Quercetin and Its Lecithin-Based Formulation: Potential Applications for Allergic Diseases Based on a Narrative Review
Abstract
1. Introduction
2. Methods
3. Pharmacokinetics of Quercetin
4. Quercetin and the Potential Effect on Allergic Disease
5. Quercetin and Allergic Diseases: In Vitro Studies
6. Quercetin and Allergic Diseases: Animal Studies
7. In Vivo Studies: Multicomponent Nutraceuticals (Containing Quercetin Among Ingredients) Studies on Humans
8. In Vivo Studies: Single-Component Nutraceuticals (Containing Only Quercetin) Studies on Humans: A New Formulation
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercitin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 243S–255S. [Google Scholar] [CrossRef]
- Jafarinia, M.; Sadat Hosseini, M.; Kasiri, N.; Fazel, N.; Fathi, F.; Ganjalikhani Hakemi, M.; Eskandari, N. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods; Release 3.1; US Department of Agriculture: Beltsville, MD, USA, 2014. Available online: https://www.ars.usda.gov/ARSUserFiles/80400535/Data/Flav/Flav_R03-1.pdf (accessed on 10 April 2025).
- Terao, J. Potential Role of Quercetin Glycosides as Anti-Atherosclerotic Food-Derived Factors for Human Health. Antioxidants 2023, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.H.; Liu, G.R.; Liu, C.; Dong, Y.M. Isoquercitrin suppresses the expression of histamine and pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells. Chin. J. Nat. Med. 2016, 14, 407–412. [Google Scholar] [CrossRef]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.Z.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef]
- Ding, Y.; Che, D.; Li, C.; Cao, J.; Wang, J.; Ma, P.; Zhao, T.; An, H.; Zhang, T. Quercetin inhibits Mrgprx2-induced pseudo-allergic reaction via PLCγ-IP3R related Ca2+ fluctuations. Int. Immunopharmacol. 2019, 66, 185–197. [Google Scholar] [CrossRef]
- Ding, Y.; Li, C.; Zhang, Y.; Ma, P.; Zhao, T.; Che, D.; Cao, J.; Wang, J.; Liu, R.; Zhang, T.; et al. Quercetin as a Lyn kinase inhibitor inhibits IgE-mediated allergic conjunctivitis. Food Chem. Toxicol. 2020, 135, 110924. [Google Scholar] [CrossRef]
- Cotin, S.; Calliste, C.A.; Mazeron, M.C.; Hantz, S.; Duroux, J.L.; Rawlinson, W.D.; Ploy, M.C.; Alain, S. Eight flavonoids and their potential as inhibitors of human cytomegalovirus replication. Antiviral Res. 2012, 96, 181–186. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2016, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Bachmetov, L.; Gal-Tanamy, M.; Shapira, A.; Vorobeychik, M.; Giterman-Galam, T.; Sathiyamoorthy, P.; Golan-Goldhirsh, A.; Benhar, I.; Tur-Kaspa, R.; Zemel, R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J. Viral Hepat. 2012, 19, e81–e88. [Google Scholar] [CrossRef] [PubMed]
- Kaul, R.; Paul, P.; Kumar, S.; Büsselberg, D.; Dwivedi, V.D.; Chaari, A. Promising Antiviral Activities of Natural Flavonoids against SARS-CoV-2 Targets: Systematic Review. Int. J. Mol. Sci. 2021, 22, 11069. [Google Scholar] [CrossRef]
- Wei, Q.; Jiang, H.; Zeng, J.; Xu, J.; Zhang, H.; Xiao, E.; Lu, Q.; Huang, G. Quercetin protected the gut barrier in ulcerative colitis by activating aryl hydrocarbon receptor. Phytomedicine 2025, 140, 156633. [Google Scholar] [CrossRef] [PubMed]
- Manikanta, K.; NaveenKumar, S.K.; Hemshekhar, M.; Thushara, R.M.; Mugesh, G.; Kemparaju, K.; Girish, K.S. Quercetin inhibits platelet activation and ER-stress mediated autophagy in response to extracellular histone. Phytomedicine 2025, 138, 156386. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Das, D.; Banerjee, A.; Mukherjee, S.; Maji, B.K. Quercetin inhibits NF-κB and JAK/STAT signaling via modulating TLR in thymocytes and splenocytes during MSG-induced immunotoxicity: An in vitro approach. Mol. Biol. Rep. 2024, 51, 277. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, X.; Zhu, G.; Liu, H.; Chen, J.; Wang, Y.; He, X. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-κB and AP-1 signaling pathway in vitro. Medicine 2020, 99, e22241. [Google Scholar] [CrossRef]
