegetable protein | Usual CKD diet 1 | 8 |
| Miraghajani, 2013 [24] | Iran | RC | 25 | None | 240 mL soy milk/d | 240 mL cow milk/d | 4 |
| Soroka, 1998 [25] | Israel | RC | 9 | None | >50% soy protein | >50% animal protein | 24 |
| Tabibi, 2010 [34] | Iran | RP | 36 | Peritoneal dialysis | 14 g soy protein/d | Usual CKD diet 1 | 8 |
| Teixeira, 2004 [30] | USA | RC | 14 | None | 50% soy protein | 50% animal protein | 8 |
3.1. Risk of Bias Assessment
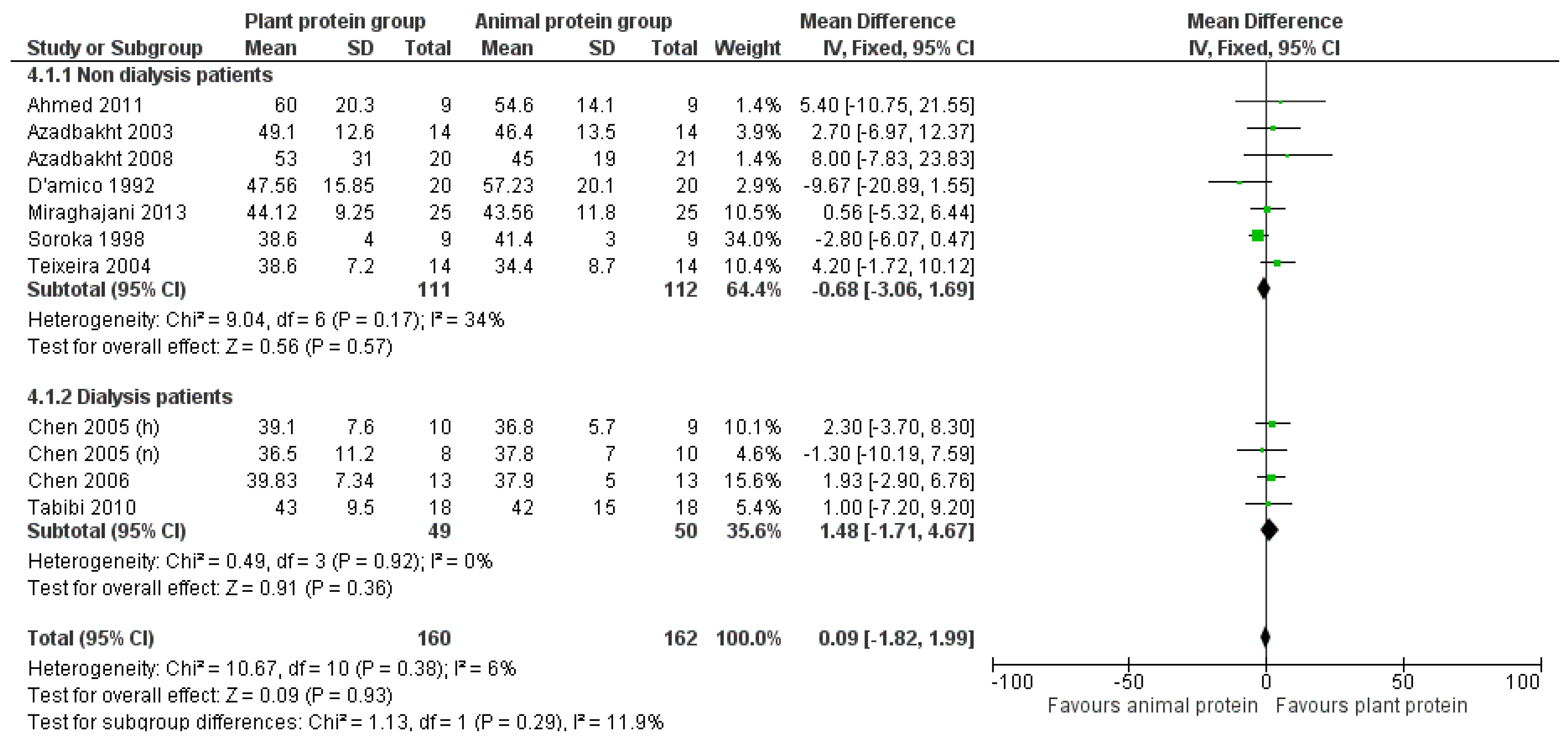
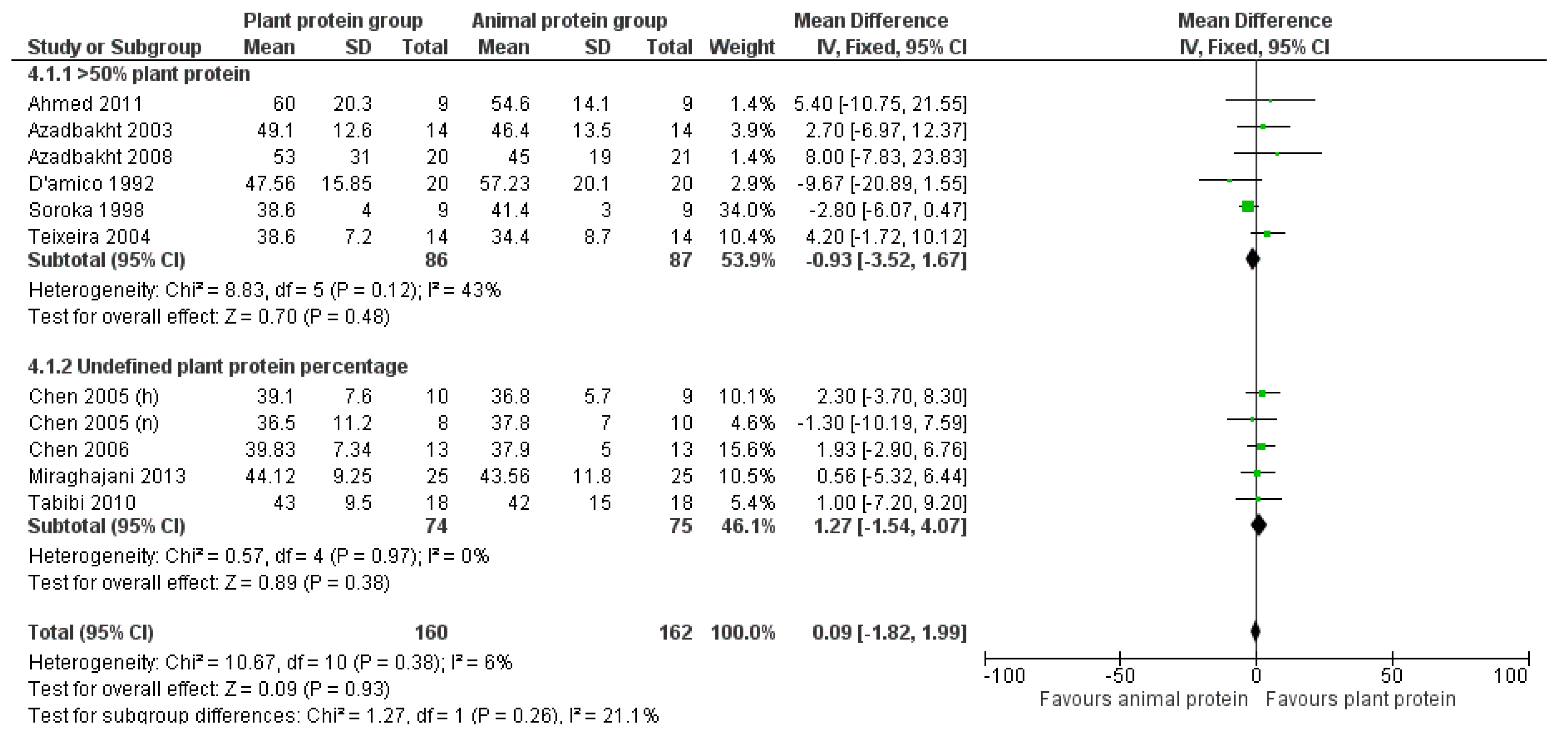
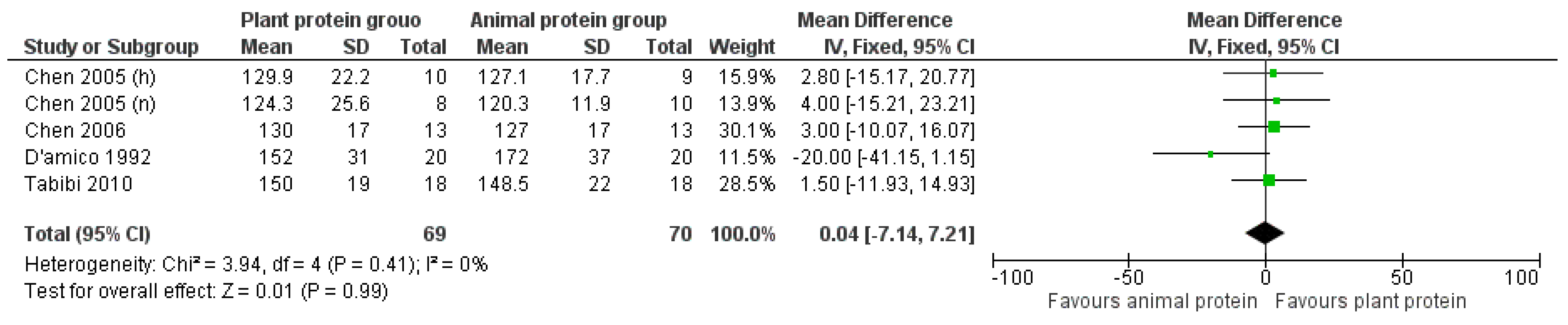
3.2. Outcomes
3.2.1. Total Cholesterol
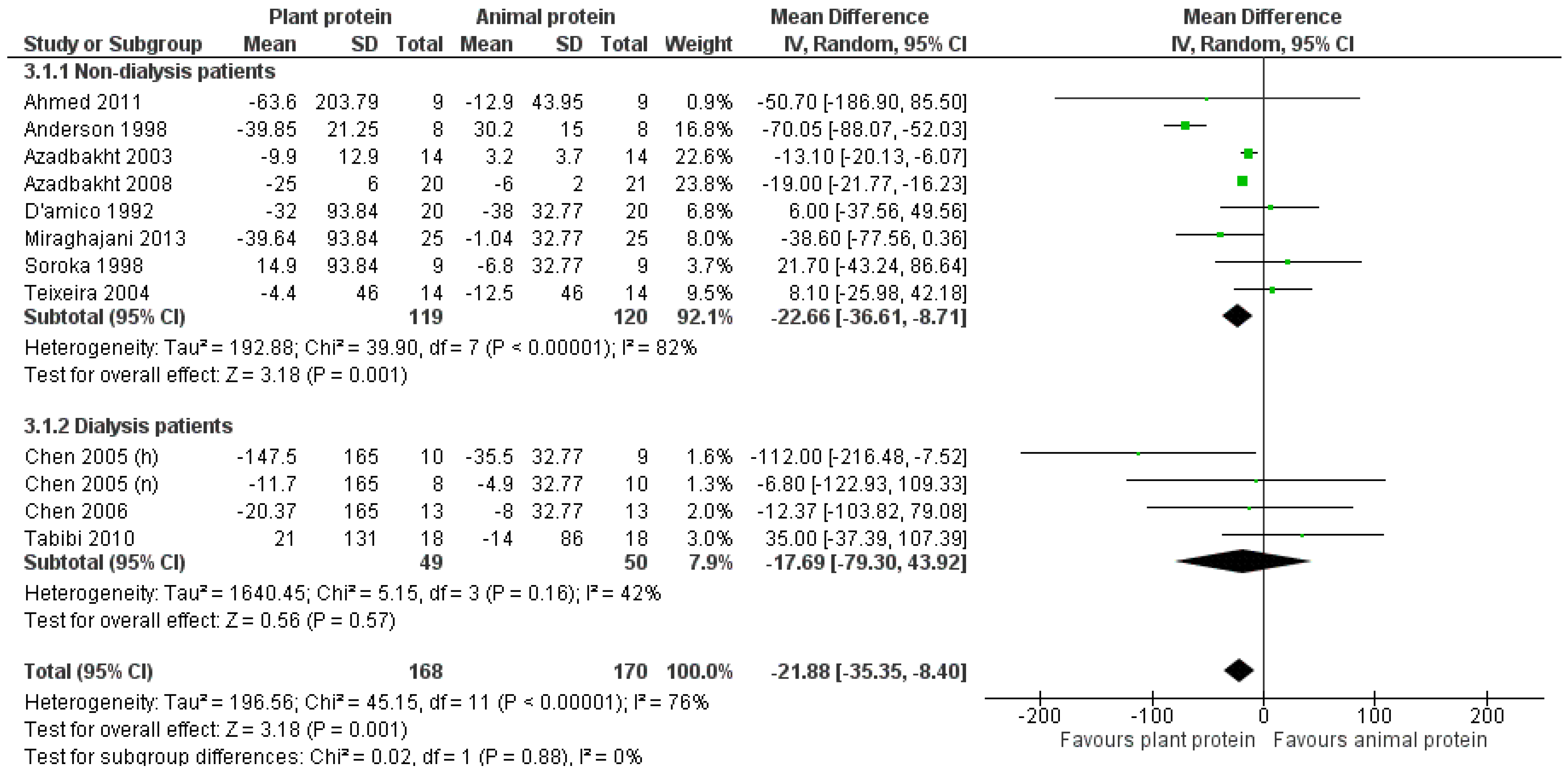
3.2.2. Triglycerides
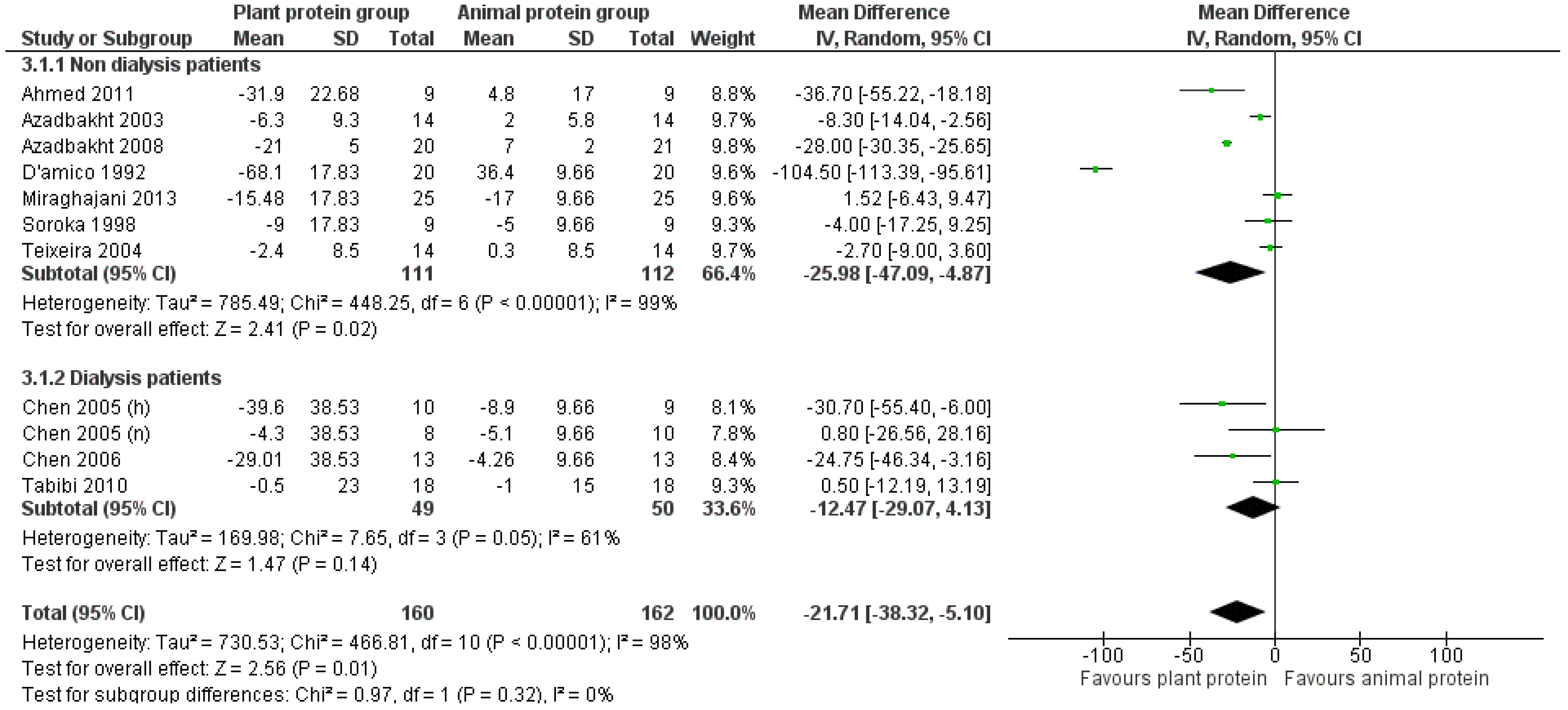
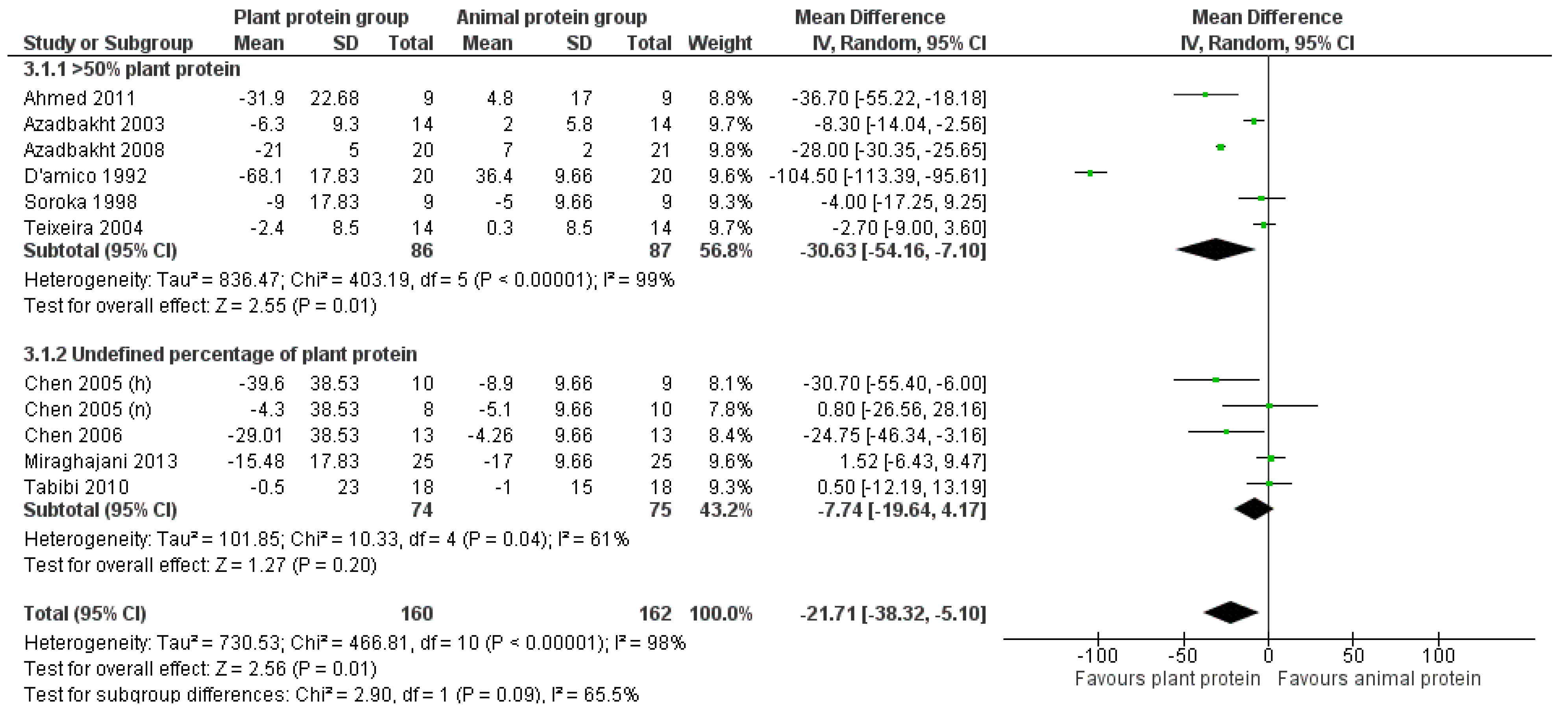
3.2.3. LDL Cholesterol
3.2.4. HDL Cholesterol
3.2.5. Apolipoprotein A
3.2.6. Apolipoprotein B
4. Publication Bias
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414–422d. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global health estimates: Leading causes of death. Cause-specific mortality, 2010. In Global Health Observatory; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Jadoul, M.; Aoun, M.; Masimango Imani, M. The major global burden of chronic kidney disease. Lancet Glob. Health 2024, 12, e342–e343. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Mitesh, S.; Gkogkou, A.; Geladari, E. Chronic Kidney Disease and Cardiovascular Disease: Is there Any Relationship? Curr. Cardiol. Rev. 2019, 15, 55–63. [Google Scholar] [CrossRef]
- Matsushita, K.; van der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef]
- Bello, A.K.; Alrukhaimi, M.; Ashuntantang, G.E.; Basnet, S.; Rotter, R.C.; Douthat, W.G.; Kazancioglu, R.; Köttgen, A.; Nangaku, M.; Powe, N.R.; et al. Complications of chronic kidney disease: Current state, knowledge gaps, and strategy for action. Kidney Int. Suppl. 2017, 7, 122–129. [Google Scholar] [CrossRef]
- Cho, G.Y. Diastolic dysfunction and chronic kidney disease. Korean J. Intern. Med. 2013, 28, 22–24. [Google Scholar] [CrossRef]
