Multilevel Comparison of Indian Naja Venoms and Their Cross-Reactivity with Indian Polyvalent Antivenoms
Abstract
1. Introduction
2. Results
2.1. Sampling
2.2. Comparative Analysis of HPLC Profiles Reveals Variation
2.3. Venom Proteomes of Different Naja Venoms
2.4. Cross-Reactivity of Indian Polyvalent Antivenoms
2.5. Immunoaffinity Chromatographic Profiling of Indian Polyvalent Antivenoms
2.6. Identification of Non-Immunoretained Proteins
3. Discussion
4. Materials and Methods
4.1. Venoms and Antivenoms
4.2. Venom Protein Profiling Using Reverse-Phase HPLC
4.3. Whole Venom in-Solution Tryptic Digestion and Protein Identification by Mass Spectrometry
4.4. Cross-Neutralization of Venom PLA2 by Polyvalent Antivenoms
4.5. Immunochemical Analysis
4.6. Immunological Profiling of Polyvalent Antivenoms Using an Antivenomics Approach
4.7. Identification of Non-Retained Proteins by Tandem Mass Spectrometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suraweera, W.; Warrell, D.; Whitaker, R.; Menon, G.; Rodrigues, R.; Fu, S.H.; Begum, R.; Sati, P.; Piyasena, K.; Bhatia, M.; et al. Trends in snakebite deaths in India from 2000 to 2019 in a nationally representative mortality study. Elife 2020, 9, e54076. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.A. Snake bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef]
- Vaiyapuri, S.; Vaiyapuri, R.; Ashokan, R.; Ramasamy, K.; Nattamaisundar, K.; Jeyaraj, A.; Chandran, V.; Gajjeraman, P.; Baksh, M.F.; Gibbins, J.; et al. Snakebite and Its Socio-Economic Impact on the Rural Population of Tamil Nadu, India. PLoS ONE 2013, 8, e80090. [Google Scholar] [CrossRef] [PubMed]
- Pore, S.M.; Ramanand, S.J.; Patil, P.T.; Gore, A.D.; Pawar, M.P.; Gaidhankar, S.L.; Ghanghas, R.R. A retrospective study of use of polyvalent anti-snake venom and risk factors for mortality from snake bite in a tertiary care setting. Indian J. Pharmacol. 2015, 47, 270–274. [Google Scholar] [PubMed]
- Maduwage, K.; Silva, A.; O’Leary, M.A.; Hodgson, W.C.; Isbister, G.K. Efficacy of Indian polyvalent snake antivenoms against Sri Lankan snake venoms: Lethality studies or clinically focussed in vitro studies. Sci. Rep. 2016, 6, 26778. [Google Scholar] [CrossRef]
- Kumar, V.; Sabitha, P. Inadequacy of Present Polyspecific Anti Snakevenom—A Study from Central Kerala. Indian J. Pediatr. 2011, 78, 1225–1228. [Google Scholar] [CrossRef]
- Whitaker, R. Common Indian Snakes: A Field Guide; Macmillan India: New Delhi, India, 2006. [Google Scholar]
- Whitaker, R.; Captain, A. Snakes of India: The Field Guide; Draco Books: Chennai, India, 2004. [Google Scholar]
- Warrell, D.A.; Gutiérrez, J.M.; Calvete, J.J.; Williams, D. New approaches & technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J. Med. Res. 2013, 138, 38–59. [Google Scholar]
- Wüster, W. Taxonomic changes and toxinology: Systematic revisions of the asiatic cobras (Najanaja species complex). Toxicon 1996, 34, 399–406. [Google Scholar] [CrossRef]
- Wüster, W.; Thorpe, R.S. Asiatic Cobras: Population Systematics of the Najanaja Species Complex (Serpentes: Elapidae) in India and Central Asia. Herpetologica 1992, 48, 69–85. [Google Scholar]
- Wüster, W.; Thorpe, R.S. Asiatic cobras: Systematics and snakebite. Experientia 1991, 47, 205–209. [Google Scholar] [CrossRef]
