Magnetically Driven Biopsy Capsule Robot with Spring Mechanism
Abstract
1. Introduction
2. The Development of the Needle-Based Biopsy Capsule Robot
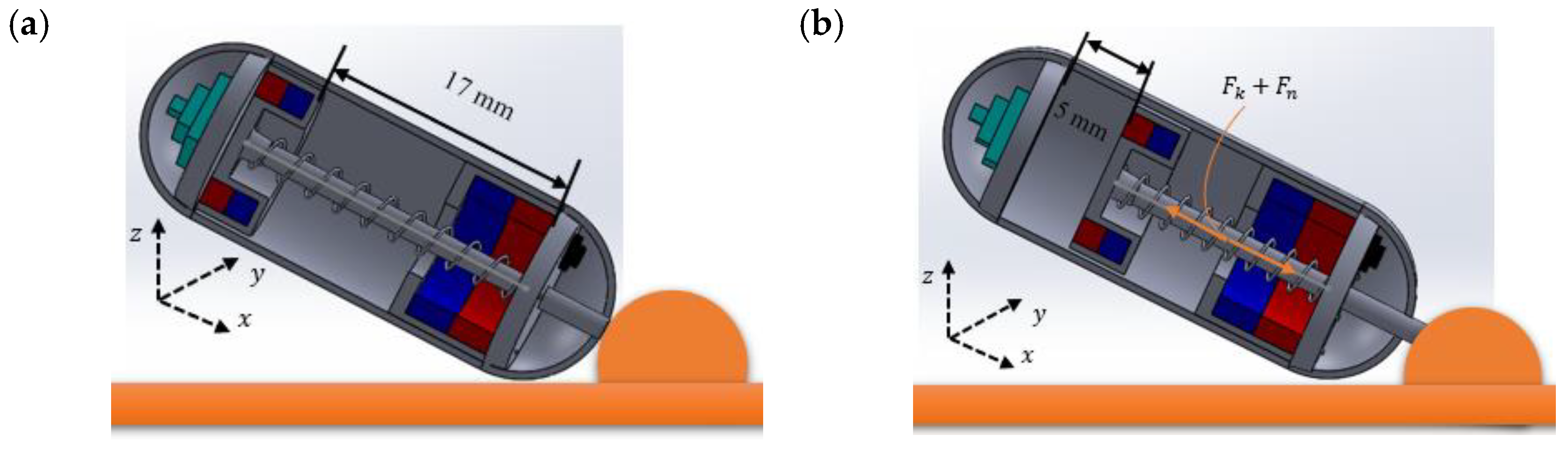
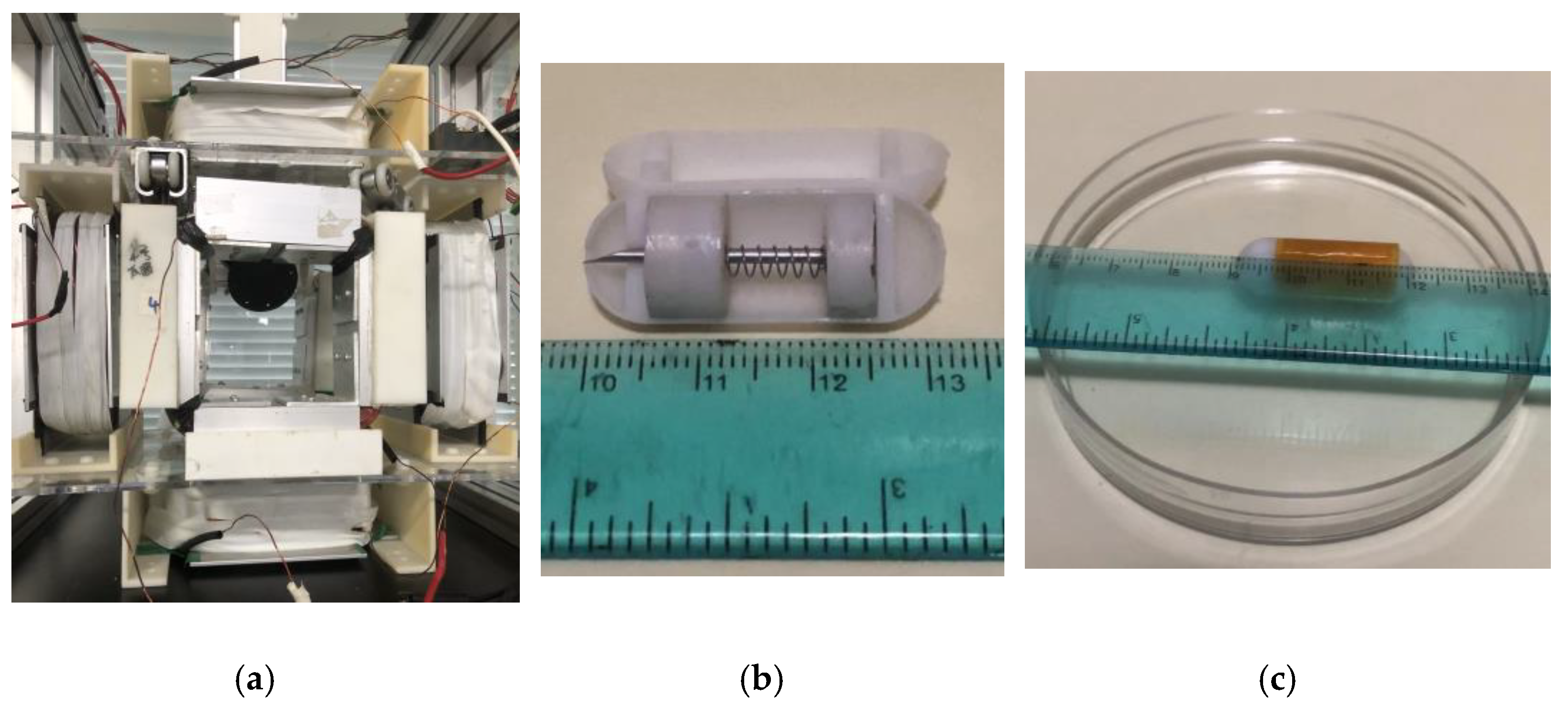
2.1. Design Structure of the Capsule Robot
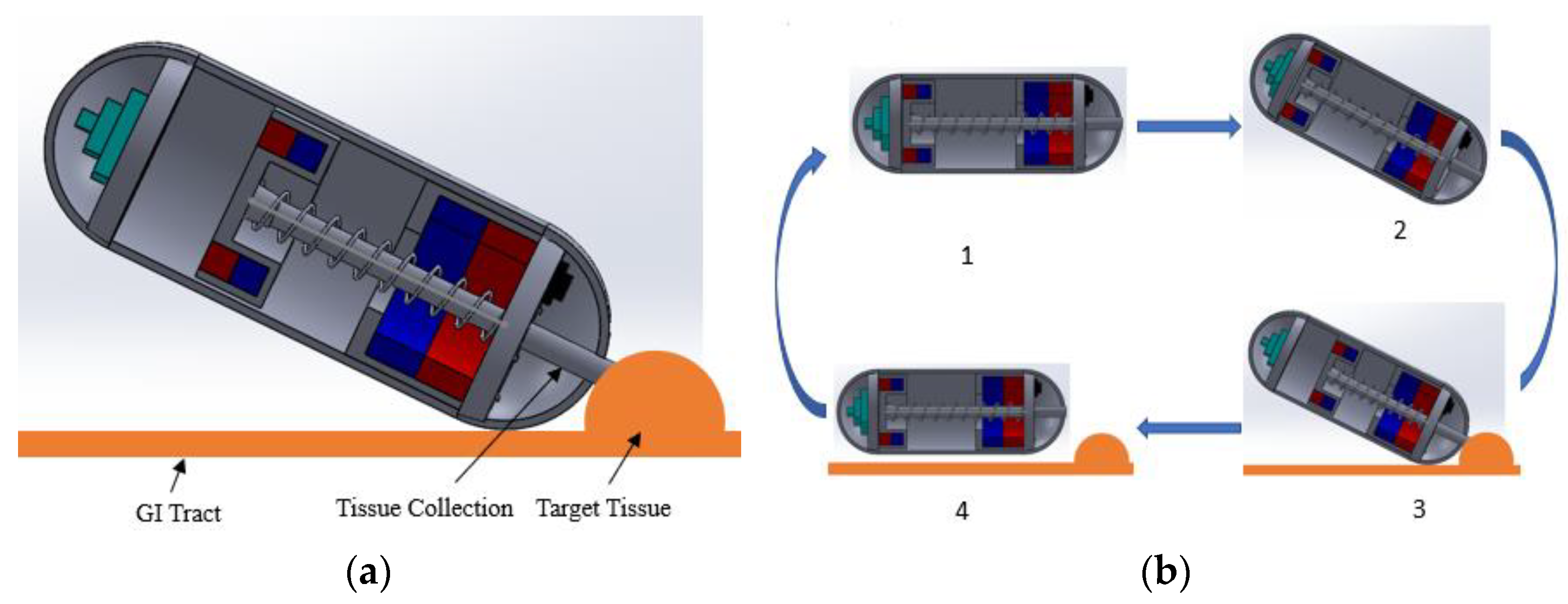
2.2. Working Principles of the Capsule Robot
3. Control Strategy of the Capsule Robot
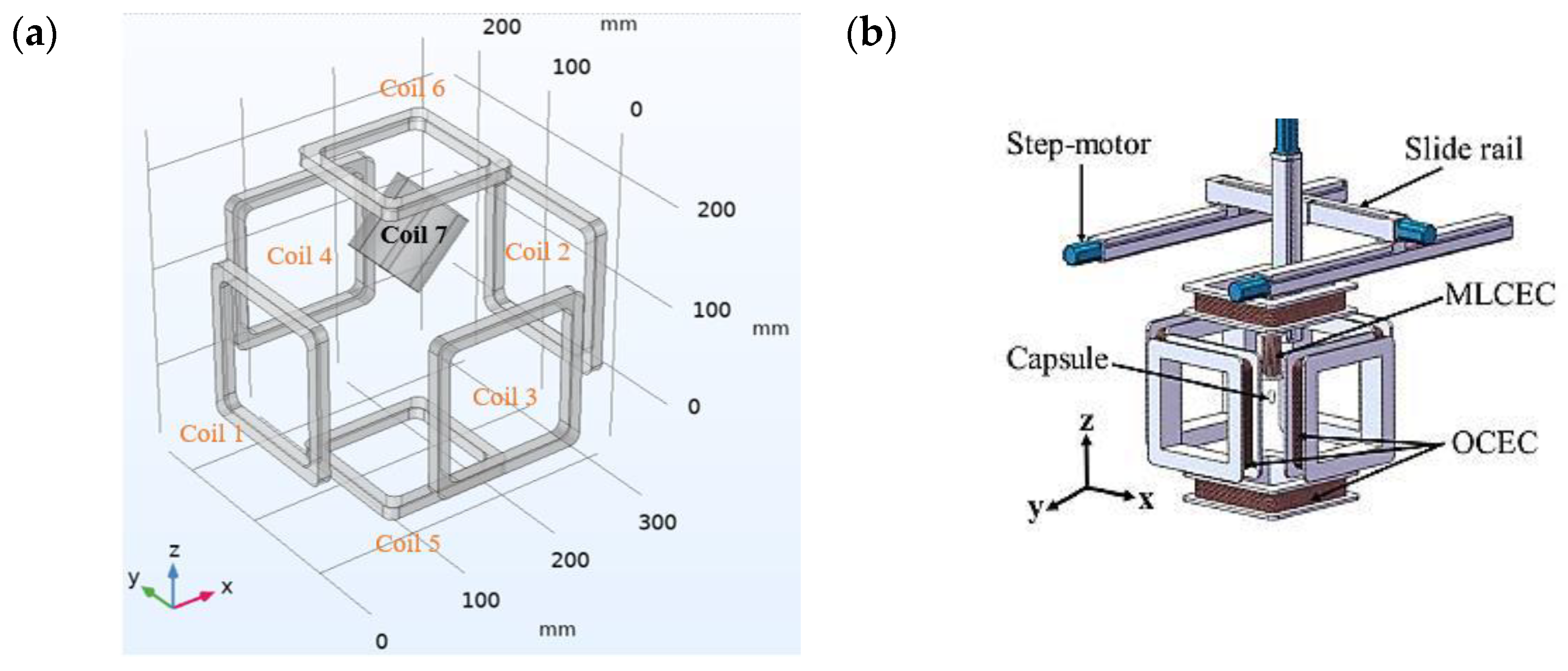
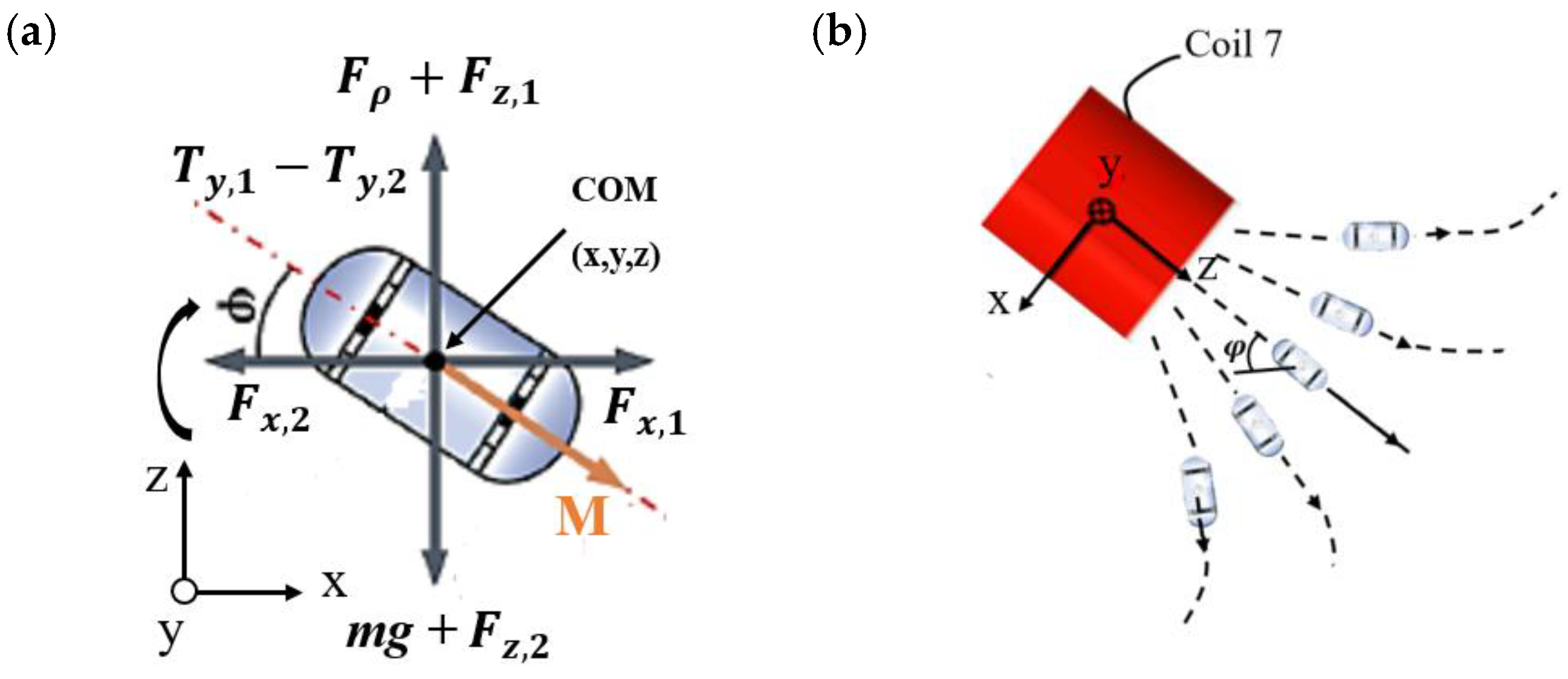
3.1. The Electromagnetic Actuation System
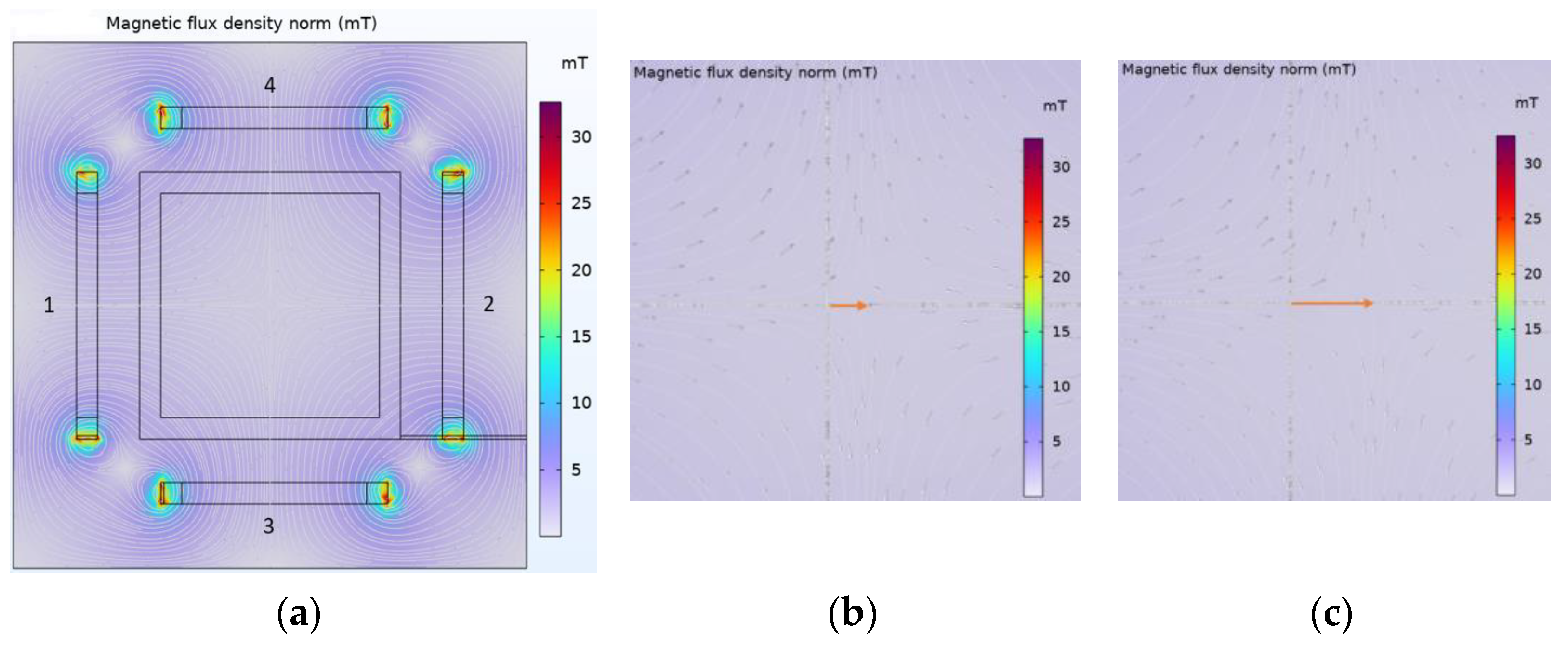
3.2. Magnetic Field Analysis
3.3. Magnetic Force Analysis
3.4. Cutting Force and Sampling
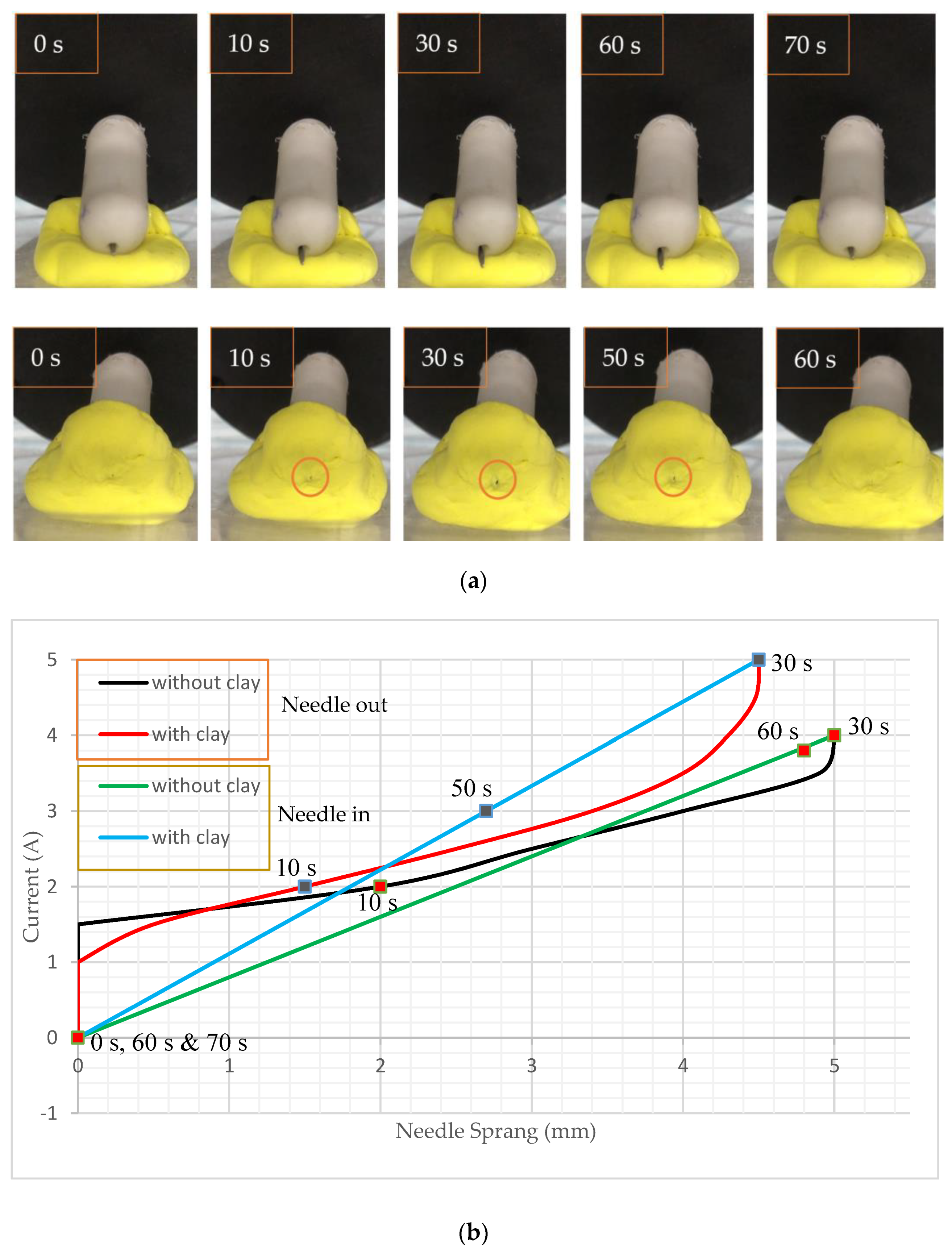
4. Experiments and Results
4.1. Experimental Process
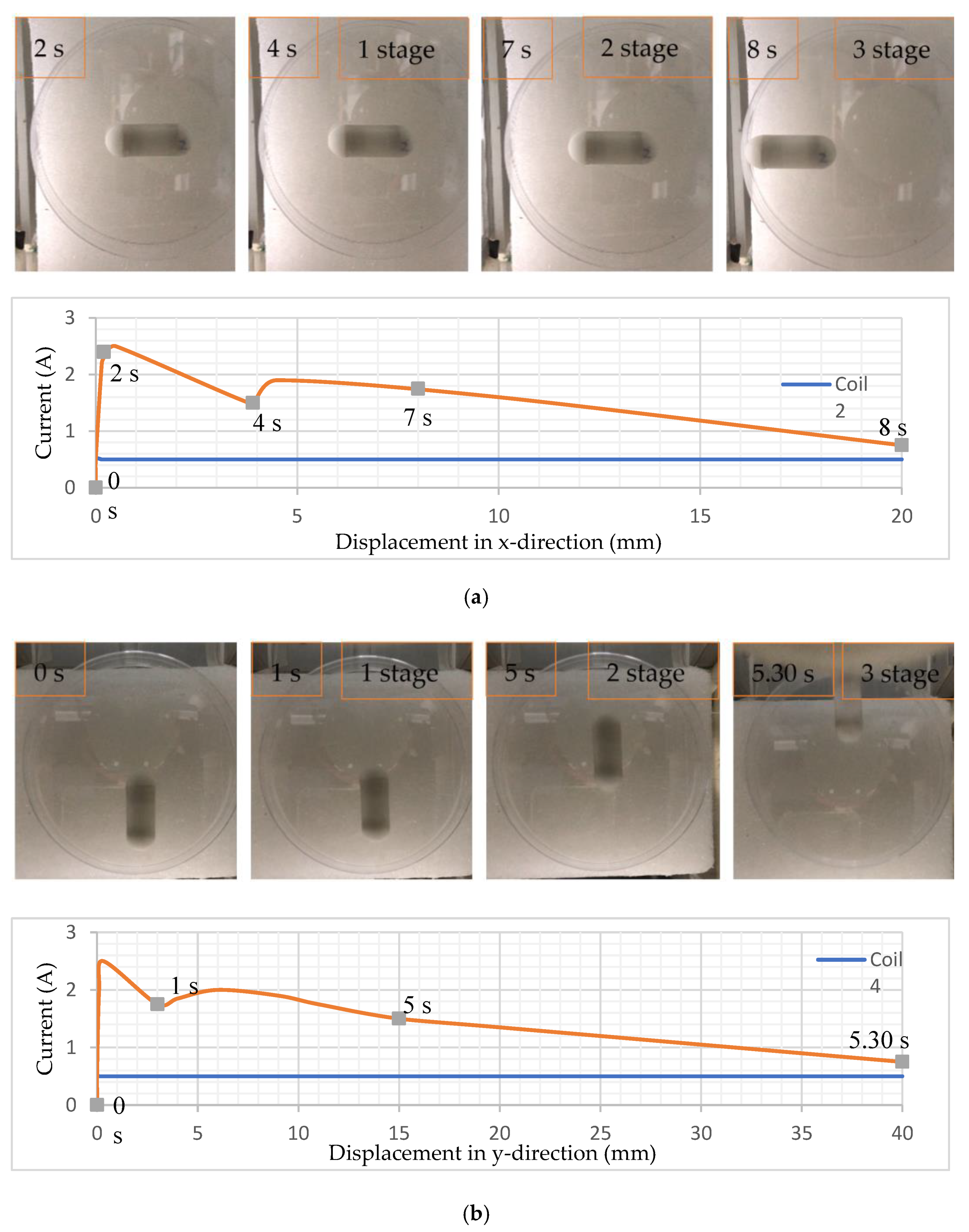
4.2. Translational Motion and Control Locomotion
4.3. Rotating the Capsule and Performing Biopsy Functions
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Toennies, J.L.; Tortora, G.; Simi, M.; Valdastri, P.; Webster, R.J. Swallowable medical devices for diagnosis and surgery: The state of the art. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2010, 224, 1397–1414. [Google Scholar] [CrossRef]
