Research on Key Technologies of Dual-Light-Type Photoelectric Colorimetric Method for Phosphate Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
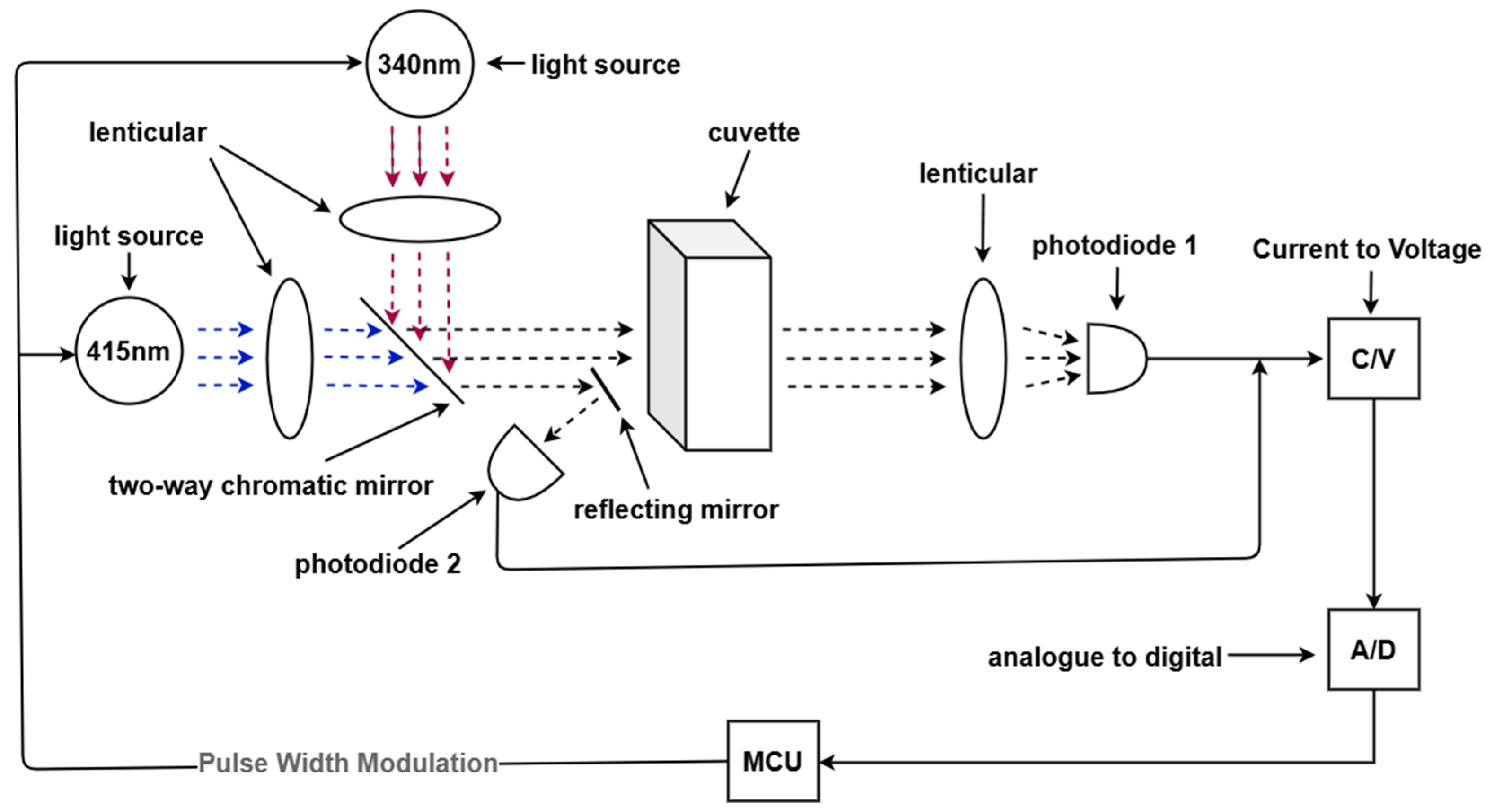
2.2. Overall Design Solution
2.3. Experimental Methods
2.3.1. Linear Experimental Program
2.3.2. Repeatability Experimental Program
2.3.3. Comparison of the Experimental Programs
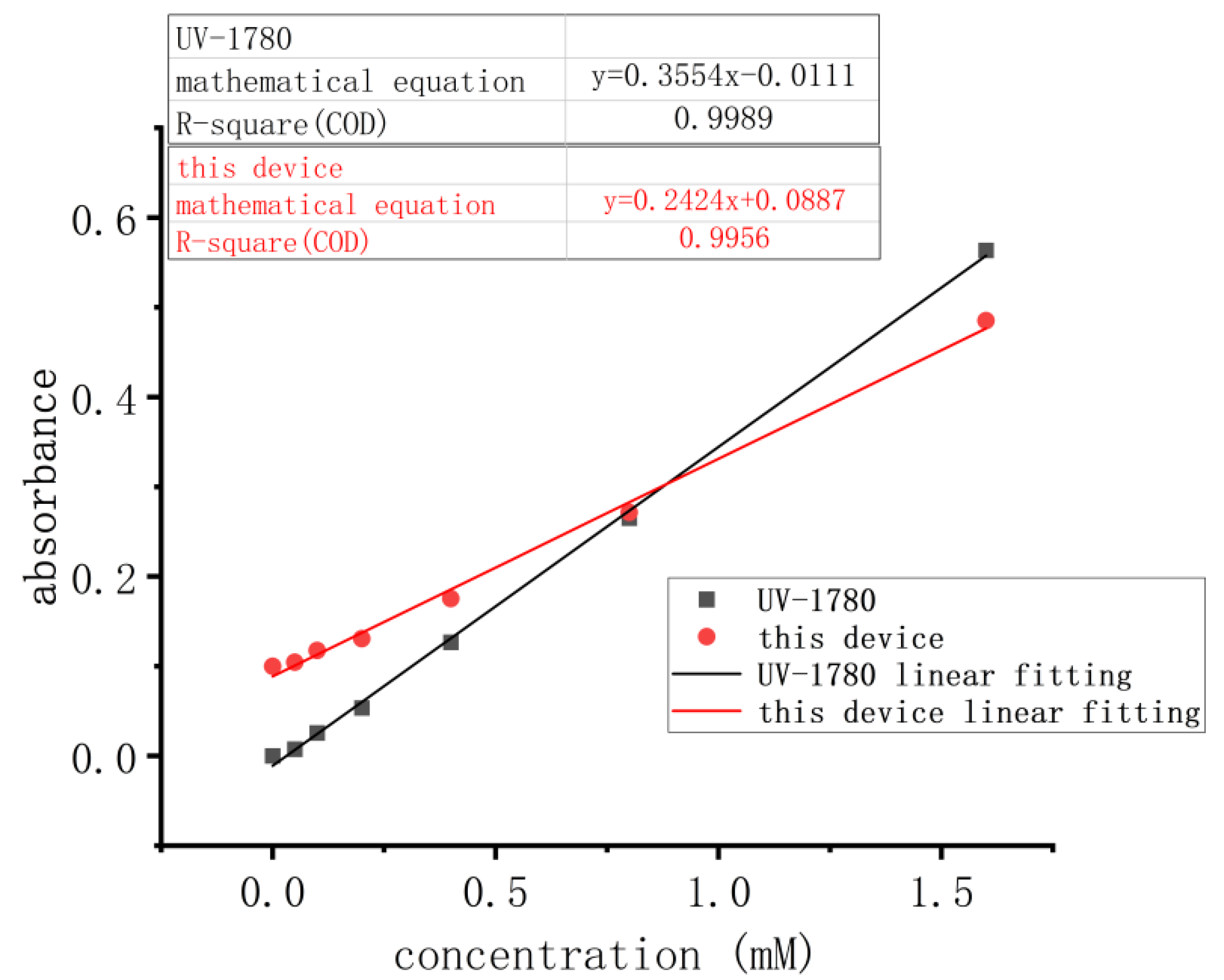
- According to the experimental steps outlined in Table 3, the samples of 7 different concentrations mentioned in Section 2.3.1 were measured using both the device described in this study and the UV-1780 UV–Visible spectrophotometer. A graph was plotted with the sample concentration on the x-axis and the corresponding absorbance on the y-axis, for both instruments, in the same coordinate system. Linear fitting was then performed.
- Following the experimental steps outlined in Table 3, the CHO cell suspension samples from 23 different batches were measured using both the device described in this study and the UV-1780 UV–Visible spectrophotometer. A graph was plotted with the sample batch number on the x-axis and the absorbance of each batch’s sample on the y-axis.
