Biocatalytic Performance of β-Glucosidase Immobilized on 3D-Printed Single- and Multi-Channel Polylactic Acid Microreactors
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
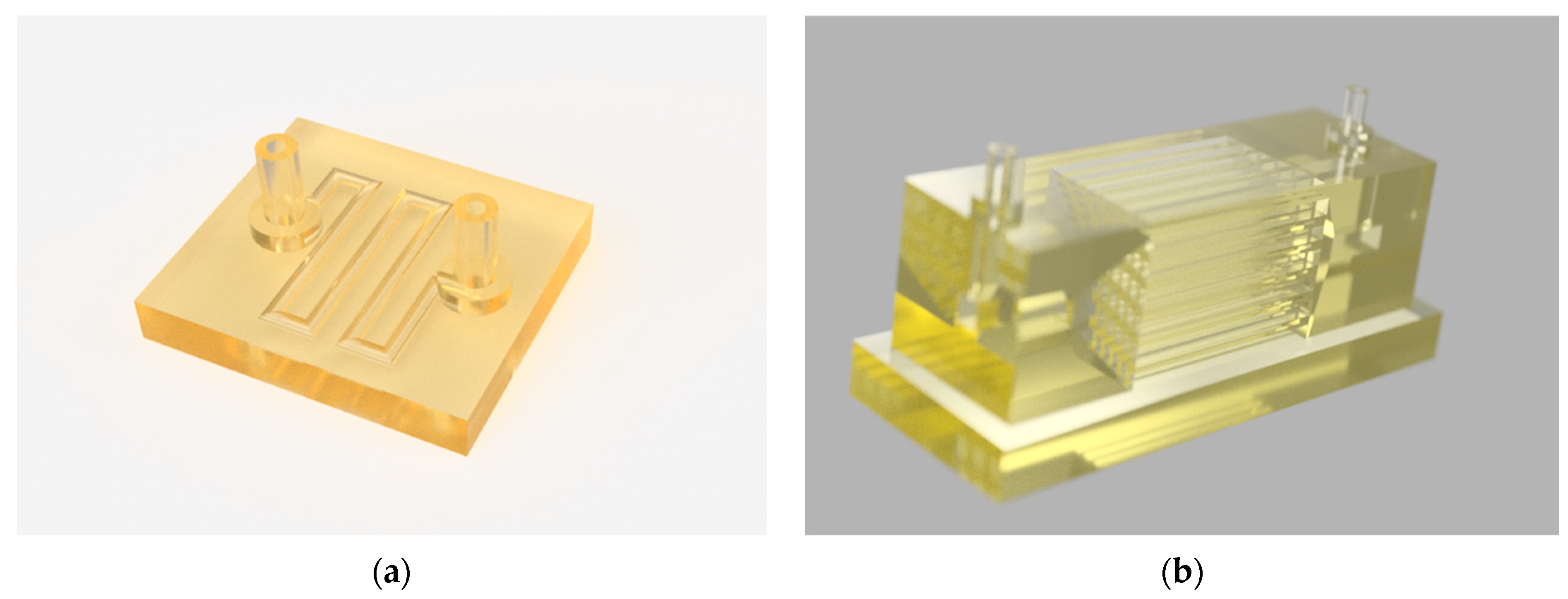
2.2. Design and Fabrication of the 3D Scaffolds
2.3. Surface Modification of the PLA Scaffolds
2.4. Immobilization of β-Glucosidase in the Microreactors
2.5. β-Glucosidase Activity Studies
2.6. Kinetic Studies of β-Glucosidase
2.7. Thermal and Operational Stability of β-Glucosidase
2.8. Computational Fluid Dynamics Simulation
2.9. Statistical Analysis
3. Results
3.1. Optimization of the Immobilization Procedure in the Single-Channel Microreactor
3.2. Biocatalytic Characterization of the Immobilized Single-Channel Microreactor
3.3. Development of a Multi-Channel Microreactor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gkantzou, E.; Chatzikonstantinou, A.V.; Fotiadou, R.; Giannakopoulou, A.; Patila, M.; Stamatis, H. Trends in the Development of Innovative Nanobiocatalysts and Their Application in Biocatalytic Transformations. Biotechnol. Adv. 2021, 51, 107738. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Shafreen, M.M.; Achudhan, A.B.; Gupta, A.; Saleena, L.M. A Review on Applications of β-Glucosidase in Food, Brewery, Pharmaceutical and Cosmetic Industries. Carbohydr. Res. 2023, 530, 108855. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.R.K.; Esen, A. β-Glucosidases. Cell. Mol. Life Sci. 2010, 67, 3389–3405. [Google Scholar] [CrossRef] [PubMed]
- Mól, P.C.G.; Júnior, J.C.Q.; Veríssimo, L.A.A.; Boscolo, M.; Gomes, E.; Minim, L.A.; Da Silva, R. β-Glucosidase: An Overview on Immobilization and Some Aspects of Structure, Function, Applications and Cost. Process Biochem. 2023, 130, 26–39. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Q.; Shao, L.; Jia, Y.; Zhang, X. Microfluidic Immobilized Enzyme Reactors for Continuous Biocatalysis. React. Chem. Eng. 2020, 5, 9–32. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Pessoa, A.C.S.N.; Bastos, R.G.; de la Torre, L.G. Microfluidic Tools toward Industrial Biotechnology. Biotechnol. Prog. 2016, 32, 1372–1389. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.; Grünberger, A.; Bahnemann, J. Towards Small Scale: Overview and Applications of Microfluidics in Biotechnology. Mol. Biotechnol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ortseifen, V.; Viefhues, M.; Wobbe, L.; Grünberger, A. Microfluidics for Biotechnology: Bridging Gaps to Foster Microfluidic Applications. Front. Bioeng. Biotechnol. 2020, 8, 589074. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Tang, H.; Zong, N.; Jiang, X. Microfluidics for Biomedical Analysis. Small Methods 2020, 4, 1900451. [Google Scholar] [CrossRef]
- Nielsen, A.V.; Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. 3D Printed Microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 45–65. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Fu, J.Z.; Gao, Q.; Qiu, J.J. Developments of 3D Printing Microfluidics and Applications in Chemistry and Biology: A Review. Electroanalysis 2016, 28, 1658–1678. [Google Scholar] [CrossRef]
