Designer Oncolytic Adenovirus: Coming of Age
Abstract
:1. Introduction
2. Replication-Selective Adenoviruses
Combination of Oncolytic Adenoviruses with Chemotherapy
3. Oncolytic Immunotherapy
4. Tropism Modification Strategies
4.1. Native Adenoviral Receptor Interactions
4.2. Ablation of Natural Adenoviral Tropisms
4.2.1. CAR (Coxsackie and Adenovirus Receptor)
4.2.2. CD46/MCP (Membrane Cofactor Protein)
4.2.3. Desmoglein 2
4.2.4. GD1a Glycan and Sialic Acid
4.2.5. Blood Coagulation Factor X (FX) and Heparan Sulphate Proteoglycans (HSPG)
4.2.6. Integrins, ανβ3/5
4.2.7. Scavenger Receptor (SR-A6)/Macrophage Receptor with Collagenous Structure (MARCO)
5. Retargeting of Adenovirus by Engineered Receptor Tropism
5.1. Chimeric Fusion Proteins
5.1.1. Single Chain Antibodies
5.1.2. Affibodies—FGFR2
5.1.3. DARPins
5.1.4. scTCR Chimeric Fiber Proteins
5.2. Peptide Based Retargeting of Adenovirus
Targeting αvβ6
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medawar, P.B.; Medawar, J.S. Aristotle to Zoos: A Philosophical Dictionary of Biology; Harvard University Press: Cambridge, MA, USA, 1985; ISBN 978-0-674-04537-8. [Google Scholar]
- Koprowski, H.; Koprowska, I.; Love, R. Ascites Tumor-Virus System as a Biological Tool. Proc. Natl. Acad. Sci. USA 1953, 39, 1147–1148. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Vile, R.G.; Melcher, A.; Chester, J.; Pandha, H.S. Clinical trials with oncolytic reovirus: Moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010, 21, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchala, D.S.; Bhatt, L.K.; Prabhavalkar, K.S. Oncolytic Herpes Simplex Viral Therapy: A Stride toward Selective Targeting of Cancer Cells. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Search of: Adenovirus OR Adv OR Adenoviral OR Ad|Recruiting, Not yet Recruiting, Active, Not Recruiting, Enrolling by Invitation Studies|Cancer NOT Communicable—List Results—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=Cancer+NOT+communicable&term=adenovirus+OR+adv+OR+adenoviral+OR+ad&type=&rslt=&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&lupd_s=&lupd_e= (accessed on 18 March 2018).
- Russell, W.C. Adenoviruses: Update on structure and function. J. Gen. Virol. 2009, 90, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fields Virology—NLM Catalog—NCBI. Available online: https://www.ncbi.nlm.nih.gov/nlmcatalog/101601028 (accessed on 18 March 2018).
- Robinson, C.M.; Singh, G.; Lee, J.Y.; Dehghan, S.; Rajaiya, J.; Liu, E.B.; Yousuf, M.A.; Betensky, R.A.; Jones, M.S.; Dyer, D.W.; et al. Molecular evolution of human adenoviruses. Sci. Rep. 2013, 3, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HAdV Working Group. Available online: http://hadvwg.gmu.edu/ (accessed on 14 June 2018).
- Seto, D.; Chodosh, J.; Brister, J.R.; Jones, M.S.; Adenovirus Research Community. Using the Whole-Genome Sequence to Characterize and Name Human Adenoviruses. J. Virol. 2011, 85, 5701–5702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twumasi-Boateng, K.; Pettigrew, J.L.; Kwok, Y.Y.E.; Bell, J.C.; Nelson, B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Ernst, P.; Honegger, A.; Suomalainen, M.; Zimmermann, M.; Braun, L.; Stauffer, S.; Thom, C.; Dreier, B.; Eibauer, M.; et al. Adenoviral vector with shield and adapter increases tumor specificity and escapes liver and immune control. Nat. Commun. 2018, 9, 450. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, C.R.; Song, A. PEGylated adenovirus for targeted gene therapy. Methods Mol. Biol. 2008, 434, 133–160. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, C.R.; Lachapelle, A.; Delgado, C.; Parkes, V.; Wadsworth, S.C.; Smith, A.E.; Francis, G.E. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999, 10, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Wonganan, P.; Croyle, M.A. PEGylated Adenoviruses: From Mice to Monkeys. Viruses 2010, 2, 468–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Al-Jamal, K.T.; Lacerda, L.; Kostarelos, K. Nanoengineering artificial lipid envelopes around adenovirus by self-assembly. ACS Nano 2008, 2, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.S.; Ostapchuk, P.; Hearing, P.; Carrico, I.S. Unnatural Amino Acid Incorporation onto Adenoviral (Ad) Coat Proteins Facilitates Chemoselective Modification and Retargeting of Ad Type 5 Vectors. J. Virol. 2011, 85, 7546–7554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapet, C.; Pellegrino, C.; Laurent, N.; Sicard, F.; Zelphati, O. Magnetic Nanoparticles Enhance Adenovirus Transduction In Vitro and In Vivo. Pharm. Res. 2012, 29, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Pandori, M.W.; Hobson, D.A.; Sano, T. Adenovirus–Microbead Conjugates Possess Enhanced Infectivity: A New Strategy for Localized Gene Delivery. Virology 2002, 299, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Everts, M.; Saini, V.; Leddon, J.L.; Kok, R.J.; Stoff-Khalili, M.; Preuss, M.A.; Millican, C.L.; Perkins, G.; Brown, J.M.; Bagaria, H.; et al. Covalently Linked Au Nanoparticles to a Viral Vector: Potential for Combined Photothermal and Gene Cancer Therapy. Nano Lett. 2006, 6, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Puumalainen, A.-M.; Vapalahti, M.; Agrawal, R.S.; Kossila, M.; Laukkanen, J.; Lehtolainen, P.; Viita, H.; Paljärvi, L.; Vanninen, R.; Ylä-Herttuala, S. β-Galactosidase Gene Transfer to Human Malignant Glioma In Vivo Using Replication-Deficient Retroviruses and Adenoviruses. Hum. Gene Ther. 1998, 9, 1769–1774. [Google Scholar] [CrossRef] [PubMed]
- Vogels, R.; Zuijdgeest, D.; Rijnsoever, R.; van Hartkoorn, E.; Damen, I.; Béthune, M.-P.; de Kostense, S.; Penders, G.; Helmus, N.; Koudstaal, W.; et al. Replication-Deficient Human Adenovirus Type 35 Vectors for Gene Transfer and Vaccination: Efficient Human Cell Infection and Bypass of Preexisting Adenovirus Immunity. J. Virol. 2003, 77, 8263–8271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemckert, A.A.C.; Grimbergen, J.; Smits, S.; Hartkoorn, E.; Holterman, L.; Berkhout, B.; Barouch, D.H.; Vogels, R.; Quax, P.; Goudsmit, J.; et al. Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: Manufacture on PER.C6 cells, tropism and immunogenicity. J. Gen. Virol. 2006, 87, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.J.; McSharry, B.P.; Armstrong, M.; Tomasec, P.; Wilkinson, G.W.G. Re-engineering adenovirus vector systems to enable high-throughput analyses of gene function. BioTechniques 2008, 45, 659–662, 664–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, I.; Harden, P.; Bauzon, M.; Chartier, C.; Nye, J.; Thorne, S.; Reid, T.; Ni, S.; Lieber, A.; Fisher, K.; et al. Directed Evolution Generates a Novel Oncolytic Virus for the Treatment of Colon Cancer. PLoS ONE 2008, 3, e2409. [Google Scholar] [CrossRef] [PubMed]
- Bressy, C.; Benihoud, K. Association of oncolytic adenoviruses with chemotherapies: An overview and future directions. Biochem. Pharmacol. 2014, 90, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Tong, A.W.; Nemunaitis, M.; Senzer, N.; Phadke, A.P.; Bedell, C.; Adams, N.; Zhang, Y.A.; Maples, P.B.; Chen, S.; et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol. Ther. 2010, 18, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Höti, N.; Li, Y.; Chen, C.-L.; Chowdhury, W.H.; Johns, D.C.; Xia, Q.; Kabul, A.; Hsieh, J.-T.; Berg, M.; Ketner, G.; et al. Androgen Receptor Attenuation of Ad5 Replication: Implications for the Development of Conditionally Replication Competent Adenoviruses. Mol. Ther. 2007, 15, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Heise, C.; Sampson-Johannes, A.; Williams, A.; McCormick, F.; Von Hoff, D.D.; Kirn, D.H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat. Med. 1997, 3, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Ries, S.J.; Brandts, C.H.; Chung, A.S.; Biederer, C.H.; Hann, B.C.; Lipner, E.M.; McCormick, F.; Korn, W.M. Loss of p14ARF in tumor cells facilitates replication of the adenovirus mutant dl1520 (ONYX-015). Nat. Med. 2000, 6, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.-H.; Yuan, Z.-Y.; Guan, Z.-Z.; Cao, Y.; Wang, H.-Q.; Hu, X.-H.; Feng, J.-F.; Zhang, Y.; Li, F.; Chen, Z.-T.; et al. Phase II clinical study of intratumoral H101, an E1B deleted adenovirus, in combination with chemotherapy in patients with cancer. Ai Zheng Aizheng Chin. J. Cancer 2003, 22, 1307–1310. [Google Scholar]
