Principles for Controlling the Shape Recovery and Degradation Behavior of Biodegradable Shape-Memory Polymers in Biomedical Applications
Abstract
:1. Introduction
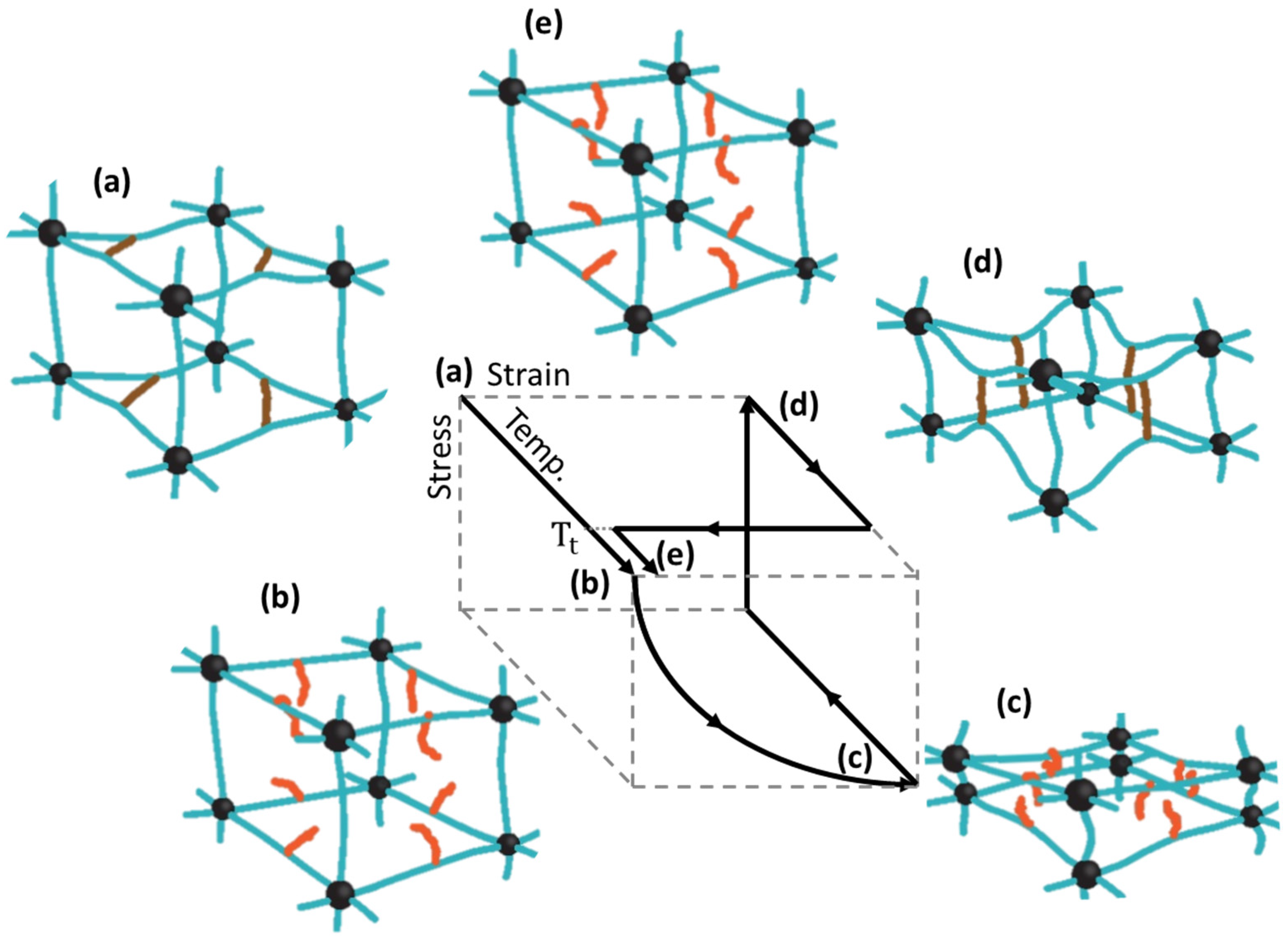
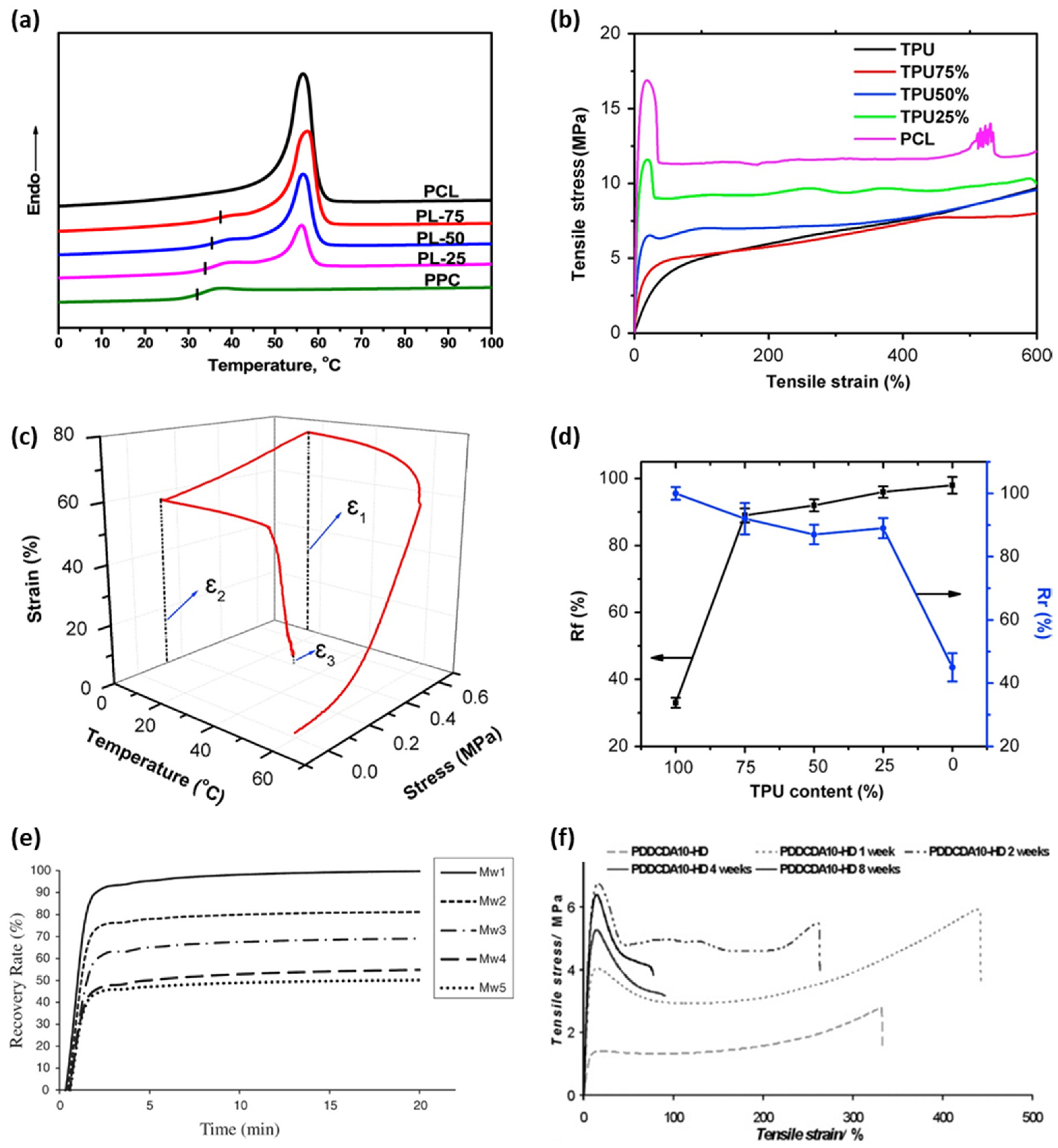
2. Control of properties of BSMPs
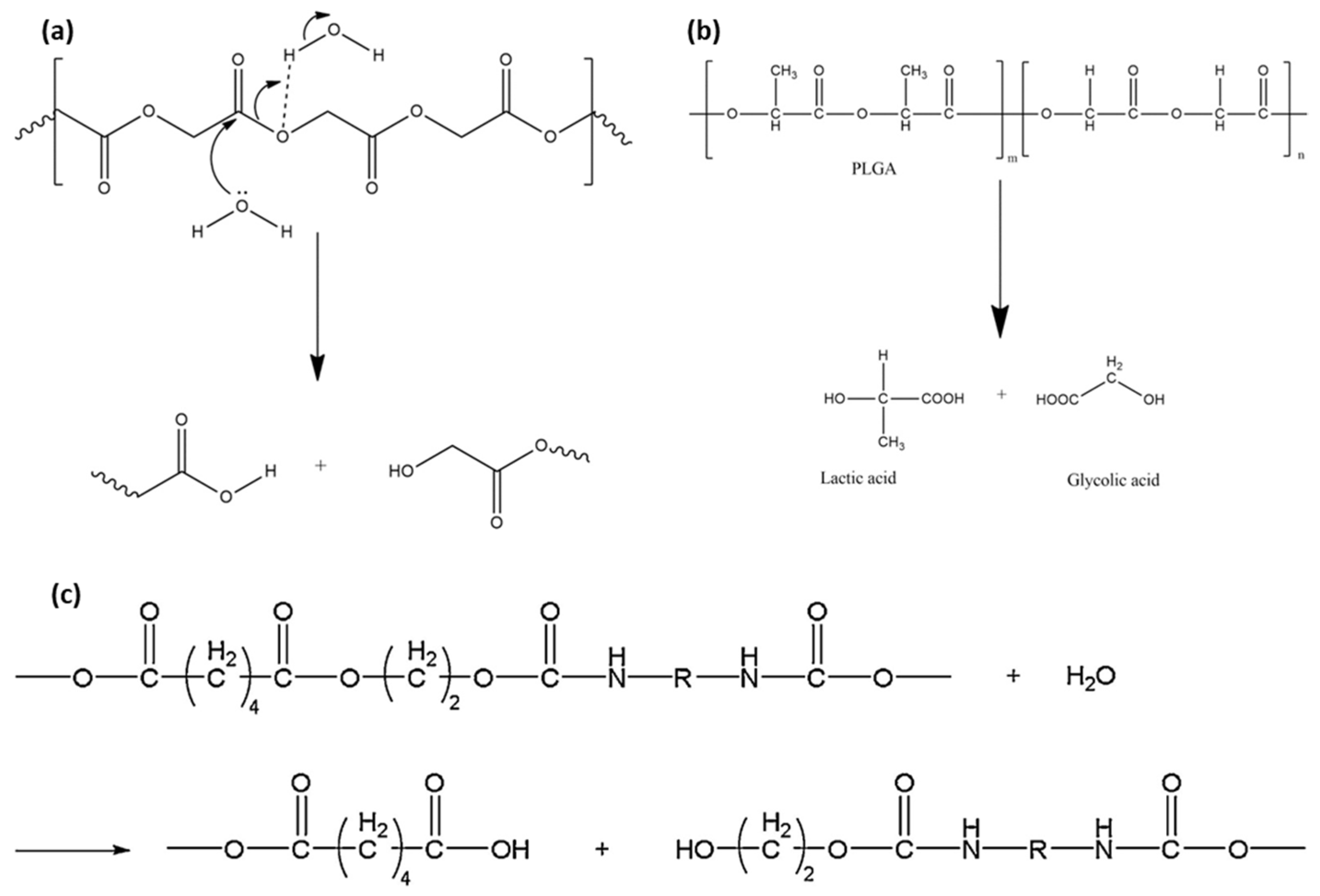
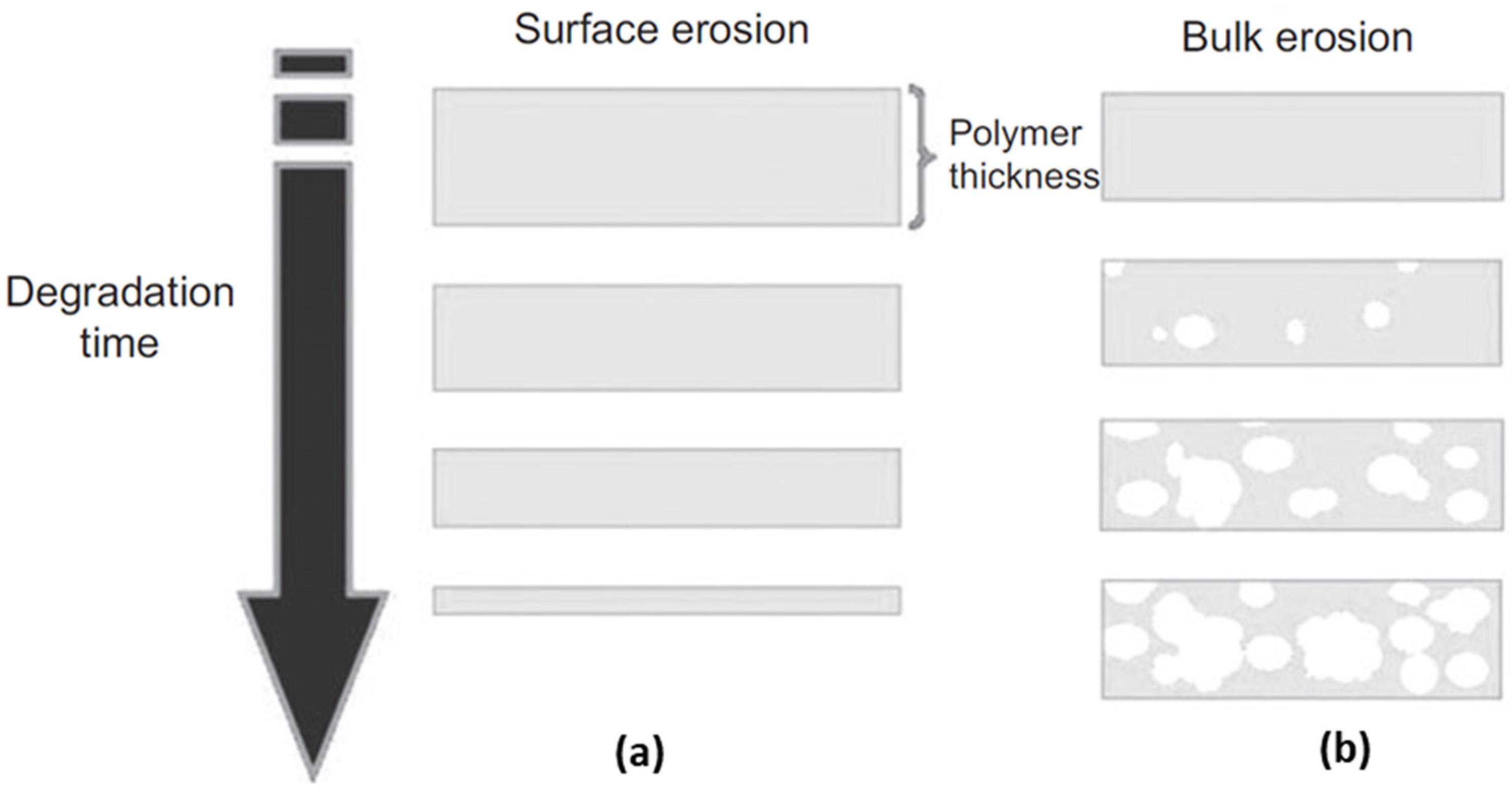
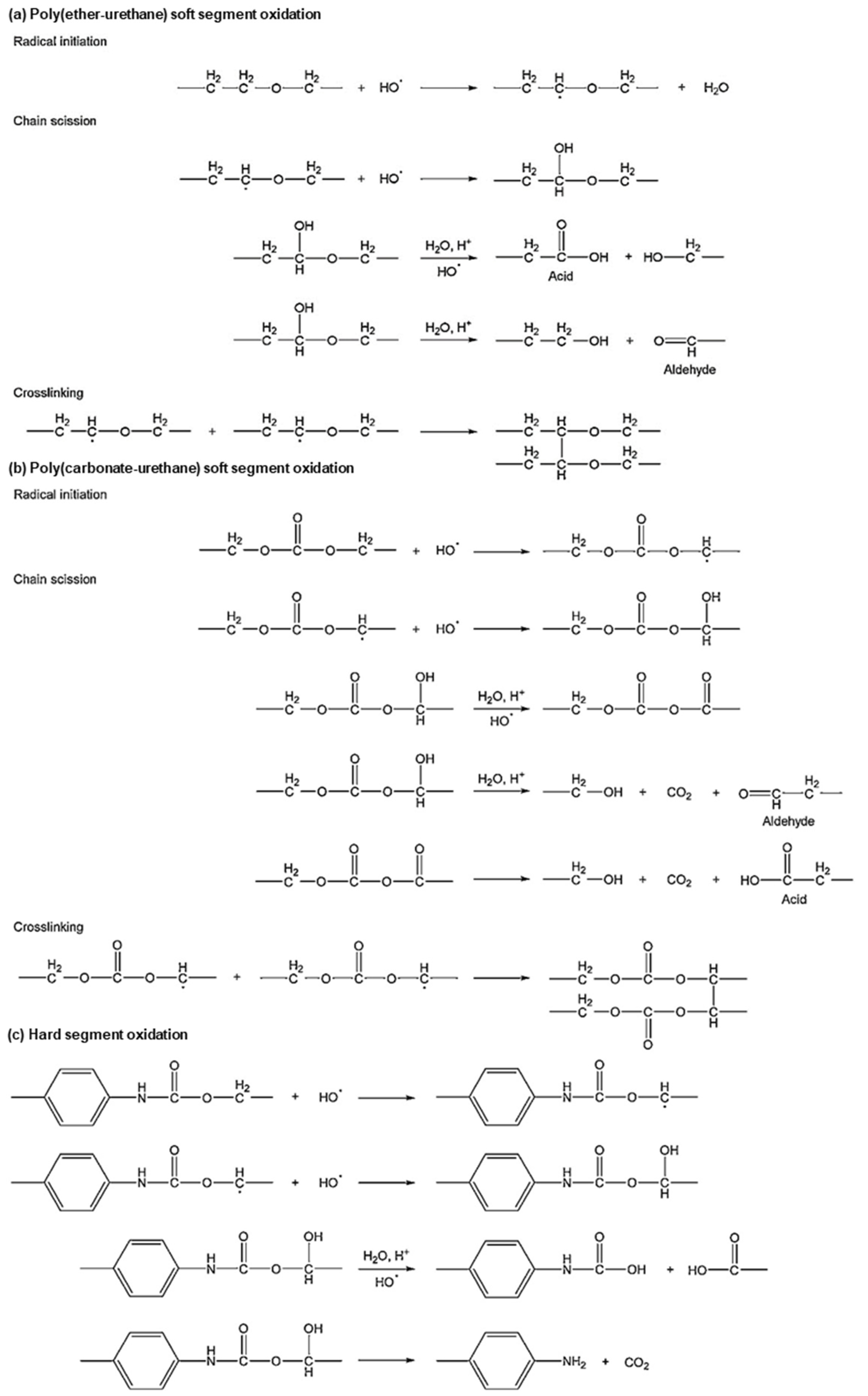
3. Biodegradable Behaviors of BSMPs
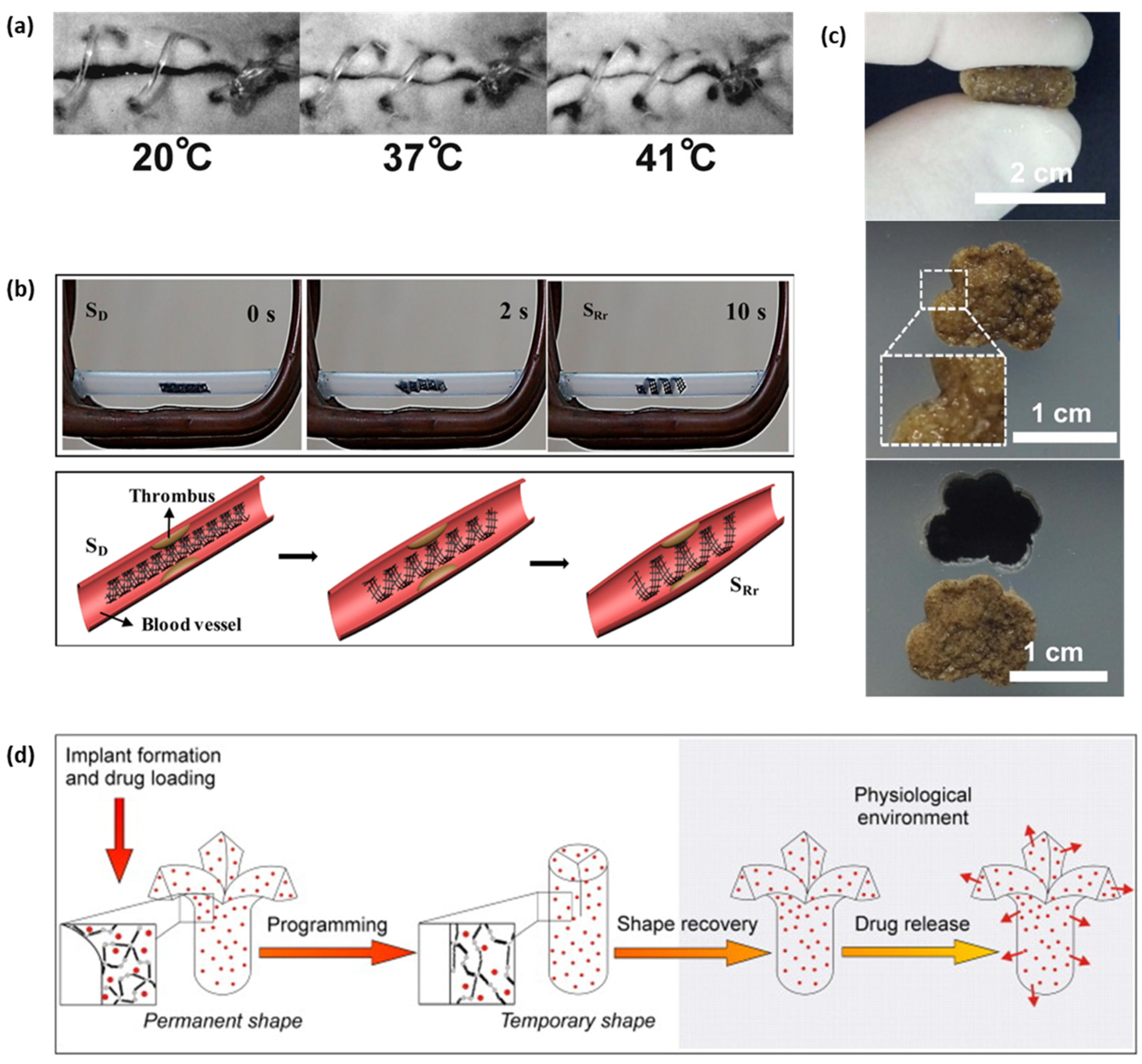
4. Applications of BSMPs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chang, L.; Read, T. Plastic deformation and diffusionless phase changes in metals—The gold-cadmium beta phase. JOM 1951, 3, 47–52. [Google Scholar] [CrossRef]
- Buehler, W.J.; Gilfrich, J.V.; Wiley, R. Effect of low-temperature phase changes on the mechanical properties of alloys near composition TiNi. J. Appl. Phys. 1963, 34, 1475–1477. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Liu, L.; Leng, J. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 023001. [Google Scholar] [CrossRef]
- Chan Vili, Y.Y.F. Investigating Smart Textiles Based on Shape Memory Materials. Text. Res. J. 2007, 77, 290–300. [Google Scholar] [CrossRef]
- Scalet, G. Two-Way and Multiple-Way Shape Memory Polymers for Soft Robotics: An Overview. Actuators 2020, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Delaey, J.; Dubruel, P.; Van Vlierberghe, S. Shape-Memory Polymers for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909047. [Google Scholar] [CrossRef]
- El Feninat, F.; Laroche, G.; Fiset, M.; Mantovani, D. Shape Memory Materials for Biomedical Applications. Adv. Eng. Mater. 2002, 4, 91–104. [Google Scholar] [CrossRef]
- Peterson, G.I.; Dobrynin, A.V.; Becker, M.L. Biodegradable Shape Memory Polymers in Medicine. Adv. Healthc. Mater. 2017, 6, 1700694. [Google Scholar] [CrossRef] [Green Version]
- Ramaraju, H.; Akman, R.E.; Safranski, D.L.; Hollister, S.J. Designing Biodegradable Shape Memory Polymers for Tissue Repair. Adv. Funct. Mater. 2020, 30, 2002014. [Google Scholar] [CrossRef]
- Fan, X.; Chung, J.Y.; Lim, Y.X.; Li, Z.; Loh, X.J. Review of Adaptive Programmable Materials and Their Bioapplications. ACS Appl. Mater. Interfaces 2016, 8, 33351–33370. [Google Scholar] [CrossRef]
- Hearon, K.; Wierzbicki, M.A.; Nash, L.D.; Landsman, T.L.; Laramy, C.; Lonnecker, A.T.; Gibbons, M.C.; Ur, S.; Cardinal, K.O.; Wilson, T.S.; et al. A Processable Shape Memory Polymer System for Biomedical Applications. Adv. Healthc. Mater. 2015, 4, 1386–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samal, S.; Molnárová, O.; Průša, F.; Kopeček, J.; Heller, L.; Šittner, P.; Škodová, M.; Abate, L.; Blanco, I. Net-shape NiTi shape memory alloy by spark plasma sintering method. Appl. Sci. 2021, 11, 1802. [Google Scholar] [CrossRef]
- Ghosh, P.; Rao, A.; Srinivasa, A.R. Design of multi-state and smart-bias components using Shape Memory Alloy and Shape Memory Polymer composites. Mater. Des. 2013, 44, 164–171. [Google Scholar] [CrossRef]
- Samal, S.; Tyc, O.; Heller, L.; Šittner, P.; Malik, M.; Poddar, P.; Catauro, M.; Blanco, I. Study of interfacial adhesion between nickel-titanium shape memory alloy and a polymer matrix by laser surface pattern. Appl. Sci. 2020, 10, 2172. [Google Scholar] [CrossRef] [Green Version]
