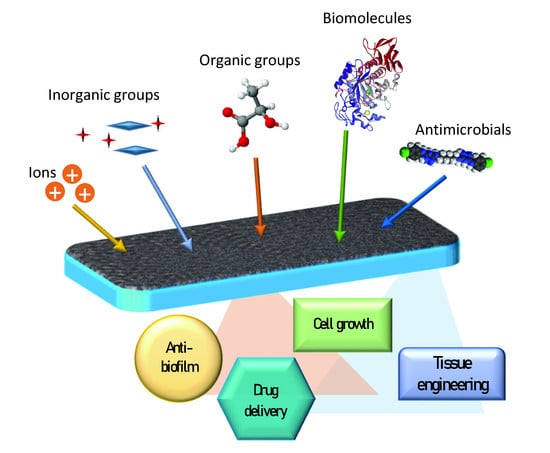
Functionalized Mesoporous Thin Films for Biotechnology
Abstract
:1. Introduction
2. Functionalization Protocols
3. Functional Groups
3.1. Change of Hydrophilicity of the Surface
3.2. Protection against Dissolution
3.3. Drug Loading
4. Antimicrobials
5. Biofunctionalization
6. Ions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soler-Illia, G.J.D.A.A.; Sanchez, C.; Lebeau, B.; Patarin, J. Chemical Strategies To Design Textured Materials: From Microporous and Mesoporous Oxides to Nanonetworks and Hierarchical Structures. Chem. Rev. 2002, 102, 4093–4138. [Google Scholar] [CrossRef]
- Sanchez, C.; Arribart, H.; Guille, M.M.G. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005, 4, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Ordered porous materials for emerging applications. Nat. Cell Biol. 2002, 417, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Baca, H.K.; Ashley, C.; Carnes, E.; Lopez, D.; Flemming, J.; Dunphy, D.; Singh, S.; Chen, Z.; Liu, N.; Fan, H.; et al. Cell-Directed Assembly of Lipid-Silica Nanostructures Providing Extended Cell Viability. Science 2006, 313, 337–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallet-Regí, M.; Balas, F. Silica Materials for Medical Applications. Open Biomed. Eng. J. 2008, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M. Mesoporous Silica Nanoparticles: Their Projection in Nanomedicine. ISRN Mater. Sci. 2012, 2012, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Functionalized TiO2Based Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Wu, S.-H.; Hung, Y.; Mou, C.-Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Sábio, R.M.; Meneguin, A.B.; dos Santos, A.M.; Monteiro, A.S.; Chorilli, M. Exploiting mesoporous silica nanoparticles as versatile drug carriers for several routes of administration. Microporous Mesoporous Mater. 2021, 312, 110774. [Google Scholar] [CrossRef]
- Pourshahrestani, S.; Kadri, N.A.; Zeimaran, E.; Towler, M.R. Well-ordered mesoporous silica and bioactive glasses: Promise for improved hemostasis. Biomater. Sci. 2019, 7, 31–50. [Google Scholar] [CrossRef]
- Karlsson, J.; Atefyekta, S.; Andersson, M. Controlling drug delivery kinetics from mesoporous titania thin films by pore size and surface energy. Int. J. Nanomed. 2015, 10, 4425–4436. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.; Grandfield, K.; Hoess, A.; Ballo, A.; Cai, Y.; Engqvist, H. Mesoporous titanium dioxide coating for metallic implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 100, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Ruiz-González, L.; Doadrio, J.C.; Gonzalez-Calbet, J.M.; Vallet-Regí, M. Tissue regeneration: A new property of mesoporous materials. Solid State Sci. 2005, 7, 983–989. [Google Scholar] [CrossRef]
- Peltola, T.; Pätsi, M.; Rahiala, H.; Kangasniemi, I.; Yli-Urpo, A. Calcium phosphate induction by sol-gel-derived titania coatings on titanium substrates in vitro. J. Biomed. Mater. Res. 1998, 41, 504–510. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Pezzoni, M.; Catalano, P.N.; Pizarro, R.A.; DeSimone, M.F.; Soler-Illia, G.J.; Bellino, M.G.; Costa, C.S. Antibiofilm effect of supramolecularly templated mesoporous silica coatings. Mater. Sci. Eng. C 2017, 77, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Bindini, E.; Chehadi, Z.; Faustini, M.; Albouy, P.-A.; Grosso, D.; Cattoni, A.; Chanéac, C.; Azzaroni, O.; Sanchez, C.; Boissière, C. Following in Situ the Degradation of Mesoporous Silica in Biorelevant Conditions: At Last, a Good Comprehension of the Structure Influence. ACS Appl. Mater. Interfaces 2020, 12, 13598–13612. [Google Scholar] [CrossRef]