- Lu, W.; Zheng, S.; Li, X.-F.; Huang, J.-J.; Zheng, X.; Li, Z. Intra-tumor injection of H101, a recombinant adenovirus, in combination with chemotherapy in patients with advanced cancers: A pilot phase II clinical trial. World J. Gastroenterol. 2004, 10, 3634–3638. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.J.; Chang, J.H.; Zhang, L.; Jiang, W.Q.; Guan, Z.Z.; Liu, J.W.; Zhang, Y.; Hu, X.H.; Wu, G.H.; Wang, H.Q.; et al. Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus. Ai Zheng 2004, 23, 1666–1670. [Google Scholar] [PubMed]
- White, E. Mechanisms of apoptosis regulation by viral oncogenes in infection and tumorigenesis. Cell Death Differ. 2006, 13, 1371–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodrum, F.D.; Ornelles, D.A. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J. Virol. 1998, 72, 9479–9490. [Google Scholar] [PubMed]
- O’Shea, C.C.; Johnson, L.; Bagus, B.; Choi, S.; Nicholas, C.; Shen, A.; Boyle, L.; Pandey, K.; Soria, C.; Kunich, J.; et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell 2004, 6, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr. Cancer Drug Targets 2018, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Berk, A.J. Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 2005, 24, 7673–7685. [Google Scholar] [CrossRef] [PubMed]
- Frolov, M.V.; Dyson, N.J. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J. Cell Sci. 2004, 117, 2173–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heise, C.; Hermiston, T.; Johnson, L.; Brooks, G.; Sampson-Johannes, A.; Williams, A.; Hawkins, L.; Kirn, D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat. Med. 2000, 6, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Fueyo, J.; Gomez-Manzano, C.; Alemany, R.; Lee, P.S.; McDonnell, T.J.; Mitlianga, P.; Shi, Y.X.; Levin, V.A.; Yung, W.K.; Kyritsis, A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000, 19, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Vassilev, L.; Ranki, T.; Hemminki, A.; Karioja-Kallio, A.; Levalampi, O.; Vuolanto, A.; Cerullo, V.; Pesonen, S. Toxicological and bio-distribution profile of a GM-CSF-expressing, double-targeted, chimeric oncolytic adenovirus ONCOS-102—Support for clinical studies on advanced cancer treatment. PLoS ONE 2017, 12, e0182715. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.J.; Cascallo, M.; Guedan, S.; Gros, A.; Martinez-Quintanilla, J.; Hemminki, A.; Alemany, R. A modified E2F-1 promoter improves the efficacy to toxicity ratio of oncolytic adenoviruses. Gene Ther. 2009, 16, 1441–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nokisalmi, P.; Pesonen, S.; Escutenaire, S.; Sarkioja, M.; Raki, M.; Cerullo, V.; Laasonen, L.; Alemany, R.; Rojas, J.; Cascallo, M.; et al. Oncolytic adenovirus ICOVIR-7 in patients with advanced and refractory solid tumors. Clin. Cancer Res. 2010, 16, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, A.; Gimenez-Alejandre, M.; Rojas, J.J.; Moreno, R.; Bazan-Peregrino, M.; Cascallo, M.; Alemany, R. Safety and efficacy of VCN-01, an oncolytic adenovirus combining fiber HSG-binding domain replacement with RGD and hyaluronidase expression. Clin. Cancer Res. 2015, 21, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; van Ginkel, J.W.; Au, K.Y.; Alemany, R.; Meulenberg, J.J.; van Beusechem, V.W. ORCA-010, a novel potency-enhanced oncolytic adenovirus, exerts strong antitumor activity in preclinical models. Hum. Gene Ther. 2014, 25, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Öberg, D.; Yanover, E.; Adam, V.; Sweeney, K.; Costas, C.; Lemoine, N.R.; Halldén, G. Improved potency and selectivity of an oncolytic E1ACR2 and E1B19K deleted adenoviral mutant (AdΔΔ) in prostate and pancreatic cancers. Clin. Cancer Res. 2010, 16, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, G.; Kallin, C.; Mozetic, A.; Hammaren-Busch, K.; Muller, H.; Lemoine, N.R.; Hallden, G. The oncolytic adenovirus AdDeltaDelta enhances selective cancer cell killing in combination with DNA-damaging drugs in pancreatic cancer models. Gene Ther. 2011, 18, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Hernandez, C.; Maya-Pineda, H.; Millan, J.S.; Man, Y.K.S.; Lu, Y.J.; Hallden, G. Sensitisation to mitoxantrone-induced apoptosis by the oncolytic adenovirus Ad through Bcl-2-dependent attenuation of autophagy. Oncogenesis 2018, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Pantelidou, C.; Cherubini, G.; Lemoine, N.R.; Hallden, G. The E1B19K-deleted oncolytic adenovirus mutant AdDelta19K sensitizes pancreatic cancer cells to drug-induced DNA-damage by down-regulating Claspin and Mre11. Oncotarget 2016, 7, 15703–15724. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.K.S.; Davies, J.A.; Coughlan, L.; Pantelidou, C.; Blázquez-Moreno, A.; Marshall, J.F.; Parker, A.L.; Halldén, G. The Novel Oncolytic Adenoviral Mutant Ad5-3Δ-A20T Retargeted to αvβ6 Integrins Efficiently Eliminates Pancreatic Cancer Cells. Mol. Cancer Ther. 2018, 17, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Bates, R.C.; Bellovin, D.I.; Brown, C.; Maynard, E.; Wu, B.; Kawakatsu, H.; Sheppard, D.; Oettgen, P.; Mercurio, A.M. Transcriptional activation of integrin beta6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J. Clin Investig. 2005, 115, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Thomas, G.J.; Clark, S.; Dawoud, M.M.; Vallath, S.; Payne, S.J.; Gomm, J.J.; Dreger, S.A.; Dickinson, S.; Edwards, D.R.; et al. Altered microenvironment promotes progression of preinvasive breast cancer: Myoepithelial expression of alphavbeta6 integrin in DCIS identifies high-risk patients and predicts recurrence. Clin. Cancer Res. 2014, 20, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, R.C.; Di, Y.; Cerny, A.M.; Sonnen, A.F.-P.; Sim, R.B.; Green, N.K.; Subr, V.; Ulbrich, K.; Gilbert, R.J.C.; Fisher, K.D.; et al. Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood 2009, 113, 1909–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayakhmetov, D.M.; Gaggar, A.; Ni, S.; Li, Z.-Y.; Lieber, A. Adenovirus Binding to Blood Factors Results in Liver Cell Infection and Hepatotoxicity. J. Virol. 2005, 79, 7478–7491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coughlan, L.; Vallath, S.; Gros, A.; Giménez-Alejandre, M.; Van Rooijen, N.; Thomas, G.J.; Baker, A.H.; Cascalló, M.; Alemany, R.; Hart, I.R. Combined Fiber Modifications Both to Target αvβ6 and Detarget the Coxsackievirus–Adenovirus Receptor Improve Virus Toxicity Profiles In Vivo but Fail to Improve Antitumoral Efficacy Relative to Adenovirus Serotype 5. Hum. Gene Ther. 2012, 23, 960–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, Y.; Seymour, L.; Fisher, K. Activity of a group B oncolytic adenovirus (ColoAd1) in whole human blood. Gene Ther. 2014, 21, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.; Di, Y.; Calderon, H.; Illingworth, S.; Kueberuwa, G.; Tedcastle, A.; Jakeman, P.; Chia, S.L.; Brown, A.; Silva, M.A.; et al. Oncolytic Group B Adenovirus Enadenotucirev Mediates Non-apoptotic Cell Death with Membrane Disruption and Release of Inflammatory Mediators. Mol. Ther. Oncolytics 2017, 4, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Salazar, R.; Duran, I.; Osman-Garcia, I.; Paz-Ares, L.; Bozada, J.M.; Boni, V.; Blanc, C.; Seymour, L.; Beadle, J.; et al. Phase 1 study of intravenous administration of the chimeric adenovirus enadenotucirev in patients undergoing primary tumor resection. J. Immunother. Cancer 2017, 5, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhn, I.; Bauzon, M.; Green, N.; Seymour, L.; Fisher, K.; Hermiston, T. OvAd1, a Novel, Potent, and Selective Chimeric Oncolytic Virus Developed for Ovarian Cancer by 3D-Directed Evolution. Mol. Ther. Oncolytics 2017, 4, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Schuur, E.R.; Lim, H.Y.; Henderson, G.A.; Simons, J.W.; Henderson, D.R. Prostate Attenuated Replication Competent Adenovirus (ARCA) CN706: A Selective Cytotoxic for Prostate-specific Antigen-positive Prostate Cancer Cells. Cancer Res. 1997, 57, 2559–2563. [Google Scholar] [PubMed]
- Huang, T.-G.; Savontaus, M.J.; Shinozaki, K.; Sauter, B.V.; Woo, S.L.C. Telomerase-dependent oncolytic adenovirus for cancer treatment. Gene Ther. 2003, 10, 1241–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, E.J.; Carducci, M.A.; Burke, J.M.; Rodriguez, R.; Fong, L.; van Ummersen, L.; Yu, D.C.; Aimi, J.; Ando, D.; Working, P.; et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol. Ther. 2006, 14, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Goldufsky, J.; Sivendran, S.; Harcharik, S.; Pan, M.; Bernardo, S.; Stern, R.H.; Friedlander, P.; Ruby, C.E.; Saenger, Y.; Kaufman, H.L. Oncolytic Virus Therapy for Cancer. Available online: https://www.dovepress.com/oncolytic-virus-therapy-for-cancer-peer-reviewed-article-OV (accessed on 16 May 2018).