- Lester, B.T.; Baxevanis, T.; Chemisky, Y.; Lagoudas, D.C. Review and perspectives: Shape memory alloy composite systems. Acta Mech. 2015, 226, 3907–3960. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, A.; Desfaits, A.-C.; Salazkin, I.; Sokolowski, W.M.; Raymond, J. Cold hibernated elastic memory foams for endovascular interventions. Biomaterials 2003, 24, 491–497. [Google Scholar] [CrossRef]
- Yang, C.-S.; Wu, H.-C.; Sun, J.-S.; Hsiao, H.-M.; Wang, T.-W. Thermo-Induced Shape-Memory PEG-PCL Copolymer as a Dual-Drug-Eluting Biodegradable Stent. ACS Appl. Mater. Interfaces 2013, 5, 10985–10994. [Google Scholar] [CrossRef]
- Shih, C.-C.; Lin, S.-J.; Chen, Y.-L.; Su, Y.-Y.; Lai, S.-T.; Wu, G.J.; Kwok, C.-F.; Chung, K.-H. The cytotoxicity of corrosion products of nitinol stent wire on cultured smooth muscle cells. J. Biomed. Mater. Res. 2000, 52, 395–403. [Google Scholar] [CrossRef]
- Hiebl, B.; Mrowietz, C.; Goers, J.; Bahramsoltani, M.; Plendl, J.; Kratz, K.; Lendlein, A.; Jung, F. In vivo evaluation of the angiogenic effects of the multiblock copolymer PDC using the hen’s egg chorioallantoic membrane test. Clin. Hemorheol. Microcirc. 2010, 46, 233–238. [Google Scholar] [CrossRef]
- Zhai, X.; Hou, C.; Pan, H.; Lu, W.W.; Liu, W.; Ruan, C. Nanoclay Incorporated Polyethylene-Glycol Nanocomposite Hydrogels for Stimulating In Vitro and In Vivo Osteogenesis. J. Biomed. Nanotechnol. 2018, 14, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Singh, A.P.; Rana, D.; Aswal, V.K.; Maiti, P. Biodegradable toughened nanohybrid shape memory polymer for smart biomedical applications. Nanoscale 2018, 10, 9917–9934. [Google Scholar] [CrossRef]
- Lendlein, A.; Langer, R. Biodegradable, Elastic Shape-Memory Polymers for Potential Biomedical Applications. Science 2002, 296, 1673. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Sharma, C.P. Review Paper: Absorbable Polymeric Surgical Sutures: Chemistry, Production, Properties, Biodegradability, and Performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef]
- Kashif, M.; Yun, B.-m.; Lee, K.-S.; Chang, Y.-W. Biodegradable shape-memory poly(ε-caprolactone)/polyhedral oligomeric silsequioxane nanocomposites: Sustained drug release and hydrolytic degradation. Mater. Lett. 2016, 166, 125–128. [Google Scholar] [CrossRef]
- Han, J.; Fei, G.; Li, G.; Xia, H. High Intensity Focused Ultrasound Triggered Shape Memory and Drug Release from Biodegradable Polyurethane. Macromol. Chem. Phys. 2013, 214, 1195–1203. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, S.; Wang, L.; Zheng, X.; Gong, T. Crosslinked poly(ε-caprolactone)/poly(sebacic anhydride) composites combining biodegradation, controlled drug release and shape memory effect. Compos. Part. B Eng. 2010, 41, 537–542. [Google Scholar] [CrossRef]
- Nagahama, K.; Ueda, Y.; Ouchi, T.; Ohya, Y. Biodegradable Shape-Memory Polymers Exhibiting Sharp Thermal Transitions and Controlled Drug Release. Biomacromolecules 2009, 10, 1789–1794. [Google Scholar] [CrossRef]
