Penetrance of the TP53 R337H Mutation and Pediatric Adrenocortical Carcinoma Incidence Associated with Environmental Influences in a 12-Year Observational Cohort in Southern Brazil
Abstract
:1. Introduction
2. Results
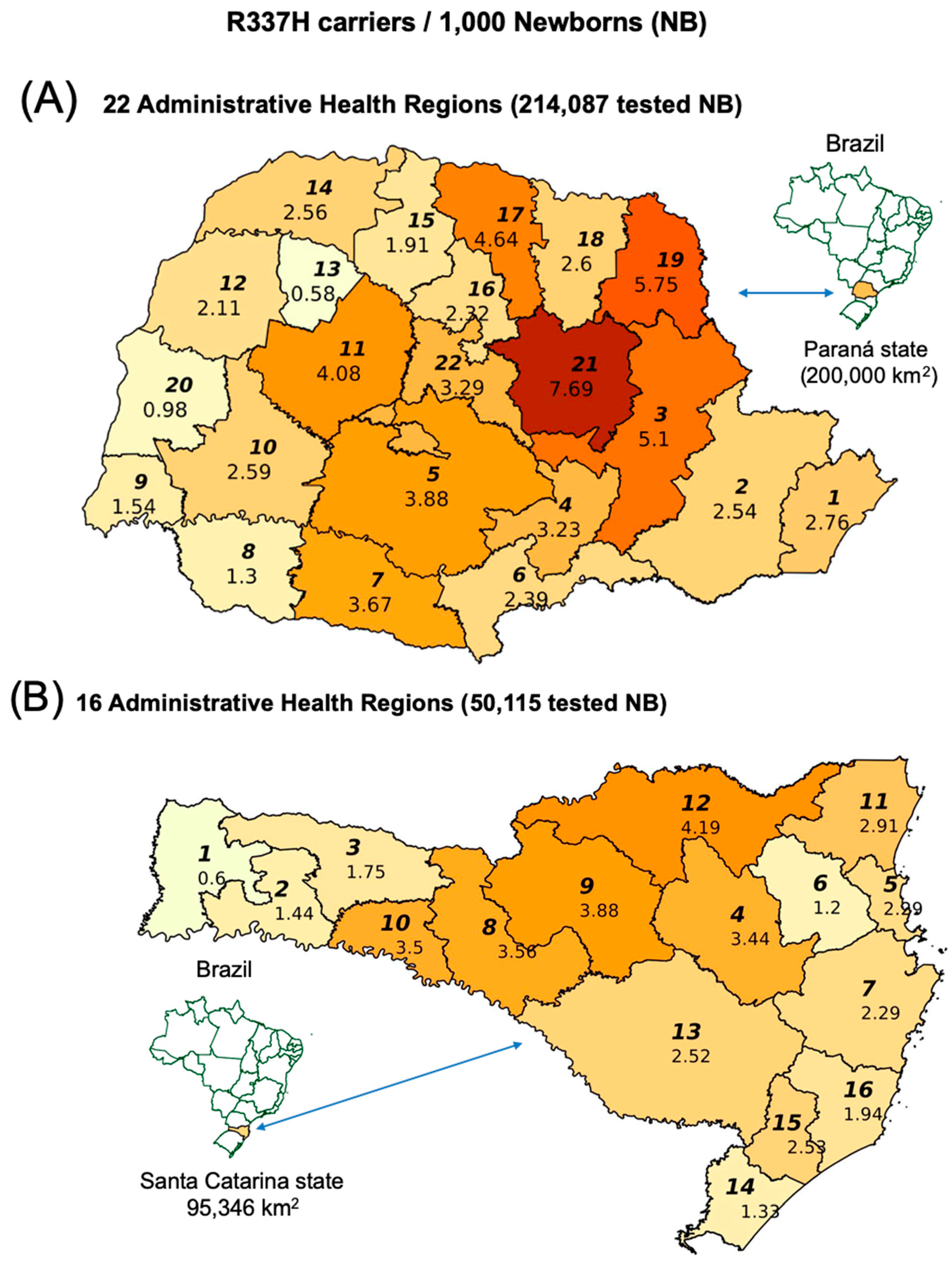
2.1. TP53 R337H Frequency in the Southern Brazilian Population
2.2. TP53 R337H Frequency in Adrenocortical Tumors from Santa Catarina
2.3. Cumulative Incidence of Pediatric ACT in the PR 2006-C Cohort According to Subregion
2.4. Pediatric ACT Incidence Rates in Paraná and Santa Catarina
2.5. Linear Regression (LR) for R337H Frequencies: General Population versus ACT Patients
2.6. ACT Surveillance in Paraná: 2006-C and 2016-C Cohorts
2.7. Revised Estimate of R337H Penetrance in the Paraná 2006-C Cohort
2.8. Age of Pediatric ACT Onset in the PR 2006-C Cohort According to Generation
3. Discussion
4. Materials and Methods
4.1. Genotyping of Newborns for TP53 R337H and Their Cohorts
4.2. TP53 R337H Assay
4.3. ACT Cases from the Cohorts and Public Registry Used to Calculate Incidence
4.4. LR for R337H Frequencies in Three Brazilian States
4.5. TP53 R337H Penetrance in 2006-C Cohort
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. SEER Cancer Statistics Review, 1975–2007; Altekruse, D.F., Kosary, C.L., Krapcho, M., Neyman, N., Aminou, R., Waldron, W., Ruhl, J., Howlader, N., Tatalovich, Z., Cho, H., et al., Eds.; National Cancer Institute: Bethesda, MD, USA, 2010. [Google Scholar]
- Bernstein, L.; Gurney, J.G. Carcinomas and other malignant epithelial neoplasms. In Cancer Incidence and Survival among Children and Adolescents: United States Seer Program 1975–1995; Ries, L.A.G., Smith, M.A., Gurney, J.G., Liney, M., Tamra, T., Young, J.L., Bunin, G.R., Eds.; National Cancer Institute, SEER Program: Bethesda, MD, USA, 1999; pp. 139–147. [Google Scholar]
- Custódio, G.; Parise, G.A.; Kiesel Filho, N.; Komechen, H.; Sabbaga, C.C.; Rosati, R.; Grisa, L.; Parise, I.Z.; Pianovski, M.A.; Fiori, C.M.; et al. Impact of neonatal screening and surveillance for the TP53 R337H mutation on early detection of childhood adrenocortical tumors. J. Clin. Oncol. 2013, 31, 2619–2626. [Google Scholar] [CrossRef]
- IARC Scientific Publication. International Incidence of Childhood Cancer; Parkin, D.M., Kramarova, E., Draper, G.J., Masuyer, E., Michaelis, J., Neglia, J., Qureshi, S., Stiller, C.A., Eds.; IARC Scientific Publication: Lyon, France, 1998; Volume 2. [Google Scholar]