3. Results and Discussion
3.1. Simulation Analysis
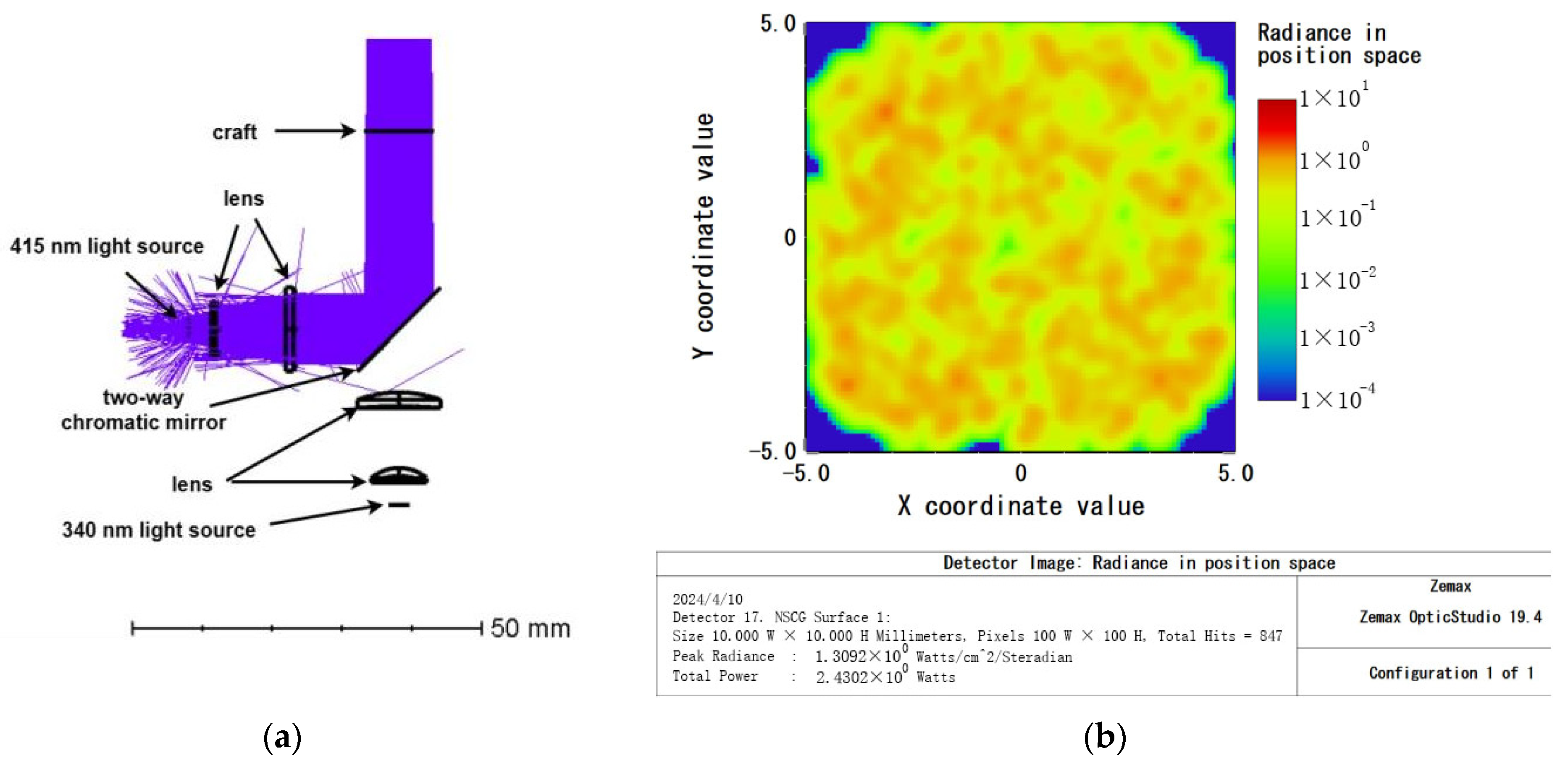
3.1.1. Excitation Light Path Simulation
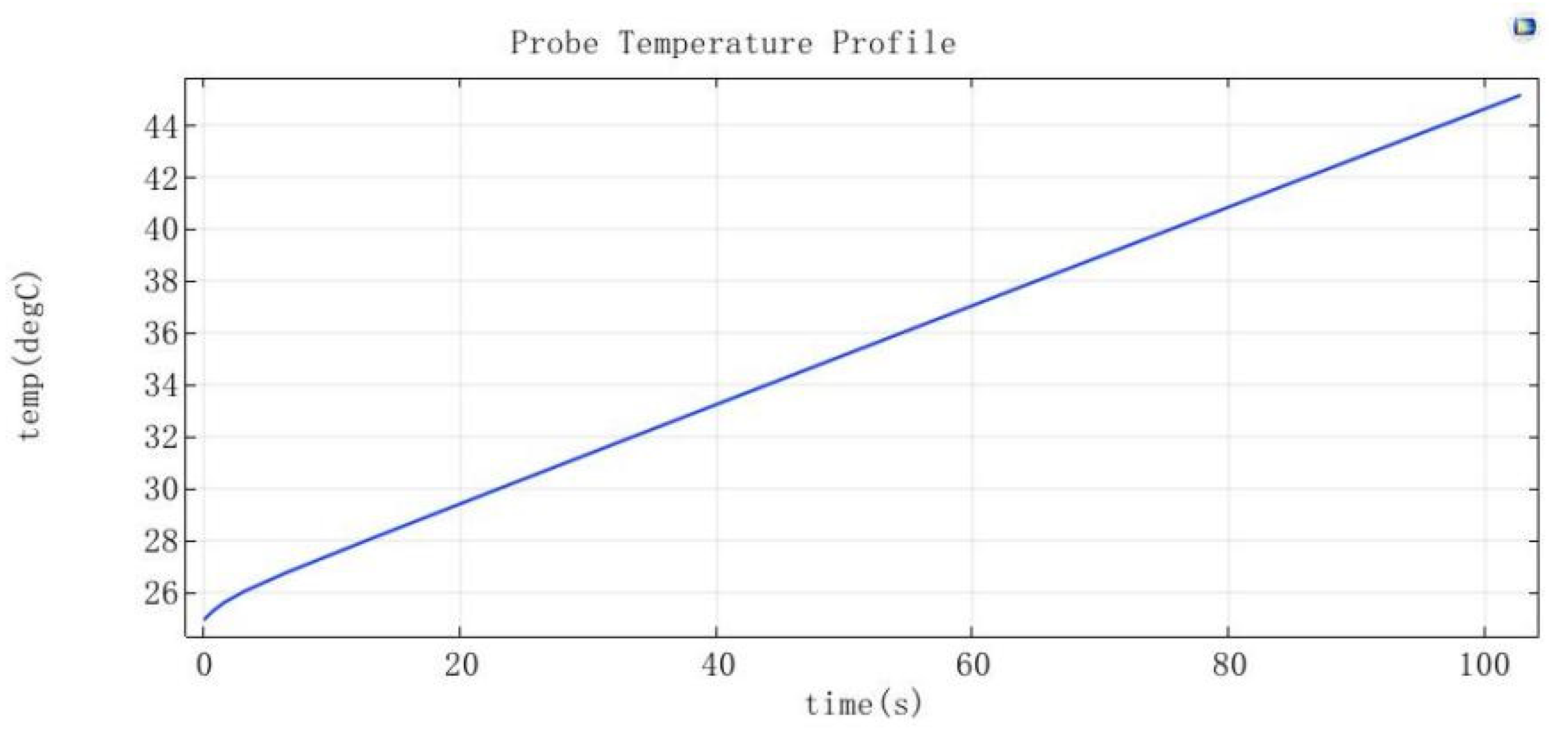
3.1.2. Temperature Control Module Simulation
3.2. Experimental Results and Discussion
3.2.1. Linear Experimental Results
3.2.2. Repeatability Experiment Results
3.2.3. Comparison of the Experimental Results
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berchmans, S.; Issa, T.B.; Singh, P. Determination of inorganic phosphate by electroanalytical methods: A review. Anal. Chim. Acta 2012, 729, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Du, Z.; Qian, Y.; Li, F. A specific colorimetric probe for phosphate detection based on anti-aggregation of gold nanoparticles. Sens. Actuators B Chem. 2013, 176, 927–931. [Google Scholar] [CrossRef]
- Wu, H.; Tong, C. A Specific Turn-On Fluorescent Sensing for Ultrasensitive and Selective Detection of Phosphate in Environmental Samples Based on Antenna Effect-Improved FRET by Surfactant. ACS Sens. 2018, 3, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, J.; Lu, Y.; Sun, J.; Yang, X. Fluorescence Immunoassay Based on the Phosphate-Triggered Fluorescence Turn-on Detection of Alkaline Phosphatase. Anal. Chem. 2018, 90, 3505–3511. [Google Scholar] [CrossRef]
- Ogata, F.; Imai, D.; Toda, M.; Otani, M.; Kawasaki, N. Adsorption of phosphate ion in aqueous solutions by calcined cobalt hydroxide at different temperatures. J. Environ. Chem. Eng. 2015, 3, 1570–1577. [Google Scholar] [CrossRef]
- Morgan, S.; Luy, E.; Furlong, A.; Sieben, V. A submersible phosphate analyzer for marine environments based on inlaid microfluidics. Anal. Methods 2022, 14, 22–33. [Google Scholar] [CrossRef]
