Liver Cancer: Current and Future Trends Using Biomaterials
Abstract
:1. Introduction
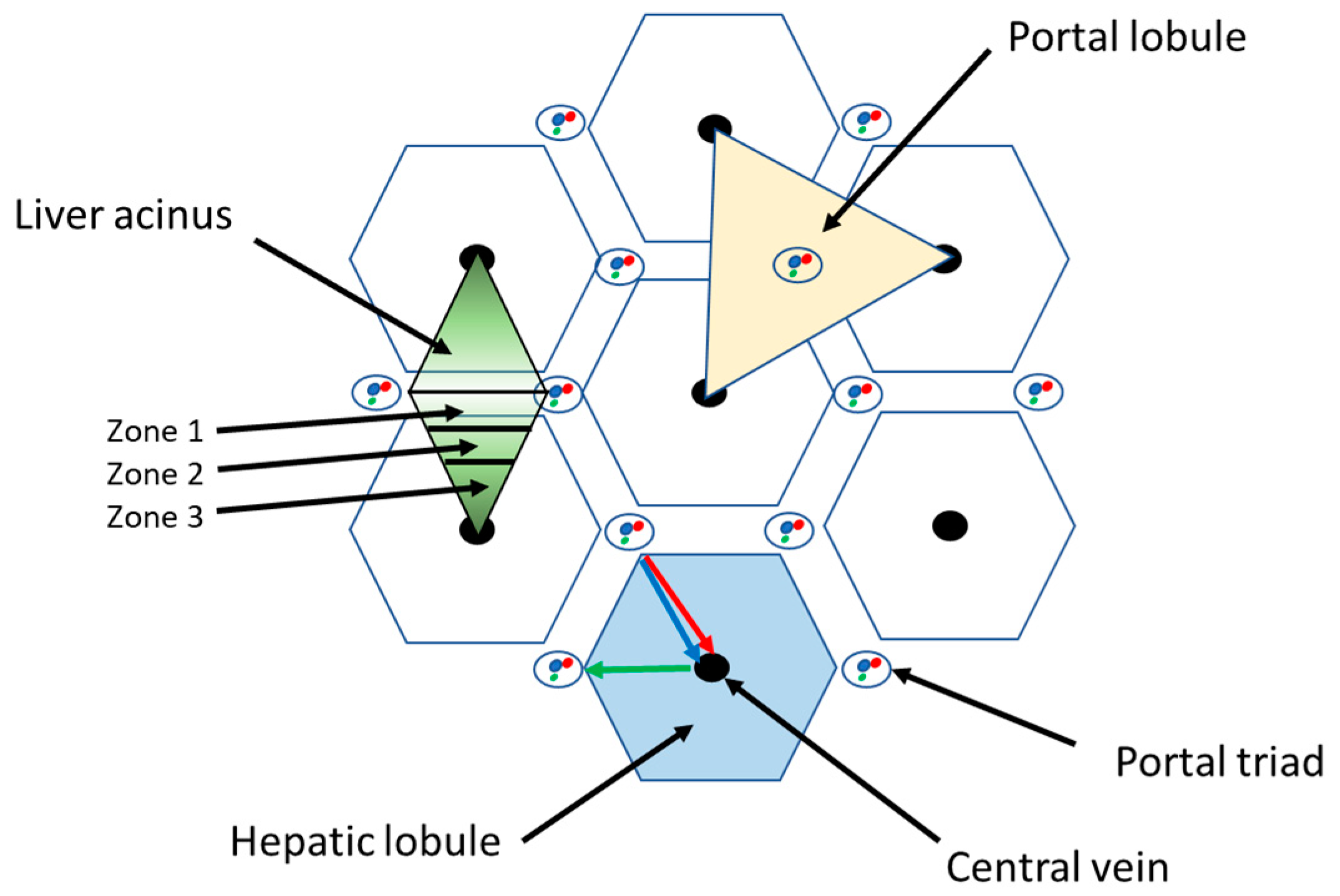
2. Liver Structure and Function
3. Liver Neoplasia
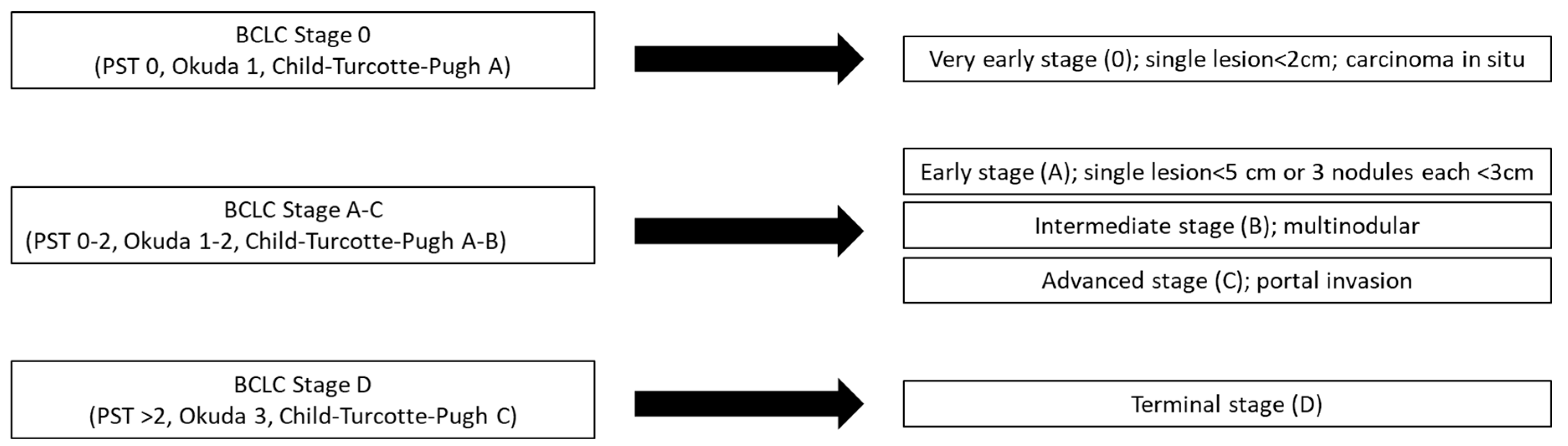
3.1. Diagnosis
3.2. Current Treatment Methods
3.2.1. Surgical Resection
3.2.2. Liver Transplant
3.2.3. Ablation
3.2.4. Chemoembolization
3.2.5. Chemotherapy
3.2.6. Immunotherapy
3.2.7. Gene Therapy
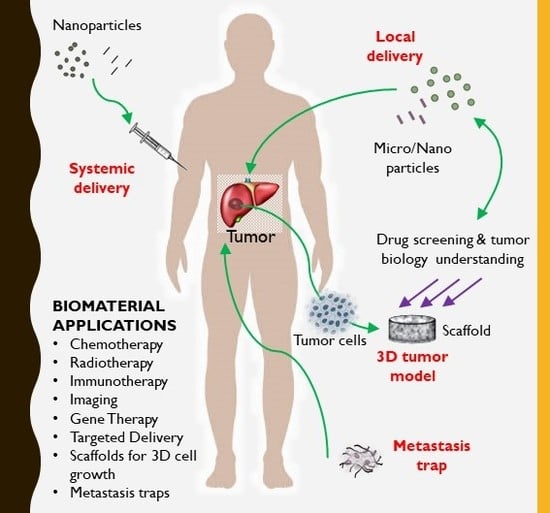
4. Biomaterials for Drug Delivery in Liver Cancer
4.1. Particles
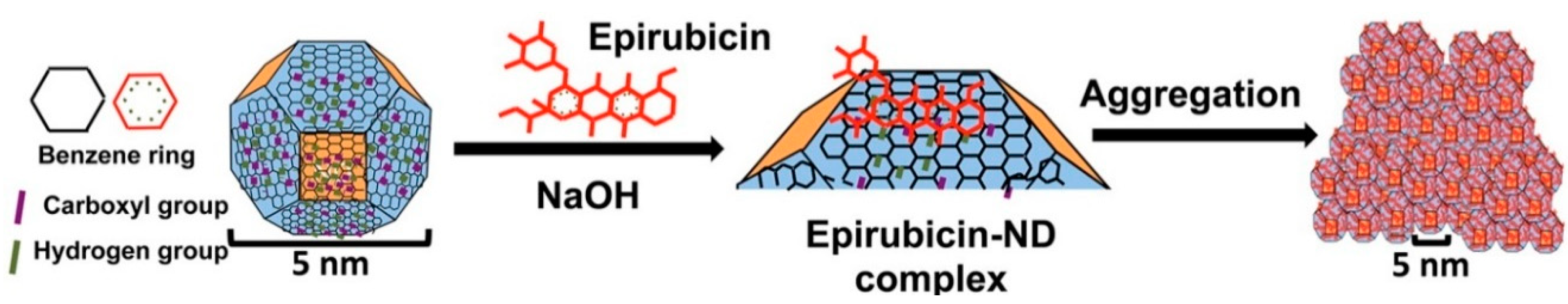
4.1.1. Nanodiamonds
4.1.2. PLGA-Based NPs
4.1.3. Natural Polymers Particles
4.1.4. Metallic NPs
4.1.5. Lipid-Based Particles
4.1.6. Other Particles
4.2. Local Delivery
4.3. Local Implantation
5. Biomaterials for Bioactive Factor Delivery in Liver Cancer Therapy and Imaging
5.1. Chemotherapy
5.2. Radiotherapy
5.3. Immunotherapy
5.4. Natural Occurring Compounds
5.5. Gene Therapy
5.5.1. Plasmid DNA
5.5.2. siRNAs
5.5.3. miRNAs
5.6. Imaging
6. Targeted Delivery
6.1. Antibodies
6.2. Carbohydrate-Targeting Agents
6.3. Peptide/Protein-Targeting Agents
6.4. Enhanced Permeability and Retention Effect (EPR)
7. Biomaterial-Based 3D In Vitro Models to Study Liver Cancer