- Pan, G.; Wang, L. Swallowable wireless capsule endoscopy: Progress and technical challenges. Gastroenterol. Res. Pract. 2012, 2012, 841691. [Google Scholar] [CrossRef] [PubMed]
- Meron, G.D. (Ed.) The development of the swallowable video capsule (M2A). Gastrointest. Endosc. 2000, 52, 817–819. [Google Scholar] [CrossRef]
- Chen, W.; Sui, J.; Wang, C. Magnetically Actuated Capsule Robots: A Review. IEEE Access 2022, 10, 88398–88420. [Google Scholar] [CrossRef]
- Catassi, C.; Fasano, A. Celiac Disease Diagnosis: Simple Rules Are Better Than Complicated Algorithms. Am. J. Med. 2010, 123, 691–693. [Google Scholar] [CrossRef]
- Hoang, M.C.; Le, V.H.; Nguyen, K.T.; Du Nguyen, V.; Kim, J.; Choi, E.; Bang, S.; Kang, B.; Park, J.-O.; Kim, C.-S. A robotic biopsy endoscope with magnetic 5-DOF locomotion and a retractable biopsy punch. Micromachines 2020, 11, 98. [Google Scholar] [CrossRef]
- Son, D.; Gilbert, H.; Sitti, M. Magnetically Actuated Soft Capsule Endoscope for Fine-Needle Biopsy. Soft Robot. 2020, 7, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Xue, J.; Yuan, S.; Zhang, F.; Song, S.; Wang, J.; Meng, M.Q.-H. Design and Control of a Magnetically-Actuated Capsule Robot with Biopsy Function. IEEE Trans. Biomed. Eng. 2022, 69, 2905–2915. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Jeon, D. Design of a biopsy module for the capsule type endoscope. Adv. Bioeng. BED 2005, 57, 19–22. [Google Scholar]