- Rajizadeh, M.A.; Bejeshk, M.A.; Doustimotlagh, A.H.; Najafipour, H.; Eftekhari, M.; Mahmoodi, M.; Azizi, M.; Rostamabadi, F.; Pourghadamyari, H. The alleviating impacts of quercetin on inflammation and oxidant-antioxidant imbalance in rats with allergic asthma. Iran J. Allergy Asthma Immunol. 2023, 22, 138–149. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Manach, C. Part of Quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am. J. Physiol. 1999, 277, G120–G126. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Texier, O.; Favier, M.L.; Agullo, G.; Demigné, C.; Régérat, F.; Rémésy, C. Quercetin metabolites in plasma of rats fed diets containing rutin or Quercetin. J. Nutr. 1995, 125, 1911–1922. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercitin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Katan, M.B. Absorption, metabolism and bioavailability of flavonoids. In Flavonoids in Health and Disease; Rice-Evans, C.A., Packer, L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1998; pp. 483–522. [Google Scholar]
- Day, A.J.; Bao, Y.; Morgan, M.R.; Williamson, G. Conjugation position of Quercetin glucuronides and effect on biological activity. Free Radic. Biol. Med. 2000, 29, 1234–1243. [Google Scholar] [CrossRef]
- Manach, C.; Texier, O.; Morand, C.; Crespy, V.; Régérat, F.; Demigné, C.; Rémésy, C. Comparison of the bioavailability of quercetin and catechin in rats. Free Radic. Biol. Med. 1999, 27, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, G.; Wang, X.; Liu, F.; Xu, Y.; Ma, J. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int. J. Pharm. 2011, 404, 231–237. [Google Scholar] [CrossRef]
- Liu, L.; Barber, E.; Kellow, N.J.; Williamson, G. Improving quercetin bioavailability: A systematic review and meta-analysis of human intervention studies. Food Chem. 2025, 477, 143630. [Google Scholar] [CrossRef]
- Schoettler, N. Advances in asthma and allergic disease genetics. Curr. Opin. Allergy Clin. Immunol. 2025, 25, 58–65. [Google Scholar] [CrossRef]
- Kopp, E.B.; Agaronyan, K.; Licona-Limón, I.; Nish, S.A.; Medzhitov, R. Modes of type 2 immune response initiation. Immunity 2023, 56, 687–694. [Google Scholar] [CrossRef]
- Ciprandi, G. Self-Management in Allergic Rhinitis: Strategies, Outcomes and Integration into Clinical Care. J. Asthma Allergy 2023, 16, 1087–1095. [Google Scholar] [CrossRef]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Marozkina, N. Ciliary Function, Antigen Stasis and Asthma. Int. J. Mol. Sci. 2024, 25, 10043. [Google Scholar] [CrossRef] [PubMed]
- Johri, R.; Zutshi, U.; Kameshwaran, L.; Atal, C. Effect of quercetin and albizzia saponins on rat mast cell. Ind. J. Physiol. Pharm. 1984, 29, 43–46. [Google Scholar]
- Chirumbolo, S. Quercetin as a potential anti-allergic drug: Which perspectives? Iran. J. Allergy Asthma Immunol. 2011, 10, 139–140. [Google Scholar]
- Fewtrell, C.; Gomperts, B. Quercetin: A novel inhibitors of Ca2+ influx and exocytosis in rat peritoneal mast cells. Bioch. Biophys. Acta 1977, 469, 52–60. [Google Scholar] [CrossRef]
- Kimata, M.; Shichijo, M.; Miura, T.; Serizawa, I.; Inagaki, N.; Nagai, H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy 2000, 30, 501–508. [Google Scholar] [CrossRef]
- Shaik, Y.; Castellani, M.; Perrella, A.; Conti, F.; Salini, V.; Tete, S.; Madhappan, B.; Vecchiet, J.; de Lutiis, M.; Caraffa, A.; et al. Role of quercetin (a natural herbal compound) in allergy and inflammation. J. Biol. Reg. Homeost. Agents 2005, 20, 47–52. [Google Scholar]
- Park, H.J.; Lee, C.M.; Jung, I.D.; Lee, J.S.; Jeong, Y.I.; Chang, J.H.; Chun, S.H.; Kim, M.J.; Choi, I.W.; Ahn, S.C.; et al. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int. Immunopharmacol. 2009, 9, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets 2010, 9, 263–285. [Google Scholar] [CrossRef]
- Kumazawa, Y.; Takimoto, H.; Matsumoto, T.; Kawaguchi, K. Potential use of dietary natural products, especially polyphenols, for improving type-1 allergic symptoms. Curr. Pharm. Design 2014, 20, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and Its Anti-Allergic Immune Response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Edo, Y.; Otaki, A.; Asano, K. Quercetin enhances the thioredoxin production of nasal epithelial cells in vitro and in vivo. Medicines 2018, 5, 124. [Google Scholar] [CrossRef]
- Irie, S.; Kashiwabara, M.; Yamada, A.; Asano, K. Suppressive Activity of quercetin on Periostin Functions In Vitro. In vivo 2016, 30, 17–25. [Google Scholar]
- Ebihara, N.; Takahashi, K.; Takemura, H.; Akanuma, Y.; Asano, K.; Sunagawa, M. Suppressive Effect of quercetin on Nitric Oxide Production from Nasal Epithelial Cells In Vitro. Evid. Based Complement Altern. Med. 2018, 2018, 6097625. [Google Scholar] [CrossRef]