7.1. 2D Versus 3D Cellular Models for HCC
7.2. Hydrogel-Aided Spheroids for HCC
7.3. Biomaterial-Based Scaffolds for HCC
8. Conclusion and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Dedication
Appendix A
| Form | Biomaterial | Bioactive Factor | Targeting Agent | Type of Therapy | Source, Year |
|---|---|---|---|---|---|
| Nanodiamonds | |||||
| Nanodiamond | Carbon | Epirubicin | Chemotherapy | Wang et al., 2014 [53] | |
| PLGA particles | |||||
| Nanoparticles (NPs) | Charge reversible pullulan-based (CAPL) shell and poly(β-amino ester) (PBAE)/poly(lactic-co-glycolic acid) (PLGA) core | Paclitaxel (PTX) and combretastatin A4 (CA4) | Polysaccharide pullulan backbone | Anti-angiogenesis and chemotherapy | Zhang et al., 2016 [57] |
| NPs | Poly d,l (lactide-coglycolide) (PLA) | 5-fluorouracil (5-FU) | Anti-SM5-1 | Chemotherapy | Ma et al., 2014 [56] |
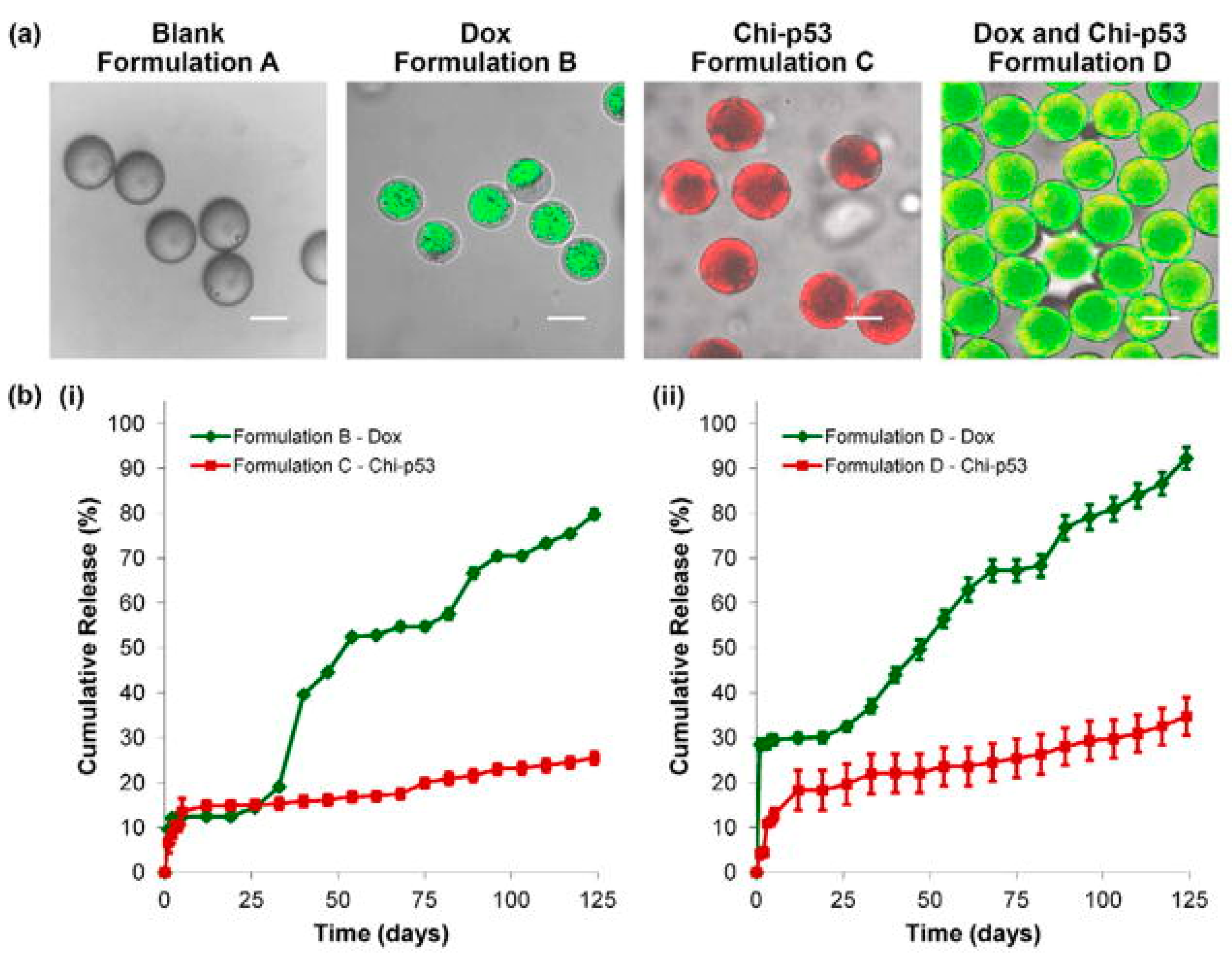
| Microspheres | Poly(d,l-lactic-co-glycolic acid) (PLGA) core surrounded by a poly(L-lactic acid) (PLLA) shell layer | Doxorubicin (DOX) and/or chitosan-DNA NPs (chi-p53) | Chemotherapy and gene therapy | Xu et al., 2013 [58] | |