- Theakston, R.D.G.; Phillips, R.E.; Warrell, D.A.; Galagedera, Y.; Abeysekera, D.T.D.J.; Dissanayaka, P.; De Silva, A.; Aloysius, D. Envenoming by the common krait (Bungarus caeruleus) and Sri Lankan cobra (Naja naja naja): Efficacy and complications of therapy with Haffkine antivenom. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 301–308. [Google Scholar] [CrossRef]
- Kularatne, S.A.M.; Budagoda, B.; Gawarammana, I.; Kularatne, W. Epidemiology, clinical profile and management issues of cobra (Naja naja) bites in Sri Lanka: First authenticated case series. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 924–930. [Google Scholar] [CrossRef]
- Mukherjee, A.K. Species-specific and geographical variation in venom composition of two major cobras in Indian subcontinent: Impact on polyvalent antivenom therapy. Toxicon 2020, 188, 150–158. [Google Scholar] [CrossRef]
- Senji Laxme, R.R.; Attarde, S.; Khochare, S.; Suranse, V.; Martin, G.; Casewell, N.R.; Whitaker, R.; Sunagar, K. Biogeographical venom variation in the Indian spectacled cobra (Naja naja) underscores the pressing need for pan-India efficacious snakebite therapy. PLoS Negl. Trop. Dis. 2021, 15, e0009150. [Google Scholar] [CrossRef]
- Shashidharamurthy, R.; Jagadeesha, D.; Girish, K.; Kemparaju, K. Variation in biochemical and pharmacological properties of Indian cobra (Naja naja naja) venom due to geographical distribution. Mol. Cell. Biochem. 2002, 229, 93–101. [Google Scholar] [CrossRef]
- Shashidharamurthy, R.; Kemparaju, K. Region-specific neutralization of Indian cobra (Naja naja) venom by polyclonal antibody raised against the eastern regional venom: A comparative study of the venoms from three different geographical distributions. Int. Immunopharmacol. 2007, 7, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Shashidharamurthy, R.; Mahadeswaraswamy, Y.; Ragupathi, L.; Vishwanath, B.; Kemparaju, K. Systemic pathological effects induced by cobra (Naja naja) venom from geographically distinct origins of Indian peninsula. Exp. Toxicol. Pathol. 2010, 62, 587–592. [Google Scholar] [CrossRef]
- Wong, K.Y.; Tan, C.H.; Tan, K.Y.; Quraishi, N.H.; Tan, N.H. Elucidating the biogeographical variation of the venom of Naja naja (spectacled cobra) from Pakistan through a venom-decomplexing proteomic study. J. Proteom. 2018, 175, 156–173. [Google Scholar] [CrossRef]
- Faiz, M.A.; Ahsan, M.F.; Ghose, A.; Rahman, M.R.; Amin, R.; Hossain, M.; Tareq, M.N.; Jalil, M.A.; Kuch, U.; Theakston, R.D.; et al. Bites by the Monocled Cobra, Naja kaouthia, in Chittagong Division, Bangladesh: Epidemiology, Clinical Features of En-venoming and Management of 70 Identified Cases. Am. J. Trop. Med. Hyg. 2017, 96, 876–884. [Google Scholar] [CrossRef]
- Wongtongkam, N.; Wilde, H.; Sitthi-Amorn, C.; Ratanabanangkoon, K. A Study of Thai Cobra (Naja kaouthia) Bites in Thailand. Mil. Med. 2005, 170, 336–341. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Tan, C.H.; Sim, S.M.; Fung, S.Y.; Tan, N.H. Geographical venom variations of the Southeast Asian monocled cobra (Naja kaouthia): Venom-induced neuromuscular depression and antivenom neutralization. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 185–186, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Tan, C.H.; Chanhome, L.; Tan, N.H. Comparative venom gland transcriptomics of Naja kaouthia(monocled cobra) from Malaysia and Thailand: Elucidating geographical venom variation and insights into sequence novelty. PeerJ 2017, 5, e3142. [Google Scholar] [CrossRef] [PubMed]