- Sonawane, V.C.; More, M.P.; Pandey, A.P.; Patil, P.O.; Deshmukh, P.K. Fabrication and characterization of shape memory polymers based bioabsorbable biomedical drug eluting stent. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1740–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Gu, S.Y.; Chang, K. 3D printed self-expandable vascular stents from biodegradable shape memory polymer. Adv. Polym. Technol. 2018, 37, 3222–3228. [Google Scholar] [CrossRef]
- Ansari, M.; Golzar, M.; Baghani, M.; Abbasishirsavar, M.; Taghavimehr, M. Force recovery evaluation of thermo-induced shape-memory polymer stent: Material, process and thermo-viscoelastic characterization. Smart Mater. Struct. 2019, 28, 095022. [Google Scholar] [CrossRef]
- Gu, S.-Y.; Chang, K.; Jin, S.-P. A dual-induced self-expandable stent based on biodegradable shape memory polyurethane nanocomposites (PCLAU/Fe3O4) triggered around body temperature. J. Appl. Polym. Sci. 2018, 135, 45686. [Google Scholar] [CrossRef]
- Xue, L.; Dai, S.; Li, Z. Biodegradable shape-memory block co-polymers for fast self-expandable stents. Biomaterials 2010, 31, 8132–8140. [Google Scholar] [CrossRef]
- Bao, M.; Lou, X.; Zhou, Q.; Dong, W.; Yuan, H.; Zhang, Y. Electrospun Biomimetic Fibrous Scaffold from Shape Memory Polymer of PDLLA-co-TMC for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2014, 6, 2611–2621. [Google Scholar] [CrossRef]
- Montgomery, M.; Ahadian, S.; Davenport Huyer, L.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S.; et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Neuss, S.; Blomenkamp, I.; Stainforth, R.; Boltersdorf, D.; Jansen, M.; Butz, N.; Perez-Bouza, A.; Knüchel, R. The use of a shape-memory poly(ε-caprolactone)dimethacrylate network as a tissue engineering scaffold. Biomaterials 2009, 30, 1697–1705. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Zhang, F.; Liu, Y.; Leng, J. A Review of Shape Memory Polymers and Composites: Mechanisms, Materials, and Applications. Adv. Mater. 2021, 33, 2000713. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Kelch, S. Shape-memory polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Hu, J. Shape Memory Polymers and Textiles; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Hu, J.; Chen, S. A review of actively moving polymers in textile applications. J. Mater. Chem. 2010, 20, 3346–3355. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Huang, H.-X.; Turng, L.-S. Shape memory thermoplastic polyurethane (TPU)/poly(ε-caprolactone) (PCL) blends as self-knotting sutures. J. Mech. Behav. Biomed. Mater. 2016, 64, 94–103. [Google Scholar] [CrossRef]
- Nagata, M.; Yamamoto, Y. Synthesis and characterization of photocrosslinked poly(ε-caprolactone)s showing shape-memory properties. J. Polym. Sci. Part. A Polym. Chem. 2009, 47, 2422–2433. [Google Scholar] [CrossRef]
- Kelch, S.; Choi, N.Y.; Wang, Z.; Lendlein, A. Amorphous, Elastic AB Copolymer Networks from Acrylates and Poly[(L-lactide)-ran-glycolide]dimethacrylates. Adv. Eng. Mater. 2008, 10, 494–502. [Google Scholar] [CrossRef]
