Research Progress in the Preparation of Transition Metal Sulfide Materials and Their Supercapacitor Performance
Abstract
:1. Introduction
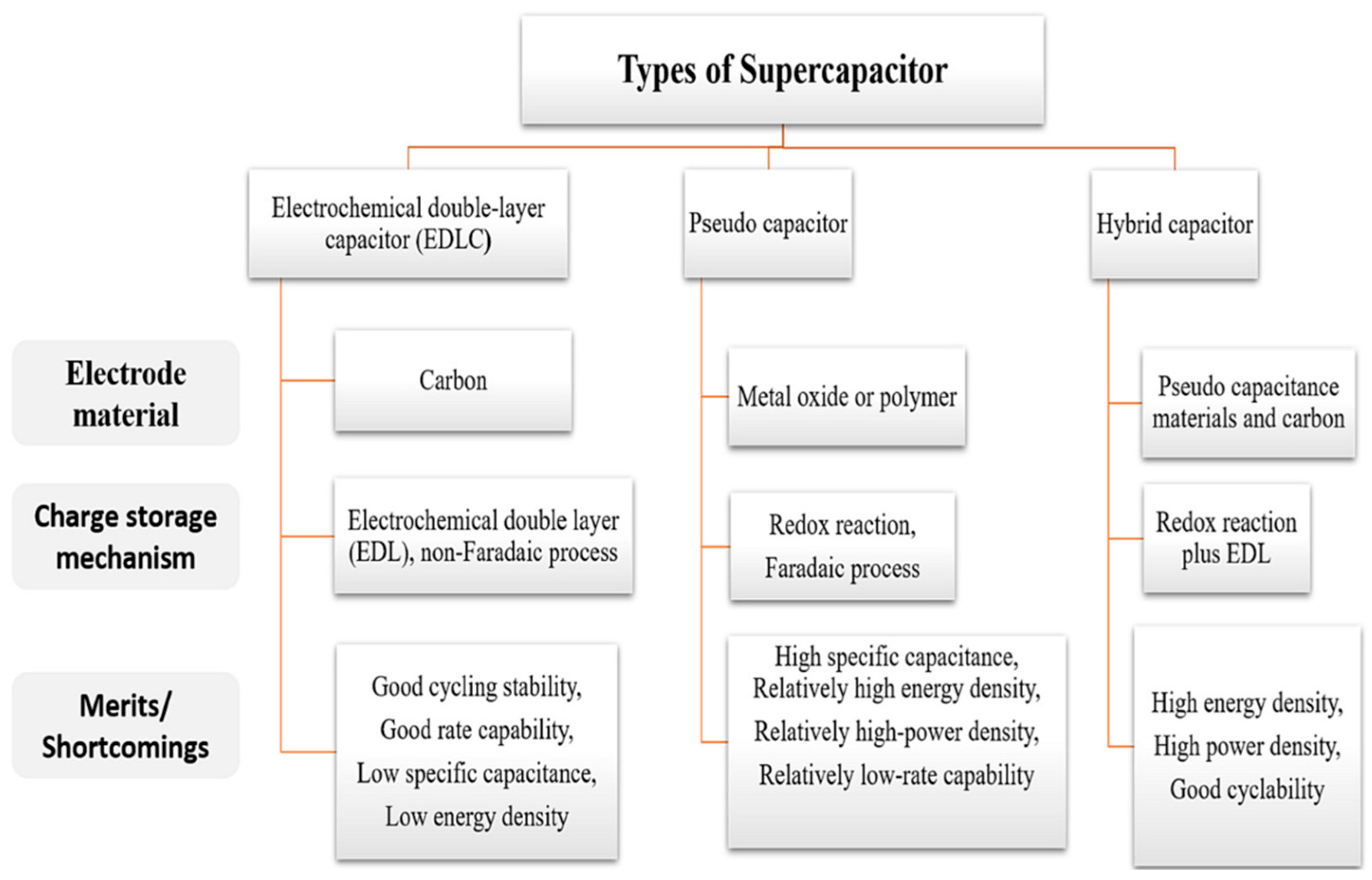
2. Classification and Energy Storage Principle of Supercapacitors
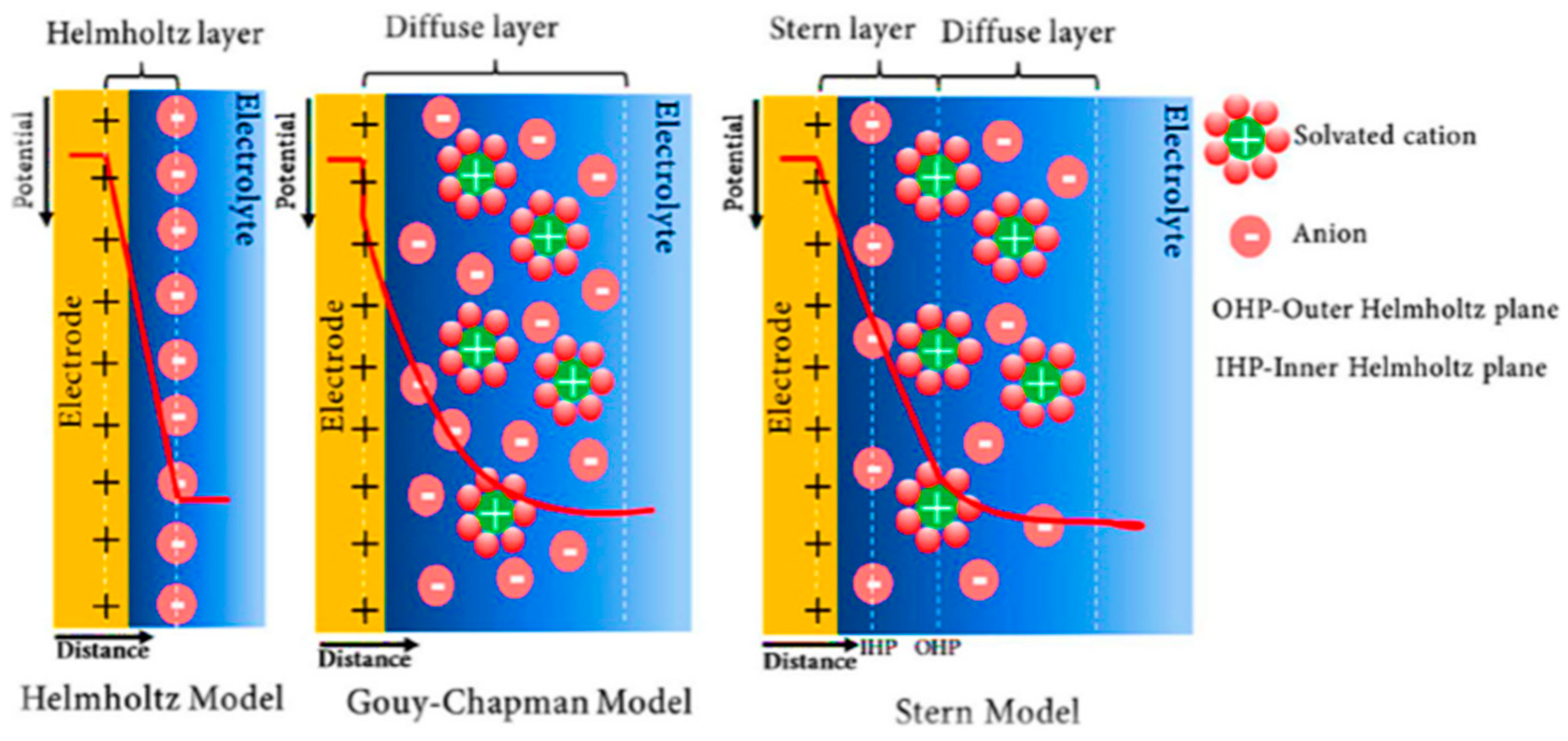
2.1. Electrochemical Double-Layer Capacitor (EDLC)
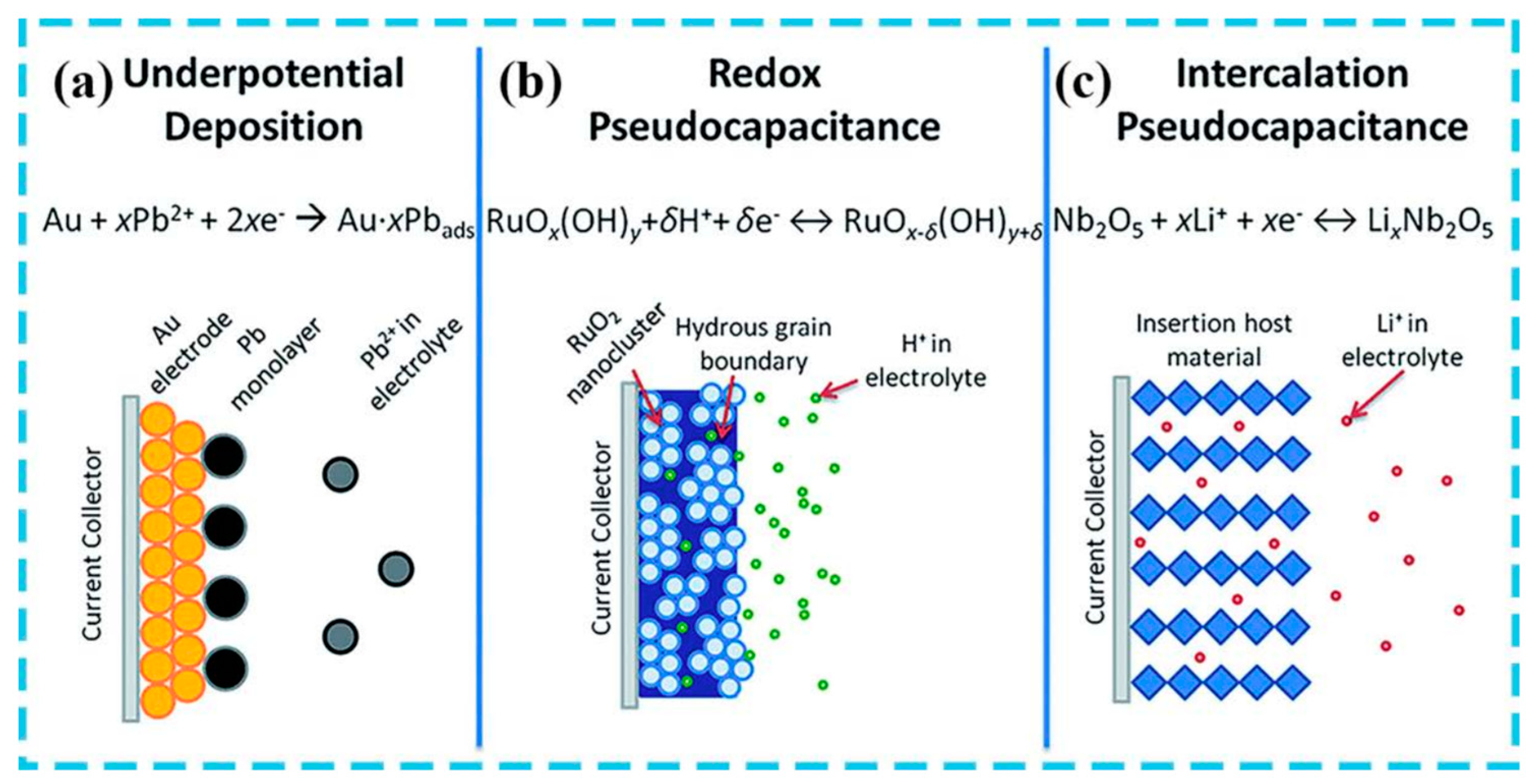
2.2. Pseudocapacitors
2.3. Hybrid Supercapacitors
3. Supercapacitor Electrode Material
3.1. Synthesis Method of Transition Metal Sulfide Electrode Materials
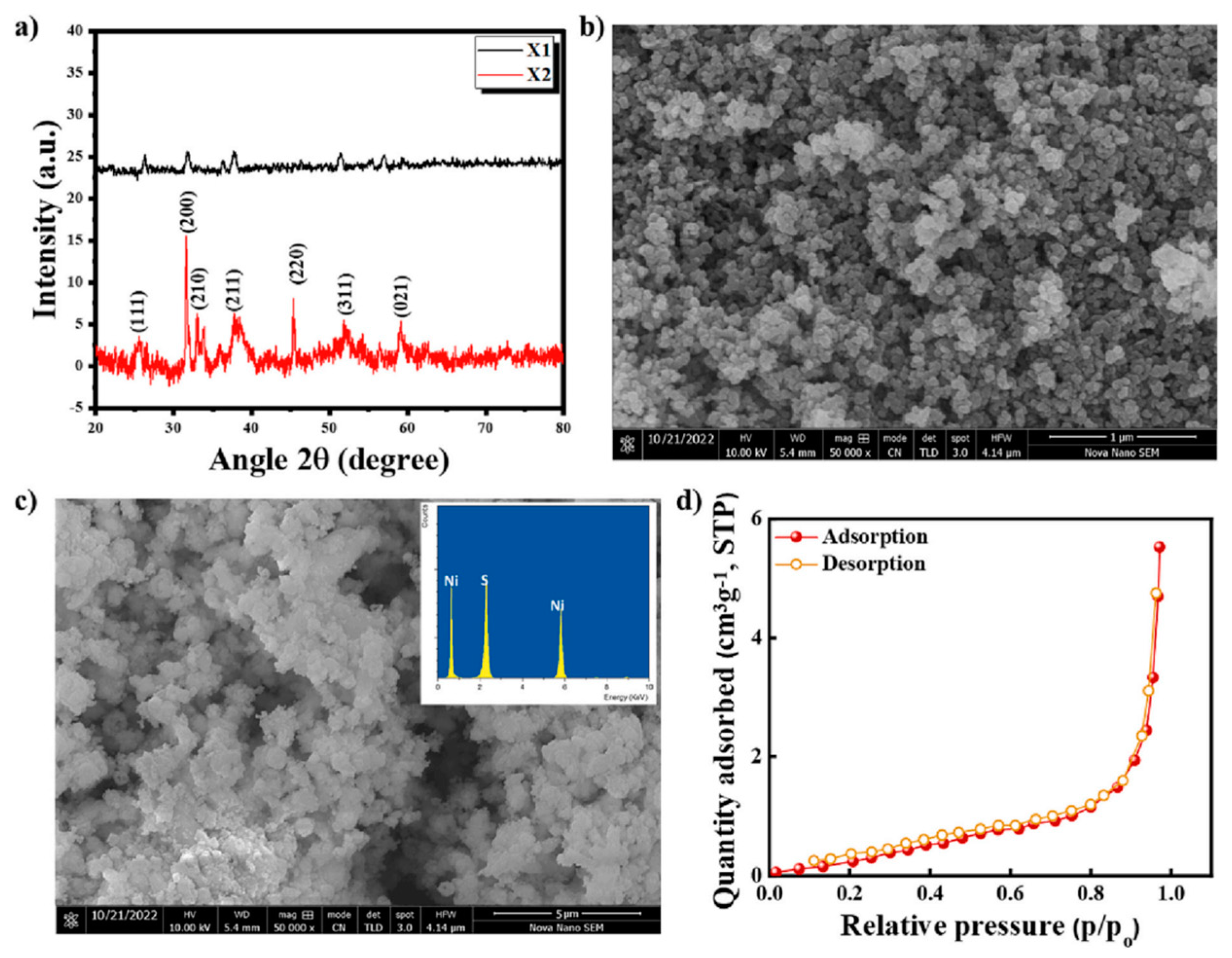
3.1.1. Hydrothermal Method
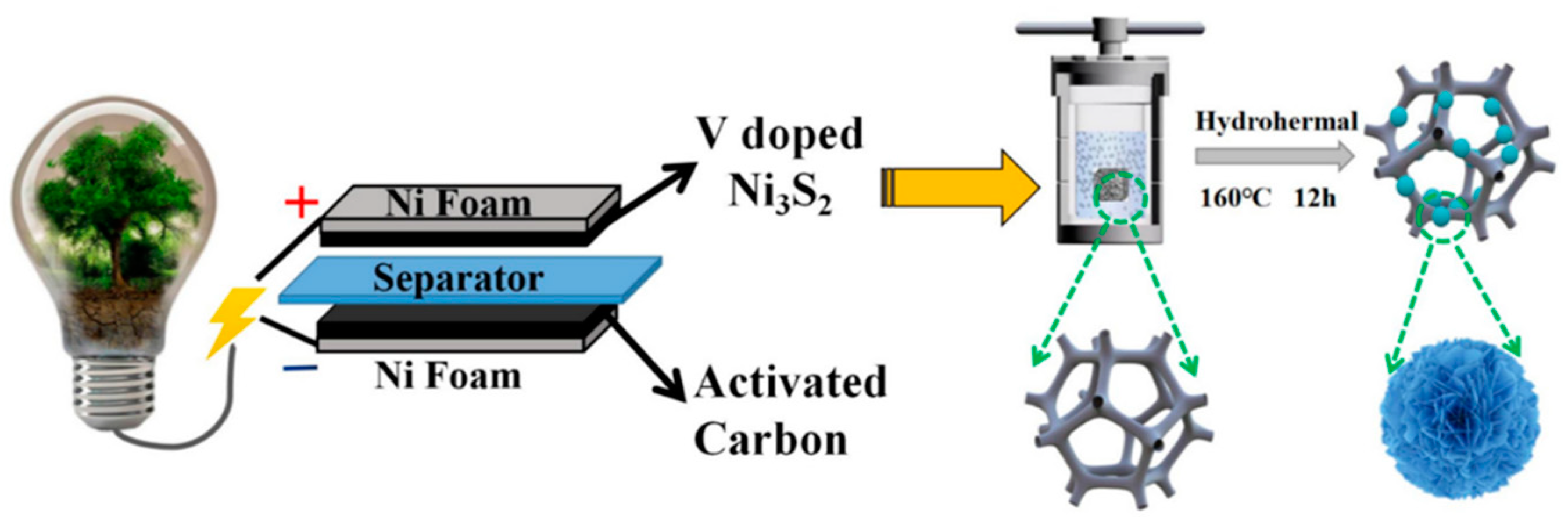
3.1.2. Electrochemical Method
3.1.3. Comparison of Hydrothermal Method and Electrochemical Method
3.2. Study on the Modification of Transition Metal Sulfide Electrode Materials
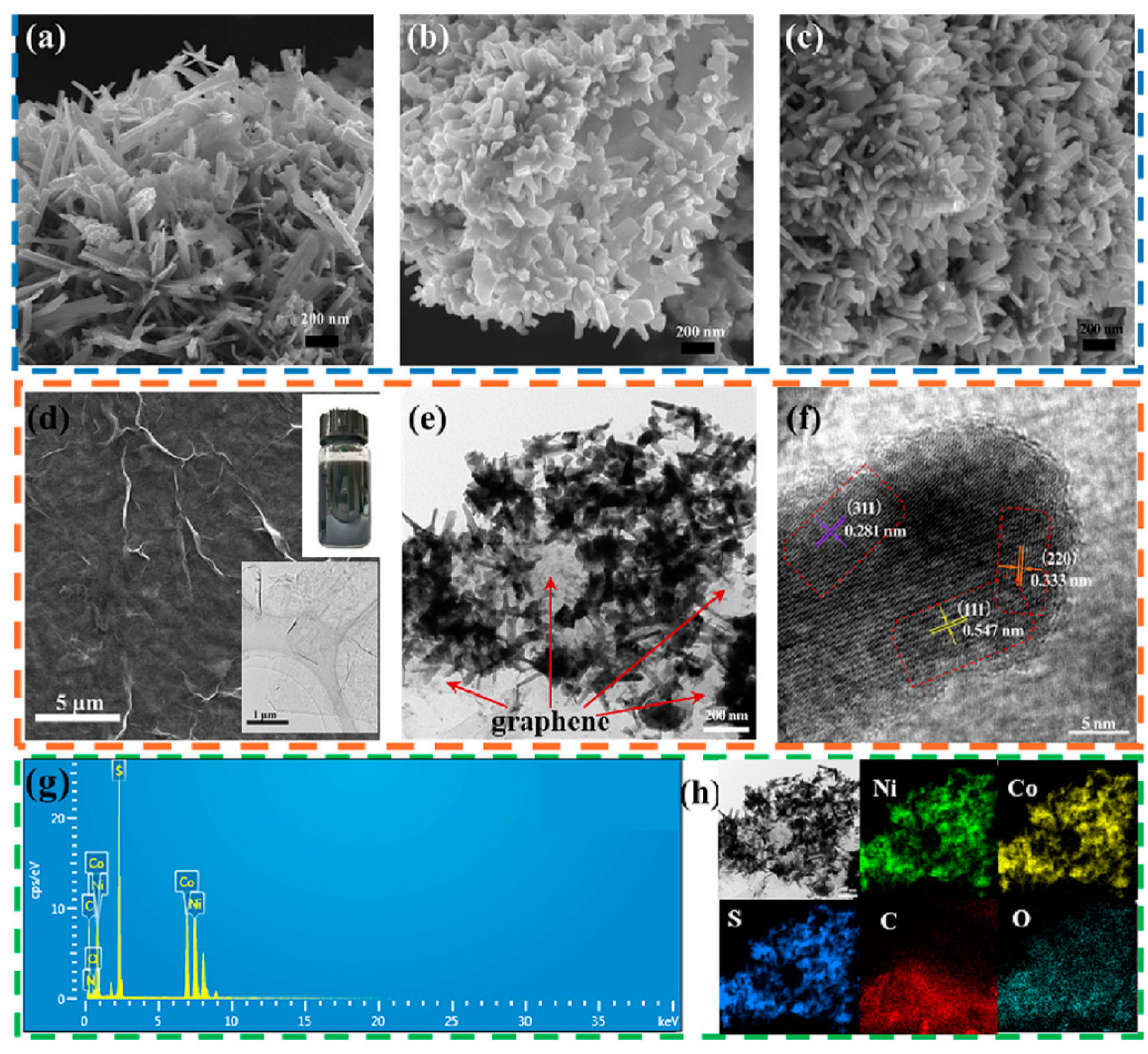
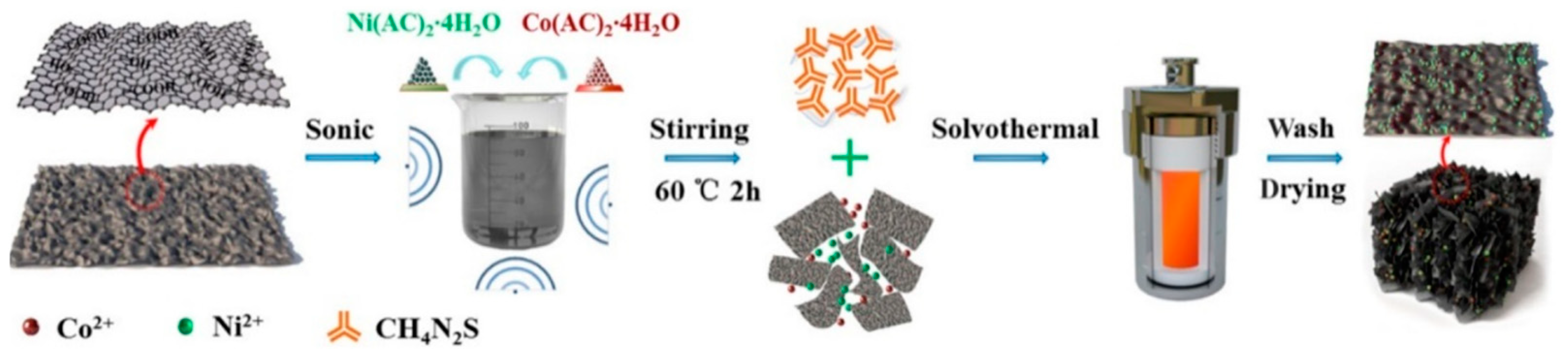
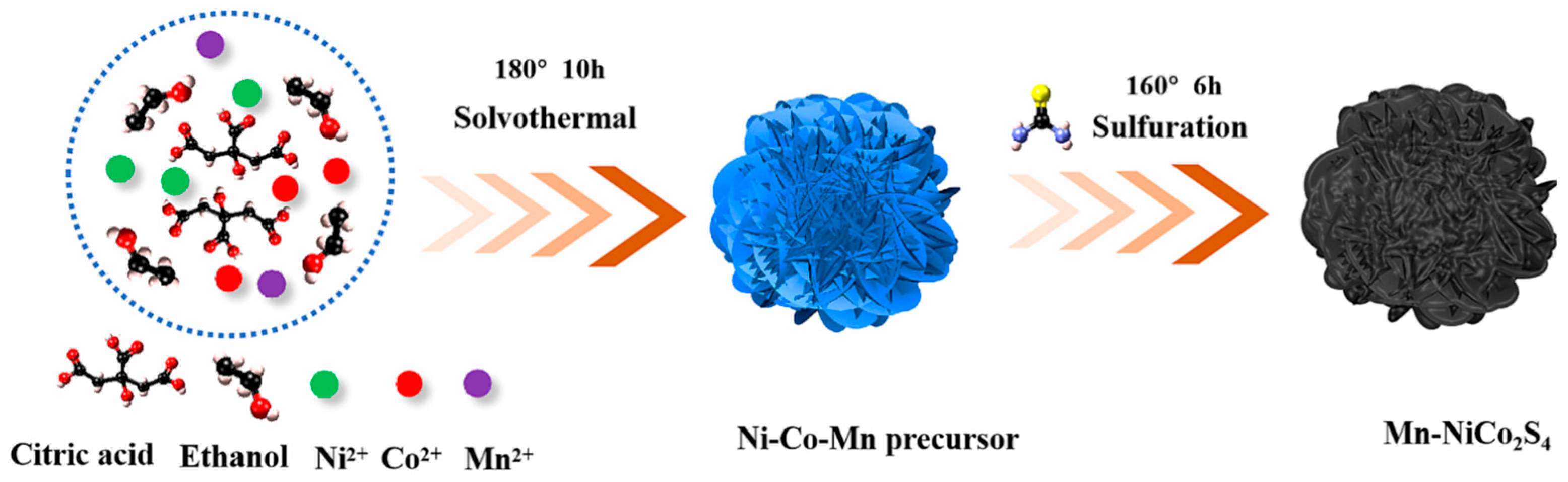
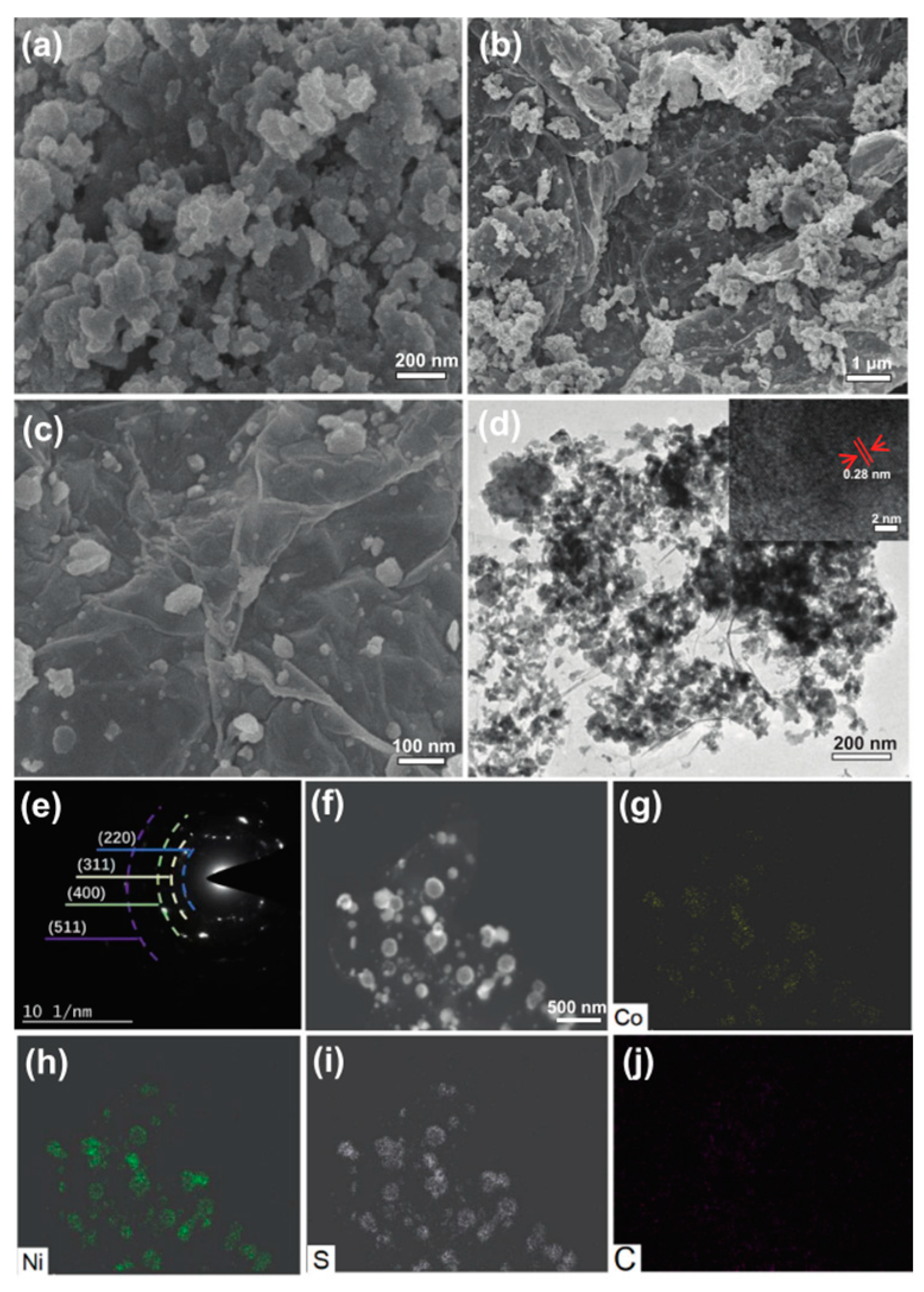
3.2.1. Morphology Control of Transition Metal Sulfide Electrode Materials
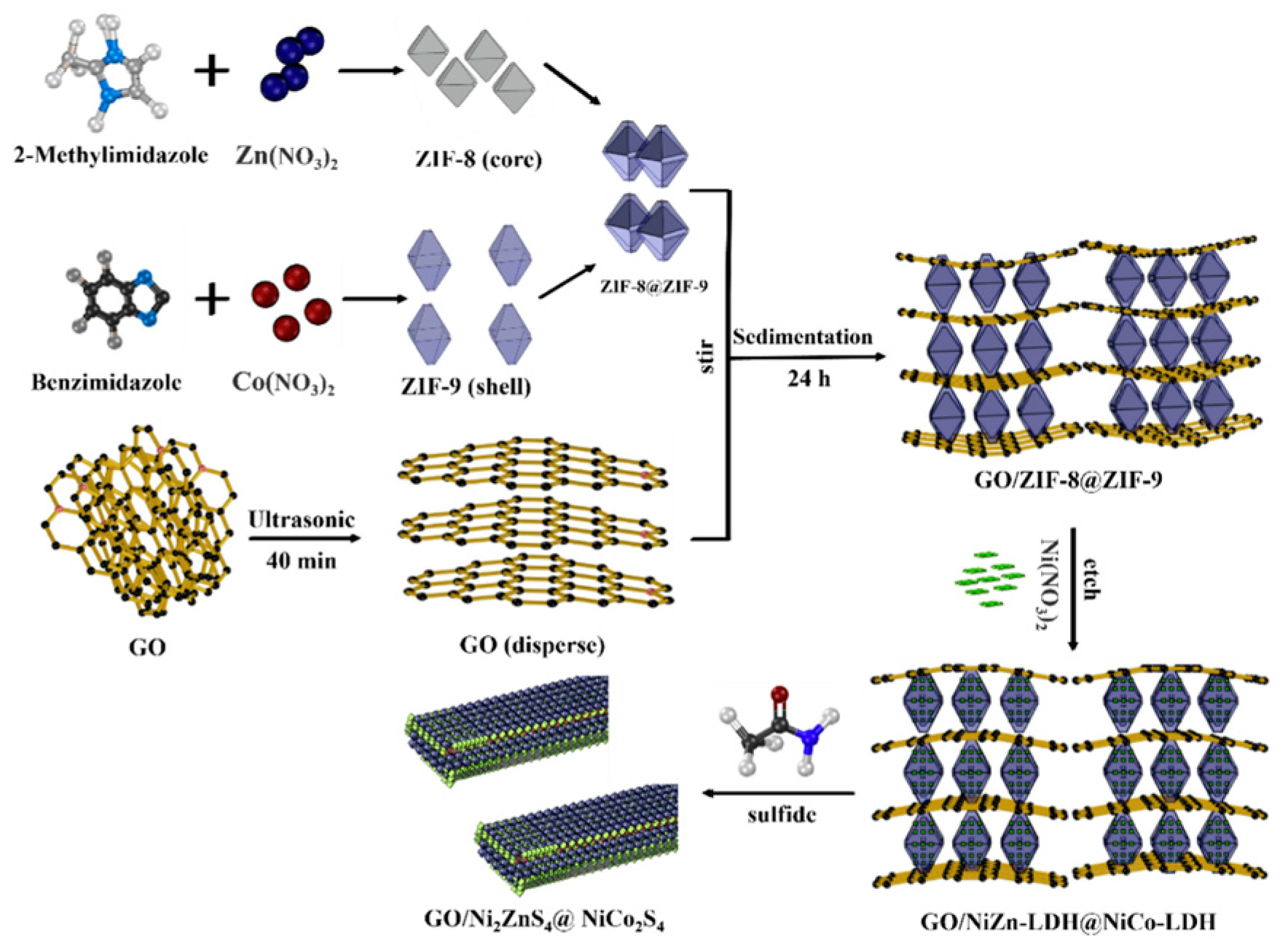
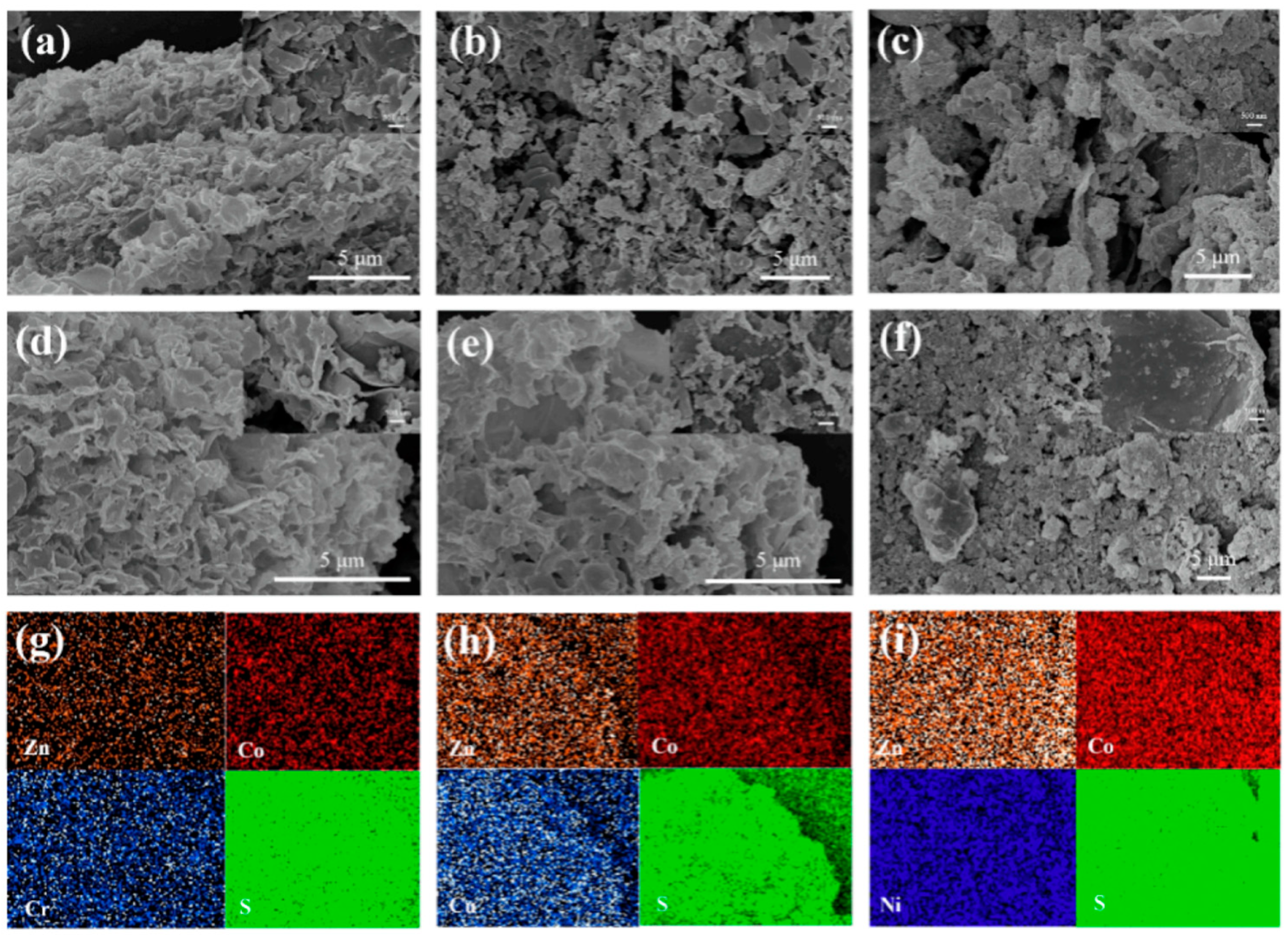
3.2.2. Composite of Metal Sulfide Electrode Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sugimoto, W.; Yokoshima, K.; Murakami, Y.; Takasu, Y. Charge storage mechanism of nanostructured anhydrous and hydrous ruthenium-based oxides. Electrochim. Acta 2006, 52, 1742–1748. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Reddy, A.L.; Rodrigues, M.T.; Gullapalli, H.; Balakrishnan, K.; Silva, G.G.; Ajayan, P.M. Supercapacitor operating at 200 degrees celsius. Sci. Rep. 2013, 3, 2572. [Google Scholar] [CrossRef]
- Qu, D. Studies of the activated carbons used in double-layer supercapacitors. J. Power Sources 2002, 109, 403–411. [Google Scholar] [CrossRef]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.Y.; Hashmi, S.A.; Khan, M.; Choi, D.; Qurashi, A. Frontiers and recent developments on supercapacitor’s materials, design, and applications: Transport and power system applications. J. Energy Storage 2023, 58, 106104. [Google Scholar] [CrossRef]
- Gouy, M. Sur la constitution de la charge électrique à la surface d’un électrolyte. J. Phys. Théorique Appliquée 1910, 9, 457–468. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, G.Z. Redox electrode materials for supercapatteries. J. Power Sources 2016, 326, 604–612. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Zhao, X.; Sánchez, B.M.; Dobson, P.J.; Grant, P.S. The role of nanomaterials in redox-based supercapacitors for next generation energy storage devices. Nanoscale 2011, 3, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, K.; Cheng, K.; Yin, J.; Cao, D.; Wang, G. Electrodeposition of nickel sulfide on graphene-covered make-up cotton as a flexible electrode material for high-performance supercapacitors. J. Power Sources 2015, 274, 943–950. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, X.; Ji, X.; Zhu, Y.; Jing, M.; Chen, Q.; Jiao, F. High energy density asymmetric supercapacitors from mesoporous NiCo2S4 nanosheets. Electrochim. Acta 2015, 174, 238–245. [Google Scholar] [CrossRef]
- Cherusseri, J.; Pandey, D.; Thomas, J. Symmetric, asymmetric, and battery-type supercapacitors using two-dimensional nanomaterials and composites. Batter. Supercapacitor 2020, 3, 860–875. [Google Scholar] [CrossRef]
- Phor, L.; Kumar, A.; Chahal, S. Electrode materials for supercapacitors: A comprehensive review of advancements and performance. J. Energy Storage 2024, 84, 110698. [Google Scholar]