- Pezzoni, M.; Catalano, P.N.; Delgado, D.C.; Pizarro, R.A.; Bellino, M.G.; Costa, C.S. Antibiofilm effect of mesoporous titania coatings on Pseudomonas aeruginosa biofilms. J. Photochem. Photobiol. B Biol. 2020, 203, 111762. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nat. Cell Biol. 1992, 359, 710–712. [Google Scholar] [CrossRef]
- David, M.; Antonelli, J.Y.Y. Synthesis of Hexagonally Packed Mesoporous TiO2 by a Modified Sol–Gel Method. Angew. Chemie Int. Ed. Engl. 1995, 34, 2014–2017. [Google Scholar]
- Kumar, S.; Malik, M.; Purohit, R. Synthesis Methods of Mesoporous Silica Materials. Mater. Today Proc. 2017, 4, 350–357. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, Y.; He, H.; Xu, L.; Li, W.; Zhao, D. Recent advances in the synthesis of hierarchically mesoporous TiO2 materials for energy and environmental applications. Natl. Sci. Rev. 2020, 7, 1702–1725. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Shen, Y.-W.; Guan, Y.-Y.; Jiang, Y.-X.; Wu, Y.; Rahman, K.; Zhang, L.-J.; Liu, H.-J.; Luan, X. Mesoporous silica nanoparticles: Facile surface functionalization and versatile biomedical applications in oncology. Acta Biomater. 2020, 116, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science the Physics and Chemistry of Sol-Gel Processing; Academic Press, I., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-Induced Self-Assembly: Nanostructures Made Easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Velev, O.; Jede, T.A.; Lobo, R.F.; Lenhoff, A. Porous silica via colloidal crystallization. Nat. Cell Biol. 1997, 389, 447–448. [Google Scholar] [CrossRef]
- Holland, B.T.; Blanford, C.F.; Stein, A. Synthesis of Macroporous Minerals with Highly Ordered Three-Dimensional Arrays of Spheroidal Voids. Science 1998, 281, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Deng, T.; Zhao, D.; Feng, P.; Pine, D.; Chmelka, B.F.; Whitesides, G.M.; Stucky, G.D. Hierarchically Ordered Oxides. Science 1998, 282, 2244–2246. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, L.; Koodali, R. Versatility of Evaporation-Induced Self-Assembly (EISA) Method for Preparation of Mesoporous TiO2 for Energy and Environmental Applications. Materials 2014, 7, 2697–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, D.; Balkenende, A.R.; Albouy, P.A.; Lavergne, M.; Mazerolles, L.; Babonneau, F. Highly oriented 3D-hexagonal silica thin films produced with cetyltrimethylammonium bromide. J. Mater. Chem. 2000, 10, 2085–2089. [Google Scholar] [CrossRef]
- Soler-Illia, G.J.A.A.; Angelomé, P.; Fuertes, M.C.; Grosso, D.; Boissiere, C. Critical aspects in the production of periodically ordered mesoporous titania thin films. Nanoscale 2012, 4, 2549–2566. [Google Scholar] [CrossRef]
- Lionello, D.F.; Steinberg, P.Y.; Zalduendo, M.; Soler-Illia, G.J.A.A.; Angelomé, P.C.; Fuertes, M.C. Structural and Mechanical Evolution of Mesoporous Films with Thermal Treatment: The Case of Brij 58 Templated Titania. J. Phys. Chem. C 2017, 121, 22576–22586. [Google Scholar] [CrossRef] [Green Version]
- Innocenzi, P.; Malfatti, L.; Falcaro, P. Hard X-rays meet soft matter: When bottom-up and top-down get along well. Soft Matter 2012, 8, 3722–3729. [Google Scholar] [CrossRef]
- Schwenzer, B.; Wang, L.; Swensen, J.S.; Padmaperuma, A.B.; Silverman, G.; Korotkov, R.; Gaspar, D.J. Tuning the Optical Properties of Mesoporous TiO2 Films by Nanoscale Engineering. Langmuir 2012, 28, 10072–10081. [Google Scholar] [CrossRef]