- Wong, Y.S.; Salvekar, A.V.; Zhuang, K.D.; Liu, H.; Birch, W.R.; Tay, K.H.; Huang, W.M.; Venkatraman, S.S. Bioabsorbable radiopaque water-responsive shape memory embolization plug for temporary vascular occlusion. Biomaterials 2016, 102, 98–106. [Google Scholar] [CrossRef]
- Alavijeh, R.Z.; Shokrollahi, P.; Barzin, J. A thermally and water activated shape memory gelatin physical hydrogel, with a gel point above the physiological temperature, for biomedical applications. J. Mater. Chem. B 2017, 5, 2302–2314. [Google Scholar] [CrossRef]
- Li, G.; Fei, G.; Xia, H.; Han, J.; Zhao, Y. Spatial and temporal control of shape memory polymers and simultaneous drug release using high intensity focused ultrasound. J. Mater. Chem. 2012, 22, 7692–7696. [Google Scholar] [CrossRef]
- Hribar, K.C.; Metter, R.B.; Ifkovits, J.L.; Troxler, T.; Burdick, J.A. Light-Induced Temperature Transitions in Biodegradable Polymer and Nanorod Composites. Small 2009, 5, 1830–1834. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Lv, J.; Li, Y.; Zhang, F.; Li, J.; Liu, Y.; Liu, L.; Leng, J. 4D-Printed Biodegradable and Remotely Controllable Shape Memory Occlusion Devices. Adv. Funct. Mater. 2019, 29, 1906569. [Google Scholar] [CrossRef]
- Kumar Patel, K.; Purohit, R. Future Prospects of shape memory polymer nano-composite and epoxy based shape memory polymer- A review. Mater. Today: Proc. 2018, 5, 20193–20200. [Google Scholar] [CrossRef]
- Hu, J. Advances in Shape Memory Polymers; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Behl, M.; Lendlein, A. Shape-memory polymers. Mater. Today 2007, 10, 20–28. [Google Scholar] [CrossRef]
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49-50, 79–120. [Google Scholar] [CrossRef] [Green Version]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49–50, 3–33. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Hu, X.; Shen, J.; Guo, S. Biocompatible Shape Memory Blend for Self-Expandable Stents with Potential Biomedical Applications. ACS Appl. Mater. Interfaces 2017, 9, 13988–13998. [Google Scholar] [CrossRef]
- Ben Abdallah, A.; Gamaoun, F.; Kallel, A.; Tcharkhtchi, A. Molecular weight influence on shape memory effect of shape memory polymer blend (poly(caprolactone)/styrene-butadiene-styrene). J. Appl. Polym. Sci. 2021, 138, 49761. [Google Scholar] [CrossRef]
- Pfau, M.R.; McKinzey, K.G.; Roth, A.A.; Grunlan, M.A. PCL-Based Shape Memory Polymer Semi-IPNs: The Role of Miscibility in Tuning the Degradation Rate. Biomacromolecules 2020, 21, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Tan, B.H.; Li, Z.; Loh, X.J. Control of PLA Stereoisomers-Based Polyurethane Elastomers as Highly Efficient Shape Memory Materials. ACS Sustain. Chem. Eng. 2017, 5, 1217–1227. [Google Scholar] [CrossRef]
- Zhang, B.; Filion, T.M.; Kutikov, A.B.; Song, J. Facile Stem Cell Delivery to Bone Grafts Enabled by Smart Shape Recovery and Stiffening of Degradable Synthetic Periosteal Membranes. Adv. Funct. Mater. 2017, 27, 1604784. [Google Scholar] [CrossRef]
- Choi, N.Y.; Kelch, S.; Lendlein, A. Synthesis, Shape-Memory Functionality and Hydrolytical Degradation Studies on Polymer Networks from Poly(rac-lactide)-b-poly(propylene oxide)-b-poly(rac-lactide) dimethacrylates. Adv. Eng. Mater. 2006, 8, 439–445. [Google Scholar] [CrossRef]