- Dong, Z.; Wen, Z.; Zhao, F.; Kuhn, S.; Noël, T. Scale-up of Micro- and Milli-Reactors: An Overview of Strategies, Design Principles and Applications. Chem. Eng. Sci. X 2021, 10, 100097. [Google Scholar] [CrossRef]
- Su, A.; Al’Aref, S.J. History of 3D Printing. In 3D Printing Applications in Cardiovascular Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–10. ISBN 9780128039175. [Google Scholar]
- Su, C.K. Review of 3D-Printed Functionalized Devices for Chemical and Biochemical Analysis. Anal. Chim. Acta 2021, 1158, 338348. [Google Scholar] [CrossRef] [PubMed]
- Berman, B. 3-D Printing: The New Industrial Revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Attaran, M. The Rise of 3-D Printing: The Advantages of Additive Manufacturing over Traditional Manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Reinicke, T. On the Environmental Impacts of 3D Printing Technology. Appl. Mater. Today 2020, 20, 100689. [Google Scholar] [CrossRef]
- Al-Dulimi, Z.; Wallis, M.; Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. 3D Printing Technology as Innovative Solutions for Biomedical Applications. Drug Discov. Today 2021, 26, 360–383. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, Y.; Karayel, E. 3D Printing Technology; Methods, Biomedical Applications, Future Opportunities and Trends. J. Mater. Res. Technol. 2021, 14, 1430–1450. [Google Scholar] [CrossRef]
- Prabhakar, P.; Sen, R.K.; Dwivedi, N.; Khan, R.; Solanki, P.R.; Srivastava, A.K.; Dhand, C. 3D-Printed Microfluidics and Potential Biomedical Applications. Front. Nanotechnol. 2021, 3, 609355. [Google Scholar] [CrossRef]
- Gkantzou, E.; Weinhart, M.; Kara, S. 3D Printing for Flow Biocatalysis. RSC Sustain. 2023, 1, 1672–1685. [Google Scholar] [CrossRef]
- Ye, J.; Chu, T.; Chu, J.; Gao, B.; He, B. A Versatile Approach for Enzyme Immobilization Using Chemically Modified 3D-Printed Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 18048–18054. [Google Scholar] [CrossRef]
- Peris, E.; Okafor, O.; Kulcinskaja, E.; Goodridge, R.; Luis, S.V.; Garcia-Verdugo, E.; O’Reilly, E.; Sans, V. Tuneable 3D Printed Bioreactors for Transaminations under Continuous-Flow. Green Chem. 2017, 19, 5345–5349. [Google Scholar] [CrossRef]
- Eixenberger, D.; Kumar, A.; Klinger, S.; Scharnagl, N.; Dawood, A.W.H.; Liese, A. Polymer-Grafted 3D-Printed Material for Enzyme Immobilization—Designing a Smart Enzyme Carrier. Catalysts 2023, 13, 1130. [Google Scholar] [CrossRef]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Ul Haq, M.I. 3D Printing—A Review of Processes, Materials and Applications in Industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Chen, C.; Mehl, B.T.; Munshi, A.S.; Townsend, A.D.; Spence, D.M.; Martin, R.S. 3D-Printed Microfluidic Devices: Fabrication, Advantages and Limitations—A Mini Review. Anal. Methods 2016, 8, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Potdar, A.; Thomassen, L.C.J.; Kuhn, S. Scalability of 3D Printed Structured Porous Milli-Scale Reactors. Chem. Eng. J. 2019, 363, 337–348. [Google Scholar] [CrossRef]