- Luo, X.; Xue, L.; Xu, H.; Zhao, Q.Y.; Wang, Q.; She, Y.S.; Zang, D.A.; Shen, J.; Peng, Y.B.; Zhao, P.; et al. Polygonum aviculare L. extract and quercetin attenuate contraction in airway smooth muscle. Sci. Rep. 2018, 8, 3114. [Google Scholar] [CrossRef]
- Otaki, A.; Furuta, A.; Asano, K. Quercetin-Induced Enhancement of Nasal Epithelial Cells’ Ability to Produce Clara Cell 10-kD Protein In Vitro and In Vivo. Medicines 2023, 10, 28. [Google Scholar] [CrossRef]
- Tanaka, Y.; Furuta, A.; Asano, K.; Kobayashi, H. Modulation of Th1/Th2 Cytokine Balance by quercetin In Vitro. Medicines 2020, 7, 46. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Kashiwabara, M.; Asano, K.; Mizuyoshi, T.; Kobayashi, H. Suppression of neuropeptide production by quercetin in allergic rhinitis model rats. BMC Complement Altern Med. 2016, 16, 132. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Zhou, L.; Shi, L.; Liu, T.; Guo, Y.; Chen, H.; Luo, H.; Ma, J.; Zhang, H.; Xiong, P.; et al. Quercetin-crosslinked chitosan nanoparticles: A potential treatment for allergic rhinitis. Sci. Rep. 2024, 14, 4021. [Google Scholar] [CrossRef]
- Sagit, M.; Polat, H.; Gurgen, S.G.; Berk, E.; Guler, S.; Yasar, M. Effectiveness of quercetin in an experimental rat model of allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2017, 274, 3087–3095. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Ziqi, C.; Xiaoqiang, W.; Houyong, K.; Suling, H. Quercetin improves the imbalance of Th1/Th2 cells and Treg/Th17 cells to attenuate allergic rhinitis. Autoimmunity 2023, 56, 2189133. [Google Scholar] [CrossRef]
- Ma, J.; Tong, P.; Chen, Q.; Liu, J.; Li, H.; Long, F. Covalent conjugation with polyphenol reduced the sensitization of walnut and ameliorated allergy by enhancing intestinal epithelial barrier in mice. Food Chem. 2024, 439, 138191. [Google Scholar] [CrossRef]
- Sozmen, S.C.; Karaman, M.; Micili, S.C.; Isik, S.; Bagriyanik, A.; Ayyildiz, Z.A.; Uzuner, N.; Anal, O.; Karaman, O. Effects of quercetin treatment on epithelium-derived cytokines and epithelial cell apoptosis in allergic airway inflammation mice model. Iran J. Allergy Asthma Immunol. 2016, 15, 487–497. [Google Scholar]
- Hu, G.; Walls, R.S.; Bass, D.; Ramon, B.; Grayson, D.; Jones, M.; Gebski, V. The Chinese herbal formulation biminne in management of perennial allergic rhinitis: A randomized, double-blind, placebo-controlled, 12-week clinical trial. Ann. Allergy Asthma Immunol. 2002, 88, 478–487. [Google Scholar] [CrossRef]
- Arpornchayanon, W.; Klinprung, S.; Chansakaow, S.; Hanprasertpong, N.; Chaiyasate, S.; Tokuda, M.; Tamura, H. Antiallergic activities of shallot (Allium ascalonicum L.) and its therapeutic effects in allergic rhinitis. Asian Pac. J. Allergy Immunol. 2022, 40, 393–400. [Google Scholar] [CrossRef]
- Ariano, R. Efficacy of a novel food supplement in the relief of the signs and symptoms of seasonal allergic rhinitis and in the reduction of the consumption of anti-allergic drugs. Acta Biomed. 2015, 86, 53–58. [Google Scholar]
- Marseglia, G.L.; Licari, A.; Ciprandi, G.; Italian Study Group on Pediatric Allergic Rhinoconjunctivitis. A polycentric, randomized, double-blind, parallel-group, placebo-controlled study on Lertal®, a multicomponent nutraceutical, as add-on treatment in children with allergic rhinoconjunctivitis: Phase I during active treatment. J. Biol. Regul. Homeost. Agents 2019, 33, 617–622. [Google Scholar]
- Marseglia, G.; Licari, A.; Leonardi, S.; Papale, M.; Zicari, A.M.; Schiavi, L.; Ciprandi, G.; Italian Study Group on Pediatric Allergic Rhinoconjunctivitis. A polycentric, randomized, parallel-group, study on Lertal®, a multicomponent nutraceutical, as preventive treatment in children with allergic rhinoconjunctivitis: Phase II. Ital. J. Pediatr. 2019, 45, 84. [Google Scholar] [CrossRef] [PubMed]
- Tosca, M.A.; Olcese, R.; Marinelli, G.; Papale, M.; Zicari, A.M.; Marseglia, G.; Ciprandi, G. Lertal®, a multicomponent nutraceutical, could reduce the use of antihistamines in children with allergic rhinoconjunctivitis. Acta Biomed. 2020, 91, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, S.; Parisi, G.; Papale, M.; Zicari, A.M.; Olcese, R.; Licari, A.; Marseglia, G.; Ciprandi, G. Small airways in children with allergic rhinoconjunctivitis: The potential role of a multicomponent nutraceutical. Acta Biomed. 2020, 91, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Zicari, A.M.; De Castro, G.; Brindisi, G.; Papale, M.; Marinelli, G.; Licari, A.; Ciprandi, G. Respiratory infections in allergic children: The preventive role of a multicomponent nutraceutical. Acta Biomed. 2020, 91, e2020072. [Google Scholar] [CrossRef]