- Kong, K.C.; Cha, J.; Jeon, D.; Cho, D.I.D. A rotational micro biopsy device for the capsule endoscope. In Proceedings of the 2005 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Edmonton, AB, Canada, 2–6 August 2005; pp. 1839–1843. [Google Scholar]
- Kong, K.; Yim, S.; Choi, S.; Jeon, D. A Robotic Biopsy Device for Capsule Endoscopy. J. Med. Devices 2012, 6, 031004. [Google Scholar] [CrossRef]
- Simi, M.; Gerboni, G.; Menciassi, A.; Valdastri, P. Magnetic torsion spring mechanism for a wireless biopsy capsule. J. Med. Devices 2013, 7, 041009. [Google Scholar] [CrossRef]
- Le, V.H.; Hernando, L.-R.; Lee, C.; Choi, H.; Jin, Z.; Nguyen, K.T.; Go, G.; Ko, S.-Y.; Park, J.-O.; Park, S. Shape memory alloy-based biopsy device for active locomotive intestinal capsule endoscope. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Le, V.H.; Jin, Z.; Leon-Rodriguez, H.; Lee, C.; Choi, H.; Du Nguyen, V.; Go, G.; Ko, S.-Y.; Park, J.-O.; Park, S. Electromagnetic field intensity triggered micro-biopsy device for active locomotive capsule endoscope. Mechatronics 2016, 36, 112–118. [Google Scholar] [CrossRef]