- Ratnarathorn, N.; Harnyuttanakorn, P.; Chanhome, L.; Evans, S.E.; Day, J.J. Geographical differentiation and cryptic diversity in the monocled cobra, Naja kaouthia (Elapidae), from Thailand. Zool. Scr. 2019, 48, 711–726. [Google Scholar] [CrossRef]
- Deka, A.; Reza, A.; Hoque, K.M.F.; Deka, K.; Saha, S.; Doley, R. Comparative analysis of Naja kaouthia venom from North-East India and Bangladesh and its cross reactivity with Indian polyvalent antivenoms. Toxicon 2019, 164, 31–43. [Google Scholar] [CrossRef]
- Das, D.; Urs, N.; Hiremath, V.; Vishwanath, B.S.; Doley, R. Biochemical and biological characterization of Naja kaouthia venom from North-East India and its neutralization by polyvalent antivenom. J. Venom Res. 2013, 4, 31–38. [Google Scholar]
- Senji Laxme, R.R.; Khochare, S.; de Souza, H.F.; Ahuja, B.; Suranse, V.; Martin, G.; Whitaker, R.; Sunagar, K. Beyond the “big four”: Venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Negl. Trop. Dis. 2019, 13, e0007899. [Google Scholar] [CrossRef]
- Santra, V.; Owens, J.B.; Graham, S.; Wüster, W.; Kuttalam, S.; Bharti, O.; Selvan, M.; Mukherjee, N.; Malhotra, A. Confirmation of Naja oxiana in Himachal Pradesh, India. Herpetol. Bull. 2019, 150, 26–28. [Google Scholar] [CrossRef]
- Heiner, J.D.; Bebarta, V.S.; Varney, S.M.; Bothwell, J.D.; Cronin, A.J. Clinical Effects and Antivenom Use for Snake Bite Victims Treated at Three US Hospitals in Afghanistan. Wilderness Environ. Med. 2013, 24, 412–416. [Google Scholar] [CrossRef]
- Angaji, S.; Houshmandi, A.; ZareMirakabadi, A. Acute Effects of the Iranian Snake (Naja naja oxiana) Venom on Heart. Biomacromol. J. 2016, 2, 97–101. [Google Scholar]
- Latifi, M. Variation in yield and lethality of venoms from Iranian snakes. Toxicon 1984, 22, 373–380. [Google Scholar] [CrossRef]
- Rashmi, U.; Khochare, S.; Attarde, S.; Senji Laxme, R.R.; Suranse, V.; Martin, G.; Sunagar, K. Remarkable intrapopulation venom vari-ability in the monocellate cobra (Naja kaouthia) unveils neglected aspects of India’s snakebite problem. J. Proteom. 2021, 242, 104256. [Google Scholar] [CrossRef]
- Chippaux, J.-P.; Williams, V.; White, J. Snake venom variability: Methods of study, results and interpretation. Toxicon 1991, 29, 1279–1303. [Google Scholar] [CrossRef]
- Furtado, M.F.; Travaglia-Cardoso, S.R.; Rocha, M.M. Sexual dimorphism in venom of Bothrops jararaca (Serpentes: Viperidae). Toxicon 2006, 48, 401–410. [Google Scholar] [CrossRef]
- Deka, A.; Gogoi, A.; Das, D.; Purkayastha, J.; Doley, R. Proteomics of Naja kaouthia venom from North East India and assessment of Indian polyvalent antivenom by third generation antivenomics. J. Proteom. 2019, 207, 103463. [Google Scholar] [CrossRef]
- Chanda, A.; Patra, A.; Kalita, B.; Mukherjee, A.K. Proteomics analysis to compare the venom composition between Najanaja and Naja kaouthia from the same geographical location of eastern India: Correlation with pathophysiology of envenomation and im-munological cross-reactivity towards commercial polyantivenom. Expert Rev. Proteom. 2018, 15, 949–961. [Google Scholar]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007–2015. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 12 January 2023).