- Russo, V.; Stella, A.; Appezzati, L.; Barone, A.; Stagni, E.; Roszkowska, A.; Delle Noci, N. Clinical efficacy of a Ginkgo biloba extract in the topical treatment of allergic conjunctivitis. Eur. J. Ophthalmol. 2009, 19, 331–336. [Google Scholar] [CrossRef]
- Remberg, P.; Björk, L.; Hedner, T.; Sterner, O. Characteristics, clinical effect profile and tolerability of a nasal spray preparation of Artemisia abrotanum L. for allergic rhinitis. Phytomedicine 2004, 11, 36–42. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Q.B.; Jing, W. Astragalus membranaceus improves therapeutic efficacy of asthmatic children by regulating the balance of Treg/Th17 cells. Chin. J. Nat. Med. 2019, 17, 252–263. [Google Scholar] [CrossRef]
- Marseglia, G.L.; Cirillo, I.; Vizzaccaro, A.; Klersy, C.; Tosca, M.A.; La Rosa, M.; Ciprandi, G. Role of FEF25-75 as early marker of small airways impairment in subjects with allergic rhinitis. Allergy Asthma Proc. 2007, 28, 74–78. [Google Scholar] [CrossRef]
- Yamada, S.; Shirai, M.; Inaba, Y.; Takara, T. Effects of repeated oral intake of a quercetin-containing supplement on allergic reaction: A randomized, placebo-controlled, double-blind parallel-group study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4331–4345. [Google Scholar] [CrossRef]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved oral absorption of quercetin from quercetin phytosome®, a new delivery system based on food grade lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Cesarone, M.R.; Belcaro, G.; Hu, S.; Dugall, M.; Hosoi, M.; Ledda, A.; Feragalli, B.; Maione, C.; Cotellese, R. Supplementary prevention and management of asthma with quercetin phytosome: A pilot registry. Minerva Med. 2019, 110, 524–529. [Google Scholar] [CrossRef]
- Riva, A.; Vitale, J.A.; Belcaro, G.; Hu, S.; Feragalli, B.; Vinciguerra, G.; Cacchio, M.; Bonanni, E.; Giacomelli, L.; Eggenhöffner, R.; et al. Quercetin phytosome® in triathlon athletes: A pilot registry study. Minerva Med. 2018, 109, 285–289. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.; Scipione, V.; Scipione, M.; Dugall, S.; Hu, B.; Feragalli, M.; Hosoi, C.; Maione, R.; Cotellese, R.; et al. Quercetin phytosome reduces the wheal response to histamine injection. Esperienze Dermatol. 2020, 22, 5–9. [Google Scholar] [CrossRef]
- Di Pede, G.; Bresciani, L.; Calani, L.; Petrangolini, G.; Riva, A.; Allegrini, P.; Del Rio, D.; Mena, P. The human microbial metabolism of quercetin in different formulations: An in vitro evaluation. Foods 2020, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Sultana, R.; Eusaph, A.Z.; Abrar, S.; Bugti, M.; Afridi, F.; Farooq, U.; Iqtadar, S.; Ghauri, F.; Makhduma, S.; et al. Effect of berberine phytosome on reproductive, dermatologic, and metabolic characteristics in women with polycystic ovary syndrome: A controlled, randomized, multi-centric, open-label clinical trial. Front. Pharmacol. 2023, 14, 1269605. [Google Scholar] [CrossRef]
- Petrangolini, G.; Ronchi, M.; Frattini, E.; De Combarieu, E.; Allegrini, P.; Riva, A. A new food-grade coenzyme Q10 formulation improves bioavailability: Single and repeated pharmacokinetic studies in healthy volunteers. Curr. Drug Deliv. 2019, 16, 759–767. [Google Scholar] [CrossRef]
- Bano, A.; Hepsomali, P.; Rabbani, F.; Farooq, U.; Kanwal, A.; Saleem, A.; Bugti, A.A.; Khan, A.A.; Khalid, Z.; Bugti, M.; et al. The possible “calming effect” of subchronic supplementation of a standardized phospholipid carrier-based Melissa officinalis L. extract in healthy adults with emotional distress and poor sleep conditions: Results from a prospective, randomized, double-blinded, placebo-controlled clinical trial. Front. Pharmacol. 2023, 14, 1250560. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin supplementation (Meriva®) modulates inflammation, lipid peroxidation and gut microbiota composition in chronic kidney disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef]