- Giménez, G.; Ybarra, G.; Soler-Illia, G.J.A.A. Preparation of mesoporous silica thin films at low temperature: A comparison of mild structure consolidation and template extraction procedures. J. Sol-Gel Sci. Technol. 2020, 96, 287–296. [Google Scholar] [CrossRef]
- Solveyra, E.G.; Fuertes, M.C.; Soler-Illia, G.J.A.A.; Angelomé, P.C. 2D-SAXS In Situ Measurements as a Tool To Study Elusive Mesoporous Phases: The Case of p6mm TiO2. J. Phys. Chem. C 2017, 121, 3623–3631. [Google Scholar] [CrossRef] [Green Version]
- Chemin, N.; Klotz, M.; Rouessac, V.; Ayral, A.; Barthel, E. Mechanical properties of mesoporous silica thin films: Effect of the surfactant removal processes. Thin Solid Films 2006, 495, 210–213. [Google Scholar] [CrossRef]
- Imai, H.; Hirashima, H.; Awazu, K. Alternative modification methods for sol–gel coatings of silica, titania and silica–titania using ultraviolet irradiation and water vapor. Thin Solid Films 1999, 351, 91–94. [Google Scholar] [CrossRef]
- Doshi, D.A.; Huesing, N.K.; Lu, M.; Fan, H.; Lu, Y.; Simmons-Potter, K., Jr.; Hurd, A.J.; Brinker, C.J. Optically Defined Multifunctional Patterning of Photosensitive Thin-Film Silica Mesophases. Science 2000, 290, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Dattelbaum, A.M.; Amweg, M.L.; Ecke, L.E.; Yee, C.K.; Shreve, A.P.; Parikh, A.N. Photochemical Pattern Transfer and Enhancement of Thin Film Silica Mesophases. Nano Lett. 2003, 3, 719–722. [Google Scholar] [CrossRef]
- Della Giustina, G.; Prasciolu, M.; Brusatin, G.; Guglielmi, M.; Romanato, F. Electron beam lithography of hybrid sol–gel negative resist. Microelectron. Eng. 2009, 86, 745–748. [Google Scholar] [CrossRef]
- Marmiroli, B.; Amenitsch, H. X-ray lithography and small-angle X-ray scattering: A combination of techniques merging biology and materials science. Eur. Biophys. J. 2012, 41, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.; Malfatti, L.; Marmiroli, B.; Falcaro, P. Hard X-rays and soft-matter: Processing of sol–gel films from a top down route. J. Sol-Gel Sci. Technol. 2014, 70, 236–244. [Google Scholar] [CrossRef]
- Covarrubias, C.; Mattmann, M.; Von Marttens, A.; Caviedes, P.; Arriagada, C.; Valenzuela, F.; Rodríguez, J.P.; Corral, C. Osseointegration properties of titanium dental implants modified with a nanostructured coating based on ordered porous silica and bioactive glass nanoparticles. Appl. Surf. Sci. 2016, 363, 286–295. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, D.; Choi, Y.S.; Jeon, H.B.; Lee, C.-H.; Moon, J.-H.; Kwon, I.K. Mesoporous TiO2 implants for loading high dosage of antibacterial agent. Appl. Surf. Sci. 2014, 303, 140–146. [Google Scholar] [CrossRef]
- Wu, P.-H.; Mäkie, P.; Odén, M.; Björk, E.M. Growth and Functionalization of Particle-Based Mesoporous Silica Films and Their Usage in Catalysis. Nanomaterials 2019, 9, 562. [Google Scholar] [CrossRef] [Green Version]
- Caricato, A.; Arima, V.; Catalano, M.; Cesaria, M.; Cozzoli, P.; Martino, M.; Taurino, A.; Rella, R.; Scarfiello, R.; Tunno, T.; et al. MAPLE deposition of nanomaterials. Appl. Surf. Sci. 2014, 302, 92–98. [Google Scholar] [CrossRef]
- Rădulescu, D.; Voicu, G.; Oprea, A.E.; Andronescu, E.; Grumezescu, V.; Holban, A.M.; Vasile, B.S.; Surdu, V.-A.; Grumezescu, A.M.; Socol, G.; et al. Mesoporous silica coatings for cephalosporin active release at the bone-implant interface. Appl. Surf. Sci. 2016, 374, 165–171. [Google Scholar] [CrossRef]