- Daltry, J.C.; Ponnudurai, G.; Shin, C.K.; Tan, N.-H.; Thorpe, R.S.; Wolfgang, W. Electrophoretic profiles and biological activities: Intraspecific variation in the venom of the Malayan pit viper (Calloselasma rhodostoma). Toxicon 1996, 34, 67–79. [Google Scholar] [CrossRef]
- Fry, B.G.; Vidal, N.; van der Weerd, L.; Kochva, E.; Renjifo, C. Evolution and diversification of the Toxicofera reptile venom system. J. Proteom. 2009, 72, 127–136. [Google Scholar] [CrossRef]
- Malhotra, A. Mutation, Duplication, and More in the Evolution of Venomous Animals and Their Toxins. In Evolution of Venomous Animals and Their Toxins; Malhotra, A., Gopalakrishnakone, P., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 33–45. [Google Scholar]
- Queiroz, G.P.; Pessoa, L.A.; Portaro, F.C.; Furtado, M.D.F.D.; Tambourgi, D.V. Interspecific variation in venom composition and toxicity of Brazilian snakes from Bothrops genus. Toxicon 2008, 52, 842–851. [Google Scholar] [CrossRef]
- Sunagar, K.; Undheim, E.A.; Scheib, H.; Gren, E.C.; Cochran, C.; Person, C.E.; Koludarov, I.; Kelln, W.; Hayes, W.K.; King, G.F.; et al. Intraspecific venom variation in the medically significant Southern Pacific Rattlesnake (Crotalus oreganus helleri): Biodis-covery, clinical and evolutionary implications. J. Proteom. 2014, 99, 68–83. [Google Scholar] [CrossRef]
- Alape-Giron, A.; Sanz, L.; Escolano, J.; Flores-Diaz, M.; Madrigal, M.; Sasa, M.; Calvete, J.J. Snake venomics of the lancehead pitviper Bothrop sasper: Geographic, individual, and ontogenetic variations. J. Proteome Res. 2008, 7, 3556–3571. [Google Scholar] [CrossRef] [PubMed]
- Mora-Obando, D.; Guerrero-Vargas, J.A.; Prieto-Sánchez, R.; Beltrán, J.; Rucavado, A.; Sasa, M.; Gutiérrez, J.M.; Ayerbe, S.; Lomonte, B. Proteomic and functional profiling of the venom of Bothrops ayerbei from Cauca, Colombia, reveals striking interspecific variation with Bothrop sasper venom. J. Proteom. 2014, 96, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Saravia, P.; Rojas, E.; Arce, V.; Guevara, C.; López, J.C.; Chaves, E.; Velásquez, R.; Rojas, G.; Gutiérrez, J.M. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: Pathophysiological and therapeutic implications. Rev. de Biol. Trop. 2002, 50, 337–346. [Google Scholar]