- Li, Y.; Xin, S.; Bian, Y.; Dong, Q.; Han, C.; Xu, K.; Dong, L. Stereocomplex crystallite network in poly (D, L-lactide): Formation, structure and the effect on shape memory behaviors and enzymatic hydrolysis of poly (D, L-lactide). RSC Adv. 2015, 5, 24352–24362. [Google Scholar] [CrossRef]
- Zhang, D.; George, O.J.; Petersen, K.M.; Jimenez-Vergara, A.C.; Hahn, M.S.; Grunlan, M.A. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014, 10, 4597–4605. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.-Q.; Yang, K.-K.; Wang, Y.-Z. Shape-memory poly (p-dioxanone)–poly (ɛ-caprolactone)/sepiolite nanocomposites with enhanced recovery stress. Chin. Chem. Lett. 2015, 26, 1221–1224. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, B.H.; Li, Z. Biodegradable polyester shape memory polymers: Recent advances in design, material properties and applications. Mater. Sci. Eng. C 2018, 92, 1061–1074. [Google Scholar] [CrossRef]
- Le, D.M.; Kulangara, K.; Adler, A.F.; Leong, K.W.; Ashby, V.S. Dynamic Topographical Control of Mesenchymal Stem Cells by Culture on Responsive Poly (ϵ-caprolactone) Surfaces. Adv. Mater. 2011, 23, 3278–3283. [Google Scholar] [CrossRef]
- Neuendorf, R.E.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Adhesion between biodegradable polymers and hydroxyapatite: Relevance to synthetic bone-like materials and tissue engineering scaffolds. Acta Biomater. 2008, 4, 1288–1296. [Google Scholar] [CrossRef]
- Serrano, M.C.; Carbajal, L.; Ameer, G.A. Novel Biodegradable Shape-Memory Elastomers with Drug-Releasing Capabilities. Adv. Mater. 2011, 23, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Guan, D.; Liu, J.; Luo, Y.; Wang, Y. Synthesis and characterization of biodegradable linear shape memory polyurethanes with high mechanical performance by incorporating novel long chain diisocyanates. New J. Chem. 2020, 44, 3493–3503. [Google Scholar] [CrossRef]
- Seo, J.; Choi, J.-W.; Koh, Y.-H.; Seo, J.-H. Enhanced Mechanical Strength, Flexibility, and Shape-Restoring Rate of a Drug-Eluting Shape-Memory Polymer by Incorporation of Supramolecular Cross-Linkers. ACS Macro Lett. 2020, 9, 389–395. [Google Scholar] [CrossRef]
- Lyu, S.; Untereker, D. Degradability of Polymers for Implantable Biomedical Devices. Int. J. Mol. Sci. 2009, 10, 4033. [Google Scholar] [CrossRef] [Green Version]
- Burkersroda, F.v.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002, 23, 4221–4231. [Google Scholar] [CrossRef]
- Göpferich, A.; Tessmar, J. Polyanhydride degradation and erosion. Adv. Drug Deliv. Rev. 2002, 54, 911–931. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Pretsch, T.; Jakob, I.; Müller, W. Hydrolytic degradation and functional stability of a segmented shape memory poly (ester urethane). Polym. Degrad. Stab. 2009, 94, 61–73. [Google Scholar] [CrossRef]
- Hu, X.; He, J.; Yong, X.; Lu, J.; Xiao, J.; Liao, Y.; Li, Q.; Xiong, C. Biodegradable poly (lactic acid-co-trimethylene carbonate)/chitosan microsphere scaffold with shape-memory effect for bone tissue engineering. Colloids Surf. B Biointerfaces 2020, 195, 111218. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Balk, M.; Behl, M.; Wischke, C.; Zotzmann, J.; Lendlein, A. Recent advances in degradable lactide-based shape-memory polymers. Adv. Drug Deliv. Rev. 2016, 107, 136–152. [Google Scholar] [CrossRef] [Green Version]