- Galli, S.; Naito, Y.; Karlsson, J.; He, W.; Miyamoto, I.; Xue, Y.; Andersson, M.; Mustafa, K.; Wennerberg, A.; Jimbo, R. Local release of magnesium from mesoporous TiO 2 coatings stimulates the peri-implant expression of osteogenic markers and improves osteoconductivity in vivo. Acta Biomater. 2014, 10, 5193–5201. [Google Scholar] [CrossRef]
- Grandfield, K.; Pujari, S.; Ott, M.; Engqvist, H.; Xia, W. Effect of Calcium and Strontium on Mesoporous Titania Coatings for Implant Applications. J. Biomater. Nanobiotechnol. 2013, 04, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Björk, E.M.; Baumann, B.; Hausladen, F.; Wittig, R.; Lindén, M. Cell adherence and drug delivery from particle based mesoporous silica films. RSC Adv. 2019, 9, 17745–17753. [Google Scholar] [CrossRef] [Green Version]
- Solano-Umaña, V.; Vega-Baudrit, J.R. Micro, Meso and Macro Porous Materials on Medicine. J. Biomater. Nanobiotechnol. 2015, 6, 247–256. [Google Scholar] [CrossRef]
- Gentile, F.; La Rocca, R.; Marinaro, G.; Nicastri, A.; Toma, A.; Paonessa, F.; Cojoc, G.; Liberale, C.; Benfenati, F.; Di Fabrizio, E.; et al. Differential Cell Adhesion on Mesoporous Silicon Substrates. ACS Appl. Mater. Interfaces 2012, 4, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Atefyekta, S.; Ercan, B.; Karlsson, J.; Taylor, E.; Chung, S.; Webster, T.J. Antimicrobial performance of mesoporous titania thin films: Role of pore size, hydrophobicity, and antibiotic release. Int. J. Nanomed. 2016, 11, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium Oxide Antibacterial Surfaces in Biomedical Devices. Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef]
- Innocenzi, P.; Malfatti, L. Mesoporous thin films: Properties and applications. Chem. Soc. Rev. 2013, 42, 4198–4216. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.N.V.R.; Sameti, M.; Mohapatra, S.; Kong, X.; Lockey, R.; Bakowsky, U.; Lindenblatt, G.; Schmidt, C.H.; Lehr, C.-M. Cationic Silica Nanoparticles as Gene Carriers: Synthesis, Characterization and Transfection Efficiency In vitro and In vivo. J. Nanosci. Nanotechnol. 2004, 4, 876–881. [Google Scholar] [CrossRef]
- Jung, J.-I.; Bae, J.Y.; Bae, B.-S. Characterization and mesostructure control of mesoporous fluorinated organosilicate films. J. Mater. Chem. 2004, 14, 1988–1994. [Google Scholar] [CrossRef]
- Hu, T.; Bouamrani, A.; Tasciotti, E.; Li, L.; Liu, X.; Ferrari, M. Tailoring of the Nanotexture of Mesoporous Silica Films and Their Functionalized Derivatives for Selectively Harvesting Low Molecular Weight Protein. ACS Nano 2009, 4, 439–451. [Google Scholar] [CrossRef]
- Boullanger, A.; Alauzun, J.; Mehdi, A.; Reyé, C.; Corriu, R.J.P. Generic way for functionalised well-ordered cubic mesoporous silica via direct synthesis approach. New J. Chem. 2010, 34, 738–743. [Google Scholar] [CrossRef]
- Bass, J.D.; Grosso, D.; Boissiere, C.; Belamie, E.; Coradin, A.T.; Sanchez, C. Stability of Mesoporous Oxide and Mixed Metal Oxide Materials under Biologically Relevant Conditions. Chem. Mater. 2007, 19, 4349–4356. [Google Scholar] [CrossRef]
- He, Q.; Zhang, J.; Shi, J.; Zhu, Z.; Zhang, L.; Bu, W.; Guo, L.; Chen, Y. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials 2010, 31, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Ha, C.-S. Organic–inorganic hybrid mesoporous silicas: Functionalization, pore size, and morphology control. Chem. Rec. 2006, 6, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Tamanna, T.; Cashin, V.; Landersdorfer, C.B.; Bulitta, J.B.; Yu, A. Stability and controlled antibiotic release from thin films embedded with antibiotic loaded mesoporous silica nanoparticles. RSC Adv. 2015, 5, 107839–107846. [Google Scholar] [CrossRef]