- Hoang, M.C.; Le, V.H.; Kim, J.; Choi, E.; Kang, B.; Park, J.-O.; Kim, C.-S. Untethered Robotic Motion and Rotating Blade Mechanism for Actively Locomotive Biopsy Capsule Endoscope. IEEE Access 2019, 7, 93364–93374. [Google Scholar] [CrossRef]
- Ye, D.; Zhang, F.; Yuan, S.; Song, S.; Meng, M.Q.-H. Magnetically driven wireless capsule robot with targeting biopsy function. In Proceedings of the IEEE International Conference on Robotics and Biomimetics, (ROBIO 2019), Dali, China, 6–8 December 2019; pp. 1222–1227. [Google Scholar]
- Park, S.; Koo, K.-I.; Bang, S.M.; Park, J.Y.; Song, S.Y.; Cho, D. A novel microactuator for microbiopsy in capsular endoscopes. J. Micromechanics Microengineering 2008, 18, 025032. [Google Scholar] [CrossRef]
- Yim, S.; Gultepe, E.; Gracias, D.H.; Sitti, M. Biopsy using a magnetic capsule endoscope carrying, releasing, and retrieving untethered microgrippers. IEEE Trans. Biomed. Eng. 2014, 61, 513–521. [Google Scholar] [PubMed]
- Chen, W.W.; Yan, G.Z.; Liu, H.; Jiang, P.P.; Wang, Z.W. Design of micro biopsy device for wireless autonomous endoscope. Int. J. Precis. Eng. Manuf. 2014, 15, 2317–2325. [Google Scholar] [CrossRef]