- Ginjupalli, K.; Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Nagaraja Upadhya, P. Poly(α-hydroxy acid) based polymers: A review on material and degradation aspects. Polym. Degrad. Stab. 2017, 144, 520–535. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.; Bryant, P.; Kurusingal, V.; Colwell, J.M.; Laycock, B. Degradation and stabilization of polyurethane elastomers. Prog. Polym. Sci. 2019, 90, 211–268. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Chew, S.A.; Arriaga, M.A.; Hinojosa, V.A. Effects of surface area to volume ratio of PLGA scaffolds with different architectures on scaffold degradation characteristics and drug release kinetics. J. Biomed. Mater. Res. A 2016, 104, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Vieira, J.; Ferra, J. Mechanical study of PLA–PCL fibers during in vitro degradation. J. Mech Behav Biomed. 2011, 4, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Wang, L.; Ge, J.; Guo, B.; Ma, P.X. Strong Electroactive Biodegradable Shape Memory Polymer Networks Based on Star-Shaped Polylactide and Aniline Trimer for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 6772–6781. [Google Scholar] [CrossRef]
- Lendlein, A.; Sisson, A. Handbook of Biodegradable Polymers: Isolation, Synthesis, Characterization and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bian, L.; Mohammed, H.S.; Shipp, D.A.; Goulet, P.J.G. Raman Microspectroscopy Study of the Hydrolytic Degradation of Polyanhydride Network Polymers. Langmuir 2019, 35, 6387–6392. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Jang, W.J.; Lee, D.; Chun, H.; Chang, Y.-W. Fatigue crack growth of elastomers in the swollen state. Polymer 2000, 41, 179–183. [Google Scholar] [CrossRef]
- Rodriguez, J.N.; Miller, M.W.; Boyle, A.; Horn, J.; Yang, C.-K.; Wilson, T.S.; Ortega, J.M.; Small, W.; Nash, L.; Skoog, H. Reticulation of low density shape memory polymer foam with an in vivo demonstration of vascular occlusion. J. Mech. Behav. Biomed. Mater. 2014, 40, 102–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangiorgi, G.; Melzi, G.; Agostoni, P.; Cola, C.; Clementi, F.; Romitelli, P.; Virmani, R.; Colombo, A. Engineering aspects of stents design and their translation into clinical practice. Ann. Ist. Super. Sanita 2007, 43, 89–100. [Google Scholar] [PubMed]
- Tamai, H.; Igaki, K.; Kyo, E.; Kosuga, K.; Kawashima, A.; Matsui, S.; Komori, H.; Tsuji, T.; Motohara, S.; Uehata, H. Initial and 6-Month Results of Biodegradable Poly-l-Lactic Acid Coronary Stents in Humans. Circulation 2000, 102, 399–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatraman, S.S.; Tan, L.P.; Joso, J.F.D.; Boey, Y.C.F.; Wang, X. Biodegradable stents with elastic memory. Biomaterials 2006, 27, 1573–1578. [Google Scholar] [CrossRef]
