A Thermal Cycler Based on Magnetic Induction Heating and Anti-Freezing Water Cooling for Rapid PCR
Abstract
1. Introduction
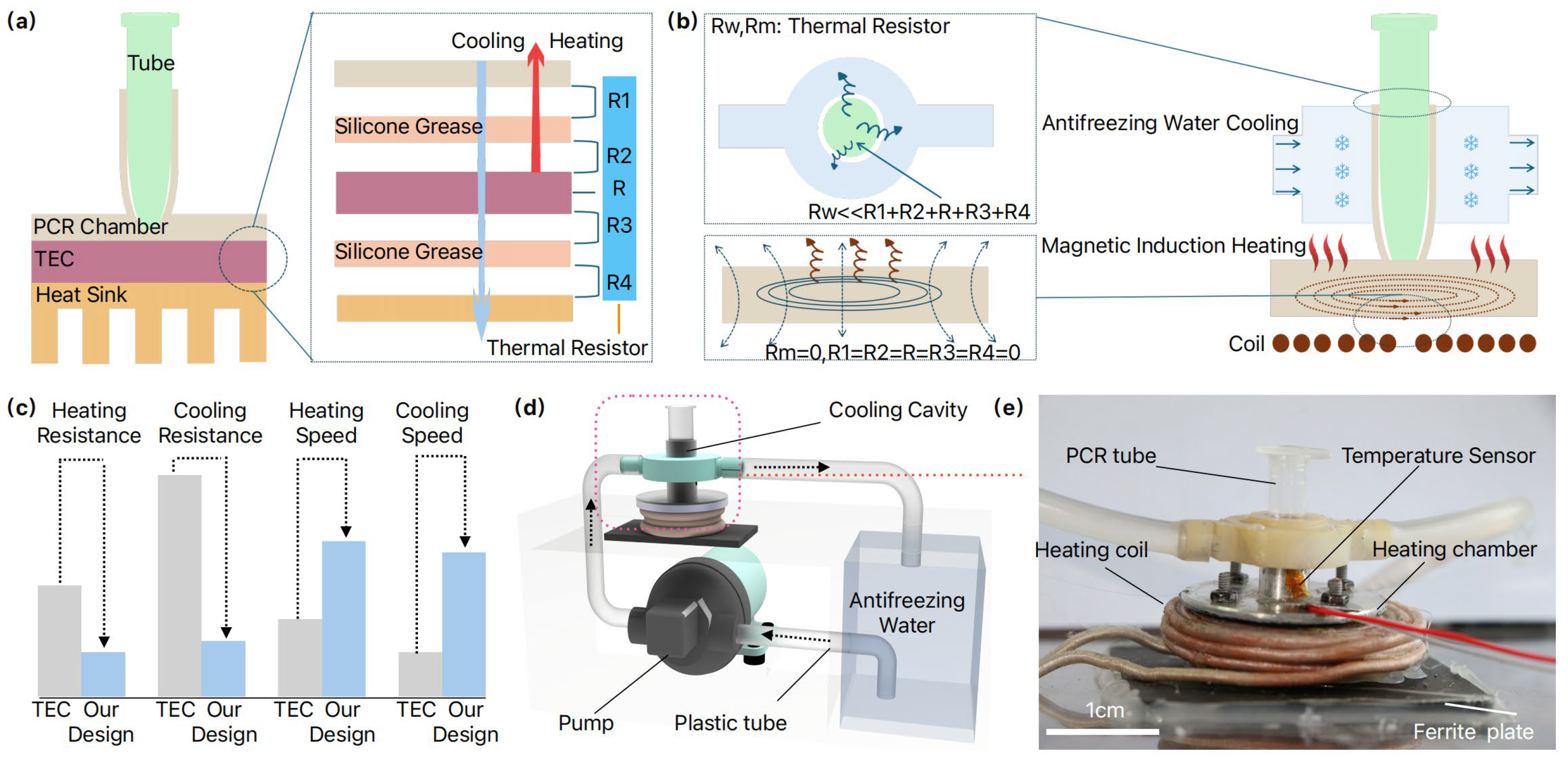
2. Principles and the Overall Design
3. Materials and Methods
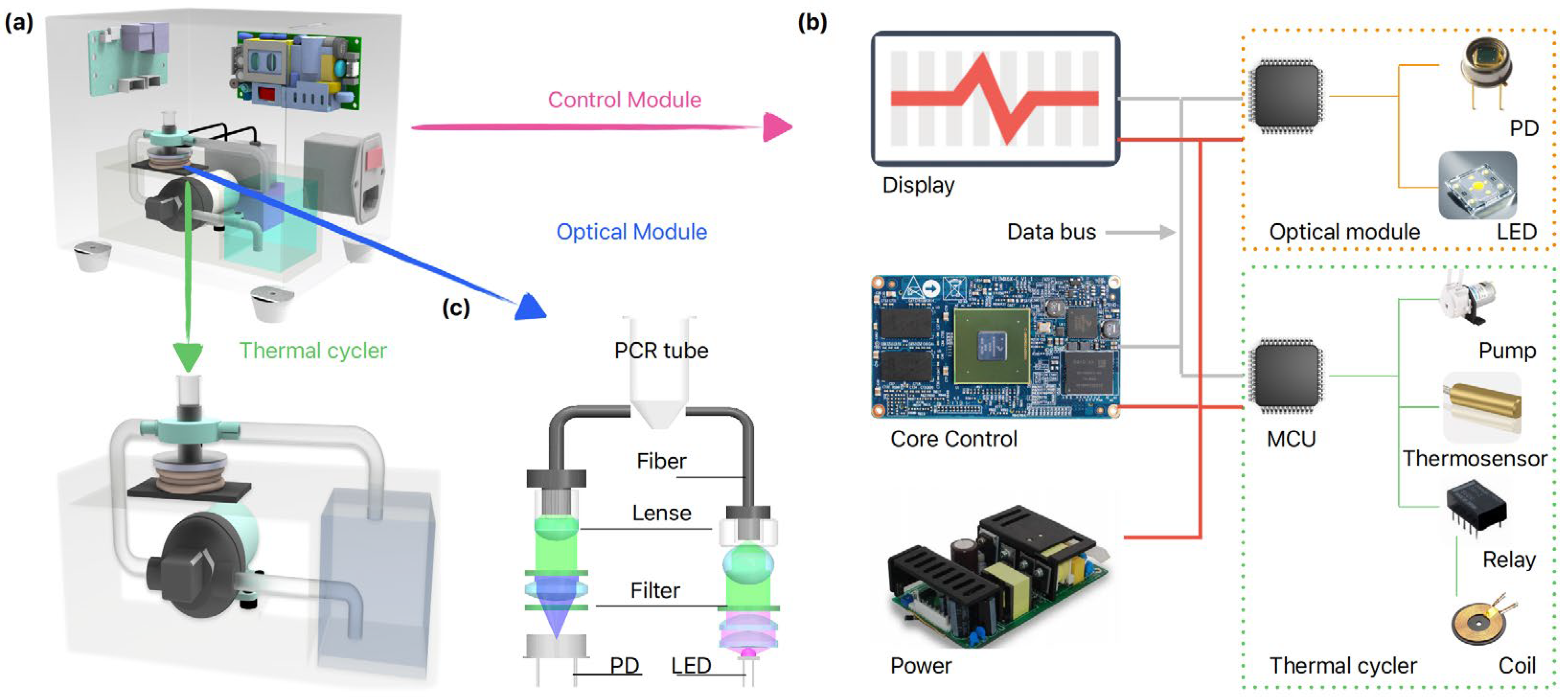
3.1. Detailed Thermal Cycler Design
3.2. Integrated qPCR Device
3.3. Amplification Program
3.4. PCR Reagents
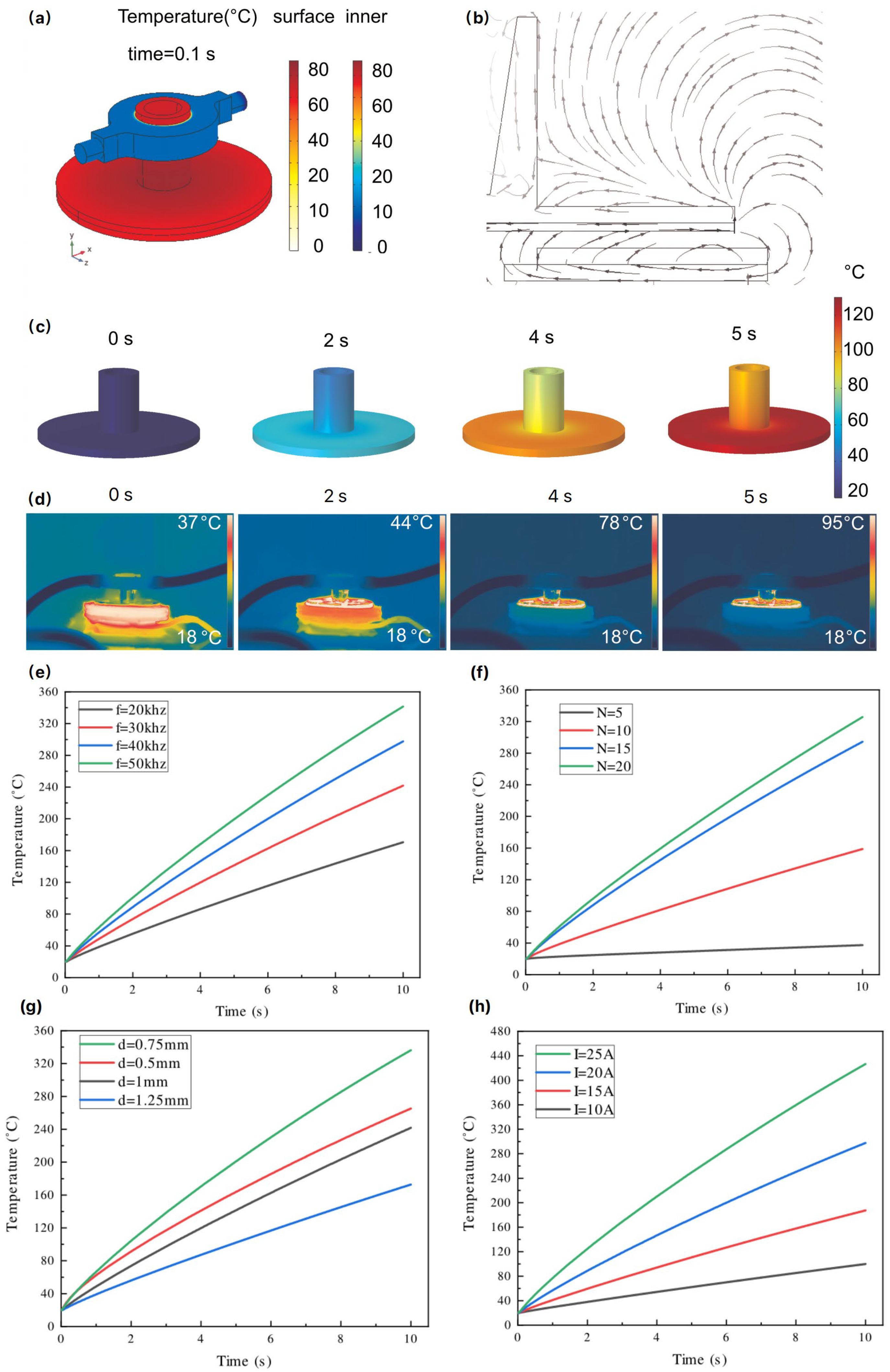
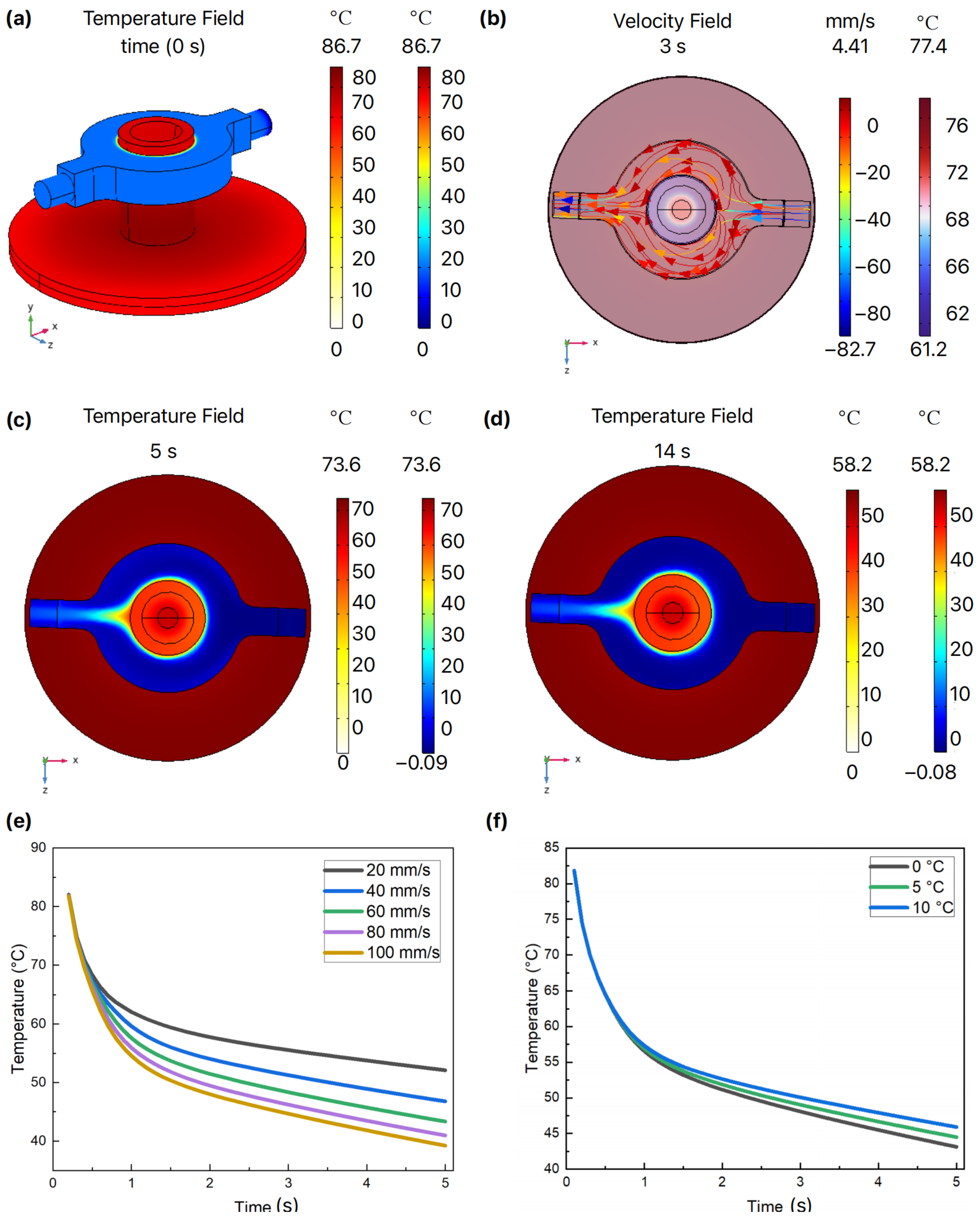
3.5. FEA Simulation
4. Results and Discussion
4.1. MIH Process Design and Simulation Analysis
4.2. AWC Process Design and Simulation Analysis
4.3. Temperature Controlling Performance
4.4. qPCR Verification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morens, D.M.; Fauci, A.S. Emerging Infectious Diseases: Threats to Human Health and Global Stability. PLoS Pathog. 2013, 9, e1003467. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Ristori, M.V.; Guarrasi, V.; Soda, P.; Petrosillo, N.; Gurrieri, F.; Longo, U.G.; Ciccozzi, M.; Riva, E.; Angeletti, S. Emerging Microorganisms and Infectious Diseases: One Health Approach for Health Shared Vision. Genes 2024, 15, 908. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Krogstad, P. SARS: The First Pandemic of the 21st Century. Pediatr. Res. 2004, 56, 1–5. [Google Scholar] [CrossRef]
- Al Hajjar, S.; Memish, Z.A.; McIntosh, K. Middle East Respiratory Syndrome Coronavirus (MERS-CoV): A Perpetual Challenge. Ann. Saudi Med. 2013, 33, 427–436. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar]
- Smith, K.M.; Machalaba, C.C.; Seifman, R.; Feferholtz, Y.; Karesh, W.B. Infectious Disease and Economics: The Case for Considering Multi-Sectoral Impacts. One Health 2019, 7, 100080. [Google Scholar] [CrossRef]
- Hauck, K. The Economics of Infectious Diseases. In Oxford Research Encyclopedia of Economics and Finance; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Ismahene, Y. Infectious Diseases, Trade, and Economic Growth: A Panel Analysis of Developed and Developing Countries. J. Knowl. Econ. 2022, 13, 2547–2583. [Google Scholar] [CrossRef]
- Sy, R.; Rothman, R.E.; Yang, S. PCR-Based Diagnostics PCR-Based Diagnostics for Infectious Diseases: Uses, Limitations, and Future Applications in Acute-Care Settings. Lancet Infect. Dis. 2004, 4, 337–348. [Google Scholar]
- Pusterla, N.; Madigan, J.E.; Leutenegger, C.M. Real-Time Polymerase Chain. Reaction: A Novel Molecular Diagnostic Tool. for Equine Infectious Diseases. J. Vet. Intern. Med. 2006, 20, 3–12. [Google Scholar]
- Wu, H.; Zhang, S.; Chen, Y.; Qian, C.; Liu, Y.; Shen, H.; Wang, Z.; Ping, J.; Wu, J.; Zhang, Y.; et al. Progress in Molecular Detection with High-Speed Nucleic Acids Thermocyclers. J. Pharm. Biomed. Anal. 2020, 190, 113489. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Zhang, L.; Zhang, J.; Ge, S.; Xia, N.; Qian, S.; Yu, D.; Qiu, X. Free Convective PCR: From Principle Study to Commercial Applications—A Critical Review. Anal. Chim. Acta 2020, 1108, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.J.; Seo, S.E.; Kim, S.J.; Lee, K.G.; Kwon, O.S. A Novel Nanoplasmonic-Based Diagnosis Platform: Advances and Emerging Technologies. Appl. Phys. Rev. 2024, 11, 021317. [Google Scholar] [CrossRef]