| NPs | Poly(gamma-glutamic acid)-poly(lactide) | PTX | Galactosamine | Chemotherapy | Liang et al., 2006 [59] |
| NPs | biotin-/lactobionic acid modified poly(ethylene glycol)-PLGA-poly(ethylene glycol) (BLPP) | Curcumin (CUR) and 5-fluorouracil (5-FU) | Biotin/lactiobionic acid | Chemotherapy/Natural therapy | Ni et al., 2018 [60] |
| Natural polymer-based particles | |||||
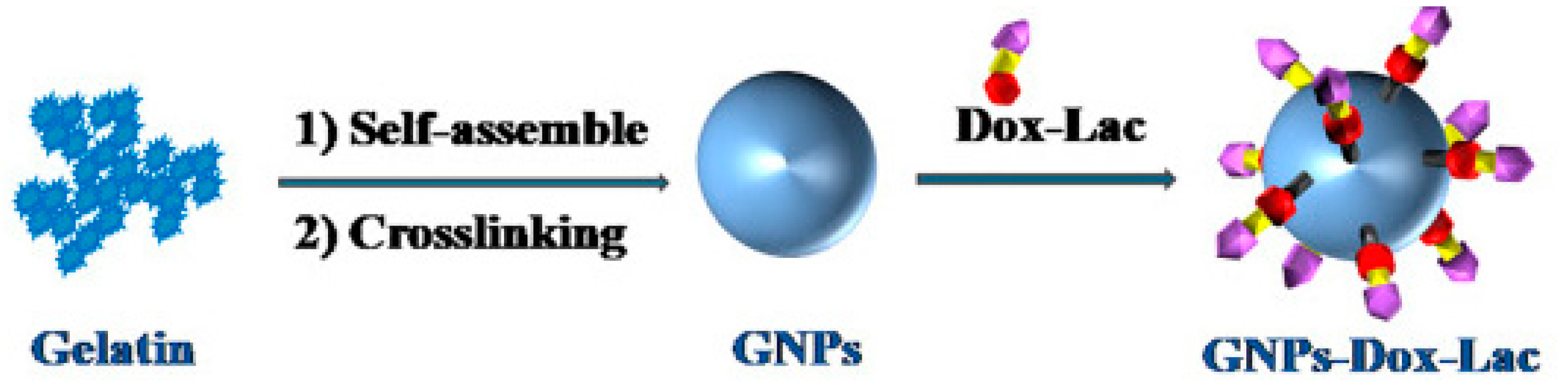
| NPs | Gelatin | DOX-lactose | Lactose | Chemotherapy | Liu et al., 2018 [65] |
| Microspheres | Gelatin and chondroitin-6-sulfate | Interleukin-2 | Immunotherapy | Hanes et al., 2001 [66] | |
| NPs | Chitosan | None | Anticancer | Qi et al., 2007 [68] | |
| NPs | Chitosan | None | Anticancer and anti-angiogenesis | Xu et al., 2010 [67] | |
| NPs | Chitosan | Trans-resveratrol | Biotin and avidin | Phytochemicals | Bu et al., 2013 [69] |
| NPs | Chitosan | Plasmid DNA with granulocyte-macrophage colony stimulating factor, interleukin 21, internal ribosome entry site, and retinoic acid early transcription factor-1 | Biotin | Gene therapy | Cheng et al., 2017 [70] |
| Metallic particles | |||||
| NPs | Galactosylated-carboxymethyl chitosan-magnetic iron oxide (Gal-CMCS-Fe3O4) | Ras Association Domain Family 1A (RASSF1A) gene | Galactose | Gene Delivery and Chemotherapy (Mitomycin injected as a free drug) | Xue et al., 2016 [72,133] |
