Sensor-on-Microtips: Design and Development of Hydrothermally Grown ZnO on Micropipette Tips as a Modified Working Electrode for Detection of Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals Used
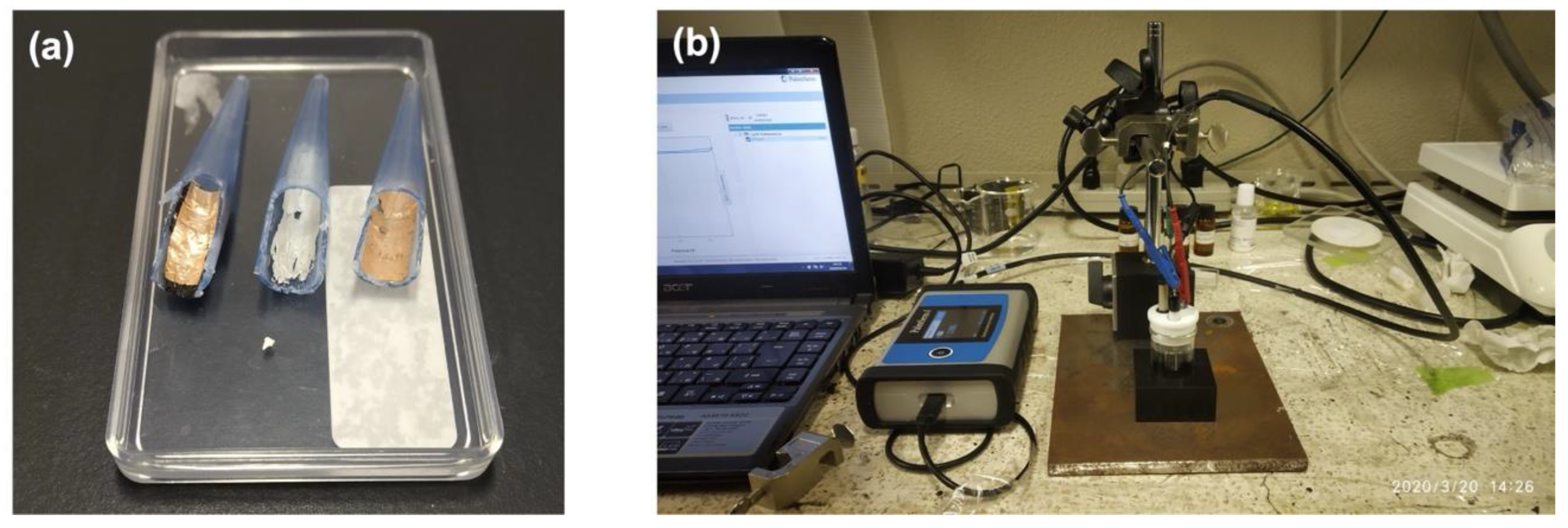
2.2. Sputtering of Ag on Bare Micropipette Tips as Electrical Contacts
2.3. Growth of ZnO Nanostructures on the Sputtered Micropipette Tips
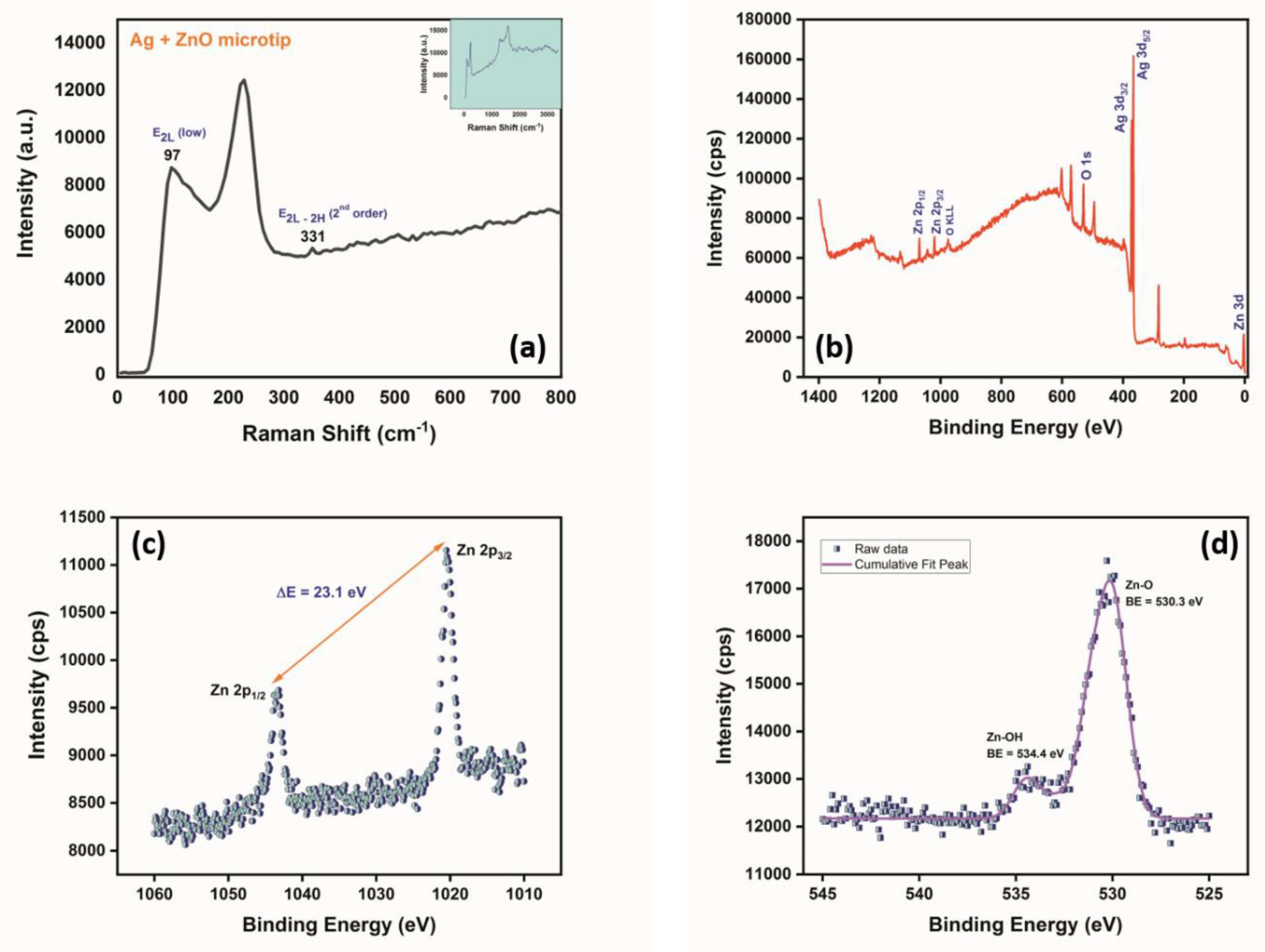
2.4. Characterization of ZnO Nanostructures
2.5. Electrochemical Analysis
3. Results and Discussion
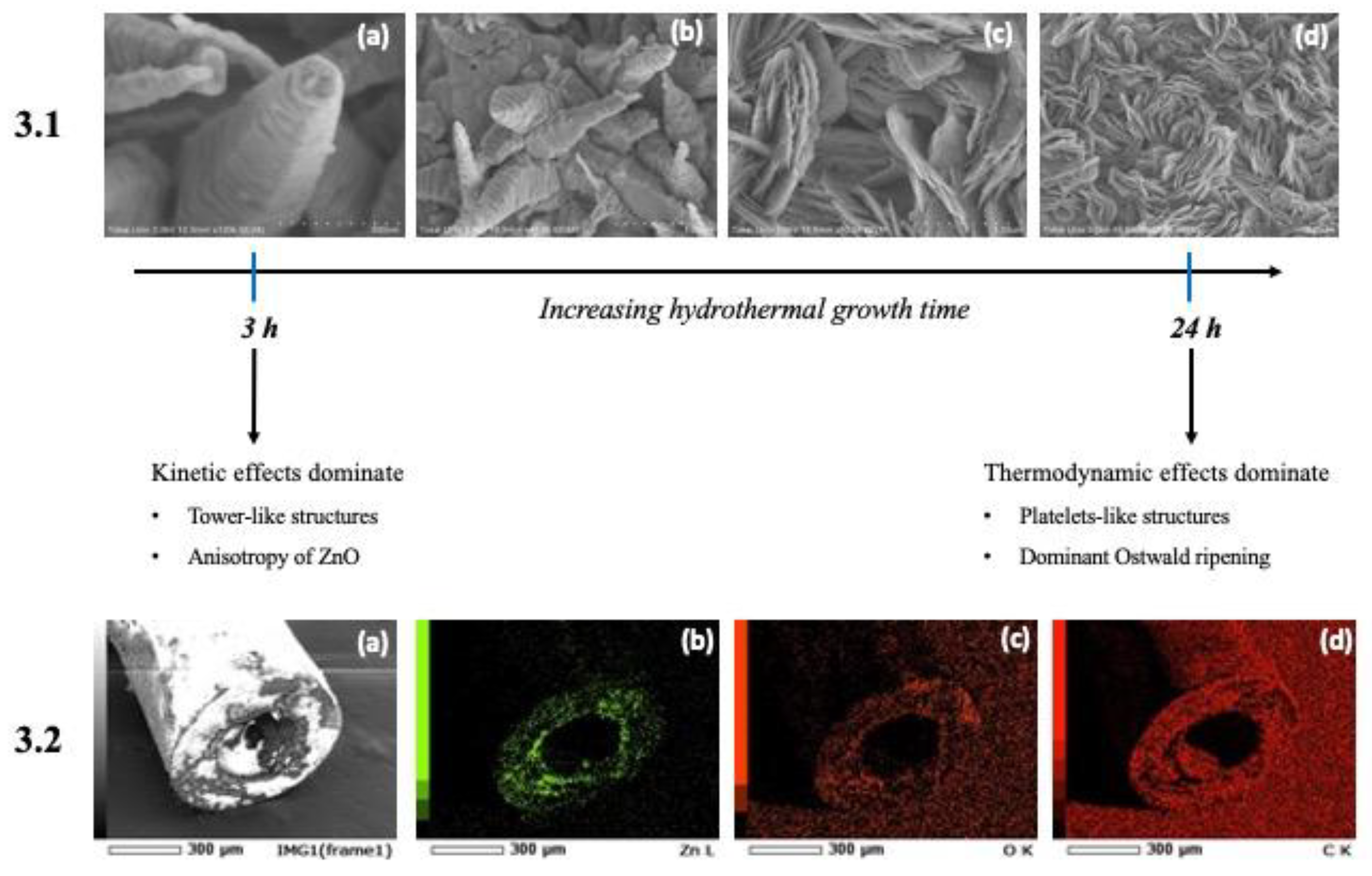
3.1. Characterization of ZnO-Modified Micropipette Tips
3.2. ZnO Nanostructures Grown on a Micropipette Tip as a Modified Working Electrode
3.2.1. Multilayer Confirmation and Stability Analysis
3.2.2. Electrochemical Detection of Glucose Using the ZnO-Modified Micropipette Tip
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, I.H.; Kim, D.H.; Park, S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef] [Green Version]
- Lopes Da Silva, A.R.; Jhones Dos Santos, A.; Martínez-Huitle, C.A. Electrochemical measurements and theoretical studies for understanding the behavior of catechol, resorcinol and hydroquinone on the boron doped diamond surface. RSC Adv. 2018, 8, 3483–3492. [Google Scholar] [CrossRef] [Green Version]
- Ball, J.C.; Scott, D.L.; Lumpp, J.K.; Daunert, S.; Wang, J.; Bachas, L.G. Electrochemistry in nanovials fabricated by combining screen printing and laser micromachining. Anal. Chem. 2000, 72, 497–501. [Google Scholar] [CrossRef]
- Paixão, T.R.L.C.; Bertotti, M. Fabrication of disposable voltammetric electronic tongues by using Prussian Blue films electrodeposited onto CD-R gold surfaces and recognition of milk adulteration. Sens. Actuators B Chem. 2009, 137, 266–273. [Google Scholar] [CrossRef]