- Calabresi, L.; Simonelli, S.; Conca, P.; Busnach, G.; Cabibbe, M.; Gesualdo, L.; Gigante, M.; Penco, S.; Veglia, F.; Franceschini, G. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J. Intern. Med. 2015, 277, 552–561. [Google Scholar] [CrossRef]
- Bulbul, M.C.; Dagel, T.; Afsar, B.; Ulusu, N.N.; Kuwabara, M.; Covic, A.; Kanbay, M. Disorders of Lipid Metabolism in Chronic Kidney Disease. Blood Purif. 2018, 46, 144–152. [Google Scholar] [CrossRef]
- Moradi, H.; Pahl, M.V.; Elahimehr, R.; Vaziri, N.D. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl. Res. 2009, 153, 77–85. [Google Scholar] [CrossRef]
- Ćwiklińska, A.; Cackowska, M.; Wieczorek, E.; Król, E.; Kowalski, R.; Kuchta, A.; Kortas-Stempak, B.; Gliwińska, A.; Dąbkowski, K.; Zielińska, J.; et al. Progression of Chronic Kidney Disease Affects HDL Impact on Lipoprotein Lipase (LPL)-Mediated VLDL Lipolysis Efficiency. Kidney Blood Press. Res. 2018, 43, 970–978. [Google Scholar] [CrossRef]
- Tsimihodimos, V.; Mitrogianni, Z.; Elisaf, M. Dyslipidemia associated with chronic kidney disease. Open Cardiovasc. Med. J. 2011, 5, 41–48. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Su, J.; Tian, F. The effects of soy protein on chronic kidney disease: A meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2014, 68, 987–993. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; De Zeeuw, D.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef]
- Chen, S.T.; Ferng, S.H.; Yang, C.S.; Peng, S.J.; Lee, H.R.; Chen, J.R. Variable effects of soy protein on plasma lipids in hyperlipidemic and normolipidemic hemodialysis patients. Am. J. Kidney Dis. 2005, 46, 1099–1106. [Google Scholar] [CrossRef]
- Chen, S.T.; Chen, J.R.; Yang, C.S.; Peng, S.J.; Feng, S.H. Effect of soya protein on serum lipid profile and lipoprotein concentrations in patients undergoing hypercholesterolaemic haemodialysis. Br. J. Nutr. 2006, 95, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Miraghajani, M.S.; Najafabadi, M.M.; Surkan, P.J.; Esmaillzadeh, A.; Mirlohi, M.; Azadbakht, L. Soy milk consumption and blood pressure among type 2 diabetic patients with nephropathy. J. Ren. Nutr. 2013, 23, 277–282.e271. [Google Scholar] [CrossRef] [PubMed]
