Poly(N-isopropylacrylamide) Hydrogel for Diving/Surfacing Device
Abstract
:1. Introduction
2. Experimental Section
2.1. Fabrication of PNIPAM Hydrogel Device
2.2. Fabrication of Electrically Controlled Device
2.3. Fabrication of Ultrasonically Controlled Device
2.4. Fabrication of PNIPAM/Carbon Buoyancy Control Device
2.5. Preparation of Submarine
2.6. Swelling Ratio Measurement
2.7. Density Measurement
2.8. Thermal Image
2.9. Characterization
3. Results and Discussion
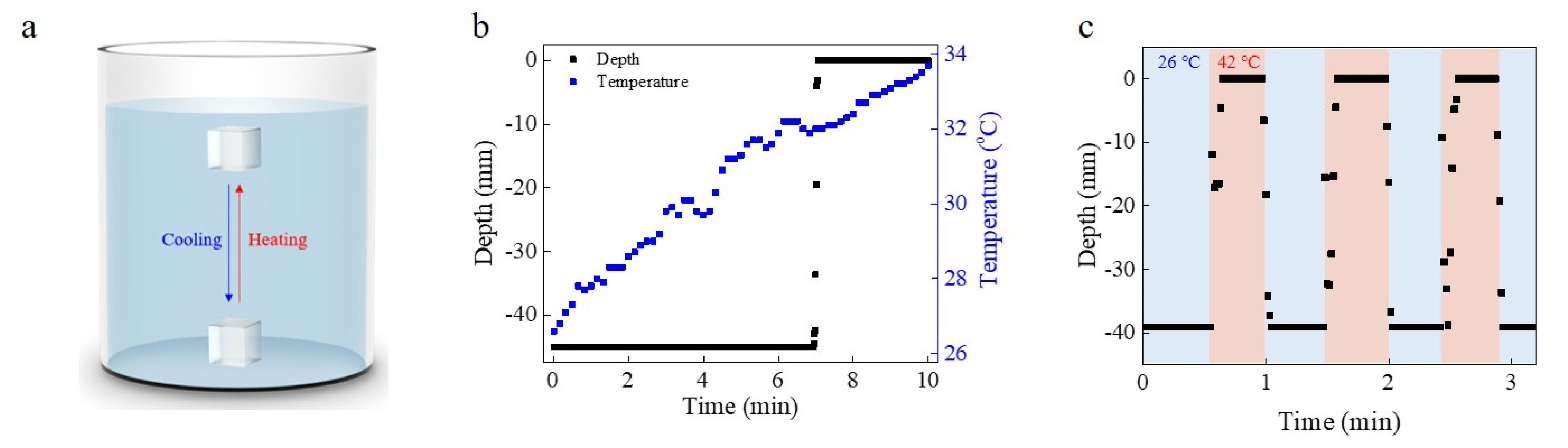
3.1. PNIPAM Hydrogel Diving/Surfacing Device
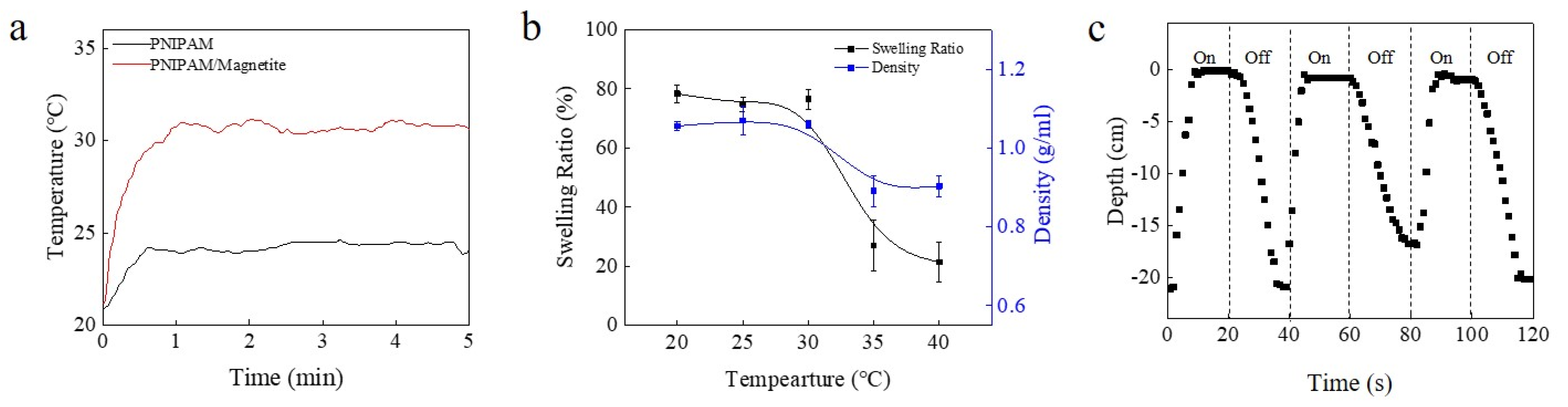
3.2. Diving/Surfacing Resulted from Swelling
3.3. Electrically and Ultrasonically Controlled Devices
3.4. Remotely Controlled Underwater Vehicle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fay, C.; Lau, K.T.; Beirne, S.; Conaire, C.O.; McGuinness, K.; Corcoran, B.; O’Connor, N.E.; Diamond, D.; McGovern, S.; Coleman, G.; et al. Wireless aquatic navigator for detection and analysis (WANDA). Sens. Actuators B Chem. 2010, 150, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Ju, G.; Cheng, M.; Xiao, M.; Xu, J.; Pan, K.; Wang, X.; Zhang, Y.; Shi, F. Smart Transportation Between Three Phases Through a Stimulus-Responsive Functionally Cooperating Device. Adv. Mater. 2013, 25, 2915–2919. [Google Scholar] [CrossRef] [PubMed]
- Solovev, A.A.; Sanchez, S.; Pumera, M.; Mei, Y.F.; Schmidt, O.G. Magnetic control of tubular catalytic microbots for the transport, assembly, and delivery of micro-objects. Adv. Funct. Mater. 2010, 20, 2430–2435. [Google Scholar] [CrossRef]
- Hoare, T.; Timko, B.P.; Santamaria, J.; Goya, G.F.; Irusta, S.; Lau, S.; Stefanescu, C.F.; Lin, D.; Langer, R.; Kohane, D.S. Magnetically triggered nanocomposite membranes: A versatile platform for triggered drug release. Nano Lett. 2011, 11, 1395–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Cheng, M.; Zhang, L.; Zhang, S.; Liu, X.; Shi, F. Electricity Generation through Light-Responsive Diving–Surfacing Locomotion of a Functionally Cooperating Smart Device. Adv. Mater. 2018, 30, 1803125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, M.; Xiao, M.; Shi, F. Diving-Surfacing Smart Locomotion Driven by a CO2-Forming Reaction, with Applications to Minigenerators. Adv. Funct. Mater. 2016, 26, 851–856. [Google Scholar] [CrossRef]
- Kumar, B.P.; Patil, A.J.; Mann, S. Enzyme-powered motility in buoyant organoclay/DNA protocells. Nat. Chem. 2018, 10, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cheng, M.; Wang, B.; Feng, Z.; Shi, F. Diving-Surfacing Cycle Within a Stimulus-responsive Smart Device towards Developing Functionally Cooperating Systems. Adv. Mater. 2010, 22, 5125–5128. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cheng, M.; Ju, G.; Zhang, Y.; Shi, F. Converting chemical energy into electricity through a functionally cooperating device with diving-surfacing cycles. Adv. Mater. 2014, 26, 7059–7063. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cheng, M.; Xiao, M.; Zhang, L.; Ju, G.; Shi, F. Biomimicking of a Swim Bladder and Its Application as a Mini-Generator. Adv. Mater. 2017, 29, 1603312. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Cheng, Y.; Cui, X.; Lian, H.; Liang, Y.; Chen, F.; Wang, H.; Guo, W.; Li, H. A Bioinspired Swimming and Walking Hydrogel Driven by Light-Controlled Local Density. Adv. Sci. 2015, 2, 1500084. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, Y.; Tian, Z. Thermal switching of thermoresponsive polymer aqueous solutions. ACS Macro Lett. 2018, 7, 53–58. [Google Scholar] [CrossRef]