- Lowinsohn, D.; Richter, E.M.; Angnes, L.; Bertotti, M. Disposable gold electrodes with reproducible area using recordable CDs and toner masks. Electroanalysis 2006, 18, 89–94. [Google Scholar] [CrossRef]
- Billon, G.; Van Den Berg, C.M.G. Gold and silver micro-wire electrodes for trace analysis of metals. Electroanalysis 2004, 16, 1583–1591. [Google Scholar] [CrossRef]
- Li, Q.S.; Ye, B.C.; Liu, B.X.; Zhong, J.J. Improvement of the performance of H2O2 oxidation at low working potential by incorporating TTF-TCNQ into a platinum wire electrode for glucose determination. Biosens. Bioelectron. 1999, 14, 327–334. [Google Scholar] [CrossRef]
- Wang, J.; Gründler, P. Electrochemical Studies of Thiol Self-Assembled Monolayers at a Heated Gold-Wire Microelectrode. Electroanalysis 2003, 15, 1756–1761. [Google Scholar] [CrossRef]
- Wang, J.; Gründler, P. The influence of temperature on the interaction between DNA and metal complex at a heated gold-wire microelectrode. J. Electroanal. Chem. 2003, 540, 153–157. [Google Scholar] [CrossRef]
- Or, V.W.; Estillore, A.D.; Tivanski, A.V.; Grassian, V.H. Lab on a tip: Atomic force microscopy–photothermal infrared spectroscopy of atmospherically relevant organic/inorganic aerosol particles in the nanometer to micrometer size range. Analyst 2018, 143, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mazzaracchio, V.; Öztürk, G.; Moscone, D.; Arduini, F. A lab-on-a-tip approach to make electroanalysis user-friendly and de-centralized: Detection of copper ions in river water. Anal. Chim. Acta 2018, 1029, 1–7. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.A.B.; Rabelo, A.C.; Munoz, R.A.A.; Richter, E.M. Three-electrode-integrated sensor into a micropipette tip. Electroanalysis 2010, 22, 2167–2171. [Google Scholar] [CrossRef]