- Miao, L.; Duan, H.; Wang, Z.; Lv, Y.; Ong, W.; Zhu, D. Improving the pore-ion size compatibility between poly (ionic liquid)-derived carbons and high-voltage electrolytes for high energy-power supercapacitors. Chem. Eng. J. 2020, 382, 122945. [Google Scholar] [CrossRef]
- Job, N.; Théry, A.; Pirard, R.; Marien, J.; Kocon, L.; Rouzaud, J.N.; Pirard, J.P. Carbon aerogels, cryogels and xerogels: Influence of the drying method on the textural properties of porous carbon materials. Carbon 2005, 43, 2481–2494. [Google Scholar] [CrossRef]
- Yang, M.; Zhong, Y.; Bao, J.; Zhou, X.; Wei, J.; Zhou, Z. Achieving battery-level energy density by constructing aqueous carbonaceous supercapacitors with hierarchical porous N-rich carbon materials. J. Mater. Chem. A 2015, 3, 11387–11394. [Google Scholar] [CrossRef]
- Stoller, M.D.; Magnuson, C.W.; Zhu, Y.; Murali, S.; Suk, J.W.; Piner, R.; Ruoff, R.S. Interfacial capacitance of single layer graphene. Energy Environ. Sci. 2011, 4, 4685–4689. [Google Scholar] [CrossRef]
- Low, W.H.; Khiew, P.S.; Lim, S.S.; Siong, C.W.; Ezeigwe, E.R. Recent development of mixed transition metal oxide and graphene/mixed transition metal oxide based hybrid nanostructures for advanced supercapacitors. J. Alloys Compd. 2019, 775, 1324–1356. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Aziz, U.; Amjad, N.; Aftab, S.; Wabaidur, S.M. Porous activated carbon and highly redox active transition metal sulfide by employing multi-synthesis approaches for battery-supercapacitor applications. Diam. Relat. Mater. 2023, 136, 110019. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.; Yu, Y.; Du, C.; Wan, L.; Ye, H.; Chen, J.; Zhang, Y. Stabilizing microstructure of Co-Ni layered double hydroxides by magnesium doping and confinement in carbonaceous mesopores for ultrahighly stable asymmetric supercapacitor. Energy Storage 2023, 59, 106422. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Faisal, M.M.; ul Hassan, H.; Afzal, A.M.; Aftab, S.; Zahid, T.; ur Rehman, A. Facile hydrothermal synthesis of high-performance binary silver-cobalt-sulfide for supercapattery devices. J. Energy Storage 2022, 52, 104847. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Ullah, A.; Aziz, U.; Asifd, M.; Wabaidur, S.M.; Ansari, M.Z. Unveiling the performance of hydrothermally synthesized transition metal sulfide with polyaniline composite for hybrid supercapacitor applications. Curr. Appl. Phys. 2023, 52, 94–100. [Google Scholar] [CrossRef]
- Wang, X. Preparation of NiCo2S4 and Porous Carbon Electrode Materials and Their Hybrid Supercapacitors. Master’s Thesis, Shandong University of Technology, Zibo, China, 2023; p. 000767. [Google Scholar]
- Sahoo, S.; Dhakal, G.; Kim, W.K.; Shim, J.J. Ternary nanohybrid of Ni3S2/CoMoS4/MnO2 on nickel foam for aqueous and solid-state high-performance supercapacitors. Nanomaterials 2022, 12, 1945. [Google Scholar] [CrossRef]
- Li, N.; Wang, D.; Liang, X.; Li, D.; Liu, G.; Sun, G.; Li, Y. Multi-stage Ordered Mesoporous Carbon-graphene Aerogel-Ni3S2/Co4S3 for Supercapacitor Electrode. Electroanalysis 2022, 34, 252–257. [Google Scholar] [CrossRef]
- Li, N.; Wang, D.; Liang, X.; Li, D.; Liu, G.; Sun, G.; Li, Y. Self-supported NiSe@Ni3S2 core-shell composite on Ni foam for a high-performance asymmetric supercapacitor. Ionics 2020, 26, 3997–4007. [Google Scholar] [CrossRef]
- Yan, C.; Yang, X.; Lu, S.; Han, E.; Chen, G.; Zhang, Z.; He, Y. Hydrothermal synthesis of vanadium doped nickel sulfide nanoflower for high-performance supercapacitor. J. Alloys Compd. 2022, 928, 167189. [Google Scholar] [CrossRef]