- Liu, T.-C.; Galanis, E.; Kirn, D. Clinical trial results with oncolytic virotherapy: A century of promise, a decade of progress. Nat. Rev. Clin. Oncol. 2007, 4, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Yang, C.-T.; Jablons, D.M. ONYX-015 Works Synergistically with Chemotherapy in Lung Cancer Cell Lines and Primary Cultures Freshly Made from Lung Cancer Patients. Cancer Res. 2000, 60, 1009–1013. [Google Scholar] [PubMed]
- Yu, D.C.; Chen, Y.; Dilley, J.; Li, Y.; Embry, M.; Zhang, H.; Nguyen, N.; Amin, P.; Oh, J.; Henderson, D.R. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 2001, 61, 517–525. [Google Scholar] [PubMed]
- Liu, D.; Kojima, T.; Ouchi, M.; Kuroda, S.; Watanabe, Y.; Hashimoto, Y.; Onimatsu, H.; Urata, Y.; Fujiwara, T. Preclinical evaluation of synergistic effect of telomerase-specific oncolytic virotherapy and gemcitabine for human lung cancer. Mol. Cancer Ther. 2009, 8, 980–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, B.; Beljin, M.; Stark, G.R.; Taylor, W.R. G2 Arrest in Response to Topoisomerase II Inhibitors: The Role of p53. Cancer Res. 2003, 63, 4074–4081. [Google Scholar] [PubMed]
- Gomez-Manzano, C.; Alonso, M.M.; Yung, W.K.; McCormick, F.; Curiel, D.T.; Lang, F.F.; Jiang, H.; Bekele, B.N.; Zhou, X.; Alemany, R.; et al. Delta-24 increases the expression and activity of topoisomerase I and enhances the antiglioma effect of irinotecan. Clin. Cancer Res. 2006, 12, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Kawamura, K.; Li, Q.; Okamoto, S.; Suzuki, N.; Kobayashi, H.; Liang, M.; Tada, Y.; Tatsumi, K.; Hiroshima, K.; et al. Combinatory cytotoxic effects produced by E1B-55kDa-deleted adenoviruses and chemotherapeutic agents are dependent on the agents in esophageal carcinoma. Cancer Gene Ther. 2010, 17, 803–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, S.; Miranda, E.; Ekblad, M.; Holford, A.; Pizarro, M.T.; Lemoine, N.R.; Hallden, G. Efficacy of oncolytic mutants targeting pRb and p53 pathways is synergistically enhanced when combined with cytotoxic drugs in prostate cancer cells and tumor xenografts. Hum. Gene Ther. 2010, 21, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- White, E. Regulation of the cell cycle and apoptosis by the oncogenes of adenovirus. Oncogene 2001, 20, 7836–7846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, E.; Maya Pineda, H.; Oberg, D.; Cherubini, G.; Garate, Z.; Lemoine, N.R.; Hallden, G. Adenovirus-mediated sensitization to the cytotoxic drugs docetaxel and mitoxantrone is dependent on regulatory domains in the E1ACR1 gene-region. PLoS ONE 2012, 7, e46617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, V.; Ekblad, M.; Sweeney, K.; Muller, H.; Busch, K.H.; Johnsen, C.T.; Kang, N.R.; Lemoine, N.R.; Hallden, G. Synergistic and Selective Cancer Cell Killing Mediated by the Oncolytic Adenoviral Mutant AdDeltaDelta and Dietary Phytochemicals in Prostate Cancer Models. Hum. Gene Ther. 2012, 23, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Rosewell Shaw, A.; Suzuki, M. Recent advances in oncolytic adenovirus therapies for cancer. Curr. Opin. Virol. 2016, 21, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, B.A.; Bell, J.C. Oncolytic viruses-immunotherapeutics on the rise. J. Mol. Med. 2016, 94, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I study with ONCOS-102 for the treatment of solid tumors—An evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer 2016, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Woller, N.; Gurlevik, E.; Fleischmann-Mundt, B.; Schumacher, A.; Knocke, S.; Kloos, A.M.; Saborowski, M.; Geffers, R.; Manns, M.P.; Wirth, T.C.; et al. Viral Infection of Tumors Overcomes Resistance to PD-1-immunotherapy by Broadening Neoantigenome-directed T-cell Responses. Mol. Ther. 2015, 23, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gomez-Manzano, C.; Rivera-Molina, Y.; Lang, F.F.; Conrad, C.A.; Fueyo, J. Oncolytic adenovirus research evolution: From cell-cycle checkpoints to immune checkpoints. Curr. Opin. Virol. 2015, 13, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; LaRocca, C.J.; Yamamoto, M. Showing the Way: Oncolytic Adenoviruses as Chaperones of Immunostimulatory Adjuncts. Biomedicines 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.T.; Bell, J.C. Oncolytic Virus Combination Therapy: Killing One Bird with Two Stones. Mol. Ther. 2018, 26. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, C.J.; Lichty, B.D.; Bell, J.C. Oncolytic Viruses: Therapeutics with an Identity Crisis. EBioMedicine 2016, 9, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Atherton, M.J.; Lichty, B.D. Evolution of oncolytic viruses: Novel strategies for cancer treatment. Immunotherapy 2013, 5, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Francis, J.; Eddouadi, A.; Lemoine, N.R.; Hallden, G. An oncolytic adenovirus defective in pRb-binding (dl922-947) can efficiently eliminate pancreatic cancer cells and tumors in vivo in combination with 5-FU or gemcitabine. Cancer Gene Ther. 2011, 18, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Bramante, S.; Kaufmann, J.K.; Veckman, V.; Liikanen, I.; Nettelbeck, D.M.; Hemminki, O.; Vassilev, L.; Cerullo, V.; Oksanen, M.; Heiskanen, R.; et al. Treatment of melanoma with a serotype 5/3 chimeric oncolytic adenovirus coding for GM-CSF: Results in vitro, in rodents and in humans. Int. J. Cancer 2015. [Google Scholar] [CrossRef] [PubMed]
- Koski, A.; Kangasniemi, L.; Escutenaire, S.; Pesonen, S.; Cerullo, V.; Diaconu, I.; Nokisalmi, P.; Raki, M.; Rajecki, M.; Guse, K.; et al. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 2010, 18, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Greig, S.L. Talimogene Laherparepvec: First Global Approval. Drugs 2016, 76, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.W.; Papayoti, M.H.; Netto, G.; Armstrong, D.T.; Ordonez, G.; Lawson, J.M.; Stone, M.J. Growth-inhibitory Effects of CD40 Ligand (CD154) and Its Endogenous Expression in Human Breast Cancer. Clin. Cancer Res. 2001, 7, 691–703. [Google Scholar] [PubMed]
- Ghamande, S.; Hylander, B.L.; Oflazoglu, E.; Lele, S.; Fanslow, W.; Repasky, E.A. Recombinant CD40 Ligand Therapy Has Significant Antitumor Effects on CD40-positive Ovarian Tumor Xenografts Grown in SCID Mice and Demonstrates an Augmented Effect with Cisplatin. Cancer Res. 2001, 61, 7556–7562. [Google Scholar] [PubMed]
- Korniluk, A.; Kemona, H.; Dymicka-Piekarska, V. Multifunctional CD40L: Pro- and anti-neoplastic activity. Tumor Biol. 2014, 35, 9447–9457. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; Vries, V.C.D.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, E.M.; Rodrigues, M.S.; Phadke, A.P.; Butcher, L.D.; Starling, C.; Chen, S.; Chang, D.; Hernandez-Alcoceba, R.; Newman, J.T.; Stone, M.J.; et al. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD154) transgene construct in human breast cancer cells. Clin. Cancer Res. 2009, 15, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, I.; Cerullo, V.; Hirvinen, M.L.M.; Escutenaire, S.; Ugolini, M.; Pesonen, S.K.; Bramante, S.; Parviainen, S.; Kanerva, A.; Loskog, A.S.I.; et al. Immune Response Is an Important Aspect of the Antitumor Effect Produced by a CD40L-Encoding Oncolytic Adenovirus. Cancer Res. 2012, 72, 2327–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesonen, S.; Diaconu, I.; Kangasniemi, L.; Ranki, T.; Kanerva, A.; Pesonen, S.K.; Gerdemann, U.; Leen, A.M.; Kairemo, K.; Oksanen, M.; et al. Oncolytic Immunotherapy of Advanced Solid Tumors with a CD40L-Expressing Replicating Adenovirus: Assessment of Safety and Immunologic Responses in Patients. Cancer Res. 2012, 72, 1621–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, E.; Moreno, R.; Milenova, I.; Liljenfeldt, L.; Dieterich, L.C.; Christiansson, L.; Karlsson, H.; Ullenhag, G.; Mangsbo, S.M.; Dimberg, A.; et al. Activation of myeloid and endothelial cells by CD40L gene therapy supports T-cell expansion and migration into the tumor microenvironment. Gene Ther. 2017, 24, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Leonard, J.P.; Sherman, M.L.; Fisher, G.L.; Buchanan, L.J.; Larsen, G.; Atkins, M.B.; Sosman, J.A.; Dutcher, J.P.; Vogelzang, N.J.; Ryan, J.L. Effects of Single-Dose Interleukin-12 Exposure on Interleukin-12–Associated Toxicity and Interferon-γ Production. Blood 1997, 90, 2541–2548. [Google Scholar] [PubMed]
- Freytag, S.O.; Barton, K.N.; Zhang, Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. Gene Ther. 2013, 20, 1131–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freytag, S.O.; Stricker, H.; Lu, M.; Elshaikh, M.; Aref, I.; Pradhan, D.; Levin, K.; Kim, J.H.; Peabody, J.; Siddiqui, F.; et al. Prospective randomized phase 2 trial of intensity modulated radiation therapy with or without oncolytic adenovirus-mediated cytotoxic gene therapy in intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; et al. Re-designing Interleukin-12 to enhance its safety and potential as an anti-tumor immunotherapeutic agent. Nat. Commun. 2017, 8, 1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cupelli, K.; Müller, S.; Persson, B.D.; Jost, M.; Arnberg, N.; Stehle, T. Structure of Adenovirus Type 21 Knob in Complex with CD46 Reveals Key Differences in Receptor Contacts among Species B Adenoviruses. J. Virol. 2010, 84, 3189–3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubczak, J.L.; Rollence, M.L.; Stewart, D.A.; Jafari, J.D.; Seggern, D.J.V.; Nemerow, G.R.; Stevenson, S.C.; Hallenbeck, P.L. Adenovirus Type 5 Viral Particles Pseudotyped with Mutagenized Fiber Proteins Show Diminished Infectivity of Coxsackie B-Adenovirus Receptor-Bearing Cells. J. Virol. 2001, 75, 2972–2981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roelvink, P.W.; Mi Lee, G.; Einfeld, D.A.; Kovesdi, I.; Wickham, T.J. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 1999, 286, 1568–1571. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liaw, Y.-C.; Stone, D.; Kalyuzhniy, O.; Amiraslanov, I.; Tuve, S.; Verlinde, C.L.M.J.; Shayakhmetov, D.; Stehle, T.; Roffler, S.; et al. Identification of CD46 binding sites within the adenovirus serotype 35 fiber knob. J. Virol. 2007, 81, 12785–12792. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yumul, R.; Cao, H.; Ran, L.; Fan, X.; Richter, M.; Epstein, F.; Gralow, J.; Zubieta, C.; Fender, P.; et al. Structural and Functional Studies on the Interaction of Adenovirus Fiber Knobs and Desmoglein 2. J. Virol. 2013, 87, 11346–11362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, E.C.; Storm, R.J.; Bauer, J.; Johansson, S.M.C.; Lookene, A.; Ångström, J.; Hedenström, M.; Eriksson, T.L.; Frängsmyr, L.; Rinaldi, S.; et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2011, 17, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Waddington, S.N.; McVey, J.H.; Bhella, D.; Parker, A.L.; Barker, K.; Atoda, H.; Pink, R.; Buckley, S.M.K.; Greig, J.A.; Denby, L.; et al. Adenovirus Serotype 5 Hexon Mediates Liver Gene Transfer. Cell 2008, 132, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyuzhniy, O.; Paolo, N.C.D.; Silvestry, M.; Hofherr, S.E.; Barry, M.A.; Stewart, P.L.; Shayakhmetov, D.M. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 5483–5488. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Bradshaw, A.C.; Parker, A.L.; Bhella, D.; Waddington, S.N.; Nicklin, S.A.; van Rooijen, N.; Custers, J.; Goudsmit, J.; Barouch, D.H.; et al. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: Effect of mutagenesis on FX interactions and gene transfer. Blood 2009, 114, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Paolo, N.C.D.; Kalyuzhniy, O.; Shayakhmetov, D.M. Fiber Shaft-Chimeric Adenovirus Vectors Lacking the KKTK Motif Efficiently Infect Liver Cells In Vivo. J. Virol. 2007, 81, 12249–12259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kritz, A.B.; Nicol, C.G.; Dishart, K.L.; Nelson, R.; Holbeck, S.; Von Seggern, D.J.; Work, L.M.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Adenovirus 5 Fibers Mutated at the Putative HSPG-binding Site Show Restricted Retargeting with Targeting Peptides in the HI Loop. Mol. Ther. 2007, 15, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Harfe, B.; Freimuth, P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J. Virol. 1993, 67, 5198–5205. [Google Scholar] [PubMed]