- Soroka, N.; Silverberg, D.S.; Greemland, M.; Birk, Y.; Blum, M.; Peer, G.; Iaina, A. Comparison of a vegetable-based (soya) and an animal-based low-protein diet in predialysis chronic renal failure patients. Nephron 1998, 79, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Anderson, J.W.; Blake, J.E.; Turner, J.; Smith, B.M. Effects of soy protein on renal function and proteinuria in patients with type 2 diabetes. Am. J. Clin. Nutr. 1998, 68, 1347s–1353s. [Google Scholar] [CrossRef]
- Azadbakht, L.; Shakerhosseini, R.; Atabak, S.; Jamshidian, M.; Mehrabi, Y.; Esmaill-Zadeh, A. Beneficiary effect of dietary soy protein on lowering plasma levels of lipid and improving kidney function in type II diabetes with nephropathy. Eur. J. Clin. Nutr. 2003, 57, 1292–1294. [Google Scholar] [CrossRef]
- Teixeira, S.R.; Tappenden, K.A.; Carson, L.; Jones, R.; Prabhudesai, M.; Marshall, W.P.; Erdman, J.W., Jr. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J. Nutr. 2004, 134, 1874–1880. [Google Scholar] [CrossRef]
- D’Amico, G.; Gentile, M.G.; Manna, G.; Fellin, G.; Ciceri, R.; Cofano, F.; Petrini, C.; Lavarda, F.; Perolini, S.; Porrini, M. Effect of vegetarian soy diet on hyperlipidaemia in nephrotic syndrome. Lancet 1992, 339, 1131–1134. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Calabria, A.C.; Kirsztajn, G.M. Short-term effects of soy protein diet in patients with proteinuric glomerulopathies. J. Bras. Nefrol. 2011, 33, 150–159. [Google Scholar] [CrossRef]
- Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: A longitudinal randomized clinical trial. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Tabibi, H.; Imani, H.; Hedayati, M.; Atabak, S.; Rahmani, L. Effects of soy consumption on serum lipids and apoproteins in peritoneal dialysis patients: A randomized controlled trial. Perit. Dial. Int. 2010, 30, 611–618. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Li, S.S.; Blanco Mejia, S.; Lytvyn, L.; Stewart, S.E.; Viguiliouk, E.; Ha, V.; de Souza, R.J.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; et al. Effect of Plant Protein on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2017, 6, e006659. [Google Scholar] [CrossRef]
- Anderson, J.W.; Bush, H.M. Soy Protein Effects on Serum Lipoproteins: A Quality Assessment and Meta-Analysis of Randomized, Controlled Studies. J. Am. Coll. Nutr. 2011, 30, 79–91. [Google Scholar] [CrossRef]
- Zhao, H.; Song, A.; Zheng, C.; Wang, M.; Song, G. Effects of plant protein and animal protein on lipid profile, body weight and body mass index on patients with hypercholesterolemia: A systematic review and meta-analysis. Acta Diabetol. 2020, 57, 1169–1180. [Google Scholar] [CrossRef]
- Barańska, A.; Błaszczuk, A.; Kanadys, W.; Baczewska, B.; Jędrych, M.; Wawryk-Gawda, E.; Polz-Dacewicz, M. Effects of Soy Protein Containing of Isoflavones and Isoflavones Extract on Plasma Lipid Profile in Postmenopausal Women as a Potential Prevention Factor in Cardiovascular Diseases: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2531. [Google Scholar] [CrossRef]
- Harland, J.I.; Haffner, T.A. Systematic review, meta-analysis and regression of randomised controlled trials reporting an association between an intake of circa 25 g soya protein per day and blood cholesterol. Atherosclerosis 2008, 200, 13–27. [Google Scholar] [CrossRef]
- Taku, K.; Umegaki, K.; Sato, Y.; Taki, Y.; Endoh, K.; Watanabe, S. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007, 85, 1148–1156. [Google Scholar] [CrossRef]
- Moradi, M.; Daneshzad, E.; Azadbakht, L. The effects of isolated soy protein, isolated soy isoflavones and soy protein containing isoflavones on serum lipids in postmenopausal women: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 3414–3428. [Google Scholar] [CrossRef]
- Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Kanadys, W.; Malm, M.; Janiszewska, M.; Jędrych, M. Effects of Soy Isoflavones on Glycemic Control and Lipid Profile in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 1886. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Khalil, D.A.; Payton, M.E.; Arjmandi, B.H. One-year soy protein supplementation does not improve lipid profile in postmenopausal women. Menopause 2010, 17, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Matthan, N.R.; Jalbert, S.M.; Ausman, L.M.; Kuvin, J.T.; Karas, R.H.; Lichtenstein, A.H. Effect of soy protein from differently processed products on cardiovascular disease risk factors and vascular endothelial function in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 960–966. [Google Scholar] [CrossRef]
- McVeigh, B.L.; Dillingham, B.L.; Lampe, J.W.; Duncan, A.M. Effect of soy protein varying in isoflavone content on serum lipids in healthy young men. Am. J. Clin. Nutr. 2006, 83, 244–251. [Google Scholar] [CrossRef]
- Najjar, R.S.; Moore, C.E.; Montgomery, B.D. Consumption of a defined, plant-based diet reduces lipoprotein(a), inflammation, and other atherogenic lipoproteins and particles within 4 weeks. Clin. Cardiol. 2018, 41, 1062–1068. [Google Scholar] [CrossRef]
- Pipe, E.A.; Gobert, C.P.; Capes, S.E.; Darlington, G.A.; Lampe, J.W.; Duncan, A.M. Soy protein reduces serum LDL cholesterol and the LDL cholesterol:HDL cholesterol and apolipoprotein B:apolipoprotein A-I ratios in adults with type 2 diabetes. J. Nutr. 2009, 139, 1700–1706. [Google Scholar] [CrossRef]
- Dominguez, J.H.; Tang, N.; Xu, W.; Evan, A.P.; Siakotos, A.N.; Agarwal, R.; Walsh, J.; Deeg, M.; Pratt, J.H.; March, K.L.; et al. Studies of renal injury III: Lipid-induced nephropathy in type II diabetes. Kidney Int. 2000, 57, 92–104. [Google Scholar] [CrossRef]