- Hwang, D.W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111417. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Rayappan, J.B.B. Investigations on room temperature dual sensitization of ZnO nanostructures towards fish quality biomarkers. Sens. Actuators B Chem. 2020, 304, 127082. [Google Scholar] [CrossRef]
- Marie, M.; Mandal, S.; Manasreh, O. An electrochemical glucose sensor based on zinc oxide nanorods. Sensors 2015, 15, 18714–18723. [Google Scholar] [CrossRef]
- Aini, B.N.; Siddiquee, S.; Ampon, K.; Rodrigues, K.F.; Suryani, S. Development of glucose biosensor based on ZnO nanoparticles film and glucose oxidase-immobilized eggshell membrane. Sens. Bio-Sens. Res. 2015, 4, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Pan, C.; Luo, Z.; Zhu, J. A single ZnO nanofiber-based highly sensitive amperometric glucose biosensor. J. Phys. Chem. C 2010, 114, 9308–9313. [Google Scholar] [CrossRef]
- Zang, J.; Chang, M.L.; Cui, X.; Wang, J.; Sun, X.; Dong, H.; Sun, C.Q. Tailoring zinc oxide nanowires for high performance amperometric glucose sensor. Electroanalysis 2007, 19, 1008–1014. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10, 013001. [Google Scholar] [CrossRef]
- Ma, S.; Kitai, A.H. Chemical vapor deposition-based growth of aligned ZnO nanowires on polycrystalline Zn2GeO4:Mn substrates. J. Mater. Sci. 2017, 52, 9324–9334. [Google Scholar] [CrossRef]
- Jayaprakasan, A.; Thangavel, A.; Ramachandra Bhat, L.; Gumpu, M.B.; Nesakumar, N.; Jayanth Babu, K.; Vedantham, S.; Rayappan, J.B.B. Fabrication of an electrochemical biosensor with ZnO nanoflakes interface for methylglyoxal quantification in food samples. Food Sci. Biotechnol. 2018, 27, 9–17. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.J. Conventional and microwave hydrothermal synthesis and application of functional materials: A review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Li, W.; Pan, L.; Zhai, D.; Wang, Y.; Li, L.; Cheng, W.; Yin, W.; Wang, X.; Xu, J.B.; et al. ZnO-nanorods/graphene