- Roche, P.J.R.; Najih, M.; Lee, S.S.; Beitel, L.K.; Carnevale, M.L.; Paliouras, M.; Kirk, A.G.; Trifiro, M.A. Real Time Plasmonic QPCR: How Fast Is Ultra-Fast? 30 Cycles in 54 Seconds. Analyst 2017, 142, 1746–1755. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, X.; Zou, T.; Miao, G.; Fu, Q.; Xiang, F.; Feng, L.; Ye, X.; Zhang, L.; Qiu, X. A Microfluidic System for Rapid Nucleic Acid Analysis Based on Real-Time Convective PCR at Point-of-Care Testing. Microfluid. Nanofluidics 2022, 26, 69. [Google Scholar] [CrossRef]
- Kopp, M.U.; De Mello, A.J.; Manz, A. Chemical Amplification: Continuous-Flow PCR on a Chip. Science 1998, 280, 1046–1048. [Google Scholar] [CrossRef]
- Mandal, P.; Dhakane, V.; Kulkarni, A.; Tallur, S. WELPCR: Low-Cost Polymerase Chain Reaction (PCR) Thermal Cycler for Nucleic Acid Amplification and Sensing. In Proceedings of the APSCON 2024, 2024 IEEE Applied Sensing Conference, Proceedings, Goa, India, 22–24 January 2024; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2024. [Google Scholar]
- Nasser, G.A.; Abdel-Mawgood, A.L.; Abouelsoud, A.A.; Mohamed, H.; Umezu, S.; El-Bab, A.M.R.F. New Cost Effective Design of PCR Heating Cycler System Using Peltier Plate without the Conventional Heating Block. J. Mech. Sci. Technol. 2021, 35, 3259–3268. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. QuantStudio™ Systems, Features. Thermo Fisher Scientific. Available online: https://www.thermofisher.cn/cn/zh/home/life-science/pcr/real-time-pcr/real-time-pcr-instruments/quantstudio-systems/features.html (accessed on 26 June 2024).
- Bio-Rad Laboratories. Bulletin 7279, T100™ Thermal Cycler (Bulletin 7279). Bio-Rad Laboratories. Available online: https://www.biorad.com/webroot/web/pdf/lsr/literature/Bulletin_7279.pdf (accessed on 26 June 2024).
- Hongshi Tech. 1203311, SLAN96S Hongshi Tech. Available online: https://www.hongshitech.com/productinfo/1203311.html (accessed on 26 June 2024).