- Zhao, H.X.; Liu, L.Q.; Liu, Z.D.; Wang, Y.; Zhao, X.J.; Huang, C.Z. Highly selective detection of phosphate in very complicated matrixes with an off–on fluorescent probe of europium-adjusted carbon dots. Chem. Commun. 2011, 47, 2604–2606. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Hasegawa, K.; Tohda, K.; Umezawa, Y. Voltammetric detection of inorganic phosphate using ion-channel sensing with self-assembled monolayers of a hydrogen bond-forming receptor. Biosens. Bioelectron. 2003, 18, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Bo, G.; Ma, J. An automated analyzer for the simultaneous determination of silicate and phosphate in seawater. Talanta 2022, 248, 123629. [Google Scholar] [CrossRef]
- Rossel, T.; Creus, M. A biomimetic cerium-based biosensor for the direct visual detection of phosphate under physiological conditions. Chem. Commun. 2019, 55, 14894–14897. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, M.; Liu, X.; Zhong, K.; Tang, L.; Li, J. A fluorescent and colorimetric dual-recognition probe based on copper(ii)-decorated carbon dots for detection of phosphate. Anal. Methods 2021, 13, 5003–5010. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified single solution method for the determination of phosphate in natural water. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Wu, T.; Huang, G. Applications of electrochemical methods in inorganic phosphate detection. Environ. Chem. 2021, 40, 2854–2863. [Google Scholar]




| Detection Methods | Detection Principle | Advantages | Disadvantages | Sensitivity Range |
|---|---|---|---|---|