- Le, V.H.; Du Nguyen, V.; Lee, C.; Go, G.; Park, J.-O.; Park, S. Miniaturized biopsy module using gripper tool for active locomotive capsule endoscope. Mechatronics 2017, 44, 52–59. [Google Scholar] [CrossRef]
- Valdastri, P.; Webster, R.J.; Quaglia, C.; Quirini, M.; Menciassi, A.; Dario, P. A New Mechanism for Mesoscale Legged Locomotion in Compliant Tubular Environments. IEEE Trans. Robot. 2009, 25, 1047–1057. [Google Scholar] [CrossRef]
- Karargyris, A.; Koulaouzidis, A. OdoCapsule: Next-generation wireless capsule endoscopy with accurate lesion localization and video stabilization capabilities. IEEE Trans. Biomed. Eng. 2015, 62, 352–360. [Google Scholar] [CrossRef]
- Zhou, H.; Alici, G.; Than, T.D.; Li, W. Modeling and Experimental Characterization of Propulsion of a Spiral-Type Microrobot for Medical Use in Gastrointestinal Tract. IEEE Trans. Biomed. Eng. 2013, 60, 1751–1759. [Google Scholar] [CrossRef]
- Gao, J.; Yan, G.; Wang, Z.; Xu, F.; Wang, W.; Jiang, P.; Liu, D. Locomotion enhancement of an inchworm-like capsule robot using long contact devices. Robot. Comput. Surg. 2017, 13, e1759. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choi, H.; Go, G.; Jeong, S.; Ko, S.Y.; Park, J.-O.; Park, S. Active Locomotive Intestinal Capsule Endoscope (ALICE) System: A Prospective Feasibility Study. IEEE/ASME Trans. Mechatron. 2015, 20, 2067–2074. [Google Scholar] [CrossRef]