- Duarah, R.; Singh, Y.P.; Gupta, P.; Mandal, B.B.; Karak, N. High performance bio-based hyperbranched polyurethane/carbon dot-silver nanocomposite: A rapid self-expandable stent. Biofabrication 2016, 8, 045013. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, Q.; Yao, Y.; Liu, L.; Liu, Y.; Leng, J. Direct-Write Fabrication of 4D Active Shape-Changing Structures Based on a Shape Memory Polymer and Its Nanocomposite. ACS Appl. Mater. Interfaces 2017, 9, 876–883. [Google Scholar] [CrossRef]
- Wischke, C.; Neffe, A.T.; Steuer, S.; Lendlein, A. Evaluation of a degradable shape-memory polymer network as matrix for controlled drug release. J. Control. Release 2009, 138, 243–250. [Google Scholar] [CrossRef]
- Baer, G.M.; Wilson, T.S.; Small Iv, W.; Hartman, J.; Benett, W.J.; Matthews, D.L.; Maitland, D.J. Thermomechanical properties, collapse pressure, and expansion of shape memory polymer neurovascular stent prototypes. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2009, 90B, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Yeazel, T.R.; Becker, M.L. Advancing Toward 3D Printing of Bioresorbable Shape Memory Polymer Stents. Biomacromolecules 2020, 21, 3957–3965. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Wei, Y.; Liu, T.; Gu, J.; Li, Z.; Wang, M.; Zhao, D.; Qiao, A.; Liu, Y. Multi-Objective Optimizations of Biodegradable Polymer Stent Structure and Stent Microinjection Molding Process. Polymers 2017, 9, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Xu, S.; Luo, X.; Liu, Z. Theoretical and Numerical Analysis of Mechanical Behaviors of a Metamaterial-Based Shape Memory Polymer Stent. Polymers 2020, 12, 1784. [Google Scholar] [CrossRef] [PubMed]
- Paonessa, S.; Barbani, N.; Rocchietti, E.C.; Giachino, C.; Cristallini, C. Design and development of a hybrid bioartificial water-induced shape memory polymeric material as an integral component for the anastomosis of human hollow organs. Mater. Sci. Eng. C 2017, 75, 1427–1434. [Google Scholar] [CrossRef] [Green Version]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni, A.; Pandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): Design concept and 4D printing feasibility. Int. J. Pharm. 2019, 559, 299–311. [Google Scholar] [CrossRef]
- Jaworska, J.; Jelonek, K.; Sobota, M.; Kasperczyk, J.; Dobrzynski, P.; Musial-Kulik, M.; Smola-Dmochowska, A.; Janeczek, H.; Jarzabek, B. Shape-memory bioresorbable terpolymer composite with antirestenotic drug. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J. A Polyurethane Based Composite with Mechanically Enhanced Performance for Bone repair. Mater. Today Proc. 2019, 16, 1399–1404. [Google Scholar] [CrossRef]
- Wendels, S.; Avérous, L. Biobased polyurethanes for biomedical applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef] [PubMed]
- Ker, D.F.E.; Wang, D.; Behn, A.W.; Wang, E.T.H.; Zhang, X.; Zhou, B.Y.; Mercado-Pagán, Á.E.; Kim, S.; Kleimeyer, J.; Gharaibeh, B.; et al. Functionally Graded, Bone- and Tendon-Like Polyurethane for Rotator Cuff Repair. Adv. Funct. Mater. 2018, 28, 1707107. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, V.; Contessi, N.; De Marco, C.; Bertoldi, S.; Tanzi, M.C.; Daidone, M.G.; Farè, S. Polyurethane foam scaffold as in vitro model for breast cancer bone metastasis. Acta Biomater. 2017, 63, 306–316. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Qian, Y.; Pan, X.; Zhu, J.; Zhang, Z.; Qian, Z.; Sun, Z.; Pi, B. Cystine dimethyl ester cross-linked PEG-poly(urethane-urea)/nano-hydroxyapatite composited biomimetic scaffold for bone defect repair. J. Biomater. Sci. Polym. Ed. 2020, 31, 407–422. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Jeng, U.S.; Hsu, S.-h. Biodegradable Water-Based Polyurethane Shape Memory Elastomers for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
| Material | Hard Segment | Soft Segment | (MPa) | Ref | ||||
|---|---|---|---|---|---|---|---|---|
| PPC/PCL | PCL | PPC | 37 | 97 | 94 | 6%/s | - | [53] |
| PCL-SBS | PCL | SBS | 58 | - | 100 | 1%/min | 116 | [54] |
| PCL/PU | PU | PCL | 45 | - | 77 | 1.3/s | 4.3–9 | [30] |
| PCL-PLLA | PLLA | PCL | 85 | - | - | - | 120–180 | [55] |
| Fe3O4-PCLAU | PLA | PCL | 40 | 99 | 82–85 | 0.2%/s | - | [31] |
| Fe3O4-PLA | Crystallites | Amorphous | 65–67 | 90–95 | 4%/s | 2–3 × 103 | [47] | |
| PLA | Crystallites | Amorphous | 70 | 99 | 87–96 | - | 4 × 103 | [29] |
| PLDU | PLLA | PDLLA | 38–46 | 99 | 72–95 | - | 1.5–2 × 103 | [56] |
| PLGA-PEG | PLGA | PEG | 37 | 99 | 99 | 3–10%/min | 206–330 | [57] |
| PLGA/PEGDA | PLGA | PEG | 37 | - | 82–92 | 0.7%/s | 1.2–1.6 × 103 | [43] |
| PCL-PEG | PCL | PEG | 41–45 | 100 | 88–100 | 9–10%/s | 28–97 | [17] |
| GelUPy | Gelatin | UPy | 100 | - | - | - | 2.42 | [44] |
| PLA-PPG | PLA | PPG | 92–96 | 87–99.5 | 2.6–3.5%/ | - | [58] | |
| PDLLA-co-TMC | DDLA | TMC | 37–44 | 99 | 94–100 | - | 175–200 | [33] |
| PCL/TspPOSS | OPD | PCL | 70 | 81 | 85 | - | - | [24] |
| TPU/PCL | TPU | PCL | 32 | 98 | 90 | - | - | [40] |
| Sc-PLA-PDLLA | Sc-PLA | PDLLA | 70 | 65–99 | 4.5–5%/s | - | [59] | |
| PCL-DA | DA | PCL | 54–60 | 100 | 95–100 | - | 0.54–4.3 | [35,60] |
| PLGA-EA | EA | PLA | 20–50 | 97–98 | 99–100 | - | 1.6–288 | [42] |
| PLGA-BA | BA | PLA | −10–40 | 93–97 | 84–100 | - | 3.3–30 | [42] |
| PLGA-HA | HA | PLA | −30–60 | 91–96 | 97–99 | - | 12–37 | [42] |
| PCL-MDI-BDO | MDI-BDO | PCL | 36–52 | 99 | 99 | 4%/s | - | [25] |
| PCL-PHBV | PHVB | PCL | 40 | 94 | 98 | 4%/s | 42–70 | [32] |
| PCL-PPDO | PPDO | PCL | 37 | 99 | 97–99 | 1.5%/s | 407–542 | [61] |
| ICM/PCL | ICM | PCL | 60 | 89–100 | 85–100 | - | - | [41] |
| OCL-HDI | HDI | OCL | 37–39 | 98 | 99 | - | - | [27] |
| OCL-ODX | ODX | OCL | 40 | 98–99.5 | 76–99 | 5%/s | - | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kang, S.-K. Principles for Controlling the Shape Recovery and Degradation Behavior of Biodegradable Shape-Memory Polymers in Biomedical Applications. Micromachines 2021, 12, 757. https://doi.org/10.3390/mi12070757
Lee J, Kang S-K. Principles for Controlling the Shape Recovery and Degradation Behavior of Biodegradable Shape-Memory Polymers in Biomedical Applications. Micromachines. 2021; 12(7):757. https://doi.org/10.3390/mi12070757
Chicago/Turabian StyleLee, Junsang, and Seung-Kyun Kang. 2021. "Principles for Controlling the Shape Recovery and Degradation Behavior of Biodegradable Shape-Memory Polymers in Biomedical Applications" Micromachines 12, no. 7: 757. https://doi.org/10.3390/mi12070757