- Baran, E.H.; Yildirim Erbil, H. Surface Modification of 3d Printed Pla Objects by Fused Deposition Modeling: A Review. Colloids Interfaces 2019, 3, 43. [Google Scholar] [CrossRef]
- Gkantzou, E.; Skonta, A.; Tsakni, A.; Polydera, A.; Moschovas, D.; Spyrou, K.; Avgeropoulos, A.; Gournis, D.; Houhoula, D.; Stamatis, H. 3D Printed PLA Enzyme Microreactors: Characterization and Application for the Modification of Bioactive Compounds. J. Biotechnol. 2022, 350, 75–85. [Google Scholar] [CrossRef]
- Schneider, M.; Fritzsche, N.; Puciul-Malinowska, A.; Baliś, A.; Mostafa, A.; Bald, I.; Zapotoczny, S.; Taubert, A. Surface Etching of 3D Printed Poly(Lactic Acid) with NaOH: A Systematic Approach. Polymers 2020, 12, 1711. [Google Scholar] [CrossRef]
- Lilly, M.D.; Hornby, W.E.; Crook, E.M. The Kinetics of Carboxymethylcellulose-Ficin in Packed Beds. Biochem. J. 1966, 100, 718–723. [Google Scholar] [CrossRef]
- Seong, G.H.; Heo, J.; Crooks, R.M. Measurement of Enzyme Kinetics Using a Continuous-Flow Microfluidic System. Anal. Chem. 2003, 75, 3161–3167. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in Bio-Catalysts Design: A Useful Crosslinker and a Versatile Tool in Enzyme Immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Adriano, W.S.; Filho, E.H.C.; Silva, J.A.; Giordano, R.L.C.; Gonçalves, L.R.B. Stabilization of penicillin G acylase by immobilization on glutaraldehyde-activated chitosan. Braz. J. Chem. Eng. 2005, 22, 529–538. [Google Scholar] [CrossRef]
- Singh, A.N.; Singh, S.; Suthar, N.; Dubey, V.K. Glutaraldehyde-Activated Chitosan Matrix for Immobilization of a Novel Cysteine Protease, Procerain B. J. Agric. Food Chem. 2011, 59, 6256–6262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Yuwen, L.X.; Peng, L.J. Parameters Affecting the Performance of Immobilized Enzyme. J. Chem. 2013, 2013, 946248. [Google Scholar] [CrossRef]
- Bellou, M.G.; Gkantzou, E.; Skonta, A.; Moschovas, D.; Spyrou, K.; Avgeropoulos, A.; Gournis, D.; Stamatis, H. Development of 3D Printed Enzymatic Microreactors for Lipase-Catalyzed Reactions in Deep Eutectic Solvent-Based Media. Micromachines 2022, 13, 1954. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xue, Y.; Lin, Y. Enhanced Catalytic Efficiency in Quercetin-4′-Glucoside Hydrolysis of Thermotoga Maritima β-Glucosidase a by Site-Directed Mutagenesis. J. Agric. Food Chem. 2014, 62, 6763–6770. [Google Scholar] [CrossRef]
- Alnadari, F.; Xue, Y.; Zhou, L.; Hamed, Y.S.; Taha, M.; Foda, M.F. Immobilization of β-Glucosidase from Thermatoga Maritima on Chitin-Functionalized Magnetic Nanoparticle via a Novel Thermostable Chitin-Binding Domain. Sci. Rep. 2020, 10, 1663. [Google Scholar] [CrossRef]