| spectrophotometry | Under acidic conditions, phosphate reacts with phenolic reagents such as ammonium molybdate and sodium molybdate to produce colored products, which can be measured for absorbance at specific wavelengths. | Easy and quick to operate. | Vulnerable to interference. | 0.01 mg/L~2.0 mg/L |
| ion chromatography | The phosphate in the solution to be measured is separated from other ions by means of an ion exchange column, and the phosphate concentration is then measured by a detection instrument. | High accuracy and detection of complex samples. | Complex operation and high instrument cost. | 0.1 ug/L~10 ug/L |
| atomic absorption | The phosphate content is determined by measuring the absorption signal of elemental phosphorus in the solution to be tested by atomic absorption spectrometry. | Highly accurate. | Complex operation and high instrument cost. | 10 ug/L~50 ug/L |
| microscopy | Combined with staining techniques under the microscope. | Phosphate morphology and distribution can be observed. | Cumbersome pre-treatment steps and an inability to accurately obtain phosphate concentrations. | Usually used for qualitative and distributional analyses. |
| spectral analysis | Measurements are made using the absorption or emission properties of phosphates at specific wavelengths, commonly UV–visible spectroscopy, fluorescence spectroscopy, and so on. | High sensitivity and accuracy. | Requires specialized instruments and is costly. | Inductively coupled plasma mass spectrometry can achieve detection limits of sub-micrograms per liter. |
| Kit Composition | Main Components in the Reagent | Concentration |
|---|---|---|