- Sintiprungrat, K.; Watcharatanyatip, K.; Senevirathne, W.; Chaisuriya, P.; Chokchaichamnankit, D.; Srisomsap, C.; Ratanabanangkoon, K. A comparative study of venomics of Naja naja from India and Sri Lanka, clinical manifestations and antivenomics of an Indian polyspecific antivenom. J. Proteom. 2016, 132, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-W.; Liu, B.-S.; Chien, K.-Y.; Chiang, L.-C.; Huang, S.-Y.; Sung, W.-C.; Wu, W.-G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef]
- Dutta, S.; Chanda, A.; Kalita, B.; Islam, T.; Patra, A.; Mukherjee, A.K. Proteomic analysis to unravel the complex venom proteome of eastern India Naja naja: Correlation of venom composition with its biochemical and pharmacological properties. J. Proteom. 2017, 156, 29–39. [Google Scholar] [CrossRef]
- Chanda, A.; Kalita, B.; Patra, A.; Senevirathne, W.D.S.T.; Mukherjee, A.K. Proteomic analysis and antivenomics study of Western India Naja naja venom: Correlation between venom composition and clinical manifestations of cobra bite in this region. Expert Rev. Proteom. 2019, 16, 171–184. [Google Scholar] [CrossRef]
- Modahl, C.M.; Doley, R.; Kini, R.M. Venom analysis of long-term captive Pakistan cobra (Naja naja) populations. Toxicon 2010, 55, 612–618. [Google Scholar] [CrossRef]
- Tan, C.H.; Wong, K.Y.; Chong, H.P.; Tan, N.H.; Tan, K.Y. Proteomic insights into short neurotoxin-driven, highly neurotoxic venom of Philippine cobra (Naja philippinensis) and toxicity correlation of cobra envenomation in Asia. J. Proteom. 2019, 206, 103418. [Google Scholar] [CrossRef]
- Amazonas, D.R.; Freitas-De-Sousa, L.A.; Orefice, D.P.; de Sousa, L.F.; Martinez, M.G.; Mourão, R.H.V.; Chalkidis, H.M.; Camargo, P.B.; Moura-Da-Silva, A.M. Evidence for Snake Venom Plasticity in a Long-Term Study with Individual Captive Bothrops atrox. Toxins 2019, 11, 294. [Google Scholar] [CrossRef]
- Xu, N.; Zhao, H.-Y.; Yin, Y.; Shen, S.-S.; Shan, L.-L.; Chen, C.-X.; Zhang, Y.-X.; Gao, J.-F.; Ji, X. Combined venomics, antivenomics and venom gland transcriptome analysis of the monocoled cobra (Naja kaouthia) from China. J. Proteom. 2017, 159, 19–31. [Google Scholar] [CrossRef]
- Kazemi, E.; Nazarizadeh, M.; Fatemizadeh, F.; Khani, A.; Kaboli, M. The phylogeny, phylogeography, and diversification history of the westernmost Asian cobra (Serpentes: Elapidae: Naja oxiana) in the Trans-Caspian region. Ecol. Evol. 2021, 11, 2024–2039. [Google Scholar] [CrossRef]
- Manuwar, A.; Dreyer, B.; Böhmert, A.; Ullah, A.; Mughal, Z.; Akrem, A.; Ali, S.A.; Schlüter, H.; Betzel, C. Proteomic Investigations of Two Pakistani Naja Snake Venoms Species Unravel the Venom Complexity, Posttranslational Modifications, and Presence of Extracellular Vesicles. Toxins 2020, 12, 669. [Google Scholar] [CrossRef]