heterostructure: A direct electron transfer glucose biosensor. Sci. Rep. 2016, 6, 32327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, X.; Zhu, X.; Dong, Y.; Chen, T.; Ye, M.; Sun, W. An amperometric glucose biosensor based on the immobilization of glucose oxidase on the platinum electrode modified with NiO doped ZnO nanorods. J. Electroanal. Chem. 2012, 676, 20–26. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Abdul Razak, K.; Lockman, Z. Fabrication and Characterization of Glucose Biosensors by Using Hydrothermally Grown ZnO Nanorods. Sci. Rep. 2018, 8, 13722. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.; Jo, E.J.; Jang, H.; Park, J.; Jung, G.Y.; Kim, M.G. Reactant/polymer hybrid films on p-n junction photodetectors for self-powered, non-invasive glucose biosensors. Biosens. Bioelectron. 2021, 175, 112855. [Google Scholar] [CrossRef] [PubMed]
- Smutok, O.; Kavetskyy, T.; Prokopiv, T.; Serkiz, R.; Wojnarowska-Nowak, R.; Sausa, O.; Novak, I.; Berek, D.; Melman, A.; Gonchar, M. New micro/nanocomposite with peroxidase-like activity in construction of oxidases-based amperometric biosensors for ethanol and glucose analysis. Anal. Chim. Acta 2021, 1143, 201–209. [Google Scholar] [CrossRef]
- Albiss, B.A.; Abdullah, H.S.; Alsaad, A.M. Structural and Electrical Properties of Glucose Biosensors Based on ZnO and ZnO-CuO Nanostructures. Curr. Nanosci. 2022, 18, 255–265. [Google Scholar] [CrossRef]
- Hossain, M.F.; Slaughter, G. Flexible electrochemical uric acid and glucose biosensor. Bioelectrochemistry 2021, 141, 107870. [Google Scholar] [CrossRef]
- Vaquer, A.; Baron, E.; de la Rica, R. Detection of low glucose levels in sweat with colorimetric wearable biosensors. Analyst 2021, 146, 3273–3279. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Dispersed Conducting Polymer Nanocomposites with Glucose Oxidase and Gold Nanoparticles for the Design of Enzymatic Glucose Biosensors. Polymers 2021, 13, 2173. [Google Scholar] [CrossRef]