- Lee, S.; Yu, Y.; Trimpert, J.; Benthani, F.; Mairhofer, M.; Richter-Pechanska, P.; Wyler, E.; Belenki, D.; Kaltenbrunner, S.; Pammer, M.; et al. Virus-induced senescence is a driver and therapeutic target in COVID-19. Nature 2025, 638, E46. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Gasparri, C.; Perna, S. Two-month period of 500 mg lecithin-based delivery form of quercetin daily dietary supplementation counterbalances chronic fatigue symptoms: A double-blind placebo controlled clinical trial. Biomed. Pharmacother 2023, 167, 115453. [Google Scholar] [CrossRef]
- Miniati, I.; Davolio, P.L.; Busi, S.; Petrangolini, G.; Galluccio, F. Quercetin Phospholipids Supplementation as a Natural Relief in Osteomuscular Health. Phys. Med. Rehabil. Int. 2024, 11, 1245. [Google Scholar] [CrossRef]
- Riva, A.; Corti, A.; Belcaro, G.; Cesarone, M.R.; Dugall, M.; Vinciguerra, G.; Feragalli, B.; Zuccarini, M.; Eggenhoffner, R.; Giacomelli, L. Interaction study between antiplatelet agents, anticoagulants, diabetic therapy and a novel delivery form of quercetin. Minerva Cardioangiol. 2019, 67, 79–83. [Google Scholar] [CrossRef]
- Gamba, P. Evaluation of Quercetin Phytosome™ and Zinc in Supporting Subjects with Chronic Allergic and Non-Allergic Rhinitis. Austin J. Otolaryngol. 2024, 10, 1137. [Google Scholar] [CrossRef]
- Tosca, M.A.; Ferrecchi, C.; Meleca, V.; Naso, M.; Ciprandi, G. A multicomponent food supplement with quercetin Phytosome®, zinc and vitamin C may be favorable in managing children with seasonal allergic rhinitis: A pilot study. Minerva Pediatr. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Ciprandi, G.; Tosca, M.A.; Fasce, L. Allergic children have more numerous and severe respiratory infections than non-allergic children. Pediatr. Allergy Immunol. 2006, 17, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Varricchio, A.; La Mantia, I.; Brunese, F.P.; Ciprandi, G. Inflammation, infection, and allergy of upper airways: New insights from national and real-world studies. Ital. J. Pediatr. 2020, 46, 18. [Google Scholar] [CrossRef]
- Yun, K.W.; Wallihan, R.; Juergensen, A.; Mejias, A.; Ramilo, O. Community-Acquired Pneumonia in Children: Myths and Facts. Am. J. Perinatol. 2019, 36, S54–S57. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Bomberger, J.M. Viral-Bacterial Co-infections in the Cystic Fibrosis Respiratory Tract. Front. Immunol. 2018, 9, 3067. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef]
- Di Pierro, F.; Derosa, G.; Maffioli, P.; Bertuccioli, A.; Togni, S.; Riva, A.; Allegrini, P.; Khan, A.; Khan, S.; Khan, B.A.; et al. Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: A prospective, randomized, controlled, and open-label study. Int. J. Gen. Med. 2021, 14, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Iqtadar, S.; Khan, A.; Ullah Mumtaz, S.; Masud Chaudhry, M.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Potential clinical benefits of quercetin in the early stage of COVID-19: Results of a second, pilot, randomized, controlled, and open-label clinical trial. Int. J. Gen. Med. 2021, 14, 2807–2816. [Google Scholar] [CrossRef] [PubMed]
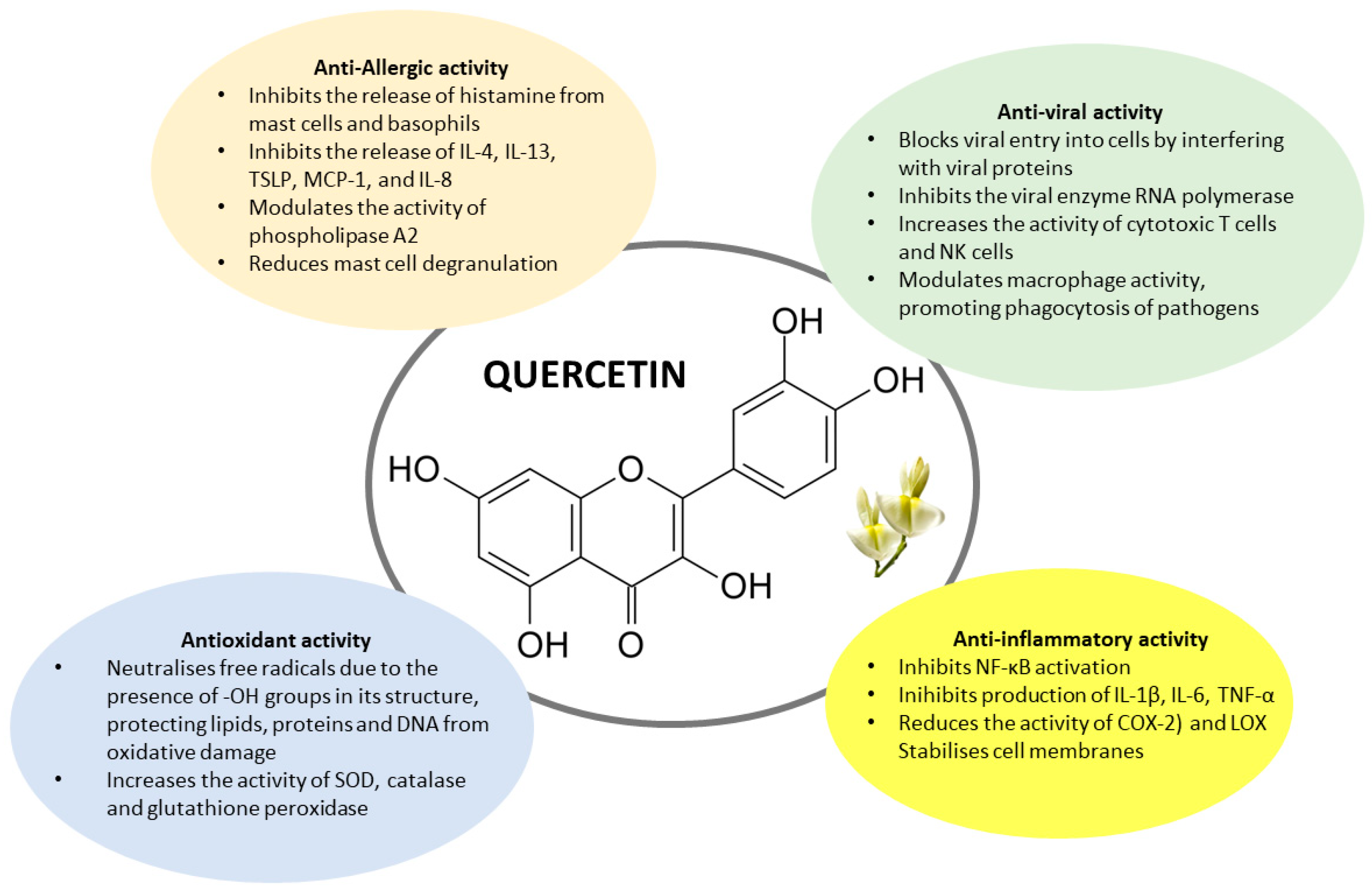
| Cell/Tissue/Species | Effect | Authors, Year [Reference] |
|---|---|---|
| IMMUNOMODULATION ACTIVITY | ||
| PMACI-stimulated human KU812 cells | Inhibition of histamine release and cytokines | Li et al., 2016 [7] |
| Human keratinocytes HaCat cells AD-like dermatitis | Inhibition of Chemokine and cytokines | Hou et al., 2019 [8] |
| LAD2 Human Mast Cells | Inhibition of Mrgprx2-induced pseudo-allergic reaction | Ding et al., 2019 [9] |
| LAD2 Human Mast Cells | Reduce MC degranulation and inhibits Lyn kinase | Ding et al., 2020 [10] |
| ANTIVIRAL ACTIVITY | ||
| Human Cytomegalovirus cell line | Inhibition of viral activity | Cotin et al., 2012 [11] |
| Influenza A virus strains | Inhibition of influenza A viral activity | Wu et al., 2016 [12] |
| Hepatitis C virus | Inhibition of hepatitis C viral activity | Bachmetov et al., 2012 [13] |
| SARS-CoV-2 | Inhibition of SARS-CoV-2 viral activity | Kaul et al., 2021 [14] |
| ANTIOXIDANT ACTIVITY | ||
| Co-culture of primary neutrophils | inhibited LPS-induced inflammatory | Wei et al., 2025 [15] |
| Mice platelets | Inhibition of platelet activation and Endoplasmic Reticulum-stress mediated autophagy | Manikanta et al., 2025 [16] |
| Review | Antioxidant activity | Xu et al., 2019 [17] |
| ANTI-INFLAMMATORY ACTIVITY | ||
| Thymocytes and splenocytes | Inhibition of NF-κB and JAK/STAT signaling | Das et al., 2024 [18] |
| HUVEC cells | Inhibition of TNF-α | Chen et al., 2020 [19] |