- Joles, J.A.; Kunter, U.; Janssen, U.; Kriz, W.; Rabelink, T.J.; Koomans, H.A.; Floege, J. Early mechanisms of renal injury in hypercholesterolemic or hypertriglyceridemic rats. J. Am. Soc. Nephrol. 2000, 11, 669–683. [Google Scholar] [CrossRef]
- Kim, H.J.; Moradi, H.; Yuan, J.; Norris, K.; Vaziri, N.D. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am. J. Physiol. Renal Physiol. 2009, 296, F1297–F1306. [Google Scholar] [CrossRef]
- Lee, H.S. Mechanisms and consequences of hypertriglyceridemia and cellular lipid accumulation in chronic kidney disease and metabolic syndrome. Histol. Histopathol. 2011, 26, 1599–1610. [Google Scholar] [CrossRef]
- Jung, H.N.; Huh, J.H.; Roh, E.; Han, K.D.; Kang, J.G.; Lee, S.J.; Ihm, S.H. High remnant-cholesterol levels increase the risk for end-stage renal disease: A nationwide, population-based, cohort study. Lipids Health Dis. 2024, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Pontremoli, R.; Desideri, G.; Arca, M.; Temporelli, P.L.; Perrone, V.; Dovizio, M.; Borghi, C.; Esposti, L.D. Hypertriglyceridemia is associated with decline of estimated glomerular filtration rate and risk of end-stage kidney disease in a real-word Italian cohort: Evidence from the TG-RENAL Study. Eur. J. Intern. Med. 2023, 111, 90–96. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, D.K.; Oh, K.H.; Joo, K.W.; Lim, C.S.; Kim, Y.S.; Han, S.S. Apolipoprotein B is a risk factor for end-stage renal disease. Clin. Kidney J. 2021, 14, 617–623. [Google Scholar] [CrossRef]
- Fanti, P.; Stephenson, T.J.; Kaariainen, I.M.; Rezkalla, B.; Tsukamoto, Y.; Morishita, T.; Nomura, M.; Kitiyakara, C.; Custer, L.J.; Franke, A.A. Serum isoflavones and soya food intake in Japanese, Thai and American end-stage renal disease patients on chronic haemodialysis. Nephrol. Dial. Transplant. 2003, 18, 1862–1868. [Google Scholar] [CrossRef][Green Version]
- Wong, J.S.; Port, F.K.; Hulbert-Shearon, T.E.; Carroll, C.E.; Wolfe, R.A.; Agodoa, L.Y.; Daugirdas, J.T. Survival advantage in Asian American end-stage renal disease patients. Kidney Int. 1999, 55, 2515–2523. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and Western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef]