- Pérez-Anguiano, O.; Wenger, B.; Pugin, R.; Hofmann, H.; Scolan, E. Controlling Mesopore Size and Processability of Transparent Enzyme-Loaded Silica Films for Biosensing Applications. ACS Appl. Mater. Interfaces 2015, 7, 2960–2971. [Google Scholar] [CrossRef] [PubMed]
- Magner, E. Immobilisation of enzymes on mesoporous silicate materials. Chem. Soc. Rev. 2013, 42, 6213–6222. [Google Scholar] [CrossRef] [Green Version]
- Soler-Illia, G.J.A.A.; Innocenzi, P. Mesoporous Hybrid Thin Films: The Physics and Chemistry Beneath. Chem. A Eur. J. 2006, 12, 4478–4494. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Yate, L.; Grzelczak, M.; Amenitsch, H.; Moya, S.E.; Bordoni, A.V.; Angelomé, P.C. One-Step Synthesis of Mesoporous Silica Thin Films Containing Available COOH Groups. ACS Omega 2017, 2, 4548–4555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athens, G.L.; Shayib, R.M.; Chmelka, B.F. Functionalization of mesostructured inorganic–organic and porous inorganic materials. Curr. Opin. Colloid Interface Sci. 2009, 14, 281–292. [Google Scholar] [CrossRef]
- Calvo, A.; Joselevich, M.; Soler-Illia, G.J.; Williams, F.J. Chemical reactivity of amino-functionalized mesoporous silica thin films obtained by co-condensation and post-grafting routes. Microporous Mesoporous Mater. 2009, 121, 67–72. [Google Scholar] [CrossRef]
- Bellino, M.G.; Tropper, I.; Durán, H.; Regazzoni, A.E.; Soler-Illia, G.J.A.A. Polymerase-Functionalized Hierarchical Mesoporous Titania Thin Films: Towards a Nanoreactor Platform for DNA Amplification. Small 2010, 6, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, M.; Weinberger, C. Selective Modification of Hierarchical Pores and Surfaces in Nanoporous Materials. Adv. Mater. Interfaces 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Marmiroli, B.; Sartori, B.; Kyvik, A.; Ratera, I.A.H. Structural study of the hydration of lipid membranes upon interaction with mesoporous supports prepared by standard methods and/or X-ray irradiation. Front. Mater. Sect. Colloid. Mater. Interfaces 2021. submitted. [Google Scholar]
- Nicole, L.; Boissière, C.; Grosso, D.; Quach, A.; Sanchez, C. Mesostructured hybrid organic–inorganic thin films. J. Mater. Chem. 2005, 15, 3598–3627. [Google Scholar] [CrossRef]
- Khalil, A.; Zimmermann, M.; Bell, A.K.; Kunz, U.; Hardt, S.; Kleebe, H.-J.; Stark, R.W.; Stephan, P.; Andrieu-Brunsen, A. Insights into the interplay of wetting and transport in mesoporous silica films. J. Colloid Interface Sci. 2020, 560, 369–378. [Google Scholar] [CrossRef]
- Fontecave, T.; Sanchez, C.; Azaïs, T.; Boissière, C. Chemical Modification As a Versatile Tool for Tuning Stability of Silica Based Mesoporous Carriers in Biologically Relevant Conditions. Chem. Mater. 2012, 24, 4326–4336. [Google Scholar] [CrossRef]
- Harmankaya, N.; Karlsson, J.; Palmquist, A.; Halvarsson, M.; Igawa, K.; Andersson, M.; Tengvall, P. Raloxifene and alendronate containing thin mesoporous titanium oxide films improve implant fixation to bone. Acta Biomater. 2013, 9, 7064–7073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izquierdo-Barba, I.; Vallet-Regí, M.; Kupferschmidt, N.; Terasaki, O.; Schmidtchen, A.; Malmsten, M. Incorporation of antimicrobial compounds in mesoporous silica film monolith. Biomaterials 2009, 30, 5729–5736. [Google Scholar] [CrossRef]