- Gonzales, W.V.; Mobashsher, A.T.; Abbosh, A. The progress of glucose monitoring—A review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, L.; Wang, G.; Hu, K.; Qian, C.; Liu, G. Advances in Biosensors for Continuous Glucose Monitoring towards Wearables. Front. Bioeng. Biotechnol. 2021, 9, 733810. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.; Luong, J. Point-of-Care Glucose Detection for Diabetic Monitoring and Management; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315366746. [Google Scholar]
- Zhou, Q.; Son, K.; Liu, Y.; Revzin, A. Biosensors for Cell Analysis. In Annual Review of Biomedical Engineering, Vol 17; Yarmush, M.L., Ed.; University of California-Davis, Department of Biomedical Engineering: Davis, CA, USA, 2015; Volume 17, pp. 165–190. ISBN 1523-9829. [Google Scholar]
- Lim, B.; Kim, Y.P. Enzymatic Glucose Biosensors Based on Nanomaterials. In Biosensors Based on Aptamers and Enzymes; Gu, M.B., Kim, H.S., Eds.; Hanyang University, Department of Life Science: Seoul, Republic of Korea, 2014; Volume 140, pp. 203–219. ISBN 0724-6145. [Google Scholar]
- Arduini, F.; Amine, A. Biosensors Based on Enzyme Inhibition. In Biosensors Based on Aptamers and Enzymes; Gu, M.B., Kim, H.S., Eds.; University of Rome Tor Vergata, Department of Chemical Science and Technology: Rome, Italy, 2014; Volume 140, pp. 299–326. ISBN 0724-6145. [Google Scholar]
- Tong, Y.; Liu, Y.; Dong, L.; Zhao, D.; Zhang, J.; Lu, Y.; Shen, D.; Fan, X. Growth of ZnO Nanostructures with Different Morphologies by Using Hydrothermal Technique. J. Phys. Chem. B 2006, 110, 20263–20267. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Truong, J.; Szychowski, B.; Xie, T.; Daniel, M.C.; Hahm, J.I. Single nanomaterial level investigation of ZnO nanorod sulfidation reactions: Via position resolved confocal Raman spectroscopy. Nanoscale 2019, 11, 1147–1158. [Google Scholar] [CrossRef]