- Gabelsberger, J.; Liebl, W.; Schleifer, K.-H. Microbiology Biotechnology Purification and Properties of Recombinant Fl-Glucosidase of the Hyperthermophilic Bacterium Thermotoga Maritima; Springer: Berlin/Heidelberg, Germany, 1993; Volume 40. [Google Scholar]
- Alnadari, F.; Xue, Y.; Alsubhi, N.H.; Alamoudi, S.A.; Alwabli, A.S.; Al-Quwaie, D.A.; Saud Hamed, Y.; Muhammad Nasiru, M.; Ebrahim, A.A.M.; El-Saadony, M.T.; et al. Reusability of Immobilized β-Glucosidase on Sodium Alginate-Coated Magnetic Nanoparticles and High Productivity Applications. J. Saudi Chem. Soc. 2022, 26, 101517. [Google Scholar] [CrossRef]
- Goyal, K.; Selvakumar, P.; Hayashi, K. Characterization of a Thermostable-Glucosidase (BglB) from Thermotoga Maritima Showing Transglycosylation Activity; 2001; Volume 15.
- Wei, C.; Zhou, Y.; Zhuang, W.; Li, G.; Jiang, M.; Zhang, H. Improving the Performance of Immobilized β-Glucosidase Using a Microreactor. J. Biosci. Bioeng. 2018, 125, 377–384. [Google Scholar] [CrossRef]
- Sokač Cvetnić, T.; Šalić, A.; Benković, M.; Jurina, T.; Valinger, D.; Gajdoš Kljusurić, J.; Zelić, B.; Jurinjak Tušek, A. A Systematic Review of Enzymatic Kinetics in Microreactors. Catalysts 2023, 13, 708. [Google Scholar] [CrossRef]
- Gkantzou, E.; Govatsi, K.; Chatzikonstantinou, A.V.; Yannopoulos, S.N.; Stamatis, H. Development of a ZnO Nanowire Continuous Flow Microreactor with β-Glucosidase Activity: Characterization and Application for the Glycosylation of Natural Products. ACS Sustain. Chem. Eng. 2021, 9, 7658–7667. [Google Scholar] [CrossRef]
- Carvalho, F.; Fernandes, P. Packed Bed Enzyme Microreactor: Application in Sucrose Hydrolysis as Proof-of-Concept. Biochem. Eng. J. 2015, 104, 74–81. [Google Scholar] [CrossRef]
- Carvalho, F.; Marques, M.P.C.; Fernandes, P. Sucrose Hydrolysis in a Bespoke Capillary Wall-Coated Microreactor. Catalysts 2017, 7, 42. [Google Scholar] [CrossRef]
- Rufer, A.C. Drug Discovery for Enzymes. Drug Discov. Today 2021, 26, 875–886. [Google Scholar] [CrossRef]
- Wang, J.; Gu, S.S.; Cui, H.S.; Yang, L.Q.; Wu, X.Y. Rapid Synthesis of Propyl Caffeate in Ionic Liquid Using a Packed Bed Enzyme Microreactor under Continuous-Flow Conditions. Bioresour. Technol. 2013, 149, 367–374. [Google Scholar] [CrossRef]
- Abd Razak, N.N.; Cognet, P.; Pérès, Y.; Gew, L.T.; Aroua, M.K. Kinetics and Hydrodynamics of Candida Antartica Lipase-Catalyzed Synthesis of Glycerol Dioleate (GDO) in a Continuous Flow Packed-Bed Millireactor. J. Clean. Prod. 2022, 373, 133816. [Google Scholar] [CrossRef]
- Gong, A.; Zhu, C.T.; Xu, Y.; Wang, F.Q.; Tsabing, D.K.; Wu, F.A.; Wang, J. Moving and Unsinkable Graphene Sheets Immobilized Enzyme for Microfluidic Biocatalysis. Sci. Rep. 2017, 7, 4309. [Google Scholar] [CrossRef]
- Bhavsar, K.V.; Yadav, G.D. N-Butyl Levulinate Synthesis Using Lipase Catalysis: Comparison of Batch Reactor versus Continuous Flow Packed Bed Tubular Microreactor. J. Flow. Chem. 2018, 8, 97–105. [Google Scholar] [CrossRef]