- Henning, P.; Andersson, K.M.E.; Frykholm, K.; Ali, A.; Magnusson, M.K.; Nygren, P.-A.; Granio, O.; Hong, S.S.; Boulanger, P.; Lindholm, L. Tumor cell targeted gene delivery by adenovirus 5 vectors carrying knobless fibers with antibody-binding domains. Gene Ther. 2005, 12, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Stichling, N.; Suomalainen, M.; Flatt, J.W.; Schmid, M.; Pacesa, M.; Hemmi, S.; Jungraithmayr, W.; Maler, M.D.; Freudenberg, M.A.; Plückthun, A.; et al. Lung macrophage scavenger receptor SR-A6 (MARCO) is an adenovirus type-specific virus entry receptor. PLoS Pathog. 2018, 14, e1006914. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.J.; Shieh, J.T.C.; Pickles, R.J.; Okegawa, T.; Hsieh, J.-T.; Bergelson, J.M. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 2001, 98, 15191–15196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, V.; Meinken, G.; Springer, K.; Srivastava, S.C.; Freimuth, P. Biodistribution of Radioiodinated Adenovirus Fiber Protein Knob Domain after Intravenous Injection in Mice. J. Virol. 2004, 78, 6431–6438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a Common Receptor for Coxsackie B Viruses and Adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Roelvink, P.W.; Lizonova, A.; Lee, J.G.M.; Li, Y.; Bergelson, J.M.; Finberg, R.W.; Brough, D.E.; Kovesdi, I.; Wickham, T.J. The Coxsackievirus-Adenovirus Receptor Protein Can Function as a Cellular Attachment Protein for Adenovirus Serotypes from Subgroups A, C, D, E, and F. J. Virol. 1998, 72, 7909–7915. [Google Scholar] [PubMed]
- Bewley, M.C.; Springer, K.; Zhang, Y.-B.; Freimuth, P.; Flanagan, J.M. Structural Analysis of the Mechanism of Adenovirus Binding to Its Human Cellular Receptor, CAR. Science 1999, 286, 1579–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiradake, E.; Lortat-Jacob, H.; Billet, O.; Kremer, E.J.; Cusack, S. Structural and Mutational Analysis of Human Ad37 and Canine Adenovirus 2 Fiber Heads in Complex with the D1 Domain of Coxsackie and Adenovirus Receptor. J. Biol. Chem. 2006, 281, 33704–33716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, I.; Davison, E.; Beavil, A.J.; Soh, C.P.C.; Wickham, T.J.; Roelvink, P.W.; Kovesdi, I.; Sutton, B.J.; Santis, G. Identification of Contact Residues and Definition of the CAR-Binding Site of Adenovirus Type 5 Fiber Protein. J. Virol. 2000, 74, 2804–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, I.; Davison, E.; Beavil, A.J.; Soh, C.P.C.; Wickham, T.J.; Roelvink, P.W.; Kovesdi, I.; Sutton, B.J.; Santis, G. Mutations in the DG Loop of Adenovirus Type 5 Fiber Knob Protein Abolish High-Affinity Binding to Its Cellular Receptor CAR. J. Virol. 1999, 73, 9508–9514. [Google Scholar] [PubMed]
- Smith, T.; Idamakanti, N.; Kylefjord, H.; Rollence, M.; King, L.; Kaloss, M.; Kaleko, M.; Stevenson, S.C. In Vivo Hepatic Adenoviral Gene Delivery Occurs Independently of the Coxsackievirus–Adenovirus Receptor. Mol. Ther. 2002, 5, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Kerttula, H.; Davies, J.; Coughlan, L.; Hulin-Curtis, S.; Jones, R.; Hanna, L.; Chester, J.D.; Parker, A.L. Pseudotyped αvβ6 integrin-targeted adenovirus vectors for ovarian cancer therapies. Oncotarget 2016, 7, 27926–27937. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; Curiel, D.T. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001, 8, 1347–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seiradake, E.; Henaff, D.; Wodrich, H.; Billet, O.; Perreau, M.; Hippert, C.; Mennechet, F.; Schoehn, G.; Lortat-Jacob, H.; Dreja, H.; et al. The cell adhesion molecule “CAR” and sialic acid on human erythrocytes influence adenovirus in vivo biodistribution. PLoS Pathog. 2009, 5, e1000277. [Google Scholar] [CrossRef] [PubMed]
- Nicol, C.G.; Graham, D.; Miller, W.H.; White, S.J.; Smith, T.A.G.; Nicklin, S.A.; Stevenson, S.C.; Baker, A.H. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol. Ther. 2004, 10, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.W.; Knowles, B.B.; Parkar, M.; Pym, B.; Stanley, K.; Goodfellow, P.N. A human cell-surface antigen defined by a monoclonal antibody and controlled by a gene on human chromosome 1. Ann. Hum. Genet. 1985, 49, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Fara, A.F.; Dasgupta, P.; Kemper, C. CD46: The ‘multitasker’ of complement proteins. Int. J. Biochem. Cell Biol. 2013, 45, 2808–2820. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.D.; Schmitz, N.B.; Santiago, C.; Zocher, G.; Larvie, M.; Scheu, U.; Casasnovas, J.M.; Stehle, T. Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens. PLoS Pathog. 2010, 6, e1001122. [Google Scholar] [CrossRef] [PubMed]
- Fleischli, C.; Sirena, D.; Lesage, G.; Havenga, M.J.E.; Cattaneo, R.; Greber, U.F.; Hemmi, S. Species B adenovirus serotypes 3, 7, 11 and 35 share similar binding sites on the membrane cofactor protein CD46 receptor. J. Gen. Virol. 2007, 88, 2925–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, B.D.; Reiter, D.M.; Marttila, M.; Mei, Y.-F.; Casasnovas, J.M.; Arnberg, N.; Stehle, T. Adenovirus type 11 binding alters the conformation of its receptor CD46. Nat. Struct. Mol. Biol. 2007, 14, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.C.; Gujer, C.; McInerney, G.; Gall, J.G.D.; Petrovas, C.; Hedestam, G.B.K.; Koup, R.A.; Loré, K. Adenovirus type-35 vectors block human CD4+ T-cell activation via CD46 ligation. Proc. Natl. Acad. Sci. USA 2011, 108, 7499–7504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, J.; Carter, D.; Lieber, A.; Astier, A.L. Recombinant Ad35 adenoviral proteins as potent modulators of human T cell activation. Immunology 2014. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [PubMed]
- Branton, P.E.; Roopchand, D.E. The role of adenovirus E4orf4 protein in viral replication and cell killing. Oncogene 2001, 20, 7855–7865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illingworth, S.; Di, Y.; Bauzon, M.; Lei, J.; Duffy, M.R.; Alvis, S.; Champion, B.; Lieber, A.; Hermiston, T.; Seymour, L.W.; et al. Preclinical Safety Studies of Enadenotucirev, a Chimeric Group B Human-Specific Oncolytic Adenovirus. Mol. Ther. Oncolytics 2017, 5, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Rhee, E.G.; Masek-Hammerman, K.; Teigler, J.E.; Abbink, P.; Barouch, D.H. Adenovirus Serotype 26 Utilizes CD46 as a Primary Cellular Receptor and Only Transiently Activates T Lymphocytes following Vaccination of Rhesus Monkeys. J. Virol. 2012, 86, 10862–10865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baden, L.R.; Walsh, S.R.; Seaman, M.S.; Johnson, J.A.; Tucker, R.P.; Kleinjan, J.A.; Gothing, J.A.; Engelson, B.A.; Carey, B.R.; Oza, A.; et al. First-in-Human Evaluation of a Hexon Chimeric Adenovirus Vector Expressing HIV-1 Env (IPCAVD 002). J. Infect. Dis. 2014, 210, 1052–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long-Term Safety Follow-Up of Participants Exposed to the Candidate Ebola Vaccines Ad26.ZEBOV and/or MVA-BN-Filo—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02661464?term=adenovirus&recrs=a&cond=ebola&rank=1 (accessed on 23 September 2017).
- Teigler, J.E.; Iampietro, M.J.; Barouch, D.H. Vaccination with Adenovirus Serotypes 35, 26, and 48 Elicits Higher Levels of Innate Cytokine Responses than Adenovirus Serotype 5 in Rhesus Monkeys. J. Virol. 2012, 86, 9590–9598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Xiang, Z.Q.; Li, Y.; Kurupati, R.K.; Jia, B.; Bian, A.; Zhou, D.M.; Hutnick, N.; Yuan, S.; Gray, C.; et al. Adenovirus-Based Vaccines: Comparison of Vectors from Three Species of Adenoviridae. J. Virol. 2010, 84, 10522–10532. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Kerttula, H.; Hulin-Curtis, S.; Davies, J.; Parker, A.L. Oncolytic Adenovirus: Strategies and Insights for Vector Design and Immuno-Oncolytic Applications. Viruses 2015, 7, 6009–6042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Li, Z.-Y.; Liu, Y.; Persson, J.; Beyer, I.; Möller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.-B.; et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011, 17, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Fender, P.; Boussaid, A.; Mezin, P.; Chroboczek, J. Synthesis, cellular localization, and quantification of penton-dodecahedron in serotype 3 adenovirus-infected cells. Virology 2005, 340, 167–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuschiotti, P.; Schoehn, G.; Fender, P.; Fabry, C.M.S.; Hewat, E.A.; Chroboczek, J.; Ruigrok, R.W.H.; Conway, J.F. Structure of the dodecahedral penton particle from human adenovirus type 3. J. Mol. Biol. 2006, 356, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.; Yumul, R.; Lara, S.; Hemminki, A.; Fender, P.; Lieber, A. Multimerization of Adenovirus Serotype 3 Fiber Knob Domains Is Required for Efficient Binding of Virus to Desmoglein 2 and Subsequent Opening of Epithelial Junctions. J. Virol. 2011, 85, 6390–6402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.-Z.; Wang, H.; Zhang, Y.; Cao, H.; Li, Z.; Fender, P.; Lieber, A. Penton-Dodecahedral Particles Trigger Opening of Intercellular Junctions and Facilitate Viral Spread during Adenovirus Serotype 3 Infection of Epithelial Cells. PLoS Pathog. 2013, 9, e1003718. [Google Scholar] [CrossRef] [PubMed]
- Fender, P.; Hall, K.; Schoehn, G.; Blair, G.E. Impact of Human Adenovirus Type 3 Dodecahedron on Host Cells and Its Potential Role in Viral Infection. J. Virol. 2012, 86, 5380–5385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Ugai, H.; Belousova, N.; Pereboev, A.; Dent, P.; Fisher, P.B.; Everts, M.; Curiel, D.T. Chimeric Adenoviral Vectors Incorporating a Fiber of Human Adenovirus 3 Efficiently Mediate Gene Transfer into Prostate Cancer Cells. Prostate 2010, 70, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Dmitriev, I.P.; Saddekni, S.; Kashentseva, E.A.; Harris, R.D.; Aurigemma, R.; Bae, S.; Singh, K.P.; Siegal, G.P.; Curiel, D.T.; et al. A phase I clinical trial of Ad5/3-Δ24, a novel serotype-chimeric, infectivity-enhanced, conditionally-replicative adenovirus (CRAd), in patients with recurrent ovarian cancer. Gynecol. Oncol. 2013, 130, 518–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ONCOS-102 (Previously CGTG-102) for Therapy of Advanced Cancers—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01598129 (accessed on 20 March 2018).
- A Phase 1/2 Study to Investigate the Safety, Biologic and Anti-Tumor Activity of ONCOS-102 in Combination with Durvalumab in Subjects with Advanced Peritoneal Malignancies—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02963831 (accessed on 20 March 2018).