| NPs | Ultrasmall superparamagnetic iron oxide | SM5-1 | Anti-SM51 | Immunotherapy | Kou et al., 2008 [73] |
| NPs | Gold | miR-375 | Gold | Gene therapy | Xue et al., 2016 [75] |
| NPs | Gold conjugated with sodium citrate or polyamidoamine dendrimers (PAMAM) | None | Gold | Chemotherapy | Paino et al., 2012 [74] |
| NPs | Gold | SM5-1 | Gold and selective binding of SM5-1 | Immunotherapy | Ma et al., 2016 [78] |
| NPs | Gold with a monolayer of L-aspartate | DOX, cisplatin, capecitabine | Gold | Chemotherapy | Tomuleasa et al., 2012 [77] |
| Lipid-based particles | |||||
| Liposomes | Soybean phosphatidylcholine/cholesterol, (PEG)ylated | DOX | Lactoferrin | Chemotherapy | Wei et al., 2015 [79] |
| Liposome | PEGylated liposome (liposome material unclear) | DOX | Targeting peptide SP94 | Chemotherapy | Lo et al., 2008 [82] |
| Immuno-liposomes | 1,2-dioleoyl-3-trimethylammonium-propane (chloride salt) (DOTAP), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide (polyethylene glycol)-2000] (DSPE-PEG-Mal), | Adriamycin (ADR) and ribonucleotide reductase M2 (RRM2) siRNA | Anti-EGFR Fab | Chemotherapy and Gene Therapy | Gao et al., 2013 [127] |