- Altaf, C.T.; Colak, T.O.; Lufrano, F.; Unal, G.S.; Sankir, N.D.; Sankir, M. Graphitic carbon nitride/zinc oxide composite electrodes for all-solid-state photo-supercapacitor with ion exchange membrane separator. J. Energy Storage 2022, 55, 105784. [Google Scholar] [CrossRef]
- Zhang, X.; Su, K.; Mohamed, A.G.; Liu, C.; Sun, Q.; Yuan, D.; Wang, Y.; Xue, W.; Wang, Y. Photo-assisted charge/discharge Li-organic battery with a charge-separated and redox-active C 60@ porous organic cage cathode. Energy Environ. Sci. 2022, 15, 780–785. [Google Scholar] [CrossRef]
- Shah, M.Z.U.; Sajjad, M.; Hou, H.; ur Rahman, S.; Shah, A. Copper sulfide nanoparticles on titanium dioxide (TiO2) nanoflakes: A new hybrid asymmetrical Faradaic supercapacitors with high energy density and superior lifespan. J. Energy Storage 2022, 55, 105651. [Google Scholar] [CrossRef]
- Momeni, M.M.; Najafi, M.; Farrokhpour, H.; Lee, B.K. Fabrication and photo/electrochem-ical properties of cobalt-manganese binary metal sulfides deposited on titania nanotubes: Efficient and stable photoelectrodes for photo-assisted charging supercapacitors. J. Energy Storage 2024, 79, 109666. [Google Scholar] [CrossRef]
- Gao, Y.P.; Huang, K.J.; Wu, X.; Hou, Z.Q.; Liu, Y.Y. MoS2 nanosheets assembling three-dimensional nanospheres for enhanced-performance supercapacitor. J. Alloys Compd. 2018, 741, 174–181. [Google Scholar] [CrossRef]
- Liang, A.; Zhang, Y.; Jiang, F.; Zhou, W.; Xu, J.; Hou, J.; Duan, X. Electrochemical self-assembly of a 3D interpenetrating porous network PEDOT-PEG-WS2 nanocomposite for high-efficient energy storage. J. Phys. Chem. C 2019, 123, 25428–25436. [Google Scholar] [CrossRef]
- Liang, A.; Li, D.; Zhou, W.; Wu, Y.; Ye, G.; Wu, J.; Du, Y. Robust flexible WS2/PEDOT: PSS film for use in high-performance miniature supercapacitors. J. Electroanal. Chem. 2018, 824, 136–146. [Google Scholar] [CrossRef]
- Volkov, A.I.; Ivanov, A.V.; Vereshchagin, A.A.; Novoselova, J.V.; Tolstopjatova, E.G.; Kondratiev, V.V. Electrochemical deposition of PEDOT/MoS2 composite films for supercapacitors. Synth. Met. 2022, 285, 117030. [Google Scholar] [CrossRef]
- Sapra, S.K.; Pati, J.; Dwivedi, P.K.; Basu, S.; Chang, J.K.; Dhaka, R.S. A comprehensive review on recent advances of polyanionic cathode materials in Na-ion batteries for cost effective energy storage applications. Wiley Interdiscip. Rev. Energy Environ. 2021, 10, e400. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar] [CrossRef]
- Vicentini, R.; Aguiar, J.P.; Beraldo, R.; Venâncio, R.; Rufino, F.; Da Silva, L.M.; Zanin, H. Ragone plots for electrochemical double-layer capacitors. Batter. Supercaps 2021, 4, 1291–1303. [Google Scholar] [CrossRef]
- Jiang, K.; Gao, M.; Dou, Z.; Wang, K.; Yu, H.; Ning, L.; Fu, M. High mass loading and additive-free prussian blue analogue based flexible electrodes for Na-ion supercapacitors. J. Colloid Interface Sci. 2023, 650, 490–497. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Yu, H.; Lv, R.; Huang, Y.; Fu, M. Construction of hairbrush-like (Ni3S2/NiSe-3:1)/carboxymethylcellulose derived carbon heterostructure as high-performance electrodes for supercapacitors. Carbon 2023, 213, 118183. [Google Scholar] [CrossRef]
- Liang, K.; Chen, Y.; Wang, D.; Wang, W.; Jia, S.; Mitsuzaki, N.; Chen, Z. Post-modified biomass derived carbon materials for energy storage supercapacitors: A review. Sustain. Energy Fuels 2023, 7, 3541–3559. [Google Scholar] [CrossRef]
- Tong, Y.; Yang, J.; Li, J.; Cong, Z.; Wei, L.; Liu, M.; An, Q. Lignin-derived electrode materials for supercapacitor applications: Progress and perspectives. J. Mater. Chem. A 2023, 11, 1061–1082. [Google Scholar] [CrossRef]