- A Randomised Phase II Open-Label Study with a Phase Ib Safety Lead-In Cohort of ONCOS-102, an Immune-Priming GM-CSF Coding Oncolytic Adenovirus, and Pemetrexed/Cisplatin in Patients with Unresectable Malignant Pleural Mesothelioma—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02879669 (accessed on 20 March 2018).
- Beyer, I.; Rensburg, R.; van Strauss, R.; Li, Z.; Wang, H.; Persson, J.; Yumul, R.; Feng, Q.; Song, H.; Bartek, J.; et al. Epithelial Junction Opener JO-1 Improves Monoclonal Antibody Therapy of Cancer. Cancer Res. 2011, 71, 7080–7090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyer, I.; Cao, H.; Persson, J.; Song, H.; Richter, M.; Feng, Q.; Yumul, R.; Rensburg, R.; van Li, Z.; Berenson, R.; et al. Coadministration of Epithelial Junction Opener JO-1 Improves the Efficacy and Safety of Chemotherapeutic Drugs. Clin. Cancer Res. 2012, 18, 3340–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, M.; Yumul, R.; Wang, H.; Saydaminova, K.; Ho, M.; May, D.; Baldessari, A.; Gough, M.; Drescher, C.; Urban, N.; et al. Preclinical safety and efficacy studies with an affinity-enhanced epithelial junction opener and PEGylated liposomal doxorubicin. Mol. Ther. Methods Clin. Dev. 2015, 2, 15005. [Google Scholar] [CrossRef] [PubMed]
- Durmort, C.; Stehlin, C.; Schoehn, G.; Mitraki, A.; Drouet, E.; Cusack, S.; Burmeister, W.P. Structure of the fiber head of Ad3, a non-CAR-binding serotype of adenovirus. Virology 2001, 285, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Beyer, I.; Persson, J.; Song, H.; Li, Z.; Richter, M.; Cao, H.; Rensburg, R.; van Yao, X.; Hudkins, K.; et al. A New Human DSG2-Transgenic Mouse Model for Studying the Tropism and Pathology of Human Adenoviruses. J. Virol. 2012, 86, 6286–6302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Brabletz, T.; Kang, Y.; Longmore, G.D.; Nieto, M.A.; Stanger, B.Z.; Yang, J.; Weinberg, R.A. Upholding a role for EMT in breast cancer metastasis. Nature 2017, 547, E1–E3. [Google Scholar] [CrossRef] [PubMed]
- Beerling, E.; Seinstra, D.; de Wit, E.; Kester, L.; van der Velden, D.; Maynard, C.; Schäfer, R.; van Diest, P.; Voest, E.; van Oudenaarden, A.; et al. Plasticity between Epithelial and Mesenchymal States Unlinks EMT from Metastasis-Enhancing Stem Cell Capacity. Cell Rep. 2016, 14, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: Necessary or permissive for metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stencel-Baerenwald, J.E.; Reiss, K.; Reiter, D.M.; Stehle, T.; Dermody, T.S. The sweet spot: Defining virus–sialic acid interactions. Nat. Rev. Microbiol. 2014, 12, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Arnberg, N.; Pring-Åkerblom, P.; Wadell, G. Adenovirus Type 37 Uses Sialic Acid as a Cellular Receptor on Chang C Cells. J. Virol. 2002, 76, 8834–8841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnberg, N.; Edlund, K.; Kidd, A.H.; Wadell, G. Adenovirus Type 37 Uses Sialic Acid as a Cellular Receptor. J. Virol. 2000, 74, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Årdahl, C.; Rajan, A.; Nilsson, E.; Bradford, W.; Kaeshammer, L.; Jones, M.S.; Frängsmyr, L.; et al. Human Adenovirus 52 Uses Sialic Acid-containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells. PLoS Pathog. 2015, 11, e1004657. [Google Scholar] [CrossRef] [PubMed]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Frängsmyr, L.; Frank, M.; Blaum, B.S.; Chai, W.; Podgorski, I.I.; Harrach, B.; Benkő, M.; et al. Polysialic acid is a cellular receptor for human adenovirus 52. Proc. Natl. Acad. Sci. USA 2018, 201716900. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L.; Gerardy-Schahn, R.; Hildebrandt, H. Sialic Acids in the Brain: Gangliosides and Polysialic Acid in Nervous System Development, Stability, Disease, and Regeneration. Physiol. Rev. 2014, 94, 461–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colley, K.J.; Kitajima, K.; Sato, C. Polysialic acid: Biosynthesis, novel functions and applications. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 498–532. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008, 9, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Galuska, C.E.; Lütteke, T.; Galuska, S.P. Is Polysialylated NCAM Not Only a Regulator during Brain Development But also during the Formation of Other Organs? Biology 2017, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Kiermaier, E.; Moussion, C.; Veldkamp, C.T.; Gerardy-Schahn, R.; Vries, I.; de Williams, L.G.; Chaffee, G.R.; Phillips, A.J.; Freiberger, F.; Imre, R.; et al. Polysialylation controls dendritic cell trafficking by regulating chemokine recognition. Science 2016, 351, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, M.; Nakayama, J.; Suzuki, A.; Angata, K.; Chen, S.; Sakai, K.; Hagihara, K.; Yamaguchi, Y.; Fukuda, M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology 2005, 15, 887–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petridis, A.K.; Wedderkopp, H.; Hugo, H.H.; Mehdorn, H.M. Polysialic acid overexpression in malignant astrocytomas. Acta Neurochir. 2009, 151, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Amoureux, M.-C.; Coulibaly, B.; Chinot, O.; Loundou, A.; Metellus, P.; Rougon, G.; Figarella-Branger, D. Polysialic Acid Neural Cell Adhesion Molecule (PSA-NCAM) is an adverse prognosis factor in glioblastoma, and regulates olig2 expression in glioma cell lines. BMC Cancer 2010, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Figarella-Branger, D.F.; Durbec, P.L.; Rougon, G.N. Differential Spectrum of Expression of Neural Cell Adhesion Molecule Isoforms and L1 Adhesion Molecules on Human Neuroectodermal Tumors. Cancer Res. 1990, 50, 6364–6370. [Google Scholar] [PubMed]
- Glüer, S.; Schelp, C.; Gerardy-Schahn, R.; von Schweinitz, D. Polysialylated neural cell adhesion molecule as a marker for differential diagnosis in pediatric tumors. J. Pediatr. Surg. 1998, 33, 1516–1520. [Google Scholar] [CrossRef]
- Lantuejoul, S.; Moro, D.; Michalides, R.J.; Brambilla, C.; Brambilla, E. Neural Cell Adhesion Molecules (ncam) and Ncam-psa Expression in Neuroendocrine Lung Tumors. Am. J. Surg. Pathol. 1998, 22, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Otake, Y.; Nakagawa, T.; Kawano, Y.; Miyahara, R.; Li, M.; Yanagihara, K.; Nakayama, J.; Fujimoto, I.; Ikenaka, K.; et al. Expression of Polysialic Acid and STX, a Human Polysialyltransferase, Is Correlated with Tumor Progression in Non-Small Cell Lung Cancer. Cancer Res. 2000, 60, 3072–3080. [Google Scholar] [PubMed]
- Schäfer, G.; Blumenthal, M.J.; Katz, A.A. Interaction of Human Tumor Viruses with Host Cell Surface Receptors and Cell Entry. Viruses 2015, 7, 2592–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlett, A.H.; Park, P.W. Proteoglycans in host–pathogen interactions: Molecular mechanisms and therapeutic implications. Expert Rev. Mol. Med. 2010, 12, e5. [Google Scholar] [CrossRef] [PubMed]
- Tuve, S.; Wang, H.; Jacobs, J.D.; Yumul, R.C.; Smith, D.F.; Lieber, A. Role of Cellular Heparan Sulfate Proteoglycans in Infection of Human Adenovirus Serotype 3 and 35. PLoS Pathog. 2008, 4, e1000189. [Google Scholar] [CrossRef] [PubMed]
- Dechecchi, M.C.; Tamanini, A.; Bonizzato, A.; Cabrini, G. Heparan Sulfate Glycosaminoglycans Are Involved in Adenovirus Type 5 and 2-Host Cell Interactions. Virology 2000, 268, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Dechecchi, M.C.; Melotti, P.; Bonizzato, A.; Santacatterina, M.; Chilosi, M.; Cabrini, G. Heparan Sulfate Glycosaminoglycans Are Receptors Sufficient To Mediate the Initial Binding of Adenovirus Types 2 and 5. J. Virol. 2001, 75, 8772–8780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruoslahti, E. Proteoglycans in cell regulation. J. Biol. Chem. 1989, 264, 13369–13372. [Google Scholar] [PubMed]