| Immuno-liposomes | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (DSPE-PEG-MAL) | DOX | Anti-CD147 antibody (Metuximab) | Chemotherapy | Wang et al., 2018 [83] |
| Nanomicelles | Casein | Berberine (BRB) and Diosmin (DSN) | lactobionic acid (LA) and folic acid (FA) | Phytochemicals | Abdelmoneem, 2018 [85] |
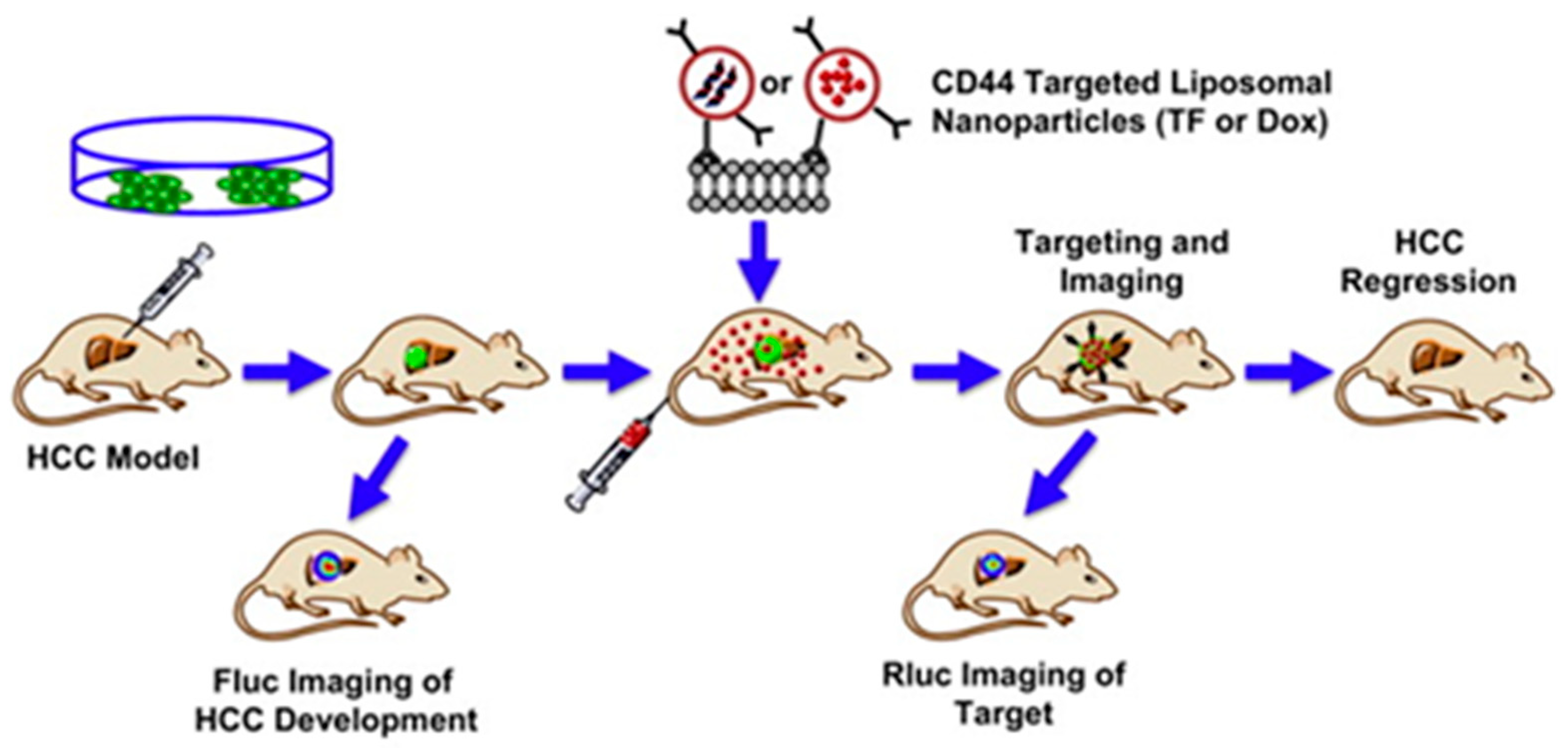
| Liposomal NPs | Distearoylphosphatidylcholine (DSPC), cholesterol, dioleoylphosphatidylethanolamine (DOPE), distearoylphosphatidylethanolamine (DSPE)-mPEG2000, and DSPE-cyclic RGDfK | DOX or triple fusion gene for molecular imaging | Anti-CD44 antibody | Chemotherapy | Wang et al., 2012 [84] |
| Lipid NPs | Cationic lipid RL01, 1,2-Distearoyl-sn-glycero-3-phosphatidylcholine (DSPC), and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DMPE-PEG2000) and cholesterol | Anti-miR-17 | Gene therapy | Huang et al., 2017 [130] | |
| Lipid NPs | Trimyristin (TM), egg yolk phosphatidylcholine (ePC), galactosylated dioleoylphosphatidyl ethanolamine (Gal-DOPE) | Docetaxel | Galactose and lactobionic acid | Chemotherapy | Xu et al., 2009 [87] |