- Fu, M.; Zhu, Z.; Chen, W.; Yu, H.; Lv, R. Carbon cloth supported flower-like porous nickel-based electrodes boosting ion/charge transfer characteristics of flexible supercapacitors. Carbon 2022, 199, 520–528. [Google Scholar] [CrossRef]
- Qin, T.; Chen, H.; Zhang, Y.; Chen, X.; Liu, L.; Yan, D.; Peng, S. Modulating surface chemistry of heteroatom-rich micropore carbon cloth electrode for aqueous 2.1 V high-voltage window all-carbon supercapacitor. J. Power Sources 2019, 431, 232–238. [Google Scholar] [CrossRef]
- Nai, J.; Lou, X.W. Hollow structures based on prussian blue and its analogs for electrochemical energy storage and conversion. Adv. Mater. 2019, 31, 1706825. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Chen, D.; Liu, J.; Zhang, Y.; Chen, J.; Du, C.; Ma, M. Facile preparation of porous carbons derived from orange peel via basic copper carbonate activation for supercapacitors. J. Alloys Compd. 2020, 823, 153747. [Google Scholar] [CrossRef]
- Wang, D.; Tian, L.; Huang, J.; Li, D.; Liu, J.; Xu, Y.; Wei, Q. “One for two” strategy to prepare MOF-derived NiCo2S4 nanorods grown on carbon cloth for high-performance Hybrid supercapacitorsand efficient oxygen evolution reaction. Electrochim. Acta 2020, 334, 135636. [Google Scholar] [CrossRef]
- Xuan, H.; Guan, Y.; Han, X.; Liang, X.; Zhu, Z.; Han, P.; Wu, Y. Hierarchical MnCo-LDH/rGO@ NiCo2S4 heterostructures on Ni foam with enhanced electrochemical properties for battery-supercapacitors. Electrochim. Acta 2020, 335, 135691. [Google Scholar] [CrossRef]
- Wan, L.; He, C.; Chen, D.; Liu, J.; Zhang, Y.; Du, C.; Chen, J. In situ grown NiFeP@ NiCo2S4 nanosheet arrays on carbon cloth for asymmetric supercapacitors. Chem. Eng. J. 2020, 399, 125778. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, X.; Wang, Y.; Zhang, C.; Qiao, X.; Akin, M.; Du, Z. Application of GO anchored mediator in a polymer electrolyte membrane for high-rate solid-state supercapacitors. J. Membr. Sci. 2023, 669, 121285. [Google Scholar] [CrossRef]
- Tamang, S.; Rai, S.; Bhujel, R.; Bhattacharyya, N.K.; Swain, B.P.; Biswas, J. A concise review on GO, rGO and metal oxide/rGO composites: Fabrication and their supercapacitor and catalytic applications. J. Alloys Compd. 2023, 947, 169588. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, Y.M.; Ding, Y.; Chen, H. Sulfidation of ZIF-derived core-shell NiCo LDH/Ni MOF heterostructure toward supercapacitor electrodes with enhanced performance. Batteries 2022, 8, 241. [Google Scholar] [CrossRef]
- Mahieddine, A.; Adnane-Amara, L. Fabrication of hierarchical Ni nanowires@ NiCo-layered double hydroxide nanosheets core-shell hybrid arrays for high-performance hybrid supercapacitors. Electrochim. Acta 2023, 439, 141622. [Google Scholar] [CrossRef]
- Patil, A.M.; Moon, S.; Roy, S.B.; Ha, J.; Chodankar, N.R.; Dubal, D.P.; Jun, S.C. Electronic Structure Engineered Heteroatom Doped All Transition Metal Sulfide Carbon Confined Heterostructure for Extrinsic Pseudocapacitor. Small 2023, 19, 2301153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, Q.; Shang, G.; Cui, J.; Zhang, Y.; He, W. Chiffon-like tulle-covered nanosheet core-shell structure of NiOOH@ nickel-iron bimetallic sulfides to enhance the supercapacitor performances. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130294. [Google Scholar] [CrossRef]
- Mohammadi Zardkhoshoui, A.; Hayati Monjoghtapeh, R.; Hosseiny Davarani, S.S. Zn–Ni–Se@ NiCo2S4 core–shell architectures: A highly efficient positive electrode for hybrid supercapacitors. Energy Fuels 2020, 34, 14934–14947. [Google Scholar] [CrossRef]