| Allergic Asthma rat model | Reduction of oxidative stress | Rajizadeh et al., 2023 [20] |
| Type of Cell, and Intervention | Dosage | Effect | Authors, Year [Reference] |
|---|---|---|---|
| HNEpC stimulated with H2O2 treated with QUE | 0.0, 0.1, 0.5, 1.0, 5.0, and 10 nM | QUE at concentrations greater than 1.0 nM induced significantly increased TRX levels in culture supernatants compared to those levels in the controls. | Edo et al., 2018 [47] |
| PMACI-stimulated human KU812 cells, incubated with isoQUE | 12.5, 25, or 50 μg·mL−1 | IsoQUE at 25 or 50 μg·mL−1 reduced the production of histamine and the pro-inflammatory cytokines (IL-6, IL-8, IL-1β, TNF-α) and suppressed MAPK and NF-κB. | Li et al. 2016 [7] |
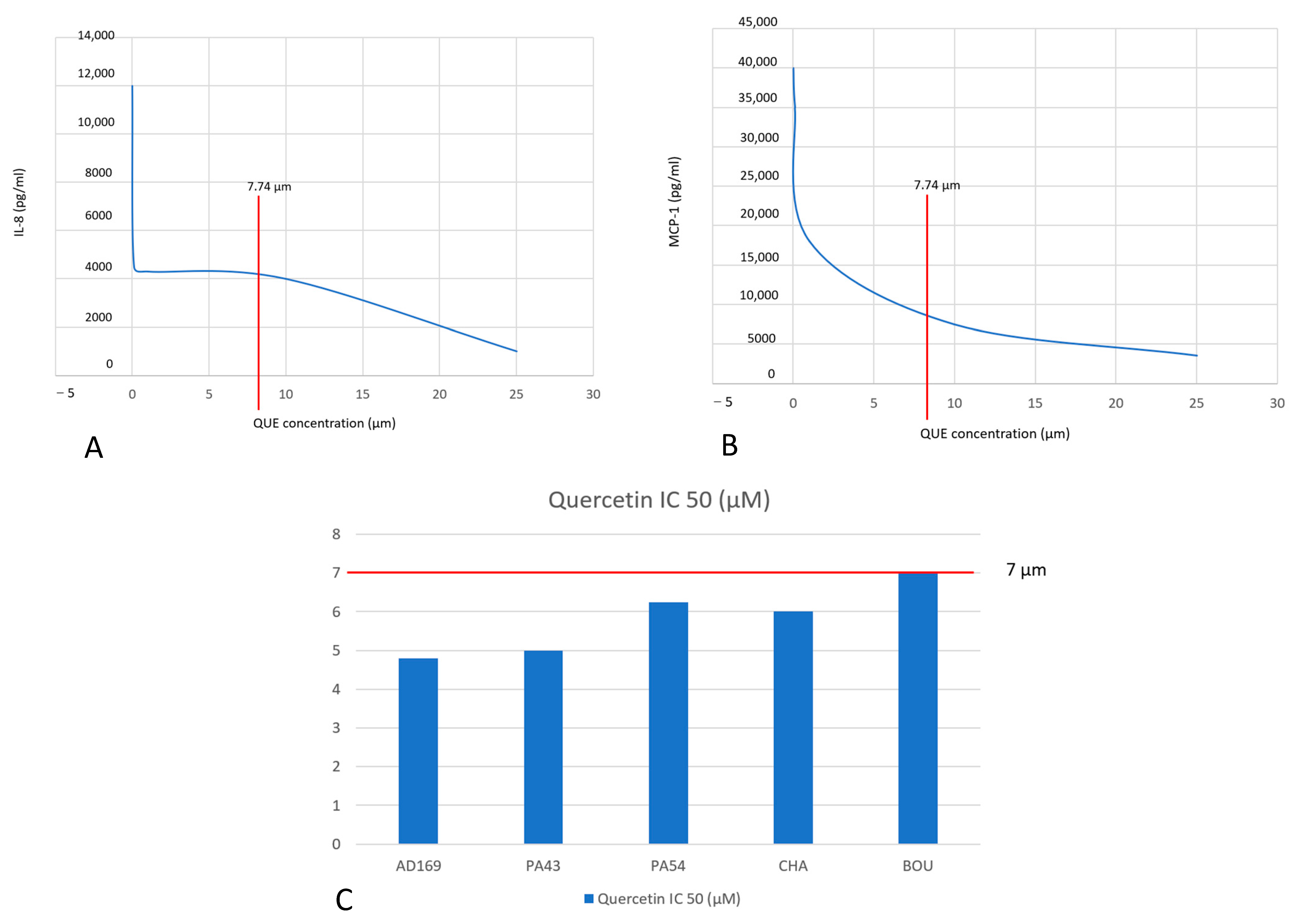
| LAD2 human MC, incubated with QUE | 0, 50, 100, 200, and 400 μM | QUE at greater concentration than 100 μM reduced Ca2+ fluxes and attenuated the release of β-hexosaminidase, histamine, MCP-1, and IL-8. | Ding et al., 2019 [9] |
| LAD2 human MC, incubated with QUE | 0, 25, 50, 100 μM | Reduced MC degranulation and inhibited Lyn kinase. | Ding et al., 2020 [10] |
| HNEpC stimulated with IL-4 in the presence of QUE | 0, 2, 4, 6, 8, 10 μM | QUE at a concentration of 4.0 μM suppressed the production of periostin in HNepC. | Irie et al., 2016 [48] |
| HNEpC stimulated with IL-4 in the presence of QUE | 100 pM, 1–25–50–100 nM, 1 µM | QUE at a concentration of 1 nM suppressed the production of NO in HNepC. | Ebihara et al., 2018 [49] |
| Contracted mice trachea treated with QUE | n.r. | QUE (IC 49.3 µM) attenuated contraction in the airway smooth muscle. | Luo et al., 2018 [50] |
| HNEpC stimulated with TNF-α, treated with QUE | 1.0, 2.5, 5.0 or 7.5 µM | QUE at 5.0 µM and higher dosages caused a significant increase in the ability ofHNEpCs to produce CC10 after TNF-α stimulation. | Otaki et al., 2023 [51] |
| T cell CD4+ stimulated with IL-4 and treated with QUE | 1.0–10.0 µM | QUE at a concentration of 5.0 µM suppressed IL-5 and IL-13 production through the suppression of transcription factor activation and cytokine mRNA expression, but abrogated the inhibitory action of IL-4 on INF-γ production. | Tanaka et al., 2020 [52] |
| Human keratinocites HaCat cells AD-like dermatitis, treated with QUE | n.r. | QUE could inhibit pro-inflammatory chemokines and cytokines. | Hou et al., 2019 [8] |
| Human keratinocites HaCat cells AD-like dermatitis, treated with QUE | 1.5 µM–25 µM | QUE at the concentration of 1.5 µM regulated expression of inflammatory mediators, supported the antioxidant defense system, promoted wound repair, and inhibited TSLP secretion. | Beken et al., 2020 [53] |
| Intervention | Concentration | Effect | Authors, Year [Reference] |
|---|---|---|---|
| QUE per os | 0, 10, 15, 20, and 25 mg/kg | QUE at a higher dosage than 20 mg/kg decreased lipid peroxide levels and increased TRX levels in the nasal lavage fluids of the mice. | Edo et al., 2018 [47] |
| QUE i.v. | 0, 1.0, 2.0, and 4.0 mg/mL | Reduce vasodilation and release of histamine and thickness of paw edema in dose-dependent manner. | Ding et al., 2019 [9] |
| QUE per os at low-medium-high dose for 7 days | 1.0, 2.0, and 4.0 mg/kg | Inhibition of secretion of IgE, Il4, Tnf-α. | Ding et al., 2020 [10] |
| QUE per os at different dosages for 5 days | 10, 20, 25, or 30 mg/kg | QUE at more than 25 mg/kg significantly increased CC10 levels in nasal lavage fluids | Otaki et al., 2023 [51] |
| Topical application of QUE | n.r | Improved AD lesions and reduced skin tissue level; of IFN-γ, CCL17, CCL22, TNF-α, IL-4, and IL-6. | Hou et al., 2019 [8] |
| QUE per os for 5 to 7 days | 10–30 mg/kg/day for 2–7 days | QUE reduced symptoms in mice AR during nasal challenge and inhibited the increase in SP, CGRP, and NGF contents in nasal lavage fluids induced by nasal challenge. | Kashiwabara et al., 2016 [54] |
| QUE nasal administration | n.r. | Decrease symptoms and mucosal levels of IgE, IL-17, TNF-α, and IL-6/ | Mu et al., 2024 [55] |
| IP QUE administration | 80 mg/kg/die | Lower specific IgE for OVA and decreased expression of COX-2 and VIP in the quercetin and nasal mometasone group. | Sagit et al., 2017 [56] |
| IP QUE for 2 weeks | 20–50 mg/kg/day for 13 days | QUE inhibited nasal symptoms of AR, promoted Th1/Th2 balance in the serum and NALF, inhibited inflammatory infltration, goblet cells, and eosinophils in nasal tissue and inhibited activation the NF-κB pathway. | Xia et al., 2023 [57] |