- Song, L.; Dai, Y.; Wang, L.; Zhang, W.; Ji, Y.; Cao, Y.; Wei, J.; Wang, F.; Zhong, J.; Yang, J.; et al. Motion Control of Capsule Robot Based on Adaptive Magnetic Levitation Using Electromagnetic Coil. IEEE Trans. Automat. Sci. Eng. 2022, 20, 2720–2731. [Google Scholar] [CrossRef]
- Hu, H.; Yang, X.; Song, L.; Wei, W.; Peng, G.; Feng, L. High Position Accuracy and 5 Degree Freedom Magnetic Driven Capsule Robot. In Proceedings of the 2019 WRC Symposium on Advanced Robotics and Automation (WRC SARA), Beijing, China, 21–22 August 2019; IEEE: Piscataway, NJ, USA, 2019; pp. 19–24. [Google Scholar]
- Yang, J.; Wei, J.; Wang, F.; Song, L.; Zhao, J.; Feng, L. A Localization Method for the Magnetic Levitation Capsule Robot. In Proceedings of the 2022 WRC Symposium on Advanced Robotics and Automation (WRC SARA), Beijing, China, 20 August 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 152–159. [Google Scholar]
- Song, L.; Yang, X.; Hu, H.; Peng, G.; Wei, W.; Dai, Y.; Feng, L. The Design of 3-D Space Electromagnetic Control System for High-Precision and Fast-Response Control of Capsule Robot with 5-DOF. In