| Reagent 1 | Oxalate | 25 mL/L |
| Reagent 2 | Ammonium molybdate | 3.5 g/L |
| Calibrator | Dipotassium hydrogen phosphate, water-based | 1.6 mmol/L |
| Serial Number | Experimental Step | Note |
|---|---|---|
| 1 | Add diluted calibrator to cuvette. | 3 uL |
| 2 | Add reagent 1 to the cuvette. | 200 uL |
| 3 | Mix well, put into the device, incubate for 3 min, and read absorbance A0. | |
| 4 | Add reagent 2 to the cuvette. | 100 uL |
| 5 | After mixing, incubate for 5 min and read the absorbance A1. | |
| 6 | Record ΔA = A1 − A0. | |
| Concentration (mM) | UV-1780 Absorbance | Absorbance of This Device |
|---|---|---|
| 1.60 | 0.5635 | 0.4852 |
| 0.80 | 0.2650 | 0.2713 |
| 0.40 | 0.1267 | 0.1753 |
| 0.20 | 0.0536 | 0.1307 |
| 0.10 | 0.0257 | 0.1176 |
| 0.05 | 0.0076 | 0.1044 |
| 0.00 | 0.0000 | 0.1000 |
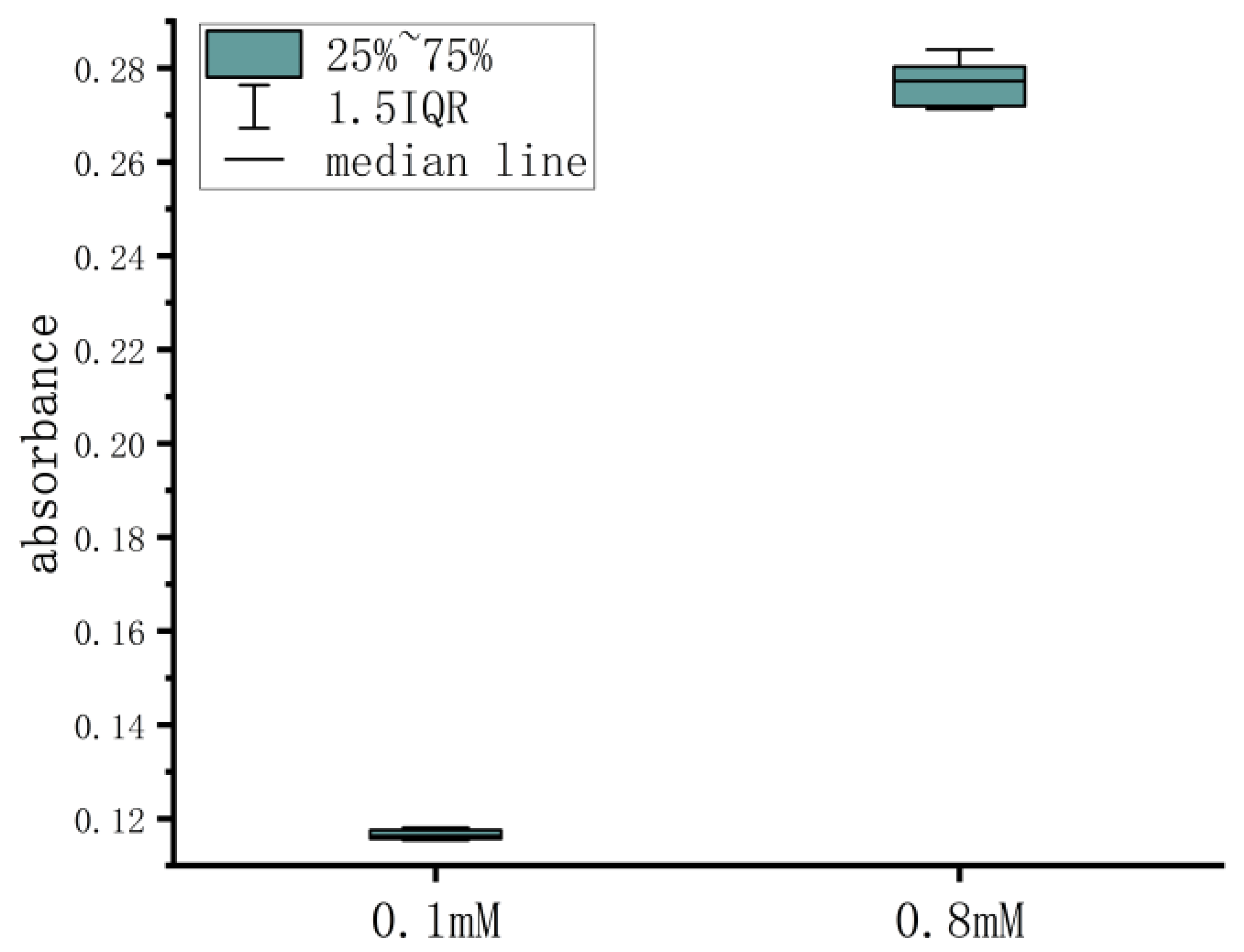
| Serial Number | 0.1 mM | 0.8 mM |
|---|---|---|
| 1 | 0.1176 | 0.2713 |
| 2 | 0.1154 | 0.2840 |
| 3 | 0.1181 | 0.2716 |
| 4 | 0.1177 | 0.2801 |
| 5 | 0.1165 | 0.2792 |
| 6 | 0.1156 | 0.2719 |
| 7 | 0.1157 | 0.2722 |
| 8 | 0.1160 | 0.2803 |
| 9 | 0.1173 | 0.2753 |
| 10 | 0.1161 | 0.2812 |
| mean | 0.1166 | 0.2767 |
| SD (standard deviation) | 0.0010 | 0.0048 |
| CV (coefficient of variation) | 0.85% | 1.72% |
| Serial Number | UV-1780 | This Device | Serial Number | UV-1780 | This Device |
|---|---|---|---|---|---|
| 1 | 0.3996 | 0.3688 | 13 | 0.2742 | 0.2833 |
| 2 | 0.0806 | 0.1513 | 14 | 0.5833 | 0.4941 |
| 3 | 0.3122 | 0.3092 | 15 | 0.6213 | 0.5200 |
| 4 | 0.5977 | 0.5040 | 16 | 0.3081 | 0.3064 |
| 5 | 0.6589 | 0.5457 | 17 | 0.4306 | 0.3900 |
| 6 | 0.2053 | 0.2363 | 18 | 0.4527 | 0.4050 |