- Arulkumar, C.; Gandhi, R.; Vadivel, S. Ultra-thin nanosheets of Ti3C2Tx MXene/MoSe2 nanocomposite electrode for asymmetric supercapacitor and electrocatalytic water splitting. Electrochim. Acta 2023, 462, 142742. [Google Scholar] [CrossRef]
- Mohammadi Zardkhoshoui, A.; Hayati Monjoghtapeh, R.; Hosseiny Davarani, S.S. ZIF-derived sulfides with tremella-like core–shell structure for high performance supercapacitors. J. Colloid Interface Sci. 2024, 660, 1010–1020. [Google Scholar]
- Hussain, I.; Ansari, M.Z.; Ahmad, M.; Ali, A.; Nawaz, T.; Hussain, T.; Zhang, K. Understanding the diffusion-dominated properties of MOF-derived Ni–Co–Se/C on CuO scaffold electrode using experimental and first principle study. Adv. Funct. Mater. 2023, 33, 2302888. [Google Scholar] [CrossRef]
- Wu, D.; Zou, J.; Chen, J.; Li, Y.; Ma, N.; Dai, W. Boosted sustained-release with iron-based MOF-derived mesoporous-carbon-spheres as a nitroimidazole drugs carrier. Particuology 2024, 86, 269–280. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, M.; Wang, S.; Zhang, S.; Wang, J.; Qiao, X.; Feng, Y. MOF-derived NiS2@ carbon microspheres wrapped with carbon nanotubes for high cycle performance supercapacitors. Electrochim. Acta 2023, 464, 142920. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Yoo, J.I.; Song, J.K.; Mittal, V. MOF-derived 3D MnO2@ graphene/CNT and Ag@ graphene/CNT hybrid electrode materials for dual-ion selective pseudocapacitive deionization. Desalination 2023, 550, 116369. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Y.; Xu, G.; Wang, X. Facile constucture of amorphous/crystalline NiCoB@ NiCo2S4 heterogeneous interface nanocomposites for enhanced supercapacitor performance. J. Power Sources 2023, 581, 233488. [Google Scholar]
- Meghanathan, K.L.; Parthibavarman, M.; Sharmila, V.; Joshua, J.R. Metal-organic framework-derived Nickle Tellurideporous structured composites electrode materials for asymmetric supercapacitor application. J. Energy Storage 2023, 72, 108665. [Google Scholar] [CrossRef]
- Wang, G.; Ding, Y.; Xu, Z.; Wang, G.; Li, Z.; Yan, Z. Co3O4@ Mn-Ni (OH)2 core–shell heterostructure for hybrid supercapacitor electrode with high utilization. Chem. Eng. J. 2023, 469, 143984. [Google Scholar] [CrossRef]
- Guo, C.; Yu, D.; Liu, L.; Hua, Y.; Zhao, X.; Liu, X. Synthesis of the sandwich-type NiMn2O4@ NC@ MnO2 core-shell nanostructured materials for the high-performance battery-supercapacitor hybrid devices. J. Energy Storage 2023, 68, 107814. [Google Scholar] [CrossRef]
- Li, C.; Yan, C.; Yang, Q.; Huo, P. Construction of MOFs derived porous carbon based core–shell hetero-structure electrode for superior asymmetric supercapacitor. Electrochim. Acta 2024, 478, 143835. [Google Scholar] [CrossRef]
- Mala, N.A.; Dar, M.A.; Sivakumar, S.; Dar, T.A.; Manikandan, E. Review article on the performance of electrochemical capacitors when altered metals doped with nickel oxide nanomaterials. J. Nanopart. Res. 2022, 24, 229. [Google Scholar] [CrossRef]
- Kate, R.S.; Pathan, H.M.; Kalubarme, R.; Kale, B.B.; Deokate, R.J. Spray pyrolysis: Approaches for nanostructured metal oxide films in energy storage application. J. Energy Storage 2022, 54, 105387. [Google Scholar] [CrossRef]
- Wickramaarachchi, K.; Minakshi, M. Status on electrodeposited manganese dioxide and biowaste carbon for hybrid capacitors: The case of high-quality oxide composites, mechanisms, and prospects. J. Energy Storage 2022, 56, 106099. [Google Scholar] [CrossRef]
- Verma, S.; Arya, S.; Gupta, V. Performance analysis, challenges and future perspectives of nickel based nanostructured electrodes for electrochemical supercapacitors. J. Mater. Res. Technol. 2021, 11, 564–599. [Google Scholar] [CrossRef]