- Abdul Halim, A.; Szita, N.; Baganz, F. Characterization and Multi-Step Transketolase-ω-Transaminase Bioconversions in an Immobilized Enzyme Microreactor (IEMR) with Packed Tube. J. Biotechnol. 2013, 168, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Lindahl, S.; Turner, C.; Karlsson, E.N. Immobilization of Thermostable β-Glucosidase Variants on Acrylic Supports for Biocatalytic Processes in Hot Water. J. Mol. Catal. B Enzym. 2012, 80, 28–38. [Google Scholar] [CrossRef]
- Goldstein, Y.; Spitz, S.; Turjeman, K.; Selinger, F.; Barenholz, Y.; Ertl, P.; Benny, O.; Bavli, D. Breaking the Third Wall: Implementing 3d-Printing Technics to Expand the Complexity and Abilities of Multi-Organ-on-a-Chip Devices. Micromachines 2021, 12, 627. [Google Scholar] [CrossRef]
- Ong, L.J.Y.; Islam, A.; Dasgupta, R.; Iyer, N.G.; Leo, H.L.; Toh, Y.C. A 3D Printed Microfluidic Perfusion Device for Multicellular Spheroid Cultures. Biofabrication 2017, 9, 045005. [Google Scholar] [CrossRef]
- Perez, C.L.; Casciatori, F.P.; Thoméo, J.C. Improving Enzyme Production by Solid-State Cultivation in Packed-Bed Bioreactors by Changing Bed Porosity and Airflow Distribution. Bioprocess. Biosyst. Eng. 2021, 44, 537–548. [Google Scholar] [CrossRef]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow Bioreactors as Complementary Tools for Biocatalytic Process Intensification. Trends Biotechnol. 2018, 36, 73–88. [Google Scholar] [CrossRef]
- Venezia, V.; Califano, V.; Pota, G.; Costantini, A.; Landi, G.; Di Benedetto, A. CFD Simulations of Microreactors for the Hydrolysis of Cellobiose to Glucose by β-Glucosidase Enzyme. Micromachines 2020, 11, 790. [Google Scholar] [CrossRef]








| Flow Rate (μL/min) | Apparent Km (mM) |
|---|---|
| 20 | 0.854 ± 0.151 a |
| 30 | 0.585 ± 0.054 b |
| 40 | 0.551 ± 0.041 b |
| 50 | 0.486 ± 0.039 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasios, A.-G.; Skonta, A.; Patila, M.; Stamatis, H. Biocatalytic Performance of β-Glucosidase Immobilized on 3D-Printed Single- and Multi-Channel Polylactic Acid Microreactors. Micromachines 2024, 15, 288. https://doi.org/10.3390/mi15020288
Vasios A-G, Skonta A, Patila M, Stamatis H. Biocatalytic Performance of β-Glucosidase Immobilized on 3D-Printed Single- and Multi-Channel Polylactic Acid Microreactors. Micromachines. 2024; 15(2):288. https://doi.org/10.3390/mi15020288
Chicago/Turabian StyleVasios, Andreas-Georgios, Anastasia Skonta, Michaela Patila, and Haralambos Stamatis. 2024. "Biocatalytic Performance of β-Glucosidase Immobilized on 3D-Printed Single- and Multi-Channel Polylactic Acid Microreactors" Micromachines 15, no. 2: 288. https://doi.org/10.3390/mi15020288
APA StyleVasios, A.-G., Skonta, A., Patila, M., & Stamatis, H. (2024). Biocatalytic Performance of β-Glucosidase Immobilized on 3D-Printed Single- and Multi-Channel Polylactic Acid Microreactors. Micromachines, 15(2), 288. https://doi.org/10.3390/mi15020288





_Stamatis.png)