| 7 | 0.5583 | 0.4770 | 19 | 0.3762 | 0.3529 |
| 8 | 0.4014 | 0.3700 | 20 | 0.0689 | 0.1433 |
| 9 | 0.0582 | 0.1360 | 21 | 0.0339 | 0.1194 |
| 10 | 0.4761 | 0.4210 | 22 | 0.2870 | 0.2920 |
| 11 | 0.2679 | 0.2790 | 23 | 0.5703 | 0.4853 |
| 12 | 0.0818 | 0.1521 |
| This Device | UV-1780 | Uicare-300 | |
|---|---|---|---|
| Sample flux | 1 | 6 | 32 |
| Sensitivity | 0.003 Abs | 0.001 Abs | 0.01 Abs |
| Detection range | 0.01 mg/L~2.0 mg/L | 0.01 mg/L~2.0 mg/L | 0.1 mg~2.0 mg/L |
| Dominance | Low price, small size, easy to operate. | The excitation wavelength is within 340 nm to 900 nm in 1 nm steps. | The sample throughput is relatively large and can be automatically spiked. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Zhang, H.; Sun, T.; Wang, X.; Gong, P. Research on Key Technologies of Dual-Light-Type Photoelectric Colorimetric Method for Phosphate Determination. Micromachines 2024, 15, 821. https://doi.org/10.3390/mi15070821
Guo H, Zhang H, Sun T, Wang X, Gong P. Research on Key Technologies of Dual-Light-Type Photoelectric Colorimetric Method for Phosphate Determination. Micromachines. 2024; 15(7):821. https://doi.org/10.3390/mi15070821
Chicago/Turabian StyleGuo, Hongzhuang, Hao Zhang, Tingting Sun, Xin Wang, and Ping Gong. 2024. "Research on Key Technologies of Dual-Light-Type Photoelectric Colorimetric Method for Phosphate Determination" Micromachines 15, no. 7: 821. https://doi.org/10.3390/mi15070821
APA StyleGuo, H., Zhang, H., Sun, T., Wang, X., & Gong, P. (2024). Research on Key Technologies of Dual-Light-Type Photoelectric Colorimetric Method for Phosphate Determination. Micromachines, 15(7), 821. https://doi.org/10.3390/mi15070821