- Naja, G.; Bouvrette, P.; Hrapovic, S.; Luong, J.H.T. Raman-based detection of bacteria using silver nanoparticles conjugated with antibodies. Analyst 2007, 132, 679–686. [Google Scholar] [CrossRef]
- Oje, A.I.; Ogwu, A.A.; Mirzaeian, M.; Tsendzughul, N. Electrochemical energy storage of silver and silver oxide thin films in an aqueous NaCl electrolyte. J. Electroanal. Chem. 2018, 829, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Kong, T.; Chen, Y.; Ye, Y.; Zhang, K.; Wang, Z.; Wang, X. An amperometric glucose biosensor based on the immobilization of glucose oxidase on the ZnO nanotubes. Sens. Actuators B Chem. 2009, 138, 344–350. [Google Scholar] [CrossRef]


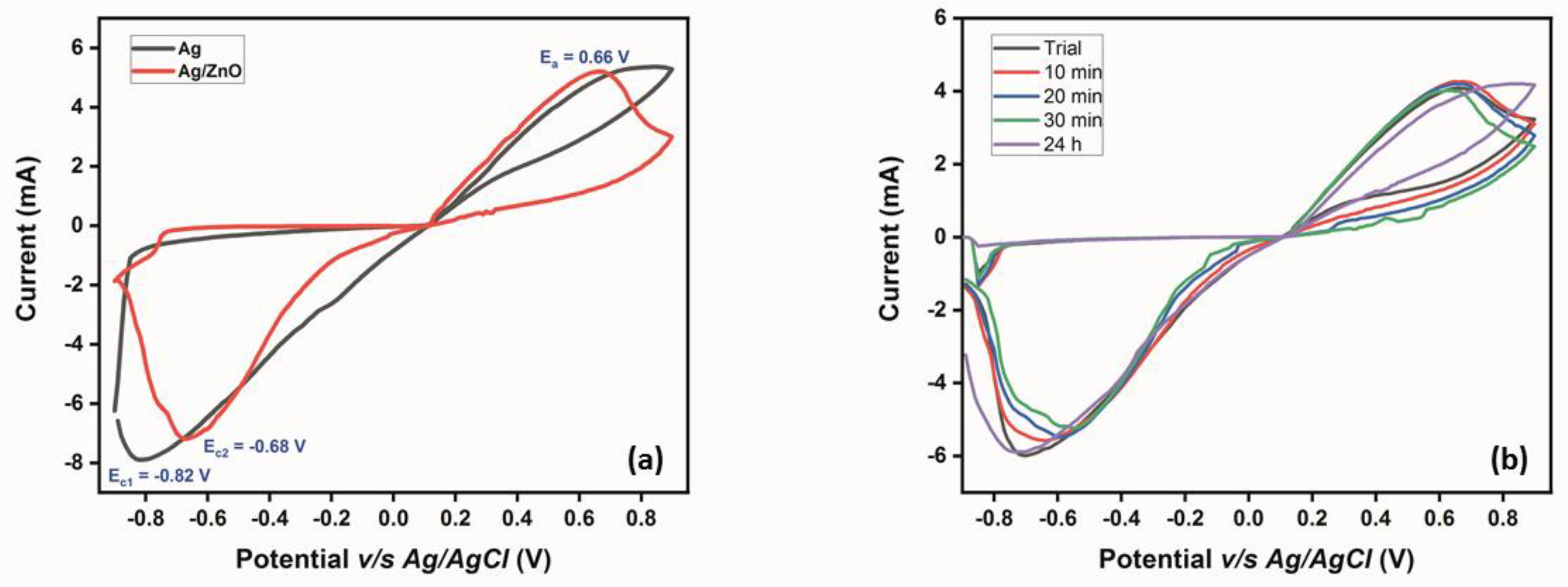

| Electrode Type | Anodic Potential (Ea) | Cathodic Potential (Ec) | Anodic Overpotential (ηa) | Cathodic Overpotential (ηc) | Gibbs Free Energy | Equilibrium Rate Constant (Ks) |
|---|---|---|---|---|---|---|
| Ag-sputtered working electrode | 0.799 V | −0.820 V | −0.192 V | 0.213 V | ~312 kJ/mol | 3.880 |
| ZnO-modified working electrode | 0.660 V | −0.680 V | 0.259 V | −0.279 V | ~517 kJ/mol | 3.811 |
| Base Electrode + Biosensor Components | Electrochemical Method Adopted | Linear Range | Detection Limit | Sensitivity | Ref. |
|---|---|---|---|---|---|
| Gold electrode + ZnO nanowires/Nafion + Glucose oxidase | Amperometry (Enzymatic) | 1–760 µM | 700 µM | 26.3 µA mM−1 cm−2 | [19] |
| Gold electrode + electrodeposited ZnO nanotubes/Nafion + Glucose oxidase | Amperometry (Enzymatic) | 50 µM–12 mM | 1 µM | 21.7 µA mM−1 cm−2 | [43] |
| Indium Tin oxide electrode + ZnO nanorods/Nafion + Glucose oxidase | Amperometry (Enzymatic) | Not reported | 0.22 µM | 10.9 mA mM−1 cm−2 | [16] |