- Escobar, A.; Muzzio, N.; Coy, E.; Liu, H.; Bindini, E.; Andreozzi, P.; Wang, G.; Angelomé, P.; Delcea, M.; Grzelczak, M.; et al. Antibacterial Mesoporous Titania Films with Embedded Gentamicin and Surface Modified with Bone Morphogenetic Protein 2 to Promote Osseointegration in Bone Implants. Adv. Mater. Interfaces 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Doherty, C.M.; Gao, Y.; Marmiroli, B.; Amenitsch, H.; Lisi, F.; Malfatti, L.; Okada, K.; Takahashi, M.; Hill, A.J.; Innocenzi, P.; et al. Microfabrication of mesoporous silica encapsulated enzymes using deep X-ray lithography. J. Mater. Chem. 2012, 22, 16191–16195. [Google Scholar] [CrossRef]
- Bellino, M.G.; Regazzoni, A.E.; Soler-Illia, G.J.A.A. Amylase-Functionalized Mesoporous Silica Thin Films as Robust Biocatalyst Platforms. ACS Appl. Mater. Interfaces 2010, 2, 360–365. [Google Scholar] [CrossRef]
- Escobar, A.; Muzzio, N.E.; Martínez-Villacorta, Á.M.; Abarrategi, A.; Bindini, E.; Grzelczak, M.; Bordoni, A.V.; Angelomé, P.; Moya, S.E. Mesoporous titania coatings with carboxylated pores for complexation and slow delivery of strontium for osteogenic induction. Appl. Surf. Sci. 2020, 510, 145172. [Google Scholar] [CrossRef]
- Chai, Y.; Yamada, S.; Kobayashi, K.; Hasegawa, K.; Tagaya, M. Surface-functionalization of mesoporous silica films for effective osteoblast-like cell culture. Microporous Mesoporous Mater. 2019, 286, 1–8. [Google Scholar] [CrossRef]
- Catalano, P.N.; Pezzoni, M.; Costa, C.; Soler-Illia, G.J.D.A.A.; Bellino, M.G.; Desimone, M.F. Optically transparent silver-loaded mesoporous thin film coating with long-lasting antibacterial activity. Microporous Mesoporous Mater. 2016, 236, 158–166. [Google Scholar] [CrossRef]
- Trindade, F.; Politi, M.J. Sol-Gel Chemistry-Deals with Sol-Gel Processes; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [Green Version]
- Rosenholm, J.; Sahlgren, C.C.; Lindén, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles–opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björk, E.M.; Söderlind, F.; Odén, M. Single-pot synthesis of ordered mesoporous silica films with unique controllable morphology. J. Colloid Interface Sci. 2014, 413, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonda-Turo, C.; Carmagnola, I.; Ciardelli, G. Quartz Crystal Microbalance With Dissipation Monitoring: A Powerful Method to Predict the in vivo Behavior of Bioengineered Surfaces. Front. Bioeng. Biotechnol. 2018, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, J.P.; Idso, M.N.; Hussain, S.; Junk, M.J.N.; Fisher, J.M.; Phan, D.D.; Han, S.; Chmelka, B.F. Functionally Active Membrane Proteins Incorporated in Mesostructured Silica Films. J. Am. Chem. Soc. 2018, 140, 3892–3906. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Lin, Z.-X.; Wu, T.-Y.; Hsu, C.-H.; Lin, H.-P.; Wu, T.-S. Effects of morphology and pore size of mesoporous silicas on the efficiency of an immobilized enzyme. RSC Adv. 2021, 11, 10010–10017. [Google Scholar] [CrossRef]
- Frančič, N.; Bellino, M.G.; Soler-Illia, G.J.A.A.; Lobnik, A. Mesoporous titania thin films as efficient enzyme carriers for paraoxon determination/detoxification: Effects of enzyme binding and pore hierarchy on the biocatalyst activity and reusability. Analyst 2014, 139, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Avnir, D.; Coradin, T.; Lev, O.; Livage, J. Recent bio-applications of sol–gel materials. J. Mater. Chem. 2006, 16, 1013–1030. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Shi, J. In Vivo Bio-Safety Evaluations and Diagnostic/Therapeutic Applications of Chemically Designed Mesoporous Silica Nanoparticles. Adv. Mater. 2013, 25, 3144–3176. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, H.; Zhang, X.; Li, G.; Chang, Q.; Zhao, J.; Qiao, Y.; Ding, X.; Yang, G.; Liu, X.; et al. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale 2016, 8, 5291–5301. [Google Scholar] [CrossRef]