- Stiller, C.A. International variations in the incidence of childhood carcinomas. Cancer Epidemiol. Biomark. Prev. 1994, 3, 305–310. [Google Scholar]
- Varley, J.M.; McGown, G.; Thorncroft, M.; James, L.A.; Margison, G.P.; Forster, G.; Evans, D.G.; Harris, M.; Kelsey, A.M.; Birch, J.M. Are there low-penetrance TP53 Alleles? evidence from childhood adrenocortical tumors. Am. J. Hum. Genet. 1999, 65, 995–1006. [Google Scholar] [CrossRef]
- Gonzalez, K.D.; Noltner, K.A.; Buzin, C.H.; Gu, D.; Wen-Fong, C.Y.; Nguyen, V.Q.; Han, J.H.; Lowstuter, K.; Longmate, J.; Sommer, S.S.; et al. Beyond Li Fraumeni Syndrome: Clinical characteristics of families with p53 germline mutations. J. Clin. Oncol. 2009, 27, 1250–1256. [Google Scholar] [CrossRef]
- Petitjean, A.; Mathe, E.; Kato, S.; Ishioka, C.; Tavtigian, S.V.; Hainaut, P.; Olivier, M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: Lessons from recent developments in the IARC TP53 database. Hum. Mutat. 2007, 28, 622–629. [Google Scholar] [CrossRef]
- Ribeiro, R.C.; Sandrini, F.; Figueiredo, B.; Zambetti, G.P.; Michalkiewicz, E.; Lafferty, A.R.; DeLacerda, L.; Rabin, M.; Cadwell, C.; Sampaio, G.; et al. An inherited p53 mutation that contributes in a tissue-specific manner to pediatric adrenal cortical carcinoma. Proc. Natl. Acad. Sci. USA 2001, 98, 9330–9335. [Google Scholar] [CrossRef]
- Pinto, E.M.; Billerbeck, A.E.; Villares, M.C.; Domenice, S.; Mendonca, B.B.; Latronico, A.C. Founder effect for the highly prevalent R337H mutation of tumor suppressor p53 in Brazilian patients with adrenocortical tumors. Arq. Bras. Endocrinol. Metabol. 2004, 48, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Paskulin, D.D.; Giacomazzi, J.; Achatz, M.I.; Costa, S.; Reis, R.M.; Hainaut, P.; dos Santos, S.E.B.; Ashton-Prolla, P. Ancestry of the Brazilian TP53 c.1010G>A (p.Arg337His, R337H) founder mutation: Clues from haplotyping of short tandem repeats on chromosome 17p. PLoS ONE 2015, 10, e0143262. [Google Scholar] [CrossRef] [PubMed]
- Lalloo, F.; Varley, J.; Ellis, D.; Moran, A.; O’Dair, L.; Pharoah, P.; Evans, D.G.R.; The Early Onset Breast Cancer Study Group. Prediction of pathogenic mutations in patients with early-onset breast cancer by family history. Lancet 2003, 361, 1101–1102. [Google Scholar] [CrossRef]
- Custodio, G.; Komechen, H.; Figueiredo, F.R.; Fachin, N.D.; Pianovski, M.A.; Figueiredo, B.C. Molecular epidemiology of adrenocortical tumors in southern Brazil. Mol. Cell. Endocrinol. 2012, 351, 44–51. [Google Scholar] [CrossRef]