Intelligent Robotics and Applications; Yu, H., Liu, J., Liu, L., Ju, Z., Liu, Y., Zhou, D., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; Volume 11745, pp. 202–212. [Google Scholar]
- Pawashe, C.; Floyd, S.; Sitti, M. Modeling and Experimental Characterization of an Untethered Magnetic Micro-Robot. Int. J. Robot. Res. 2009, 28, 1077–1094. [Google Scholar] [CrossRef]
- Elbuken, C.; Khamesee, M.B.; Yavuz, M. Design and Implementation of a Micromanipulation System Using a Magnetically Levitated MEMS Robot. IEEE/ASME Trans. Mechatron. 2009, 14, 434–445. [Google Scholar] [CrossRef]
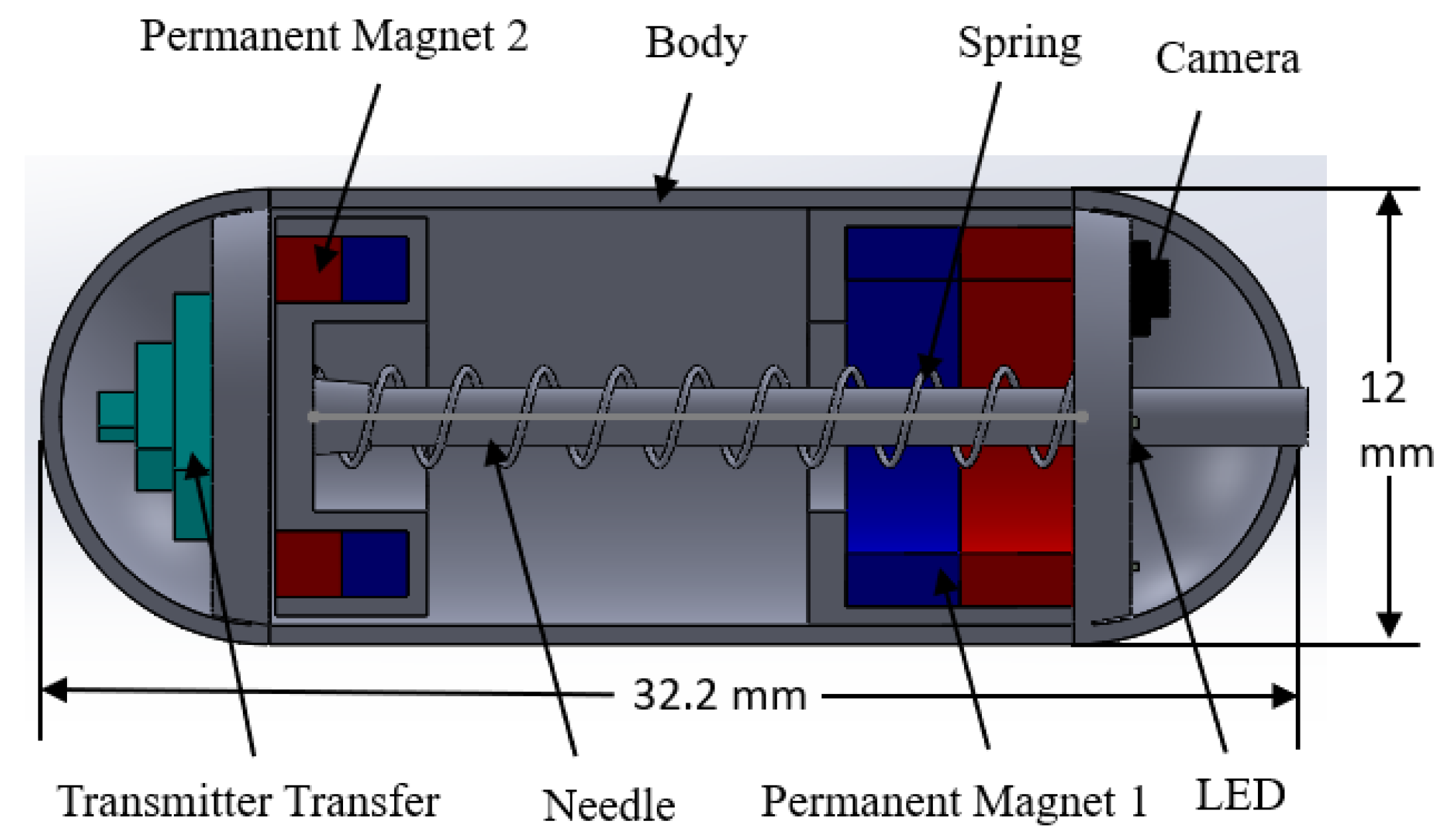


| Part’s Name | Number of Parts | Dimensions (mm) |
|---|---|---|
| Body | 1 | 12 × 32.2 |
| Permanent magnet 1 | 2 | 10-5 × 3 |
| Permanent magnet 2 | 1 | 9.5-6 × 3.5 |
| Spring | 1 | 17 |
| Needle | 1 | 1.2 × 23 |
| Camera module | 1 | |
| Transmitter module | 1 |
| OCEC | MLCEC | ||||
|---|---|---|---|---|---|
| Parameter Name | Value | Unit | Parameter Name | Value | Unit |
| Outer length | ~155 | mm | Outer length | ~75 | mm |
| Inner length | 130 | mm | Inner length | 10 | mm |
| Wire diameter | 0.85 | mm | Wire diameter | 0.87 | mm |
| Number of turns | ~1000 | - | Number of turns | ~1700 | - |