- Ikram, M.; Haider, A.; Imran, M.; Haider, J.; Naz, S. Cellulose grafted poly acrylic acid doped manganese oxide nanorods as novel platform for catalytic, antibacterial activity and molecular docking analysis. Surf. Interfaces 2023, 37, 102710. [Google Scholar] [CrossRef]
- Xu, J.; Dong, Z.; Huang, K.J.; Wang, T.; Qi, Y.; Sun, Y.; Wu, X. Preparation of large layer spacing bimetallic sulfide hollow nanosphere for high-energy battery system application. Appl. Surf. Sci. 2023, 637, 157959. [Google Scholar] [CrossRef]
- Ikram, M.; Haider, A.; Bibi, S.T.; Ul-Hamid, A.; Haider, J.; Shahzadi, I.; Kanoun, M.B. Synthesis of Al/starch co-doped in CaO nanoparticles for enhanced catalytic and antimicrobial activities: Experimental and DFT approaches. RSC Adv. 2022, 12, 32142–32155. [Google Scholar] [CrossRef]
- Shahzadi, A.; Moeen, S.; Khan, A.D.; Haider, A.; Haider, J.; Ul-Hamid, A.; Nabgan, W.; Shahzadi, I.; Ikram, M.; Al-Shanini, A. La-doped CeO2 quantum dots: Novel dye degrader, antibacterial activity, and in silico molecular docking analysis. ACS Omega 2023, 8, 8605–8616. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jin, R.; Ke, S.; Liu, S.; Li, Q.; Liu, Q.; Zhang, Y. Sulfur vacancies reinforced cobalt molybdenum sulfide nanosheets integrated cathode for high energy density hybrid supercapacitors. Electrochim. Acta 2024, 475, 143594. [Google Scholar] [CrossRef]
- Su, S.; Sun, L.; Xie, F.; Qian, J.; Zhang, Y. Phosphorus-doped Ni-Co sulfides connected by carbon nanotubes for flexible hybrid supercapacitor. Front. Chem. Sci. Eng. 2023, 17, 491–503. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Wang, P.; Cong, Z.; Shi, F.; Wei, L.; Wang, K.; Tong, Y. NiCo2S4 combined 3D hierarchical porous carbon derived from lignin for high-performance supercapacitors. Int. J. Biol. Macromol. 2023, 232, 123344. [Google Scholar] [CrossRef]
- Devi, R.K.; Ganesan, M.; Chen, T.W.; Chen, S.M.; Akilarasan, M.; Rwei, S.P.; Shaju, A. A facile strategy for the synthesis of manganese-doped nickel sulfide nanosheets and oxygen, nitrogen-enriched 3D-graphene-like porous carbon for hybrid supercapacitor. J. Alloys Compd. 2023, 944, 169261. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Ma, T.; Li, Z.; Tan, X.; Bateer, B. Design of thin-layer porous nickel cobalt sulfide for high-performance asymmetric supercapacitors. J. Alloys Compd. 2023, 945, 168902. [Google Scholar] [CrossRef]
- Jin, Y.; Sun, A.; Geng, J.; Ren, F.; Chen, Z.; Pei, L.; Ren, P.G. Rational design of nickel-cobalt sulfide nanorods grown on graphene with high performance for supercapacitors. Diam. Relat. Mater. 2023, 137, 110151. [Google Scholar] [CrossRef]
- He, X.; Bi, L.; Li, Y.; Xu, C.; Lin, D. CoS2 embedded graphitic structured N-doped carbon spheres interlinked by rGO as anode materials for high-performance sodium-ion batteries. Electrochim. Acta 2020, 332, 135453. [Google Scholar] [CrossRef]
- Beka, L.G.; Li, X.; Wang, X.; Han, C.; Liu, W. Reduced graphene oxide/CoS 2 porous nanoparticle hybrid electrode material for supercapacitor application. RSC Adv. 2019, 9, 26637–26645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, H.; Lin, Y.; Chen, J.; Li, K.; Cheng, A. Design and construction of nickel-cobalt-sulfide nanoparticles in-situ grown on graphene with enhanced performance for asymmetric supercapacitors. Diam. Relat. Mater. 2020, 108, 107925. [Google Scholar] [CrossRef]