- Li, X.; Wang, J.; Meng, Q.; Yu, D. Theoretical and Experimental Investigation about the Influence of Peltier Effect on the Temperature Loss and Performance Loss of Thermoelectric Generator. Energy Technol. 2022, 10, 2100895. [Google Scholar] [CrossRef]
- Wang, H.J.; Xiang, Y.H.; Hu, R.; Ji, R.; Wang, Y.P. Research Progress in Laboratory Detection of SARS-CoV-2. Ir. J. Med. Sci. 2022, 191, 509–517. [Google Scholar] [CrossRef]
- Tark, Z.; Hamed, A.J.; Khalifa, A.H.N. Performance Study of the Thermoelectric Personal Cooler under Different Ambient Temperatures. Int. J. Heat Technol. 2022, 40, 53–62. [Google Scholar] [CrossRef]
- Sulaiman, A.C.; Amin, N.A.M.; Basha, M.H.; Majid, M.S.A.; Nasir, N.F.B.M.; Zaman, I. Cooling Performance of Thermoelectric Cooling (TEC) and Applications: A Review. In Proceedings of the UTP-UMP-VIT Symposium on Energy Systems 2018 (SES 2018), Tamil Nadu, India, 18–19 September 2018; EDP Sciences: Les Ulis, France, 2018; Volume 225. [Google Scholar]
- Zhu, H.; Zhang, H.; Xu, Y.; Laššáková, S.; Korabečná, M.; Neužil, P. PCR Past, Present and Future. Biotechniques 2020, 69, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lucia, O.; Maussion, P.; Dede, E.J.; Burdio, J.M. Induction Heating Technology and Its Applications: Past Developments, Current Technology, and Future Challenges. IEEE Trans. Ind. Electron. 2014, 61, 2509–2520. [Google Scholar] [CrossRef]
- Elsaady, W.; Moughton, C.; Nasser, A.; Lacovides, H. Coupled Numerical Modelling and Experimental Analysis of Domestic Induction Heating Systems. Appl. Therm. Eng. 2023, 227, 120170. [Google Scholar] [CrossRef]
- Sun, K.; Fan, G.; Dong, H.; Fan, Y.; Xie, Y.; Liang, K.; Zhang, Y. Water-Cooling-Based and Low-Cost QPCR Device for Rapid Nucleic Acid Analysis. Sens. Actuators A Phys. 2024, 375, 115496. [Google Scholar] [CrossRef]
- Hurley, W.; Kassakian, J. Induction Heating of Circular Ferromagnetic Plates. IEEE Trans. Magn. 1979, 15, 1174–1181. [Google Scholar] [CrossRef]
- Wu, J.; Kodzius, R.; Xiao, K.; Qin, J.; Wen, W. Fast detection of genetic information by an optimized PCR in an interchangeable chip. Biomed. Microdev. 2012, 14, 179–186. [Google Scholar] [CrossRef]


| Our Design | SLAN96 [22] | CFX96 [21] | Qs5 [20] | ||
|---|---|---|---|---|---|
| Heating | Avg ramp rate | 14.92 °C/s | - | 3.3 °C/s | - |
| Max ramp rate | 20.29 °C/s | 4.0 °C/s | 5 °C/s | 6.5 °C/s | |
| Cooling | Avg ramp rate | 13.39 °C/s | - | 3.3 °C/s | - |
| Max ramp rate | 21.34 °C/s | 4.0 °C/s | 5 °C/s | 6.5 °C/s | |
| Accuracy | 45 °C | 0.20 | 0.10 | 0.20 | 0.25 |
| 72 °C | 0.20 | 0.10 | 0.20 | 0.25 | |
| 95 °C | 0.2 | 0.10 | 0.20 | 0.25 | |
| Precision | 45 °C | 0.07 | 0.10 | 0.20 | 0.25 |
| 72 °C | 0.19 | 0.10 | 0.20 | 0.25 | |
| 95 °C | 0.13 | 0.10 | 0.20 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Jiang, Q.; Chang, C.; Zhao, X.; Yong, H.; Ke, X.; Wu, Z. A Thermal Cycler Based on Magnetic Induction Heating and Anti-Freezing Water Cooling for Rapid PCR. Micromachines 2024, 15, 1462. https://doi.org/10.3390/mi15121462
Xie Y, Jiang Q, Chang C, Zhao X, Yong H, Ke X, Wu Z. A Thermal Cycler Based on Magnetic Induction Heating and Anti-Freezing Water Cooling for Rapid PCR. Micromachines. 2024; 15(12):1462. https://doi.org/10.3390/mi15121462
Chicago/Turabian StyleXie, Yaping, Qin Jiang, Chang Chang, Xin Zhao, Haochen Yong, Xingxing Ke, and Zhigang Wu. 2024. "A Thermal Cycler Based on Magnetic Induction Heating and Anti-Freezing Water Cooling for Rapid PCR" Micromachines 15, no. 12: 1462. https://doi.org/10.3390/mi15121462
APA StyleXie, Y., Jiang, Q., Chang, C., Zhao, X., Yong, H., Ke, X., & Wu, Z. (2024). A Thermal Cycler Based on Magnetic Induction Heating and Anti-Freezing Water Cooling for Rapid PCR. Micromachines, 15(12), 1462. https://doi.org/10.3390/mi15121462