- Yoshizawa-Smith, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Chai, C.Y.; Loh, H.-S. In vitro evaluation of osteoblast adhesion, proliferation and differentiation on chitosan-TiO2 nanotubes scaffolds with Ca2+ ions. Mater. Sci. Eng. C 2017, 76, 144–152. [Google Scholar] [CrossRef]
- Galli, S.; Naito, Y.; Karlsson, J.; He, W.; Andersson, M.; Wennerberg, A.; Jimbo, R. Osteoconductive Potential of Mesoporous Titania Implant Surfaces Loaded with Magnesium: An Experimental Study in the Rabbit. Clin. Implant Dent. Relat. Res. 2015, 17, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kim, Y.-J.; Jang, J.-H. Enhanced osteoblast response to hydrophilic strontium and/or phosphate ions-incorporated titanium oxide surfaces. Clin. Oral Implant. Res. 2010, 21, 398–408. [Google Scholar] [CrossRef]
- Akgun, B.A.; Wren, A.W.; Durucan, C.; Towler, M.R.; Mellott, N.P. Sol–gel derived silver-incorporated titania thin films on glass: Bactericidal and photocatalytic activity. J. Sol-Gel Sci. Technol. 2011, 59, 228–238. [Google Scholar] [CrossRef]
- Zernik, J.; Twarog, K.; Upholt, W.B. Regulation of alkaline phosphatase and alpha2(I) procollagen synthesis during early intramembranous bone formation in the rat mandible. Differentiation 1990, 44, 207–215. [Google Scholar] [CrossRef] [PubMed]




| Mesoporous Matrix | Primary Functionalization Group | Secondary Funct. Group | Ref | Activity | |
|---|---|---|---|---|---|
| Silica | 3-aminopropyl triethoxysilane (APTES) |  | [78] | Hydrophobicity | |
| Silica | 3-aminopropyl triethoxysilane (APTES) | [79] | Hydrophobicity | ||
| Silica | 1H,1H,2H,2H-perfluorooctyl dimethylchlorosilane (PFODMCS) |  | [80] | Hydrophobicity | |
| Silica | 3-mercaptopropyl triethoxysilane (MPTEOS) |  | [81] | Protection Against Dissolution | |
| Silica (hybrid film) | ZrCl4 | [81] | Protection Against Dissolution | ||
| Silica | Triethoxymethylsilane (MTES) |  | [81] | Protection Against Dissolution | |
| Silica | 3-aminopropyl triethoxysilane (APTES) | [81] | Protection Against Dissolution | ||
| Silica | PEG |  | [67] | Protection Against Dissolution | |
| Silica | Carboxyl group | 3,3′-dioctadecyloxacarbocyanine perchlorate | [54] | Drug Loading | |
| Titania | dichlorodimethylsilane (DDMS) |  | alendronate and raloxifene | [82] | Drug Loading |
| Titania | dichlorodimethylsilane (DDMS) | daptomycin, vancomycin, gentamicyn | [57] | Drug Loading | |
| 3-mercaptopropyl triethoxysilane (MPTEOS) | LL-37, chlorexidine | [83] | Antimicrobials | ||
| Titania | Gentamicine | hrBMP-2 | [84] | Antimicrobials | |
| Silica | 3-aminopropyltriethoxysilane (APTES) | OpdA | [85] | Bio fuctionalization | |
| Silica | alpha-amylase | [86] | Bio functionalization | ||
| Silica-titania (hybrid) | Vinyltrimethoxysilane |  | carboxyl + Sr2+ | [87] | Ions |
| Silica | Ca2+, PO43−, Mg2+ (from SBF) | fibrinogen | [88] | Ions | |
| Titania | Mg2+ | [52] | Ions | ||
| Titania | Ag+ | [89] | Ions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartori, B.; Amenitsch, H.; Marmiroli, B. Functionalized Mesoporous Thin Films for Biotechnology. Micromachines 2021, 12, 740. https://doi.org/10.3390/mi12070740
Sartori B, Amenitsch H, Marmiroli B. Functionalized Mesoporous Thin Films for Biotechnology. Micromachines. 2021; 12(7):740. https://doi.org/10.3390/mi12070740
Chicago/Turabian StyleSartori, Barbara, Heinz Amenitsch, and Benedetta Marmiroli. 2021. "Functionalized Mesoporous Thin Films for Biotechnology" Micromachines 12, no. 7: 740. https://doi.org/10.3390/mi12070740