| Coil resistance | 14.3 | Ω | Coil resistance | 5.5 | Ω |
| Distance from center | 120 | - | |||
| Operation Name | Minimum | Maximum | Operation Name | Minimum | Maximum |
|---|---|---|---|---|---|
| Translational motion in the x-direction | 0.5 A (coil 2) | 2.5 A (coil 1) | Rotational motion | 1.5 A | |
| Translational motion in the y-direction | 0.5 A (coil 4) | 2.5 A (coil 3) | (coil 5 + coil 6) | ||
| Motion control forward & reverse | 3.5 A (coil 1) | 0.5 A (coil 2) | Biopsy function | 5 A (coil 7) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.H.O.; Lin, F. Magnetically Driven Biopsy Capsule Robot with Spring Mechanism. Micromachines 2024, 15, 287. https://doi.org/10.3390/mi15020287
Rashid MHO, Lin F. Magnetically Driven Biopsy Capsule Robot with Spring Mechanism. Micromachines. 2024; 15(2):287. https://doi.org/10.3390/mi15020287
Chicago/Turabian StyleRashid, Md Harun Or, and Feng Lin. 2024. "Magnetically Driven Biopsy Capsule Robot with Spring Mechanism" Micromachines 15, no. 2: 287. https://doi.org/10.3390/mi15020287
APA StyleRashid, M. H. O., & Lin, F. (2024). Magnetically Driven Biopsy Capsule Robot with Spring Mechanism. Micromachines, 15(2), 287. https://doi.org/10.3390/mi15020287