| Lipidoid | Ionizable lipid or cationic lipid, disteroylphosphatidyl choline, cholesterol, and 1,2-dimyristoyl-sn-glycerol, methoxypolyethylene glycol | siRNA for all integrin subunits in hepatocytes | Gene delivery | Bogorad et al., 2014 [86] | |
| Other Particles | |||||
| NPs | Hydroxyapatite | Selenium | Anticancer | Wang et al., 2016 [88] | |
| NPs | Albumin | DOX | Galactosamine | Chemotherapy | Shen et al., 2011 [93] |
| NPs | N-urocanyl pullulan | Methotrexate and Combretastatin A4 | Pullulan | Anti-angiogenic and chemotherapy | Wang et al., 2013 [91] |
| NPs | Polyisohexylcyanoacrylate (PIHCA) | DOX | Chemotherapy | Barraud et al., 2005 [94] | |
| Dendrimer NPs | Lipids cholesterol, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), and lipid PEG2000 with amine core and thiol peripeheries | Let-7g microRNA | Gene delivery | Zhou et al., 2016 [95] | |
| NPs | Calcium carbonate with lipid coating | Sorafenib (Sor) and miR-375 | Chemotherapy and gene therapy | Zhao et al., 2018 [92] | |
| NPs | Insulin multi-methacrylate | DOX | Targeting peptide, Cyclic RGD | Chemotherapy | Bibby et al., 2005 [96] |
| NPs | Block copolymer PEG5k-PLA8k | SN38 prodrug | Chemotherapy | Wang et al., 2018 [61] | |
| Local Delivery | |||||