- Prasad, N.B.; Uma, B.; Bhatt, S.K.; Gowda, V.T. Comparative characterisation of Russell’s viper (Daboia/Vipera russelli) venoms from different regions of the Indian peninsula. Biochim. Biophys. Acta 1999, 1428, 121–136. [Google Scholar] [CrossRef]
- Sharma, M.; Gogoi, N.; Dhananjaya, B.L.; Menon, J.C.; Doley, R. Geographical variation of Indian Russell’s viper venom and neutralization of its coagulopathy by polyvalent antivenom. Toxin Rev. 2014, 33, 7–15. [Google Scholar] [CrossRef]
- Jayanthi, G.P.; Gowda, T.V. Geographical variation in India in the composition and lethal potency of Russell’s viper (Vipera russelli) venom. Toxicon 1988, 26, 257–264. [Google Scholar] [CrossRef]
- Ariaratnam, C.; Thuraisingam, V.; Kularatne, S.A.M.; Sheriff, M.; Theakston, R.; De Silva, A.; Warrell, D. Frequent and potentially fatal envenoming by hump-nosed pit vipers (Hypnale hypnale and H. nepa) in Sri Lanka: Lack of effective antivenom. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 1120–1126. [Google Scholar] [CrossRef]
- Joseph, J.; Simpson, I.; Menon, N.; Jose, M.; Kulkarni, K.; Raghavendra, G.; Warrell, D. First authenticated cases of life-threatening envenoming by the hump-nosed pit viper (Hypnale hypnale) in India. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 85–90. [Google Scholar] [CrossRef]
- Faiz, M.A.; Ghose, A.; Ahsan, M.F.; Rahman, M.R.; Amin, M.M.; Hassan, M.M.U.; Chowdhury, M.A.W.; Kuch, U.; Rocha, T.; Harris, J.B.; et al. The greater black krait (Bungarus niger), a newly recognized cause of neuro-myotoxic snake bite envenoming in Bangladesh. Brain 2010, 133, 3181–3193. [Google Scholar] [CrossRef]
- Sunthornandh, P.; Matangkasombut, P.; Ratanabanangkoon, K. Preparation, characterization and immunogenicity of various polymers and conjugates of elapid postsynaptic neurotoxins. Mol. Immunol. 1992, 29, 501–510. [Google Scholar] [CrossRef]
- Leong, P.K.; Fung, S.Y.; Tan, C.H.; Sim, S.M.; Tan, N.H. Immunological cross-reactivity and neutralization of the principal toxins of Najas umatrana and related cobra venoms by a Thai polyvalent antivenom (Neuro Polyvalent Snake Antivenom). Acta Trop. 2015, 149, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.; Samianifard, M.; Rabie, H.; Mirakabadi, A.Z. Recombinant antibodies against Iranian cobra venom as a new emerging therapy by phage display technology. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190099. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.M.A.; Oliveira, T.S.; Silveira, C.R.F.; Caporrino, M.C.; Rodriguez, D.; Moura-da-Silva, A.M.; Ramos, O.H.P.; Rucavado, A.; Gutiérrez, J.M.; Magalhães, G.S.; et al. A neutralizing recombinant single chain antibody, scFv, against BaP1, A PI hemorrhagic metalloproteinase from Bothrop sasper snake venom. Toxicon 2014, 87, 81–91. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immuno-Globulins; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Pla, D.; Rodríguez, Y.; Calvete, J.J. Third Generation Antivenomics: Pushing the Limits of the In Vitro Preclinical Assessment of Antivenoms. Toxins 2017, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Sintiprungrat, K.; Chaisuriya, P.; Watcharatanyatip, K.; Ratanabanangkoon, K. Immunoaffinity chromatography in antivenomics studies: Various parameters that can affect the results. Toxicon 2016, 119, 129–139. [Google Scholar] [CrossRef]
- Kazandjian, T.D.; Petras, D.; Robinson, S.D.; van Thiel, J.; Greene, H.W.; Arbuckle, K.; Barlow, A.; Carter, D.A.; Wouters, R.M.; Whiteley, G.; et al. Convergent evolution of pain-inducing defensive venom components in spitting cobras. Science 2021, 371, 386–390. [Google Scholar] [CrossRef]
- McDiarmid, R.W.; Foster, M.S.; Guyer, C.; Chernoff, N.; Gibbons, J.W. Reptile Biodiversity: Standard Methods for Inventory and Monitoring; University of California Press: Berkeley, CA, USA, 2012. [Google Scholar]
- Johnson, R. Clinical Technique: Handling and Treating Venomous Snakes. J. Exot. Pet Med. 2011, 20, 124–130. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Kinter, M.; Sherman, N.E. Protein Sequencing and Identification Using Tandem Mass Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Calvete, J.J. Next-generation snake venomics: Protein-locus resolution through venom proteome decomplexation. Expert Rev. Proteom. 2014, 11, 315–329. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, N.H.; Tan, C.H. Venom proteomics and antivenom neutralization for the Chinese eastern Russell’s viper, Daboia siamensis from Guangxi and Taiwan. Sci. Rep. 2018, 8, 8545. [Google Scholar] [CrossRef]
- Pla, D.; Gutiérrez, J.M.; Calvete, J.J. Second generation snake antivenomics: Comparing immunoaffinity and immunodepletion protocols. Toxicon 2012, 60, 688–699. [Google Scholar] [CrossRef]
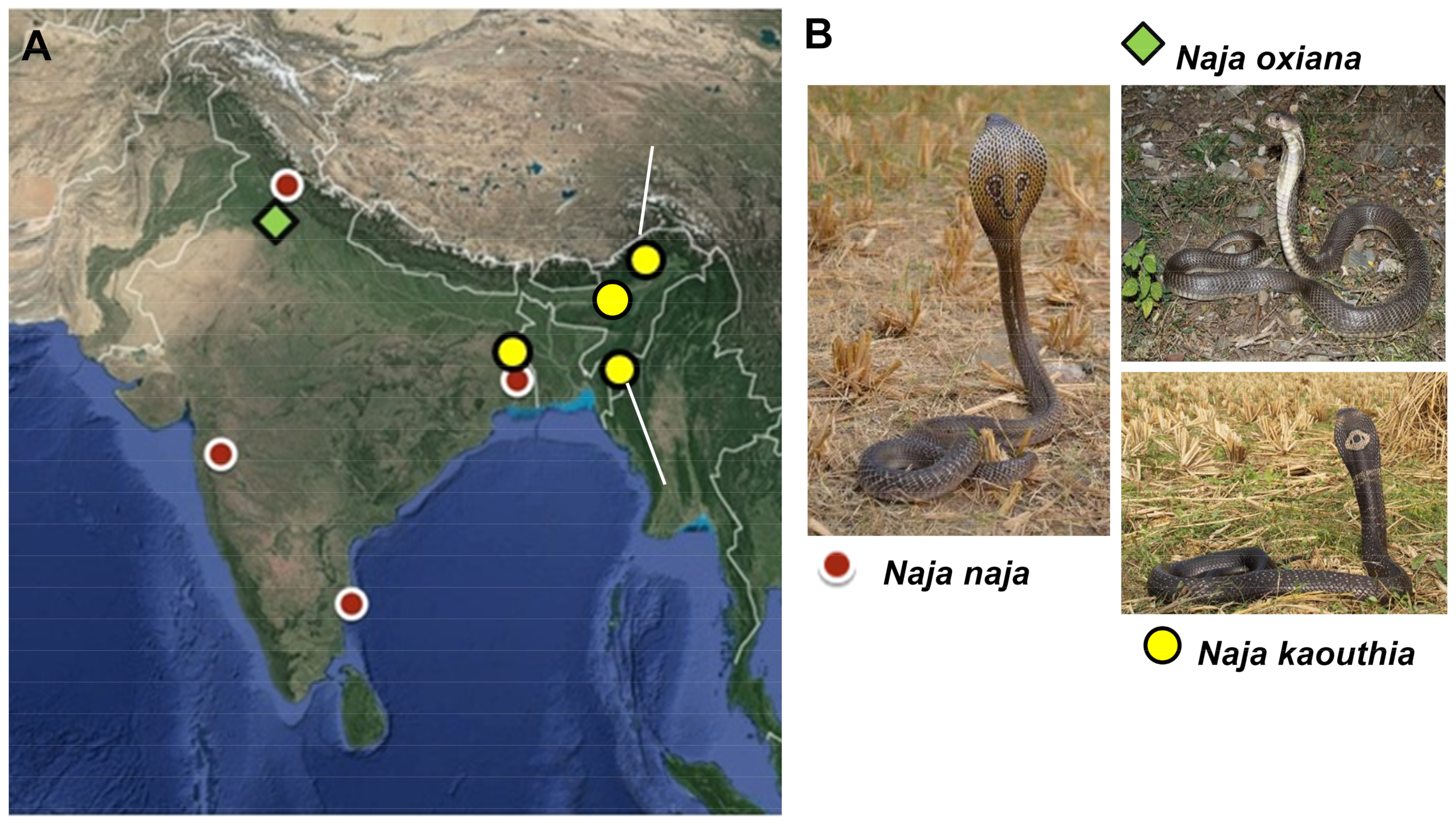









| Sample ID | Species | Location |
|---|---|---|
| 15.47 | Naja kaouthia | Papum Pare District, Arunachal Pradesh |
| 16.24 | Naja kaouthia | Aizawl District, Mizoram |