- Bayo-Puxan, N.; Gimenez-Alejandre, M.; Lavilla-Alonso, S.; Gros, A.; Cascallo, M.; Hemminki, A.; Alemany, R. Replacement of Adenovirus Type 5 Fiber Shaft Heparan Sulfate Proteoglycan-Binding Domain with RGD for Improved Tumor Infectivity and Targeting. Hum. Gene Ther. 2009, 20, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Waddington, S.N.; Nicol, C.G.; Shayakhmetov, D.M.; Buckley, S.M.; Denby, L.; Kemball-Cook, G.; Ni, S.; Lieber, A.; McVey, J.H.; et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 2006, 108, 2554–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigant, F.; Descamps, D.; Jullienne, B.; Esselin, S.; Connault, E.; Opolon, P.; Tordjmann, T.; Vigne, E.; Perricaudet, M.; Benihoud, K. Substitution of Hexon Hypervariable Region 5 of Adenovirus Serotype 5 Abrogates Blood Factor Binding and Limits Gene Transfer to Liver. Mol. Ther. 2008, 16, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Bradshaw, A.C.; Coughlan, L.; Denby, L.; McDonald, R.A.; Waddington, S.N.; Buckley, S.M.K.; Greig, J.A.; Parker, A.L.; Miller, A.M.; et al. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood 2010, 116, 2656–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Qiu, Q.; Tian, J.; Smith, J.S.; Conenello, G.M.; Morita, T.; Byrnes, A.P. Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat. Med. 2013, 19, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Doszpoly, A.; Turner, G.; Nicklin, S.A.; Baker, A.H. The relevance of coagulation factor X protection of adenoviruses in human sera. Gene Ther. 2016, 23, 592–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Duffy, M.R.; Deng, L.; Dakin, R.S.; Uil, T.; Custers, J.; Kelly, S.M.; McVey, J.H.; Nicklin, S.A.; Baker, A.H. Manipulating Adenovirus Hexon Hypervariable Loops Dictates Immune Neutralisation and Coagulation Factor X-dependent Cell Interaction In Vitro and In Vivo. PLoS Pathog. 2015, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alba, R.; Bradshaw, A.C.; Mestre-Francés, N.; Verdier, J.-M.; Henaff, D.; Baker, A.H. Coagulation factor X mediates adenovirus type 5 liver gene transfer in non-human primates (Microcebus murinus). Gene Ther. 2012, 19, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.C.; Parker, A.L.; Duffy, M.R.; Coughlan, L.; Rooijen, N. van; Kähäri, V.-M.; Nicklin, S.A.; Baker, A.H. Requirements for Receptor Engagement during Infection by Adenovirus Complexed with Blood Coagulation Factor X. PLoS Pathog. 2010, 6, e1001142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corjon, S.; Gonzalez, G.; Henning, P.; Grichine, A.; Lindholm, L.; Boulanger, P.; Fender, P.; Hong, S.-S. Cell Entry and Trafficking of Human Adenovirus Bound to Blood Factor X Is Determined by the Fiber Serotype and Not Hexon:Heparan Sulfate Interaction. PLoS ONE 2011, 6, e18205. [Google Scholar] [CrossRef] [PubMed]
- Ranki, T.; Kanerva, A.; Ristimäki, A.; Hakkarainen, T.; Särkioja, M.; Kangasniemi, L.; Raki, M.; Laakkonen, P.; Goodison, S.; Hemminki, A. A heparan sulfate-targeted conditionally replicative adenovirus, Ad5.pk7-Δ24, for the treatment of advanced breast cancer. Gene Ther. 2007, 14, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Hirvinen, M.; Garofalo, M.; Romaniuk, D.; Kuryk, L.; Sarvela, T.; Vitale, A.; Antopolsky, M.; Magarkar, A.; Viitala, T.; et al. Oncolytic adenoviruses coated with MHC-I tumor epitopes increase the antitumor immunity and efficacy against melanoma. Oncoimmunology 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Konz, J.O.; Livingood, R.C.; Bett, A.J.; Goerke, A.R.; Laska, M.E.; Sagar, S.L. Serotype Specificity of Adenovirus Purification Using Anion-Exchange Chromatography. Hum. Gene Ther. 2005, 16, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef]
- Patterson, S.; Russell, W.C. Ultrastructural and Immunofluorescence Studies of Early Events in Adenovirus-HeLa Cell Interactions. J. Gen. Virol. 1983, 64, 1091–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, E.; Stupack, D.; Klemke, R.; Cheresh, D.A.; Nemerow, G.R. Adenovirus Endocytosis via αv Integrins Requires Phosphoinositide-3-OH Kinase. J. Virol. 1998, 72, 2055–2061. [Google Scholar] [PubMed]
- Li, E.; Stupack, D.; Bokoch, G.M.; Nemerow, G.R. Adenovirus Endocytosis Requires Actin Cytoskeleton Reorganization Mediated by Rho Family GTPases. J. Virol. 1998, 72, 8806–8812. [Google Scholar] [PubMed]
- Bradshaw, A.C.; Coughlan, L.; Miller, A.M.; Alba, R.; van Rooijen, N.; Nicklin, S.A.; Baker, A.H. Biodistribution and inflammatory profiles of novel penton and hexon double-mutant serotype 5 adenoviruses. J. Control. Release 2012, 164, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Mathias, P.; Wickham, T.; Moore, M.; Nemerow, G. Multiple adenovirus serotypes use alpha v integrins for infection. J. Virol. 1994, 68, 6811–6814. [Google Scholar] [PubMed]
- Li, E.; Brown, S.L.; Stupack, D.G.; Puente, X.S.; Cheresh, D.A.; Nemerow, G.R. Integrin αvβ1 Is an Adenovirus Coreceptor. J. Virol. 2001, 75, 5405–5409. [Google Scholar] [CrossRef] [PubMed]
- Davison, E.; Kirby, I.; Whitehouse, J.; Hart, I.; Marshall, J.F.; Santis, G. Adenovirus type 5 uptake by lung adenocarcinoma cells in culture correlates with Ad5 fibre binding is mediated by alpha(v)beta1 integrin and can be modulated by changes in beta1 integrin function. J. Gene Med. 2001, 3, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kamata, T.; Takada, Y.; Ruggeri, Z.M.; Nemerow, G.R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 1996, 70, 4502–4508. [Google Scholar] [PubMed]
- Davison, E.; Diaz, R.M.; Hart, I.R.; Santis, G.; Marshall, J.F. Integrin alpha5beta1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J. Virol. 1997, 71, 6204–6207. [Google Scholar] [PubMed]
- Lindert, S.; Silvestry, M.; Mullen, T.-M.; Nemerow, G.R.; Stewart, P.L. Cryo-Electron Microscopy Structure of an Adenovirus-Integrin Complex Indicates Conformational Changes in both Penton Base and Integrin. J. Virol. 2009, 83, 11491–11501. [Google Scholar] [CrossRef] [PubMed]
- Meier, O.; Greber, U.F. Adenovirus endocytosis. J. Gene Med. 2004, 6, S152–S163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Ghosh, R.N.; Maxfield, F.R. Endocytosis. Physiol. Rev. 1997, 77, 759–803. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guan, T.; Cheresh, D.A.; Nemerow, G.R. Regulation of Adenovirus Membrane Penetration by the Cytoplasmic Tail of Integrin β5. J. Virol. 2000, 74, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.Y.; Mathias, P.; Nemerow, G.R.; Stewart, P.L. Structure of Adenovirus Complexed with Its Internalization Receptor, αvβ5 Integrin. Available online: http://jvi.asm.org (accessed on 19 April 2018).
- Acharya, R.; Fry, E.; Stuart, D.; Fox, G.; Rowlands, D.; Brown, F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 Å resolution. Nature 1989, 337, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.; Sharma, A.; Ghazaleh, R.A.; Blakemore, W.E.; Ellard, F.M.; Simmons, D.L.; Newman, J.W.; Stuart, D.I.; King, A.M. Arginine-glycine-aspartic acid-specific binding by foot-and-mouth disease viruses to the purified integrin alpha(v)beta3 in vitro. J. Virol. 1997, 71, 8357–8361. [Google Scholar] [PubMed]
- Lyle, C.; McCormick, F. Integrin αvβ5 is a primary receptor for adenovirus in CAR-negative cells. Virol. J. 2010, 7, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, E.; Pache, L.; Seggern, D.J.V.; Mullen, T.-M.; Mikyas, Y.; Stewart, P.L.; Nemerow, G.R. Flexibility of the Adenovirus Fiber Is Required for Efficient Receptor Interaction. J. Virol. 2003, 77, 7225–7235. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Dong, X.; Wu, X.; Wen, B.; Ji, G.; Cheng, L.; Liu, H. Conserved fiber-penton base interaction revealed by nearly atomic resolution cryo-electron microscopy of the structure of adenovirus provides insight into receptor interaction. J. Virol. 2012, 86, 12322–12329. [Google Scholar] [CrossRef] [PubMed]
- Lieber, A.; He, C.Y.; Meuse, L.; Schowalter, D.; Kirillova, I.; Winther, B.; Kay, M.A. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 1997, 71, 8798–8807. [Google Scholar] [PubMed]
- Zaiss, A.-K.; Liu, Q.; Bowen, G.P.; Wong, N.C.W.; Bartlett, J.S.; Muruve, D.A. Differential Activation of Innate Immune Responses by Adenovirus and Adeno-Associated Virus Vectors. J. Virol. 2002, 76, 4580–4590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolo, N.C.D.; Baldwin, L.K.; Irons, E.E.; Papayannopoulou, T.; Tomlinson, S.; Shayakhmetov, D.M. IL-1α and Complement Cooperate in Triggering Local Neutrophilic Inflammation in Response to Adenovirus and Eliminating Virus-Containing Cells. PLoS Pathog. 2014, 10, e1004035. [Google Scholar] [CrossRef] [PubMed]
- Maler, M.D.; Nielsen, P.J.; Stichling, N.; Cohen, I.; Ruzsics, Z.; Wood, C.; Engelhard, P.; Suomalainen, M.; Gyory, I.; Huber, M.; et al. Key Role of the Scavenger Receptor MARCO in Mediating Adenovirus Infection and Subsequent Innate Responses of Macrophages. mBio 2017, 8, e00670-17. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Stein, S.; Falck-Pedersen, E. Adenovirus Detection by the cGAS/STING/TBK1 DNA Sensing Cascade. J. Virol. 2014, 88, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Thorne, S.H. Adding STING to the Tale of Oncolytic Virotherapy. Trends Cancer 2016, 2, 67–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Queiroz, N.M.G.P.; Xia, T.; Barber, G.N. Defective STING signaling in ovarian cancer cells favor oncolytic virus action. J. Immunol. 2017, 198, 130.28. [Google Scholar]