- Jing, Z.; Wei-Jie, Y. Effects of soy protein containing isoflavones in patients with chronic kidney disease: A systematic review and meta-analysis. Clin. Nutr. 2016, 35, 117–124. [Google Scholar] [CrossRef]
- Siefker, K.; DiSilvestro, R.A. Safety and antioxidant effects of a modest soy protein intervention in hemodialysis patients. J. Med. Food 2006, 9, 368–372. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. Suppl. 2008, 74, S4–S9. [Google Scholar] [CrossRef]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.L.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J.B. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef]
- Hewitson, T.D.; Holt, S.G.; Smith, E.R. Progression of Tubulointerstitial Fibrosis and the Chronic Kidney Disease Phenotype—Role of Risk Factors and Epigenetics. Front. Pharmacol. 2017, 8, 520. [Google Scholar] [CrossRef]
- Javanbakht, M.H.; Sadria, R.; Djalali, M.; Derakhshanian, H.; Hosseinzadeh, P.; Zarei, M.; Azizi, G.; Sedaghat, R.; Mirshafiey, A. Soy protein and genistein improves renal antioxidant status in experimental nephrotic syndrome. Nefrologia 2014, 34, 483–490. [Google Scholar] [CrossRef]
- Jheng, H.F.; Hirotsuka, M.; Goto, T.; Shibata, M.; Matsumura, Y.; Kawada, T. Dietary low-fat soy milk powder retards diabetic nephropathy progression via inhibition of renal fibrosis and renal inflammation. Mol. Nutr. Food Res. 2017, 61, 1600461. [Google Scholar] [CrossRef]
- Guolo, A.; Varin, C. Random-effects meta-analysis: The number of studies matters. Stat. Methods Med. Res. 2017, 26, 1500–1518. [Google Scholar] [CrossRef]
- von Hippel, P.T. The heterogeneity statistic I2 can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef]
- Zhan, S.; Ho, S.C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar] [CrossRef]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-Analysis of the Effects of Soy Protein Intake on Serum Lipids. New Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef]