- Michalkiewicz, E.; Sandrini, R.; Figueiredo, B.; Miranda, E.C.; Caran, E.; Oliveira-Filho, A.G.; Marques, R.; Pianovski, M.A.D.; Lacerda, L.; Cristofani, L.M.; et al. Clinical and outcome characteristics of children with adrenocortical tumors: A report from the International Pediatric Adrenocortical Tumor Registry. J. Clin. Oncol. 2004, 22, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Overview on the management of adrenocortical carcinoma (ACC). In Adrenal and Endocrine Tumors in Children; Humphrey, G.B.; Pysher, T.; Holcombe, J.; Gross, M.; Chan, H.; Cushing, B.; D’Angio, G.J.; Schein, P.; Lemerle, J.J.A. (Eds.) Springer: Boston, MA, USA, 1983; pp. 349–358. [Google Scholar]
- Steenman, M.; Westerveld, A.; Mannens, M. Genetics of Beckwith-Wiedemann syndrome-associated tumors: Common genetic pathways. Genes Chromosomes Cancer 2000, 28, 1–13. [Google Scholar] [CrossRef]
- Pinto, E.M.; Rodriguez-Galindo, C.; Pounds, S.B.; Wang, L.; Clay, M.R.; Neale, G.; Garfinkle, E.A.; Lam, C.G.; Levy, C.F.; Pappo, A.S.; et al. Identification of clinical and biologic correlates associated with outcome in children with adrenocortical tumors without germline TP53 mutations: A St Jude Adrenocortical Tumor Registry and Children’s Oncology Group Study. J. Clin. Oncol. 2017, 35, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, H.C.; Melanda, V.S.; Gerber, V.K.Q.; Licht, O.A.B.; Ibanez, M.V.C.; Aguiar Junior, T.R.; Mello, R.G.; Komechen, H.; Andrade, D.P.; Picharski, G.L.; et al. Spatial trends in congenital malformations and stream water chemistry in Southern Brazil. Sci. Total Environ. 2019, 650 Pt 1, 1278–1291. [Google Scholar] [CrossRef]
- Figueiredo, B.C.; Sandrini, R.; Zambetti, G.P.; Pereira, R.M.; Cheng, C.; Liu, W.; Lacerda, L.; Pianovski, M.A.; Michalkiewicz, E.; Jenkins, J.; et al. Penetrance of adrenocortical tumours associated with the germline TP53 R337H mutation. J. Med. Genet. 2006, 43, 91–96. [Google Scholar] [CrossRef]
- Latronico, A.C.; Pinto, E.M.; Domenice, S.; Fragoso, M.C.; Martin, R.M.; Zerbini, M.C.; Lucon, A.M.; Mendonca, B.B. An inherited mutation outside the highly conserved DNA-binding domain of the p53 tumor suppressor protein in children and adults with sporadic adrenocortical tumors. J. Clin. Endocrinol. Metab. 2001, 86, 4970–4973. [Google Scholar] [CrossRef]