| Administratio of conjugated WP with QUE | n.r. | Conjugation of WP with quercetin reduced its allergenicity, even promoting Th1/Th2 and Treg/Th17 balance. | Ma et al., 2024 [58] |
| IP QUE administration | 16 mg/kg/day | QUE was associated with lower epithelial, and subepithelial smooth muscle thickness, and goblet and mast cell numbers compared to untreated mice with allergic airway inflammation (p < 0.05). IL-4, IL-25, IL-33, and TSLP levels in BALF and OVA-specific IgE in serum were lower in quercetin-treated mice compared to the control group (p < 0.05). | Sozmen et al., 2016 [59] |
| IP QUE administration | 50 mg/kg/day for 7 days | QUE reduced oxidative stress, TH2 citokynes levels, and the expression of GATA-3, α-SMA, IL-1β, TNFα, and TGF-β genes in lung tissue. | Rajizadeh et al., 2023 [20] |
| Type of Study | Number of Patients and Characteristics | Type of Intervention | Effects Described | Authors, Year [Reference] |
|---|---|---|---|---|
| RCDB | 58 adult patients with AR (26 treated with Biminne (11 chinese herbs, among these Ginkgo biloba) and 32 received placebo) | Biminne (Rehmannia glutinosa 460 mg, Scutellaria baicalensis 460 mg, Polygonatum sibiricum 368 mg, Epimedium sagittatum 460 mg, Psoralea corylifolia 460 mg, Wu Wei Zi Schisandra chinensis 368 mg, Prunus mume 184 mg, Ledebouriella divaricata 460 mg, Angelica dahurica 368 mg, and Astragalus membranaceus 552 mg, Ginkgo biloba) five capsules twice a day for 12 weeks | Statistically significant improvement in symptoms score and VAS. | Hu et al., 2002 [60] |
| RCDB | 16 adults with AR (8 treated with shallot capsule + cetirizine and 8 treated with placebo + cetirizine) | Cetirizine + Allium ascalonicum L. (shallot) 3 g capsule once a day for 4 weeks | Oral supplementation with shallot was safe and improve patients’ overall symptoms more than placebo, especially for itchy nose and eyes. | Arpornchayanon et al., 2022 [61] |
| OL | 23 adults with AR | Perilla frutescens 80 mg (as dry extract), quercetin 150 mg, and vitamin D3 (200 IU) once a day + SM for 4 weeks | Reduction of AR symptoms: 70% for symptom scores and 73% in use of anti-allergic drugs. | Ariano, 2015 [62] |
| RCDB | 146 children >6 y; <12 y with AR (70 treated with nutraceutical + SM vs. 66 treated with placebo + SM) | Perilla frutescens 80 mg (as dry extract), quercetin 150 mg, and vitamin D3 (200 IU) + SM for 4 weeks | Both groups signifcantly reduced TSS, without between-group difference. 24 children had total symptom score worsened: 8 in the AG and 16 in the placebo group, the difference between treatments being signifcant (p < 0.05). | Marseglia et al., 2019 [63] |
| OL | 128 children >6 y; <12 y with AR (64 treated with nutraceutical and 64 received placebo) | Perilla frutescens 80 mg (as dry extract), quercetin 150 mg, and vitamin D3 (200 IU) for 4–12 weeks | Significant difference between groups as only 16 children in the AG had an AR exacerbation vs 27 children (42.2%) of OG (p = 0.039). Rescue medication utilization was significantly lower in the AG (9.6 + 9 days and 28.5 + 27.2 days, p = 0.018). | Marseglia et al., 2019 [64] |
| O | 63 patients with AR, 32 treated with nutraceutical +SM and 31 received placebo + SM | Observation after treatment with Perilla frutescens 80 mg (as dry extract), quercetin 150 mg, and vitamin D3 (200 IU) + SM for 4 weeks | The median number of days of antihistamine therapy in the AG was 15 and 30 in the the CG (p =0.008). | Tosca et al., 2020 [65] |
| O | 53 patients with AR, 28 treated with nutraceutical +SM and 25 received placebo + SM | Observation after treatment with Perilla frutescens 80 mg (as dry extract), quercetin 150 mg, and vitamin D3 (200 IU) + SM for 4–12 weeks | After 1 year of observation, the AG had significantly higher MEF50 than the CG (p = 0.009). | Leonardi et al., 2020 [66] |
| O | 53 patients with AR, 32 treated with nutraceutical +SM and 31 received placebo + SM | Observation after treatment with Perilla frutescens 80 mg (as dry extract), quercetin 150 mg, and vitamin D3 (200 IU) + SM for 4–12 weeks | In the AG, the number of RI was lower compared to the CG (p = 0.01). In the AG, the number of antibiotic courses was lower compared to the CG (p = 0.002). | Zicari et al., 2020 [67] |