- Konno, H.; Yamauchi, S.; Berglund, A.; Putney, R.M.; Mulé, J.J.; Barber, G.N. Suppression of STING signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene 2018, 37, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Myhre, S.; Henning, P.; Granio, O.; Tylö, A.S.; Nygren, P.Å.; Olofsson, S.; Boulanger, P.; Lindholm, L.; Hong, S.-S. Decreased immune reactivity towards a knobless, affibody-targeted adenovirus type 5 vector. Gene Ther. 2007, 14, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.R.; Maxfield, L.F.; Lynch, D.M.; Iampietro, M.J.; Borducchi, E.N.; Barouch, D.H. Adenovirus Serotype 5-Specific Neutralizing Antibodies Target Multiple Hexon Hypervariable Regions. J. Virol. 2012, 86, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.R.; Lynch, D.M.; Iampietro, M.J.; Borducchi, E.N.; Barouch, D.H. Adenovirus Serotype 5 Neutralizing Antibodies Target both Hexon and Fiber following Vaccination and Natural Infection. J. Virol. 2012, 86, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, P.; Steipe, B. Intrabody construction and expression III: Engineering hyperstable V(H) domains. Protein Sci. Publ. Protein Soc. 1999, 8, 2245–2250. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.K.; Hong, S.S.; Henning, P.; Boulanger, P.; Lindholm, L. Genetic retargeting of adenovirus vectors: Functionality of targeting ligands and their influence on virus viability. J. Gene Med. 2002, 4, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Young, J.S.; Solomaha, E.; Kanojia, D.; Lesniak, M.S.; Balyasnikova, I.V. A novel single-chain antibody redirects adenovirus to IL13Rα2-expressing brain tumors. Sci. Rep. 2015, 5, 18133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulin, K.L.; Lanthier, R.M.; Smith, A.C.; Christou, C.; Quiroz, M.R.; Powell, K.L.; O’Meara, R.W.; Kothary, R.; Lorimer, I.A.; Parks, R.J. Retargeting of Adenovirus Vectors through Genetic Fusion of a Single-Chain or Single-Domain Antibody to Capsid Protein IX. J. Virol. 2010, 84, 10074–10086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasgow, J.N.; Mikheeva, G.; Krasnykh, V.; Curiel, D.T. A Strategy for Adenovirus Vector Targeting with a Secreted Single Chain Antibody. PLoS ONE 2009, 4, e8355. [Google Scholar] [CrossRef] [PubMed]
- Nettelbeck, D.M.; Miller, D.W.; Jérôme, V.; Zuzarte, M.; Watkins, S.J.; Hawkins, R.E.; Müller, R.; Kontermann, R.E. Targeting of Adenovirus to Endothelial Cells by a Bispecific Single-Chain Diabody Directed against the Adenovirus Fiber Knob Domain and Human Endoglin (CD105). Mol. Ther. 2001, 3, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Kashentseva, E.A.; Seki, T.; Curiel, D.T.; Dmitriev, I.P. Adenovirus targeting to c-erbB-2 oncoprotein by single-chain antibody fused to trimeric form of adenovirus receptor ectodomain. Cancer Res. 2002, 62, 609–616. [Google Scholar] [PubMed]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.-Å.; Löfblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef] [PubMed]
- Frejd, F.Y.; Kim, K.-T. Affibody molecules as engineered protein drugs. Exp. Mol. Med. 2017, 49, e306. [Google Scholar] [CrossRef] [PubMed]
- Henning, P.; Magnusson, M.K.; Gunneriusson, E.; Hong, S.S.; Boulanger, P.; Nygren, P.-A.; Lindholm, L. Genetic modification of adenovirus 5 tropism by a novel class of ligands based on a three-helix bundle scaffold derived from staphylococcal protein A. Hum. Gene Ther. 2002, 13, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.K.; Henning, P.; Myhre, S.; Wikman, M.; Uil, T.G.; Friedman, M.; Andersson, K.M.E.; Hong, S.S.; Hoeben, R.C.; Habib, N.A.; et al. Adenovirus 5 vector genetically re-targeted by an Affibody molecule with specificity for tumor antigen HER2/neu. Cancer Gene Ther. 2007, 14, 468–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belousova, N.; Mikheeva, G.; Gelovani, J.; Krasnykh, V. Modification of Adenovirus Capsid with a Designed Protein Ligand Yields a Gene Vector Targeted to a Major Molecular Marker of Cancer. J. Virol. 2008, 82, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.K.; Kraaij, R.; Leadley, R.M.; De Ridder, C.M.A.; van Weerden, W.M.; Van Schie, K.A.J.; Van der Kroeg, M.; Hoeben, R.C.; Maitland, N.J.; Lindholm, L. A Transductionally Retargeted Adenoviral Vector for Virotherapy of Her2/neu-Expressing Prostate Cancer. Hum. Gene Ther. 2012, 23, 70–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgeois-Daigneault, M.-C.; Roy, D.G.; Aitken, A.S.; Sayes, N.E.; Martin, N.T.; Varette, O.; Falls, T.; St-Germain, L.E.; Pelin, A.; Lichty, B.D.; et al. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 2018, 10, eaao1641. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Gujar, S. Potentiating prostate cancer immunotherapy with oncolytic viruses. Nat. Rev. Urol. 2018, 15, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Hoffner, B.; Gasal, E.; Hong, J.; Carvajal, R.D. Oncolytic immunotherapy: Unlocking the potential of viruses to help target cancer. Cancer Immunol. Immunother. 2017, 66, 1249–1264. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Liu, Z.; Kowalsky, S.; Feist, M.; Kalinski, P.; Lu, B.; Storkus, W.J.; Bartlett, D.L. Oncolytic Immunotherapy: Conceptual Evolution, Current Strategies, and Future Perspectives. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myhre, S.; Henning, P.; Friedman, M.; Ståhl, S.; Lindholm, L.; Magnusson, M.K. Re-targeted adenovirus vectors with dual specificity; binding specificities conferred by two different Affibody molecules in the fiber. Gene Ther. 2009, 16, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Belousova, N.; Mikheeva, G.; Xiong, C.; Stagg, L.J.; Gagea, M.; Fox, P.S.; Bassett, R.L.; Ladbury, J.E.; Braun, M.B.; Stehle, T.; et al. Native and engineered tropism of vectors derived from a rare species D adenovirus serotype 43. Oncotarget 2016, 7, 53414–53429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrij, J.; de Dautzenberg, I.J.C.; Hengel, S.K.; van den Magnusson, M.K.; Uil, T.G.; Cramer, S.J.; Vellinga, J.; Verissimo, C.S.; Lindholm, L.; Koppers-Lalic, D.; et al. A cathepsin-cleavage site between the adenovirus capsid protein IX and a tumor-targeting ligand improves targeted transduction. Gene Ther. 2012, 19, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Vellinga, J.; van den Wollenberg, D.J.M.; van der Heijdt, S.; Rabelink, M.J.W.E.; Hoeben, R.C. The Coiled-Coil Domain of the Adenovirus Type 5 Protein IX Is Dispensable for Capsid Incorporation and Thermostability. J. Virol. 2005, 79, 3206–3210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meulenbroek, R.A.; Sargent, K.L.; Lunde, J.; Jasmin, B.J.; Parks, R.J. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion—Generation of fluorescent virus through the incorporation of pIX-GFP. Mol. Ther. 2004, 9, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.K.; Parrott, M.B.; Barry, M.A. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol. Ther. 2004, 9, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Vellinga, J.; Rabelink, M.J.W.E.; Cramer, S.J.; van den Wollenberg, D.J.M.; der Meulen, H.V.; Leppard, K.N.; Fallaux, F.J.; Hoeben, R.C. Spacers Increase the Accessibility of Peptide Ligands Linked to the Carboxyl Terminus of Adenovirus Minor Capsid Protein IX. J. Virol. 2004, 78, 3470–3479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greber, U.F.; Willetts, M.; Webster, P.; Helenius, A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell 1993, 75, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Campos, S.K.; Barry, M.A. Comparison of adenovirus fiber, protein IX, and hexon capsomeres as scaffolds for vector purification and cell targeting. Virology 2006, 349, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binz, H.K.; Amstutz, P.; Kohl, A.; Stumpp, M.T.; Briand, C.; Forrer, P.; Grütter, M.G.; Plückthun, A. High-affinity binders selected from designed ankyrin repeat protein libraries. Nat. Biotechnol. 2004, 22, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Forrer, P.; Plückthun, A. Efficient Selection of DARPins with Sub-nanomolar Affinities using SRP Phage Display. J. Mol. Biol. 2008, 382, 1211–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plückthun, A. Designed Ankyrin Repeat Proteins (DARPins): Binding Proteins for Research, Diagnostics, and Therapy. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Guillard, S.; Kolasinska-Zwierz, P.; Debreczeni, J.; Breed, J.; Zhang, J.; Bery, N.; Marwood, R.; Tart, J.; Overman, R.; Stocki, P.; et al. Structural and functional characterization of a DARPin which inhibits Ras nucleotide exchange. Nat. Commun. 2017, 8, 16111. [Google Scholar] [CrossRef] [PubMed]
- Dreier, B.; Mikheeva, G.; Belousova, N.; Parizek, P.; Boczek, E.; Jelesarov, I.; Forrer, P.; Plückthun, A.; Krasnykh, V. Her2-specific Multivalent Adapters Confer Designed Tropism to Adenovirus for Gene Targeting. J. Mol. Biol. 2011, 405, 410–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreier, B.; Honegger, A.; Hess, C.; Nagy-Davidescu, G.; Mittl, P.R.E.; Grütter, M.G.; Belousova, N.; Mikheeva, G.; Krasnykh, V.; Plückthun, A. Development of a generic adenovirus delivery system based on structure-guided design of bispecific trimeric DARPin adapters. Proc. Natl. Acad. Sci. USA 2013, 110, E869–E877. [Google Scholar] [CrossRef] [PubMed]