- Villani, A.; Shore, A.; Wasserman, J.D.; Stephens, D.; Kim, R.H.; Druker, H.; Gallinger, B.; Naumer, A.; Kohlmann, W.; Novokmet, A.; et al. Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 year follow-up of a prospective observational study. Lancet Oncol. 2016, 17, 1295–1305. [Google Scholar] [CrossRef]
- Pinto, E.M.; Chen, X.; Easton, J.; Finkelstein, D.; Liu, Z.; Pounds, S.; Rodriguez-Galindo, C.; Lund, T.C.; Mardis, E.R.; Wilson, R.K.; et al. Genomic landscape of paediatric adrenocortical tumours. Nat. Commun. 2015, 6, 6302. [Google Scholar] [CrossRef]
- Brasil, Ministério da Saúde. DataSus, SIHSUS, SIASUS. Available online: http://www2.datasus.gov.br/DATASUS/index.php?area=0901 (accessed on 4 August 2019).
- Agência Nacional de Saúde Sumplementar. Available online: https://www.ans.gov.br/perfil-do-setor/dados-gerais (accessed on 9 May 2019).
- Pereira, R.M.; Michalkiewicz, E.; Sandrini, F.; Figueiredo, B.C.; Pianovski, M.; Franca, S.N.; Boguszewski, M.; Costa, O.; Cat, I.; Lacerda Filho, L.D.; et al. Childhood adrenocortical tumors. Arq. Bras. Endocrinol. Metabol. 2004, 48, 651–658. [Google Scholar] [CrossRef]
- Sandrini, F.; Villani, D.P.; Tucci, S.; Moreira, A.C.; de Castro, M.; Elias, L.L. Inheritance of R337H p53 gene mutation in children with sporadic adrenocortical tumor. Horm. Metab. Res. 2005, 37, 231–235. [Google Scholar] [CrossRef]
- Seidinger, A.L.; Mastellaro, M.J.; Paschoal Fortes, F.; Godoy Assumpcao, J.; Aparecida Cardinalli, I.; Aparecida Ganazza, M.; Ribeiro, R.C.; Brandalise, S.R.; dos Santos Aguiar, S.; Yunes, J.A. Association of the highly prevalent TP53 R337H mutation with pediatric choroid plexus carcinoma and osteosarcoma in Southeast Brazil. Cancer 2011, 117, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Caminha, I.P. Prevalence of Germline TP53 p.R337H Mutation at Metropolitan Area of Campinas and Surrounding Cities; Universidade Estadual de Campinas: Campinas, Brazil, 2015. [Google Scholar]
- Wu, C.C.; Shete, S.; Amos, C.I.; Strong, L.C. Joint effects of germ-line p53 mutation and sex on cancer risk in Li-Fraumeni syndrome. Cancer Res. 2006, 66, 8287–8292. [Google Scholar] [CrossRef] [PubMed]
- DiGiammarino, E.L.; Lee, A.S.; Cadwell, C.; Zhang, W.; Bothner, B.; Ribeiro, R.C.; Zambetti, G.; Kriwacki, R.W. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat. Struct. Biol. 2002, 9, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Martz, F.; Straw, J.A. The in vitro metabolism of 1-(o-chlorophenyl)-1-(p-chlorophenyl)-2,2-dichloroethane (o,p′-DDD) by dog adrenal mitochondria and metabolite covalent binding to mitochondrial macromolecules: A possible mechanism for the adrenocorticolytic effect. Drug Metab. Dispos. 1977, 5, 482–486. [Google Scholar] [PubMed]