- Chung, W.T.; Mekhemer, I.M.; Mohamed, M.G.; Elewa, A.M.; EL-Mahdy, A.F.; Chou, H.H.; Wu, K.C.W. Recent advances in metal/covalent organic frameworks based materials: Their synthesis, structure design and potential applications for hydrogen production. Coord. Chem. Rev. 2023, 483, 215066. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.R.; Xiao, Y.-Y. Electronic metal–support interaction modulates single-atom platinum catalysis for hydrogen evolution reaction. Nat. Commun. 2021, 12, 3021. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Zhu, Y.; Chen, P.; Chen, Y.; Kong, X. Mn-doped nickel–cobalt sulfides as bifunctional electrodes for high-performance supercapacitors and electrocatalysts for hydrogen evolution reactions. J. Electroanal. Chem. 2024, 955, 118048. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Son, L.T.; Thao, P.M. One-step solvothermal synthesis of mixed nickel–cobalt sulfides as high-performance supercapacitor electrode materials. J. Alloys Compd. 2020, 831, 154921. [Google Scholar] [CrossRef]
- Reddy, B.J.; Vickraman, P.; Justin, A.S. Electrochemical performance of nitrogen-doped graphene anchored nickel sulfide nanoflakes for supercapacitors. Appl. Surf. Sci. 2019, 483, 1142–1148. [Google Scholar] [CrossRef]
- Song, X.; Zhong, R.; Zeng, Y. Cobalt nickel sulfide anchored on graphene for high performance all-solid-state asymmetric supercapacitors. Diam. Relat. Mater. 2023, 140, 110447. [Google Scholar] [CrossRef]
- Paul, A.; Ghosh, S.; Kolya, H. Synthesis of nickel-tin oxide/nitrogen-doped reduced graphene oxide composite for asymmetric supercapacitor device. Chem. Eng. J. 2022, 443, 136453. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Pham, H.D.; Nanjundan, A.K. True meaning of pseudocapacitors and their performance metrics: Asymmetric versus hybrid supercapacitors. Small 2020, 16, 2002806. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, C.; Liu, G. Hydroxide ion dependent α-MnO2 enhanced via oxygen vacancies as the negative electrode for high-performance supercapacitors. J. Mater. Chem. A 2021, 9, 2872–2887. [Google Scholar] [CrossRef]
- Zheng, L.; Ye, W.; Yang, P. Manganese doping to boost the capacitance performance of hierarchical Co9S8@Co(OH)2 nanosheet arrays. Green Energy Environ. 2022, 7, 289–1297. [Google Scholar] [CrossRef]
- Zheng, D.; Wen, H.; Sun, X. Ultrathin Mn Doped Ni-MOF Nanosheet Array for Highly Capacitive and Stable Asymmetric Supercapacitor. Chem. Eur. J. 2020, 26, 17149–17155. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, T.; Van Thuy, V.; Thao, V.D. Facile synthesis of Mn-doped NiCo2O4 nanoparticles with enhanced electrochemical performance for a battery-type supercapacitor electrode. Dalton Trans. 2020, 49, 6718–6729. [Google Scholar] [CrossRef]
- Yadav, N.; Hashmi, S.A. Energy enhancement of quasi-solid-state supercapacitors based on a non-aqueous gel polymer electrolyte via a synergistic effect of dual redox additives diphenylamine and potassium iodide. J. Mater. Chem. A 2020, 8, 18266–18279. [Google Scholar] [CrossRef]
- Paul, A.; Samanta, P.; Kolya, H. Rational design of asymmetric aqueous supercapacitor based on the composites of reduced graphene oxide with manganese-doped nickel sulfide-tin sulfide as positive electrode and manganese sulfide negative electrode. J. Power Sources 2023, 580, 233352. [Google Scholar] [CrossRef]
- Ismail, K.B.M.; Arun Kumar, M.; Mahalingam, S. Recent advances in molybdenum disulfide and its nanocomposites for energy applications: Challenges and development. Materials 2023, 16, 4471. [Google Scholar] [CrossRef]
- Mohan, M.; Shetti, N.P.; Aminabhavi, T.M. Phase dependent performance of MoS2 for supercapacitor applications. J. Energy Storage 2023, 58, 106321. [Google Scholar] [CrossRef]