- Sebestyen, Z.; de Vrij, J.; Magnusson, M.; Debets, R.; Willemsen, R. An Oncolytic Adenovirus Redirected with a Tumor-Specific T-Cell Receptor. Cancer Res. 2007, 67, 11309–11316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meek, D.W.; Marcar, L. MAGE-A antigens as targets in tumour therapy. Cancer Lett. 2012, 324, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Zajac, P.; Schultz-Thater, E.; Tornillo, L.; Sadowski, C.; Trella, E.; Mengus, C.; Iezzi, G.; Spagnoli, G.C. MAGE-A Antigens and Cancer Immunotherapy. Front. Med. 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Liu, C.; Zhang, Y. T-cell receptor-engineered T cells for cancer treatment: Current status and future directions. Protein Cell. 2018, 9, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Belousova, N.; Krendelchtchikova, V.; Curiel, D.T.; Krasnykh, V. Modulation of Adenovirus Vector Tropism via Incorporation of Polypeptide Ligands into the Fiber Protein. J. Virol. 2002, 76, 8621–8631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uusi-Kerttula, H.; Legut, M.; Davies, J.; Jones, R.; Hudson, E.; Hanna, L.; Stanton, R.J.; Chester, J.D.; Parker, A.L. Incorporation of Peptides Targeting EGFR and FGFR1 into the Adenoviral Fiber Knob Domain and Their Evaluation as Targeted Cancer Therapies. Hum. Gene Ther. 2015, 26, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.K.; Hong, S.S.; Boulanger, P.; Lindholm, L. Genetic Retargeting of Adenovirus: Novel Strategy Employing “Deknobbing” of the Fiber. J. Virol. 2001, 75, 7280–7289. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Yoshida, K.; Nishimoto, T.; Hatanaka, K.; Ohnami, S.; Asaka, M.; Douglas, J.T.; Curiel, D.T.; Yoshida, T.; Aoki, K. Direct selection of targeted adenovirus vectors by random peptide display on the fiber knob. Gene Ther. 2007, 14, 1448–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimoto, T.; Yoshida, K.; Miura, Y.; Kobayashi, A.; Hara, H.; Ohnami, S.; Kurisu, K.; Yoshida, T.; Aoki, K. Oncolytic virus therapy for pancreatic cancer using the adenovirus library displaying random peptides on the fiber knob. Gene Ther. 2009, 16, 669–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böckmann, M.; Drosten, M.; Pützer, B.M. Discovery of targeting peptides for selective therapy of medullary thyroid carcinoma. J. Gene Med. 2005, 7, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Anderson, B.; Mao, Q.; Davidson, B.L. Recombinant Human Adenovirus: Targeting to the Human Transferrin Receptor Improves Gene Transfer to Brain Microcapillary Endothelium. J. Virol. 2000, 74, 11359–11366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatty, M.S.; Curiel, D.T. Adenovirus Strategies for Tissue-Specific Targeting. Adv. Cancer Res. 2012, 115, 39–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandyopadhyay, A.; Raghavan, S. Defining the Role of Integrin αvβ6 in Cancer. Curr. Drug Targets 2009, 10, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Patsenker, E.; Wilkens, L.; Banz, V.; Osterreicher, C.H.; Weimann, R.; Eisele, S.; Keogh, A.; Stroka, D.; Zimmermann, A.; Stickel, F. The alphavbeta6 integrin is a highly specific immunohistochemical marker for cholangiocarcinoma. J. Hepatol. 2010, 52, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, A.; Wang, Q.; Dong, X.; Ilca, S.L.; Ondiviela, M.; Zihe, R.; Seago, J.; Charleston, B.; Fry, E.E.; Abrescia, N.G.A.; et al. Rules of engagement between αvβ6 integrin and foot-and-mouth disease virus. Nat. Commun. 2017, 8, 15408. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.; Abu-Ghazaleh, R.; Blakemore, W.; Curry, S.; Jackson, T.; King, A.; Lea, S.; Lewis, R.; Newman, J.; Parry, N.; et al. Structure of a major immunogenic site on foot-and-mouth disease virus. Nature 1993, 362, 566–568. [Google Scholar] [CrossRef] [PubMed]
- DiCara, D.; Rapisarda, C.; Sutcliffe, J.L.; Violette, S.M.; Weinreb, P.H.; Hart, I.R.; Howard, M.J.; Marshall, J.F. Structure-Function Analysis of Arg-Gly-Asp Helix Motifs in αvβ6 Integrin Ligands. J. Biol. Chem. 2007, 282, 9657–9665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausner, S.H.; DiCara, D.; Marik, J.; Marshall, J.F.; Sutcliffe, J.L. Use of a Peptide Derived from Foot-and-Mouth Disease Virus for the Noninvasive Imaging of Human Cancer: Generation and Evaluation of 4-[18F]Fluorobenzoyl A20FMDV2 for In vivo Imaging of Integrin αvβ6 Expression with Positron Emission Tomography. Cancer Res. 2007, 67, 7833–7840. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.; Vallath, S.; Saha, A.; Flak, M.; McNeish, I.A.; Vassaux, G.; Marshall, J.F.; Hart, I.R.; Thomas, G.J. In Vivo Retargeting of Adenovirus Type 5 to αvβ6 Integrin Results in Reduced Hepatotoxicity and Improved Tumor Uptake following Systemic Delivery. J. Virol. 2009, 83, 6416–6428. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.; Uusi-Kerttula, H.; Ma, J.; Degg, B.P.; Parker, A.L.; Baker, A.H. Retargeting Adenovirus Serotype 48 Fiber Knob Domain by Peptide Incorporation. Hum. Gene Ther. 2014, 25, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Sela-Culang, I.; Kunik, V.; Ofran, Y. The Structural Basis of Antibody-Antigen Recognition. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Stumpp, M.T.; Amstutz, P. DARPins: A true alternative to antibodies. Curr. Opin. Drug Discov. Dev. 2007, 10, 153–159. [Google Scholar]
- Huang, P.-S.; Boyken, S.E.; Baker, D. The coming of age of de novo protein design. Nature 2016, 537, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.H.; McVey, J.H.; Waddington, S.N.; Di Paolo, N.C.; Shayakhmetov, D.M. The Influence of Blood on In Vivo Adenovirus Bio-distribution and Transduction. Mol. Ther. 2007, 15, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Blair, G.E.; Dixon, S.C.; Griffiths, S.A.; Blair Zajdel, M.E. Restricted replication of human adenovirus type 5 in mouse cell lines. Virus Res. 1989, 14, 339–346. [Google Scholar] [CrossRef]
- Thomas, M.A.; Spencer, J.F.; Wold, W.S.M. Use of the Syrian hamster as an animal model for oncolytic aladenovirus vectors. Methods Mol. Med. 2007, 130, 169–183. [Google Scholar] [PubMed]
- Hsu, E.C.; Dörig, R.E.; Sarangi, F.; Marcil, A.; Iorio, C.; Richardson, C.D. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J. Virol. 1997, 71, 6144–6154. [Google Scholar] [PubMed]
- Inoue, N.; Ikawa, M.; Nakanishi, T.; Matsumoto, M.; Nomura, M.; Seya, T.; Okabe, M. Disruption of Mouse CD46 Causes an Accelerated Spontaneous Acrosome Reaction in Sperm. Mol. Cell. Biol. 2003, 23, 2614–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]




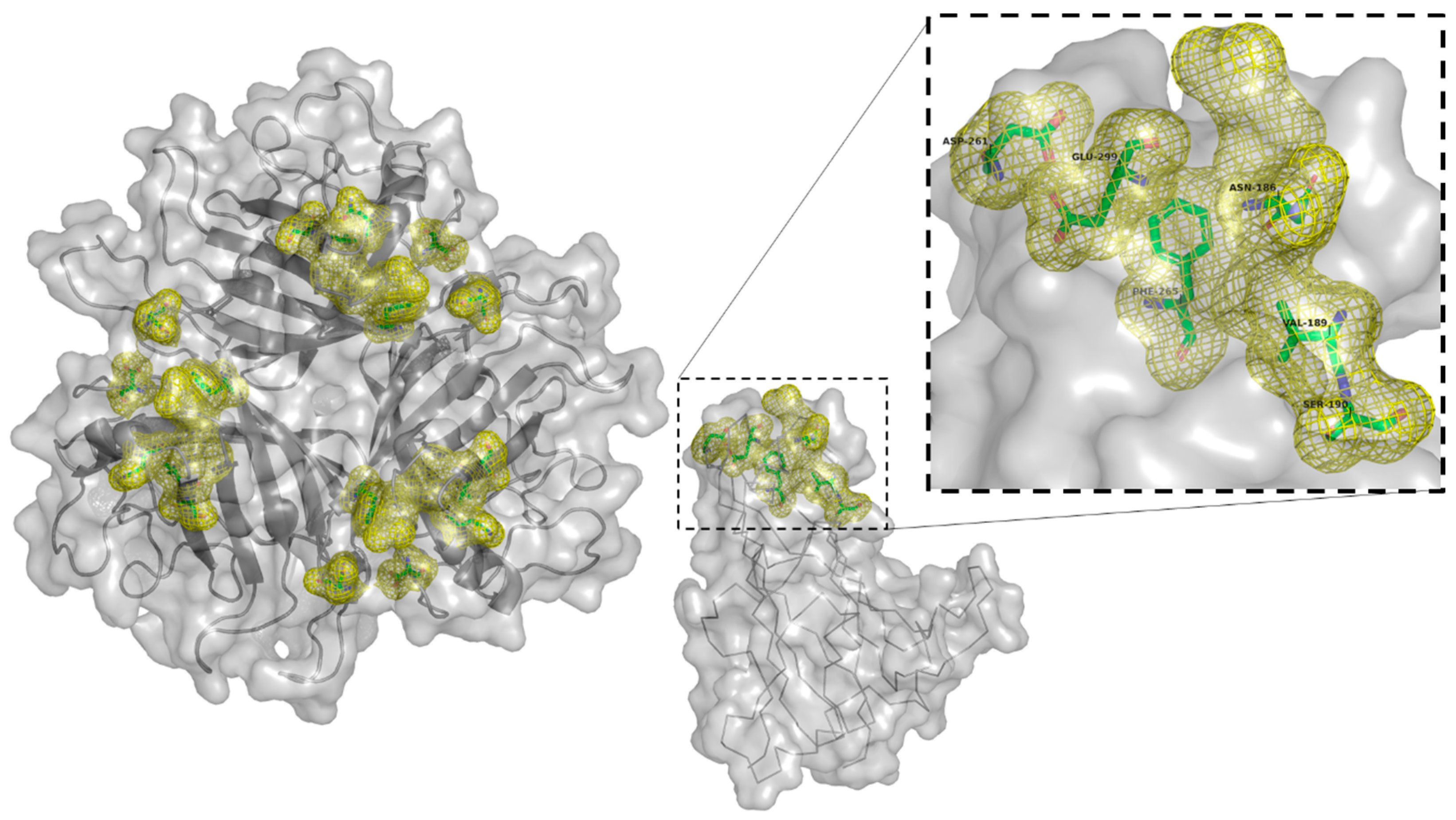


| Receptor | Prototype Viral Receptor | Receptor Binding Residues | Previously Demonstrated Tropism Aablating Mutations | References |
|---|---|---|---|---|
| CAR | Ad5—Fiber Protein knob | A406; S408; P409; R412; Y477; R481; L485; Y491 | KO1 (SP408-409EA); KO2 (ΔVK441-44s); KO3 (R460E); KO4 (ΔGK509-510); KO5 (ΔGT538-539); KO8 (N468T); KO9 (V466H); KO10 (P505A); KO11 (Δ404-581 Whole region) | Jakubczak et al., 2001 [106] |
| ΔTAYT (ΔTAYT489-492) | Roelvink et al., 1999 [107] | |||
| CD46 | Ad35—Fiber Protein knob | F132; N133; T136; R169; M170; S172; N194; E192 | F242; R279; S282 | Wang et al., 2007 [108] |
| DSG2 | Ad3—Fiber Protein Knob | N186; V189; S190; D261; F265; L292; L296; E299 | N186D; V189G; S190P; D261N; F265L; L296R; E299V; ND186-261DN; ΔD261+L296R; NDL186-261-296DNR. | Wang et al., 2013 [109] |
| GD1a/Sialic acid | Ad37—Fiber Protein knob | Y308; Y312; P317; V322; K322 | None reported | Nilsson et al., 2011 [110] |
| Blood Coagulation Factor X | Ad5—Hexon Protein HVR’s | HVR regions 3; and 7 (Individual residues not clearly defined). | Ad5HVR48 (Ad5 with the HVR’s of Ad48) | Waddington et al., 2008 [111] |
| HVR5-BAP (71aa BAP (Biofilm Associated Protein) peptide insert) | Kalyuzhniy et al., 2008 [112] | |||
| HVR5* (TE268-269AT); HVR7* (ITEL420-422-423-425GNSY); E451Q | Alba et al., 2009 [113] | |||
| HSPG | Ad5—Fiber Protein shaft | KKTK91-94 | S* (KKTK91-94GAGA); KKTK91-94RGDK | Paolo et al., 2007, Kritz et al., 2007 [114,115] |
| Integrin | Ad5—Penton Protein | R340, G341, D342 | RGE (D342E) | Bai et al., 1993 [116] |
| EGD (R340E) | Henning et al., 2005 [117] | |||
| MARCO | Ad5—Hexon Protein | HVR1; implied but not conclusively determined | None reported | Stichling et al., [118] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baker, A.T.; Aguirre-Hernández, C.; Halldén, G.; Parker, A.L. Designer Oncolytic Adenovirus: Coming of Age. Cancers 2018, 10, 201. https://doi.org/10.3390/cancers10060201
Baker AT, Aguirre-Hernández C, Halldén G, Parker AL. Designer Oncolytic Adenovirus: Coming of Age. Cancers. 2018; 10(6):201. https://doi.org/10.3390/cancers10060201
Chicago/Turabian StyleBaker, Alexander T., Carmen Aguirre-Hernández, Gunnel Halldén, and Alan L. Parker. 2018. "Designer Oncolytic Adenovirus: Coming of Age" Cancers 10, no. 6: 201. https://doi.org/10.3390/cancers10060201
APA StyleBaker, A. T., Aguirre-Hernández, C., Halldén, G., & Parker, A. L. (2018). Designer Oncolytic Adenovirus: Coming of Age. Cancers, 10(6), 201. https://doi.org/10.3390/cancers10060201