| Glassy carbon electrode + ionic liquid/ZnO nanoparticles/egg-shell membrane + Glucose oxidase | Cyclic Voltammetry (Enzymatic) | 1 pM–0.5 mM | 0.1 pM | Not reported | [17] |
| Gold electrode + electrospun ZnO nanofibers/poly(vinyl alcohol) + L-cysteine + Glucose oxidase | Amperometry (Enzymatic) | 0.25–19 mM | 1 µM | 70.2 µA mM−1 cm−2 | [18] |
| Platinum electrode + ZnO nanorods/chemically reduced Graphene film/Nafion + Glucose oxidase | Amperometry (Enzymatic) | 0.2–1.6 mM | Not reported | 17.6 µA mM−1 cm−2 | [24] |
| Platinum electrode + NiO doped ZnO nanorods + Glucose oxidase | Amperometry (Enzymatic) | 0.5–8.0 mM | 2.5 µM | 61.8 µA mM−1 cm−2 | [25] |
| Micropipette tip + Ag-sputtered + hydrothermally grown ZnO nanostructures | Amperometry (Non-enzymatic) | 10–200 µM | 67.5 µM | 69.02 µA µM−1 cm−2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasami Sundhar Baabu, P.; Mani, G.K.; Rayappan, J.B.B.; Tsuyuki, Y.; Inazu, T.; Tsuchiya, K. Sensor-on-Microtips: Design and Development of Hydrothermally Grown ZnO on Micropipette Tips as a Modified Working Electrode for Detection of Glucose. Micromachines 2023, 14, 498. https://doi.org/10.3390/mi14030498
Ramasami Sundhar Baabu P, Mani GK, Rayappan JBB, Tsuyuki Y, Inazu T, Tsuchiya K. Sensor-on-Microtips: Design and Development of Hydrothermally Grown ZnO on Micropipette Tips as a Modified Working Electrode for Detection of Glucose. Micromachines. 2023; 14(3):498. https://doi.org/10.3390/mi14030498
Chicago/Turabian StyleRamasami Sundhar Baabu, Priyannth, Ganesh Kumar Mani, John Bosco Balaguru Rayappan, Yuichiro Tsuyuki, Toshiyuki Inazu, and Kazuyoshi Tsuchiya. 2023. "Sensor-on-Microtips: Design and Development of Hydrothermally Grown ZnO on Micropipette Tips as a Modified Working Electrode for Detection of Glucose" Micromachines 14, no. 3: 498. https://doi.org/10.3390/mi14030498