| 17.25 | Naja kaouthia | Hooghly District, West Bengal |
| 18.41 (Sample A) | Naja naja | Kangra District, Himachal Pradesh |
| 18.43 (Sample B) | Naja naja | Kangra District, Himachal Pradesh |
| 15.35 (Sample A) | Naja naja | Ahmednagar District, Maharashtra |
| 15.37 (Sample B) | Naja naja | Ahmednagar District, Maharashtra |
| 15.73 (Sample A) | Naja naja | Hooghly District, West Bengal |
| 15.74 (Sample B) | Naja naja | Hooghly District, West Bengal |
| 17.24 (Sample C) | Naja naja | Hooghly District, West Bengal |
| - | Naja naja | Irula Snake Catcher’s Cooperative, Tamil Nadu |
| 17.v18 | Naja oxiana | Chamba District, Himachal Pradesh |
| Manufacturer | Batch No. & Expiry | Active Substance | Protein per Vial | Neutralization Potency per Vial (mg venom/mL Antivenom) |
|---|---|---|---|---|
| Premium Serums and Vaccines Pvt. Limited | 212013; 08/2023 | F(ab) 2 | 629.95 mg | Naja naja venom (0.6 mg), Daboia russelii venom (0.6 mg), Bungarus caeruleus (0.45 mg), Echis carinatus (0.45 mg) |
| VINS Bioproducts Limited | 01AS15007; 01/2019 | F(ab) 2 | 487.05 mg | Naja naja venom (0.6 mg), Daboia russelii venom (0.6 mg), Bungarus caeruleus (0.45 mg), Echis carinatus (0.45 mg) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deka, A.; Bhatia, S.; Santra, V.; Bharti, O.K.; Lalremsanga, H.T.; Martin, G.; Wüster, W.; Owens, J.B.; Graham, S.; Doley, R.; et al. Multilevel Comparison of Indian Naja Venoms and Their Cross-Reactivity with Indian Polyvalent Antivenoms. Toxins 2023, 15, 258. https://doi.org/10.3390/toxins15040258
Deka A, Bhatia S, Santra V, Bharti OK, Lalremsanga HT, Martin G, Wüster W, Owens JB, Graham S, Doley R, et al. Multilevel Comparison of Indian Naja Venoms and Their Cross-Reactivity with Indian Polyvalent Antivenoms. Toxins. 2023; 15(4):258. https://doi.org/10.3390/toxins15040258
Chicago/Turabian StyleDeka, Archana, Siddharth Bhatia, Vishal Santra, Omesh K. Bharti, Hmar Tlawmte Lalremsanga, Gerard Martin, Wolfgang Wüster, John B. Owens, Stuart Graham, Robin Doley, and et al. 2023. "Multilevel Comparison of Indian Naja Venoms and Their Cross-Reactivity with Indian Polyvalent Antivenoms" Toxins 15, no. 4: 258. https://doi.org/10.3390/toxins15040258
APA StyleDeka, A., Bhatia, S., Santra, V., Bharti, O. K., Lalremsanga, H. T., Martin, G., Wüster, W., Owens, J. B., Graham, S., Doley, R., & Malhotra, A. (2023). Multilevel Comparison of Indian Naja Venoms and Their Cross-Reactivity with Indian Polyvalent Antivenoms. Toxins, 15(4), 258. https://doi.org/10.3390/toxins15040258