- Berruti, A.; Terzolo, M.; Pia, A.; Angeli, A.; Dogliotti, L. Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer. Cancer 1998, 83, 2194–2200. [Google Scholar] [CrossRef]
- Zancanella, P.; Pianovski, M.A.; Oliveira, B.H.; Ferman, S.; Piovezan, G.C.; Lichtvan, L.L.; Voss, S.Z.; Stinghen, S.T.; Callefe, L.G.; Parise, G.A.; et al. Mitotane associated with cisplatin, etoposide, and doxorubicin in advanced childhood adrenocortical carcinoma: Mitotane monitoring and tumor regression. J. Pediatr. Hematol. Oncol. 2006, 28, 513–524. [Google Scholar] [CrossRef]
- Cueto, C.; Brown, J.H.; Richardson, A.P., Jr. Biological studies on an adrenocorticolytic agent and the isolation of the active components. Endocrinology 1958, 62, 334–339. [Google Scholar] [CrossRef]
- Cai, W.; Benitez, R.; Counsell, R.E.; Djanegara, T.; Schteingart, D.E.; Sinsheimer, J.E.; Wotring, L.L. Bovine adrenal cortex transformations of mitotane [1-(2-chlorophenyl)-1-(4-chlorophenyl)-2,2-dichloroethane; o,p′-DDD] and its p,p′- and m,p′-isomers. Biochem. Pharmacol. 1995, 49, 1483–1489. [Google Scholar] [CrossRef]
- Peterson, J.E.; Robison, W.H. Metabolic products of P,P′-DDT in the rat. Toxicol. Appl. Pharmacol. 1964, 6, 321–327. [Google Scholar] [CrossRef]
- Matsushima, A. A novel action of endocrine-disrupting chemicals on wildlife; DDT and its derivatives have remained in the environment. Int. J. Mol. Sci. 2018, 19, 1377. [Google Scholar] [CrossRef]
- Wasserman, J.D.; Zambetti, G.P.; Malkin, D. Towards an understanding of the role of p53 in adrenocortical carcinogenesis. Mol. Cell. Endocrinol. 2012, 351, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Ponder, B. Cancer. Gene losses in human tumours. Nature 1988, 335, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Watson, I.R.; Takahashi, K.; Futreal, P.A.; Chin, L. Emerging patterns of somatic mutations in cancer. Nat. Rev. Genet. 2013, 14, 703–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Custodio, G.; Taques, G.R.; Figueiredo, B.C.; Gugelmin, E.S.; Oliveira Figueiredo, M.M.; Watanabe, F.; Pontarolo, R.; Lalli, E.; Torres, L.F.B. Increased incidence of choroid plexus carcinoma due to the germline TP53 R337H mutation in southern Brazil. PLoS ONE 2011, 6, e18015. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2017. Available online: https://www.R-project.org/ (accessed on 15 June 2018).