| Nanofibers NPs (immobilized on the nanofibers) | Poly(ε-caprolactone) disulfide cross linked branched PEI (ssPEI) | PTX and miRNA-145 | Chemotherapy and gene delivery | Che et al., 2015 [98] | |
| Microspheres | Glass | Phosphorus-32 | Radiotherapy | Wang et al., 2008 [97] | |
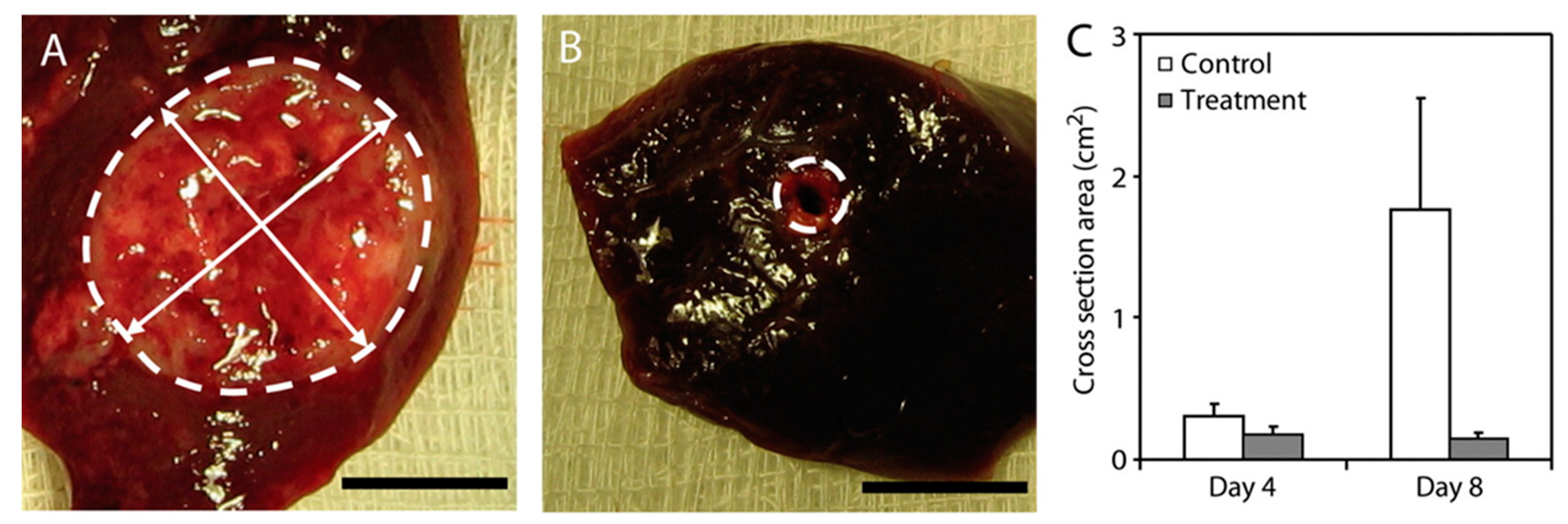
| Polymer millirods | Poly(lactic-co-glycolic acid) PLGA | DOX carboplatin 5-fluorouracil | Chemotherapy | Qian et al., 2002 & 2004 [101,103] Szymanski-Exner et al., 2003 [102]; Weinberg et al., 2007 & 2007 [99,100] | |
| Drug eluting beads | Sulfonate-modified poly(vinyl alcohol) hydrogel | DOX sor | TACE, chemotherapy | Pawlik et al., 2011 [109] | |
| Drug-eluting microspheres/beads (DEB) | Sulfonate-modified poly(vinyl alcohol) hydrogel | DOX | TACE, chemotherapy | Hong et al., 2006 [108] | |
| Drug eluting beads | Polyvinyl alcohol polymer modified with sulfonate groups to form a hyodrogel | DOX | TACE, chemotherapy | Poon et al., 2007 [111] | |
| Microspheres | Poly-lactide-co-glycolide PLGA | Mitomycin | TACE, chemotherapy | Qian et al., 2003 [110] | |
| Biomaterials to capture cells | |||||