- Herreman, L.; Nommensen, P.; Pennings, B.; Laus, M.C. Comprehensive overview of the quality of plant- And animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020, 8, 5379–5391. [Google Scholar] [CrossRef]
- van den Berg, L.A.; Mes, J.J.; Mensink, M.; Wanders, A.J. Protein quality of soy and the effect of processing: A quantitative review. Front. Nutr. 2022, 9, 1004754. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Hagström, E.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Danchin, N.; Diaz, R.; Goodman, S.G.; Harrington, R.A.; Jukema, J.W.; et al. Apolipoprotein B, Residual Cardiovascular Risk After Acute Coronary Syndrome, and Effects of Alirocumab. Circulation 2022, 146, 657–672. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaodyssea, I.; Lagiou, A.; Tzoulaki, I.; Valanou, E.; Naska, A. The Effect of Increased Plant Protein Intake on the Lipid Profile of Chronic Kidney Disease Patients: A Meta-Analysis of Controlled Clinical Trials. Nutrients 2025, 17, 1408. https://doi.org/10.3390/nu17091408
Papaodyssea I, Lagiou A, Tzoulaki I, Valanou E, Naska A. The Effect of Increased Plant Protein Intake on the Lipid Profile of Chronic Kidney Disease Patients: A Meta-Analysis of Controlled Clinical Trials. Nutrients. 2025; 17(9):1408. https://doi.org/10.3390/nu17091408
Chicago/Turabian StylePapaodyssea, Ioanna, Areti Lagiou, Ioanna Tzoulaki, Elisavet Valanou, and Androniki Naska. 2025. "The Effect of Increased Plant Protein Intake on the Lipid Profile of Chronic Kidney Disease Patients: A Meta-Analysis of Controlled Clinical Trials" Nutrients 17, no. 9: 1408. https://doi.org/10.3390/nu17091408
APA StylePapaodyssea, I., Lagiou, A., Tzoulaki, I., Valanou, E., & Naska, A. (2025). The Effect of Increased Plant Protein Intake on the Lipid Profile of Chronic Kidney Disease Patients: A Meta-Analysis of Controlled Clinical Trials. Nutrients, 17(9), 1408. https://doi.org/10.3390/nu17091408