- Kalbfleisch, J.; Prentice, R. The Statistical Analysis of Failure Time Data, 2nd ed.; Wiley: Hoboken, NJ, USA, 2002; ISBN 978-047-0471363576. [Google Scholar]
- Sahai, H.; Khurshid, A. Statistics in Epidemiology: Methods, Techniques, and Applications; CRC Press: Boca Raton, FL, USA, 1995; p. 321. [Google Scholar]


| ACT and Carriers | Subregion | |||||||
|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | Total | |||||
| ACT cases <10 years of age | 7 | 34 | 36 | 77 | ||||
| Carriers without ACT | 730 | 1819 | 1536 | 4085 | ||||
| Total | 737 | 1853 | 1572 | 4162 | ||||
| ACT | ||||||||
| Subregion | N | C.I. | Subregion | N | C.I. | RR | p Value | |
| (/1000) | (/1000) | X2 | Fischer | |||||
| C2 | 34 | 18.3 | C1 | 7 | 9.5 | 1.932 [0.86; 4.338] | 0.146 | 0.118 |
| C3 | 36 | 22.9 | C2 | 34 | 18.3 | 1.248 [0.785; 1.985] | 0.414 | 0.397 |
| C3 | 36 | 22.9 | C1 | 7 | 9.5 | 2.411 [1.078; 5.392] | 0.04 | 0.031 |
| A | ||||
| Subregion | ACT Cases <10 Years (2007–2018) | Persons <10 Years (2007–2018) | Incidence (<10 Years) /Million/Year | 95% CI |
| C0 | 9 | 956,764 | 9.41 | 4.30; 17.86 |
| C3 | 46 | 5,643,503 | 8.15 | 5.97; 10.87 |
| C2 | 50 | 7,552,735 | 6.62 | 4.91; 8.73 |
| C1 | 13 | 4,328,094 | 3.0 | 1.60; 5.14 |
| B | ||||
| Comparison | Incidence Rate Ratio (IRR) | 95% CI | Adjusted p (Bonferroni) | |
| C0/C3 | 1.154 | 0.497; 2.386 | 1.0000 | |
| C0/C2 | 1.421 | 0.614; 2.919 | 1.0000 | |
| C0/C1 | 3.132 | 1.182; 7.919 | 0.0638 | |
| C3/C2 | 1.231 | 0.807; 1.875 | 1.0000 | |
| C3/C1 | 2.714 | 1.442; 5.475 | 0.0053 | |
| C2/C1 | 2.204 | 1.180; 4.423 | 0.0514 | |
| A | |||||||
| Code | Surveillance */Interval ** | Gender/ACC Age (Y) | Clinical Features | ACT Weight (g) | Stage/Treatment | Recurrence | Outcome/Present Age (Years) |
| 1 | Regular/4 months | F/6.2 | Acne | 14 | I/CR | No | Well/8.2 |
| 2 | Irregular/9 months | M/7.3 | Acne + pubic hair | 267 | II/CR + M | No | Well/11.4 |
| 3 | Irregular/22 months | F/5.8 | EnlargedClitoris, acne, pubic hair + H | NA | IV/PR + EDPM | Remained local tumor and metastases | DD/6.4 |
| B | |||||||
| Code | Surveillance */Interval ** | Gender/ACC Age (Y) | Clinical Features | ACT Weight (g) | Stage/Treatment | Recurrence | Outcome/Present Age (Years) |
| 1 | Regular/No PE | F/0.9 | Pubic hair | 21 | I/CR | No | Well/3.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, T.E.J.; Gerber, V.K.Q.; Ibañez, H.C.; Melanda, V.S.; Parise, I.Z.S.; Watanabe, F.M.; Pianovski, M.A.D.; Fiori, C.M.C.M.; Fabro, A.L.M.R.; da Silva, D.B.; et al. Penetrance of the TP53 R337H Mutation and Pediatric Adrenocortical Carcinoma Incidence Associated with Environmental Influences in a 12-Year Observational Cohort in Southern Brazil. Cancers 2019, 11, 1804. https://doi.org/10.3390/cancers11111804
Costa TEJ, Gerber VKQ, Ibañez HC, Melanda VS, Parise IZS, Watanabe FM, Pianovski MAD, Fiori CMCM, Fabro ALMR, da Silva DB, et al. Penetrance of the TP53 R337H Mutation and Pediatric Adrenocortical Carcinoma Incidence Associated with Environmental Influences in a 12-Year Observational Cohort in Southern Brazil. Cancers. 2019; 11(11):1804. https://doi.org/10.3390/cancers11111804
Chicago/Turabian StyleCosta, Tatiana E. J., Viviane K. Q. Gerber, Humberto C. Ibañez, Viviane S. Melanda, Ivy Z. S. Parise, Flora M. Watanabe, Mara A. D. Pianovski, Carmem M. C. M. Fiori, Ana L. M. R. Fabro, Denise B. da Silva, and et al. 2019. "Penetrance of the TP53 R337H Mutation and Pediatric Adrenocortical Carcinoma Incidence Associated with Environmental Influences in a 12-Year Observational Cohort in Southern Brazil" Cancers 11, no. 11: 1804. https://doi.org/10.3390/cancers11111804