- Mohan, M.; Shetti, N.P.; Aminabhavi, T.M. Recent developments in MoS2-based flexible supercapacitors. Mater. Today Chem. 2023, 27, 101333. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, L. Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem. Eng. J. 2022, 430, 132745. [Google Scholar] [CrossRef]
- Dahiya, Y.; Hariram, M.; Kumar, M. Modified transition metal chalcogenides for high performance supercapacitors: Current trends and emerging opportunities. Coord. Chem. Rev. 2022, 451, 214265. [Google Scholar] [CrossRef]
- Mehta, S.; Samra, K.S. Metal-sulfide based nano-composites and their electrochemical performance: A Review. J. Phys. Conf. Ser. 2022, 2267, 012004. [Google Scholar] [CrossRef]
- Rehman, J.; Eid, K.; Ali, R. Engineering of transition metal sulfide nanostructures as efficient electrodes for high-performance supercapacitors. ACS Appl. Energy Mater. 2022, 5, 6481–6498. [Google Scholar] [CrossRef]
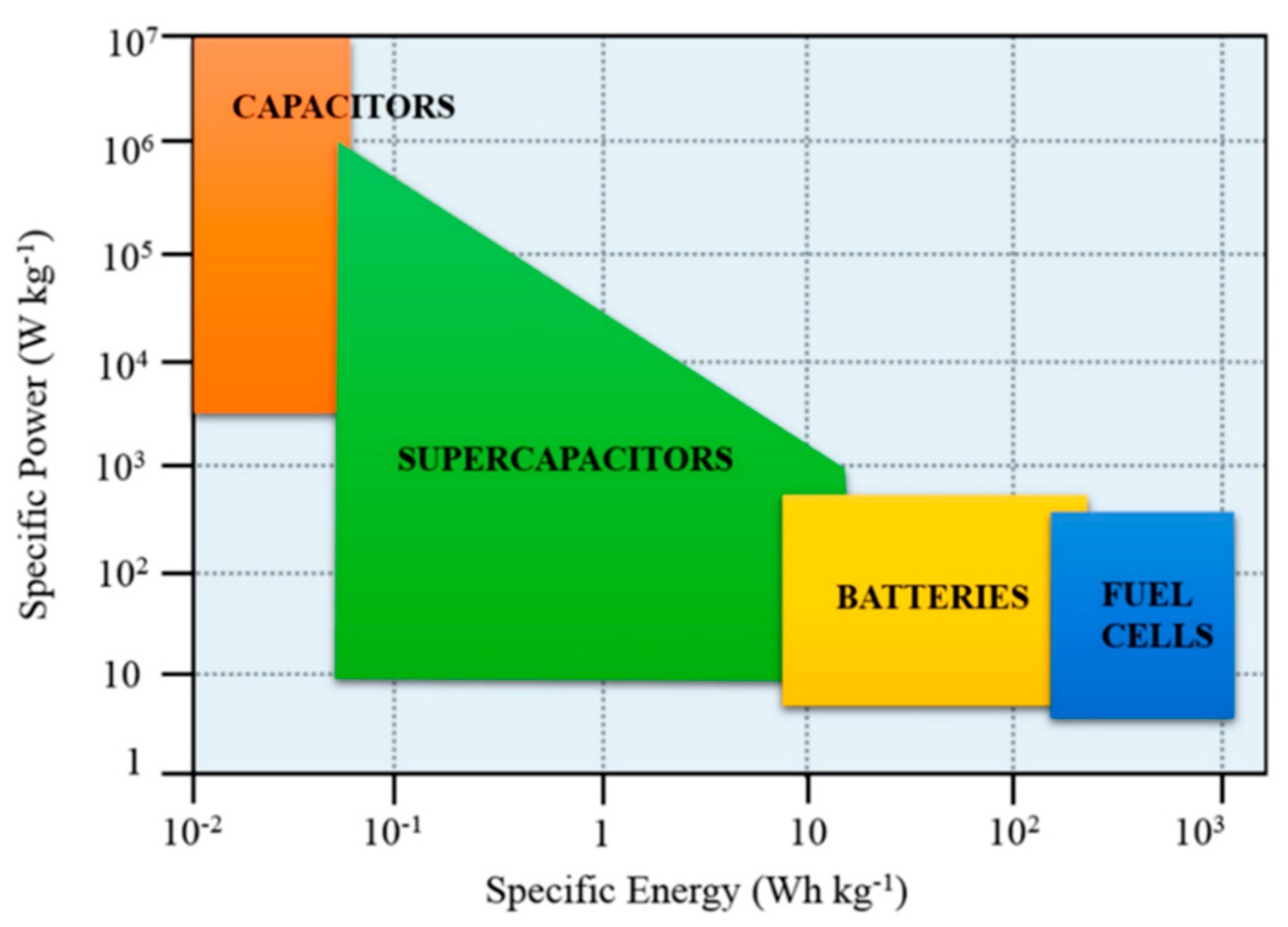

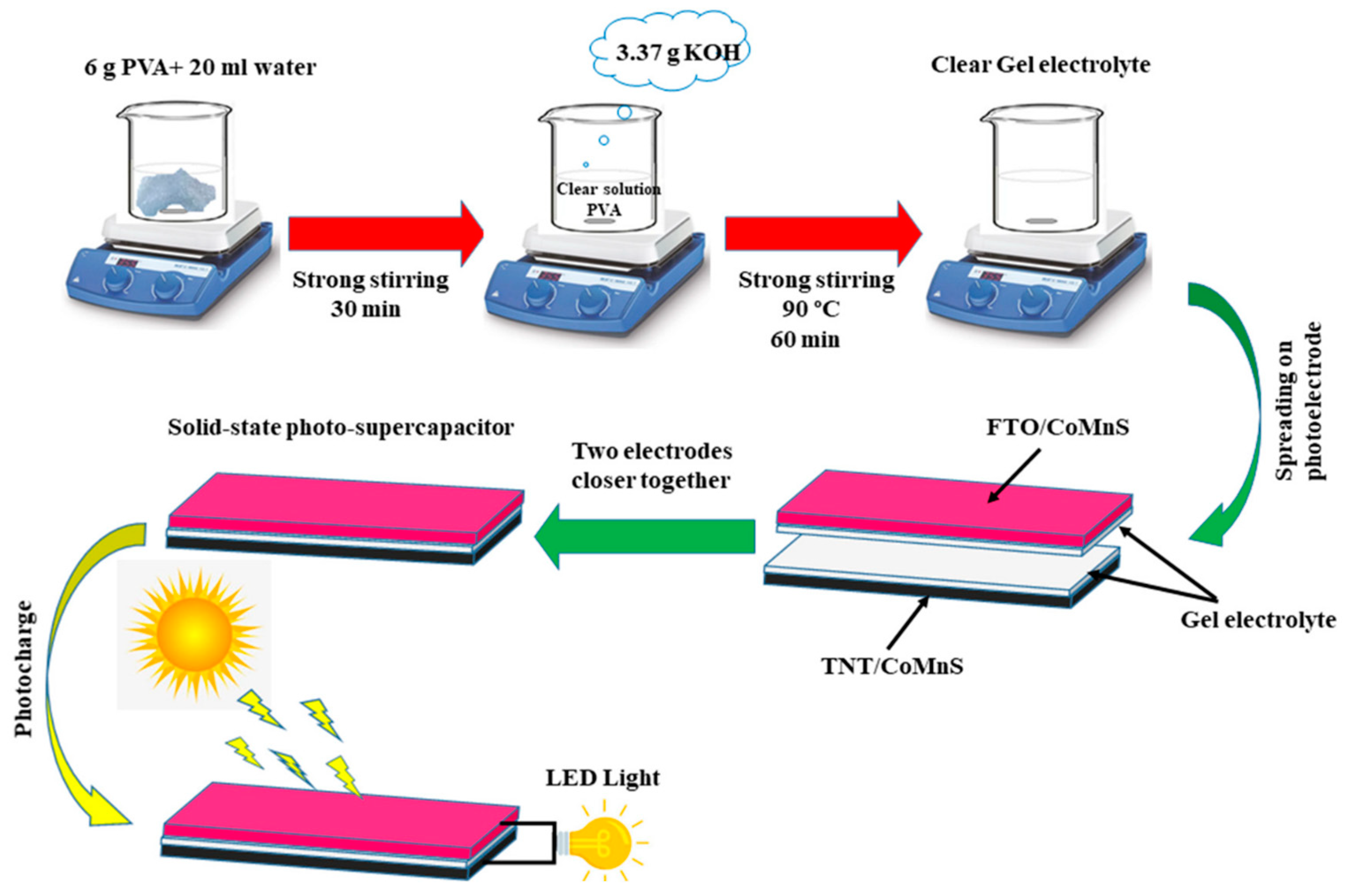



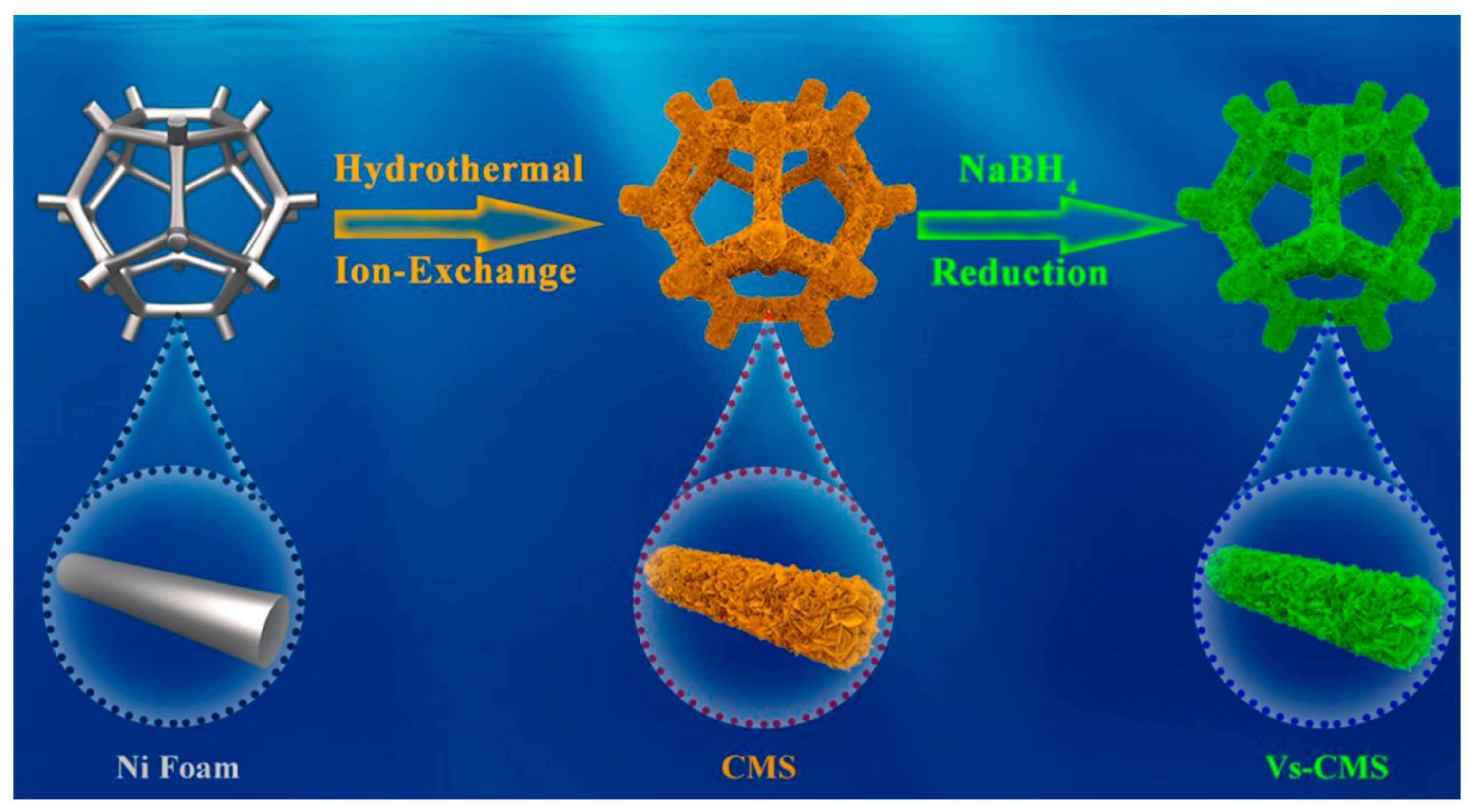
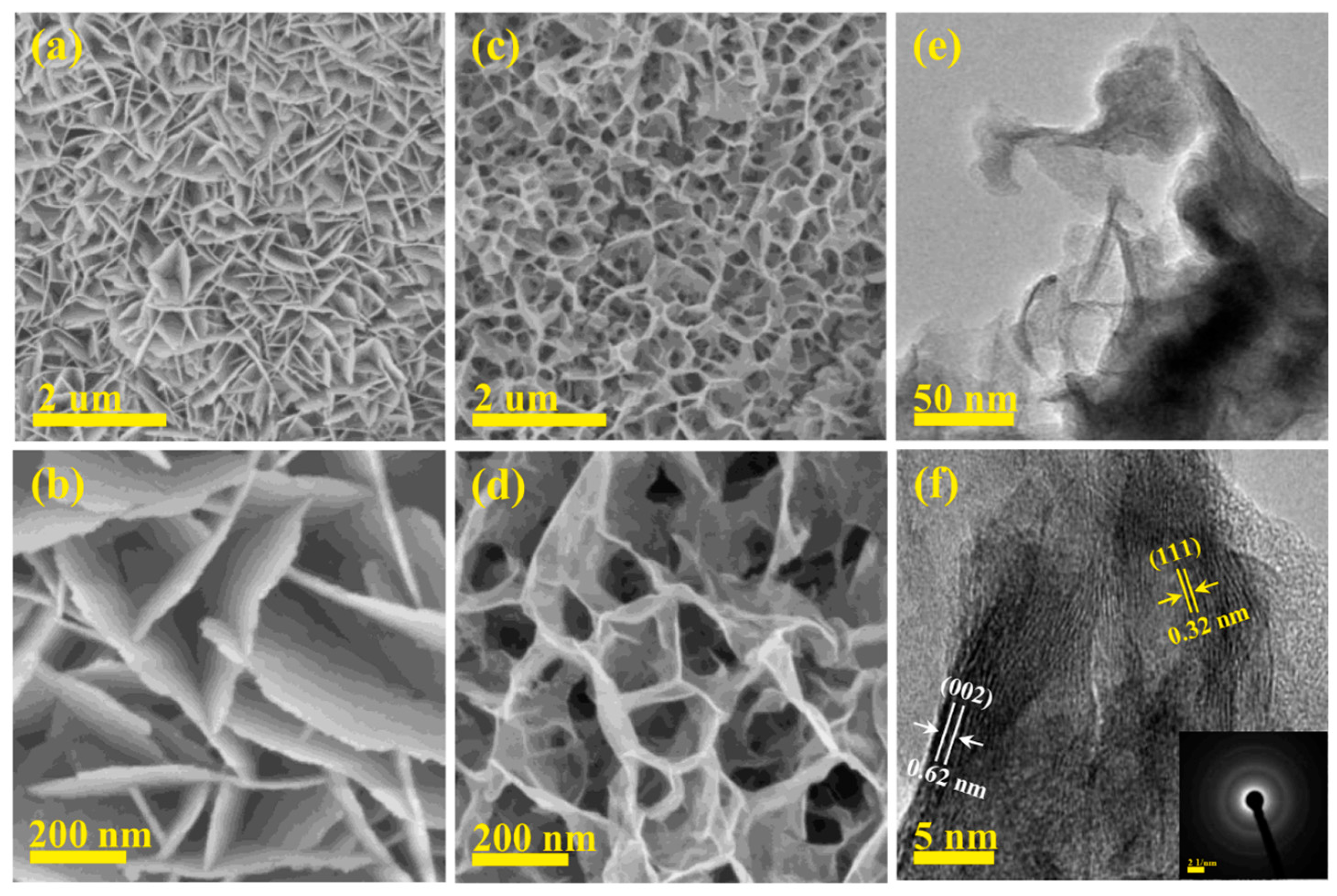
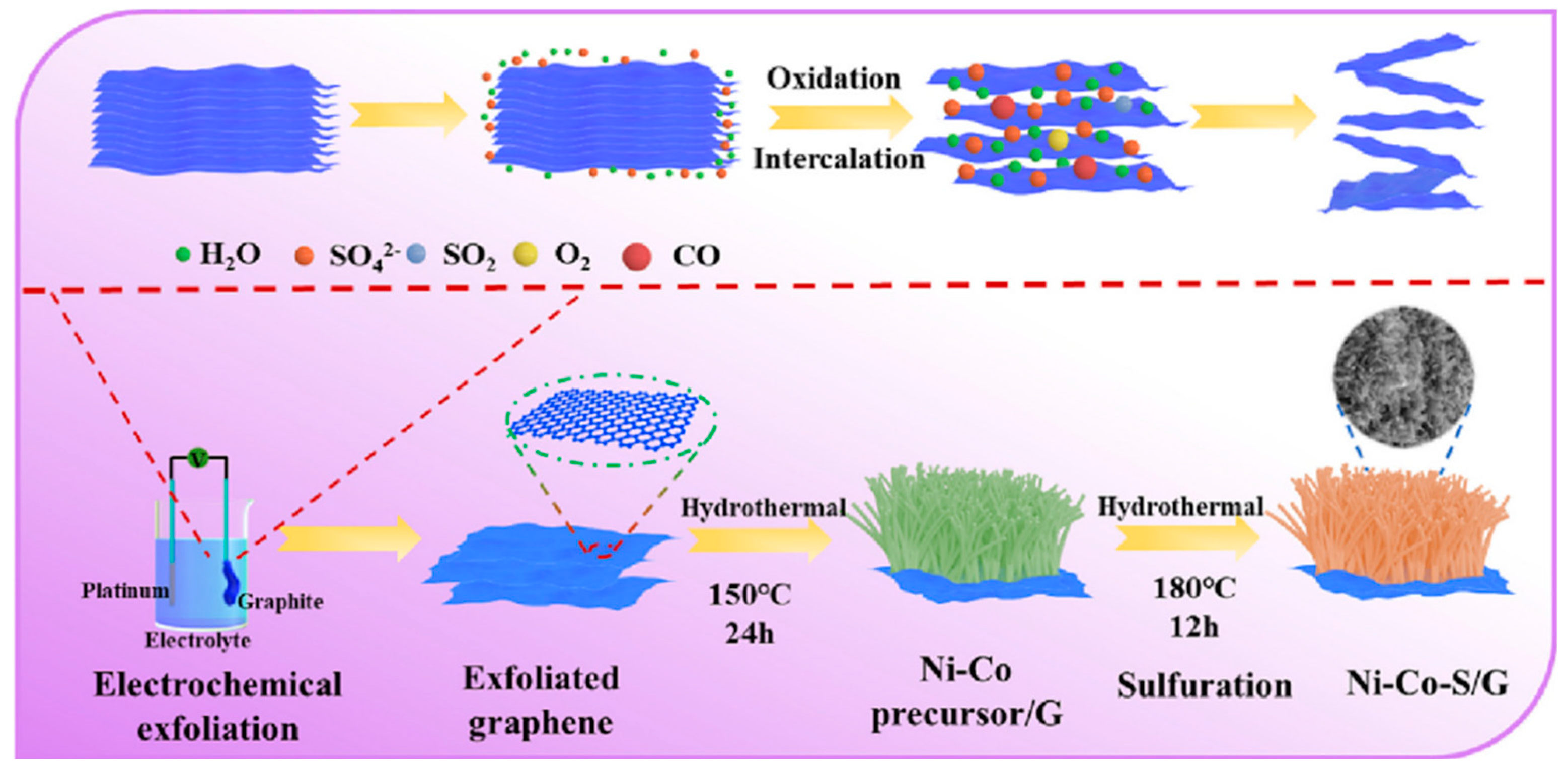

| Characteristic | Battery | Supercapacitor | Conventional Capacitor |
|---|---|---|---|
| Charging/ discharging time | 1 to 10 h | Milliseconds to seconds | Picoseconds to milliseconds |
| Energy density | 8 to 600 Wh/kg | 1 to 10 Wh/kg | 0.01 to 0.05 Wh/kg |
| Power density | 0.005 to 0.4 Wh/kg | 1 to 120 Wh/kg | 0.25 to 10,000 Wh/kg |
| Weight | 1 to >10 kg | 0.001 to 0.230 kg | 1 to 10 kg |
| Operating voltage | 1.25 to 4.2 V | 6 to 800 V | 2.3 to 2.75 V |
| Life | 150 to 1500 cycles | 50,000+ h unlimited cycles | >100,000 cycles |
| Synthetic Material | Specific Capacitance (Fg−1) | Current Density (Ag−1) | Retention (%) | Cycle (No.) | Energy Density (Whkg−1) | Power Density (Wkg−1) | Ref. |
|---|---|---|---|---|---|---|---|
| NiFeP@NiCo2S4 | 874.4 | 5 | 91.2 | 5000 | 32.1 | 18,034.2 | [51] |
| GO/Ni2ZnS4@ NiCo2S4 | 218 | 1 | 62.5 | 5000 | 168.68 | 750 | [60] |
| Co-C@NiCoB | 211 | 1 | 92.1 | 10,000 | 66.03 | 763.35 | [69] |
| Vs-CMS | 395.1 | 1 | 96.7 | 10,000 | 73.2 | 252.2 | [78] |
| Ni–Co–S/G | 1579.68 | 1 | 91.5 | 5000 | 75.3 | 1125 | [83] |
| Synthetic Material | Specific Capacitance (Fg−1) | Current Density (Ag−1) | Retention (%) | Cycle (No.) | Energy Density (Whkg−1) | Power Density (Wkg−1) | Ref. |
|---|---|---|---|---|---|---|---|
| GS-NCS-0.5//N-rGO | 2418 | 1 | 86.4 | 5000 | 34.1 | 411 | [86] |
| Mn0.5-NCS | 156 | 1 | 81 | 7000 | 55.4 | 797 | [89] |
| CoNiS-10GO | 746.7 | 1 | 80.9 | 5000 | 37.3 | —— | [92] |
| MNS2 | 810 | 2 | 85 | 10,000 | 50.37 | 1086 | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Lu, J.; Sheng, Y.; Sun, Y.; Zhang, D. Research Progress in the Preparation of Transition Metal Sulfide Materials and Their Supercapacitor Performance. Micromachines 2024, 15, 849. https://doi.org/10.3390/mi15070849
Yan J, Lu J, Sheng Y, Sun Y, Zhang D. Research Progress in the Preparation of Transition Metal Sulfide Materials and Their Supercapacitor Performance. Micromachines. 2024; 15(7):849. https://doi.org/10.3390/mi15070849
Chicago/Turabian StyleYan, Jin, Jiancheng Lu, Yuxuan Sheng, Yin Sun, and Dapeng Zhang. 2024. "Research Progress in the Preparation of Transition Metal Sulfide Materials and Their Supercapacitor Performance" Micromachines 15, no. 7: 849. https://doi.org/10.3390/mi15070849
APA StyleYan, J., Lu, J., Sheng, Y., Sun, Y., & Zhang, D. (2024). Research Progress in the Preparation of Transition Metal Sulfide Materials and Their Supercapacitor Performance. Micromachines, 15(7), 849. https://doi.org/10.3390/mi15070849