| RCDB | 60 adult patients with AR (30 treated with Ginko Biloba + HA eyedrops vs. 30 with HA eyedrops) | Ginko biloba (quercetin, ginkgetin, kaempferol, isorhamnetin, procyani-din, prodelphinidin, Ginkgolids A, B, C, J, M, and bilobalides) + HA eyedrops for 4 weeks | All patients treated with GB-HA showed a significant improvement of subjective symptoms compared to HA patients. | Russo et al., 2009 [68] |
| POC study | 12 adult patients affected by AR and/or bronchial obstruction disease | Nasal application of extract of Artemisia abrotanum L. at occurrence of AR symptoms | Significant nasal and ocular symptom relief was rapid and almost complete as evidenced by an improvement in the symptom score. | Remberg et al., 2004 [69] |
| RCDB | 148 children with asthma in remission (74 treated with A. membranaceus + SM) vs. placebo + SM | Budesonide, terbutaline and A. membranaceus (0.25 mg for patients <20 kg and 0.5 mg for patients >20 kg) aerosol for 3 months | Improved lung function, reduction exacerbation, reduction of IgE, IL-17,IL-23 in treatment group. | Wang et al., 2019 [70] |
| Type of Study | Number of Patients and Characteristics | Type of Intervention | Effects Described | Authors, Year [Reference] |
|---|---|---|---|---|
| RCDB | 66 adult patients with AR (33 received QUE phospholipids vs. 33 placebo) | 200 mg of QUE phospholipids for 4 weeks | Significant improvement in subjective evaluation (influence on sleep) and objective evaluation (severity of allergic rhinitis and nasal discharge eosinophil count). | Yamada et al., 2022 [72] |
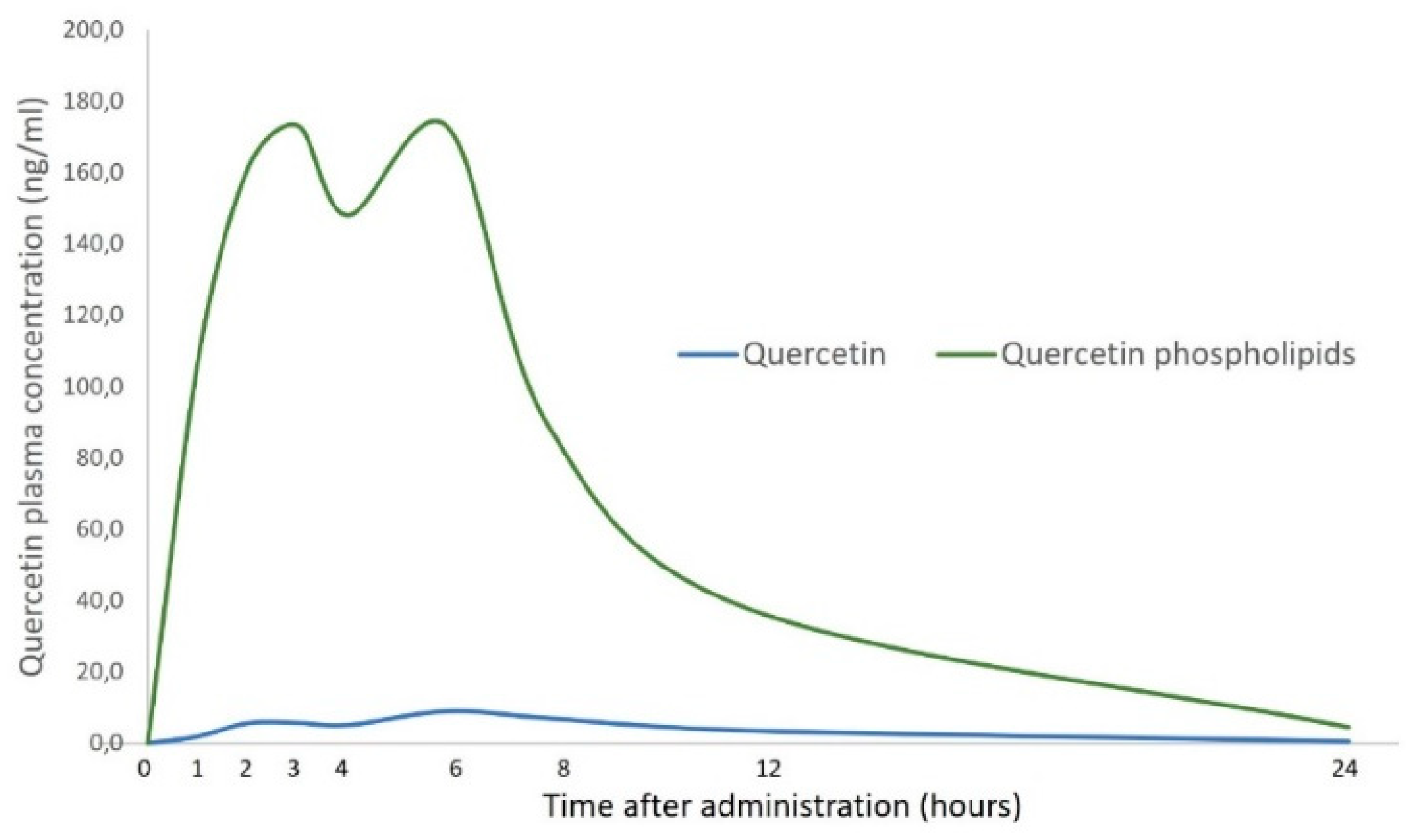
| Pilot randomized, six-sequence/three-period crossover clinical trial (3 × 3 × 3 crossover design) | 12 health adults | Oral administration of 500 mg of QUE or 250 mg of QUE phospholipids or 500 mg of QUE phospholipids | Quercetin phospholipids demonstrated a better solubility and bioavailability than QUE. | Riva et al., 2019 [73] |
| Pilot study | 58 adult patients with AA and AR (30 received nutraceutical + SM vs. 28 received placebo + SM) | 250 or 500 mg/day of quercetin phospholipids + SM for 30 days | Quercetin phospholipids + SM showed superior results compared with SM alone in controlling, preventing, and reducing daily and night symptoms; in maintaining higher PEF; and in decreasing variability. | Cesarone et al., 2019 [74] |
| Pilot study | 48 amatour healthy athletes (23 with QUE phospholipids and 25 did not) who underwent repeated triathlon sessions | 500 of quercetin phospholipids for 2 weeks | In quercetin phospholipids the improvement of time to complete the run was greater (−11.3% vs. −3.9%; p < 0.05). Training was considered more valuable (p < 0.05). Post-run muscular pain, cramps, localized pain and the post-exercise recovery time were all considered better (p < 0.05). Oxidative stress was also reduced (p < 0.05). | Riva et al., 2018 [75] |
| Pilot study | 57 healthy subjects (27 received quercetin phospholipids, 30 did not receive any treatment) received an injection of histamine, and wheal was measured | 14 received 250 mg of quercetin phospholipids, 13 received 500 mg of quercetin phospholipids in the 3 previous days | The reaction to histamine was reduced in the groups supplemented with quercetin phospholipids. The higher dose was more effective. CF in supplemented subjects was also significantly lower after 3 days of quercetin phospholipids consumption, in comparison with controls. | Belcaro et al., 2020 [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naso, M.; Trincianti, C.; Tosca, M.A.; Ciprandi, G. Quercetin and Its Lecithin-Based Formulation: Potential Applications for Allergic Diseases Based on a Narrative Review. Nutrients 2025, 17, 1476. https://doi.org/10.3390/nu17091476
Naso M, Trincianti C, Tosca MA, Ciprandi G. Quercetin and Its Lecithin-Based Formulation: Potential Applications for Allergic Diseases Based on a Narrative Review. Nutrients. 2025; 17(9):1476. https://doi.org/10.3390/nu17091476
Chicago/Turabian StyleNaso, Matteo, Chiara Trincianti, Maria Angela Tosca, and Giorgio Ciprandi. 2025. "Quercetin and Its Lecithin-Based Formulation: Potential Applications for Allergic Diseases Based on a Narrative Review" Nutrients 17, no. 9: 1476. https://doi.org/10.3390/nu17091476
APA StyleNaso, M., Trincianti, C., Tosca, M. A., & Ciprandi, G. (2025). Quercetin and Its Lecithin-Based Formulation: Potential Applications for Allergic Diseases Based on a Narrative Review. Nutrients, 17(9), 1476. https://doi.org/10.3390/nu17091476