- Ono, Y.; Shikata, T. Hydration and dynamic behavior of poly(N-isopropylacrylamide) s in aqueous solution: A sharp phase transition at the lower critical solution temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Retama, J.; Zafeiropoulos, N.E.; Serafinelli, C.; Rojas-Reyna, R.; Voit, B.; Lopez Cabarcos, E.; Stamm, M. Synthesis and characterization of thermosensitive PNIPAM microgels covered with superparamagnetic γ-Fe2O3 nanoparticles. Langmuir 2007, 23, 10280–10285. [Google Scholar] [CrossRef] [PubMed]
- Dybal, J.; Trchová, M.; Schmidt, P. The role of water in structural changes of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide) studied by FTIR, Raman spectroscopy and quantum chemical calculations. Vib. Spectrosc. 2009, 51, 44–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Sagle, L.B.; Cho, Y.; Bergbreiter, D.E.; Cremer, P.S. Effects of Hofmeister anions on the LCST of PNIPAM as a function of molecular weight. J. Phys. Chem. C 2007, 111, 8916–8924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Józefczak, A.; Kaczmarek, K.; Hornowski, T.; Kubovčíková, M.; Rozynek, Z.; Timko, M.; Skumiel, A. Magnetic nanoparticles for enhancing the effectiveness of ultrasonic hyperthermia. Appl. Phys. Lett. 2016, 108, 263701. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Hornowski, T.; Kubovcikova, M.; Timko, M.; Koralewski, M.; Joózefczak, A. Heating induced by therapeutic ultrasound in the presence of magnetic nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 11554–11564. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, C.; Piñeiro, Y.; Riminucci, A.; Bañobre, M.; Rivas, J.; Dediu, V. Regulating the thermal response of PNIPAM hydrogels by controlling the adsorption of magnetite nanoparticles. Appl. Phys. A 2014, 114, 585–590. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.G.; Gwac, H.; Jang, Y.; Richards, C.; Warren, H.; Spinks, G.; Kim, S.J. Poly(N-isopropylacrylamide) Hydrogel for Diving/Surfacing Device. Micromachines 2021, 12, 210. https://doi.org/10.3390/mi12020210
Choi JG, Gwac H, Jang Y, Richards C, Warren H, Spinks G, Kim SJ. Poly(N-isopropylacrylamide) Hydrogel for Diving/Surfacing Device. Micromachines. 2021; 12(2):210. https://doi.org/10.3390/mi12020210
Chicago/Turabian StyleChoi, Jung Gi, Hocheol Gwac, Yongwoo Jang, Christopher Richards, Holly Warren, Geoffrey Spinks, and Seon Jeong Kim. 2021. "Poly(N-isopropylacrylamide) Hydrogel for Diving/Surfacing Device" Micromachines 12, no. 2: 210. https://doi.org/10.3390/mi12020210
APA StyleChoi, J. G., Gwac, H., Jang, Y., Richards, C., Warren, H., Spinks, G., & Kim, S. J. (2021). Poly(N-isopropylacrylamide) Hydrogel for Diving/Surfacing Device. Micromachines, 12(2), 210. https://doi.org/10.3390/mi12020210