| Nanofibers | Lactobionic acid-functionalized electrospun polyvinyl alcohol/polyethyleneimine via PEG spacer | Diagnostic purposes | Cancer diagnosis | Zhao et al., 2015 [191] | |
Appendix B
| Biomaterial | Type | Model | Cells | Application | Results | Source, Year |
|---|---|---|---|---|---|---|
| Synthetic | ||||||
| PEG (8-arm PEG-SH) | Spheroid | HepG2 | Spheroid formation and recovery due to polymer biodegradation with cysteine | Increased cell function (albumin and urea) | Moriyama et al., 2016 [159] | |
| Polystyrene (PHP) | Tissue Engineered (TE) | HepG2 | Structure, function and cytotoxicity study (methotrexate) | TME bio-mimicry: bile duct formation and higher drug resistance | Bokhari et al., 2007 [172] | |
| TEOS–PDMS | TE | HepG2 | Function under dynamic flow | TME bio-mimicry: increased proliferation and aggregation capacity, higher albumin synthesis than in 2D cultures | Kataoka et al., 2005 [176] | |
| PVA | TE | HepG2 | Function under dynamic flow | TME bio-mimicry: higher albumin synthesis than in 2D cultures | Kataoka et al., 2005 [176] | |
| Biologic | ||||||
| Collagen | Hetero-spheroid | HepG2 and stromal fibroblasts | Cytotoxicity study (DOX) | TME bio-mimicry: cell function (P450 activity) and increased drug resistance | Yip et al., 2013 [161] | |
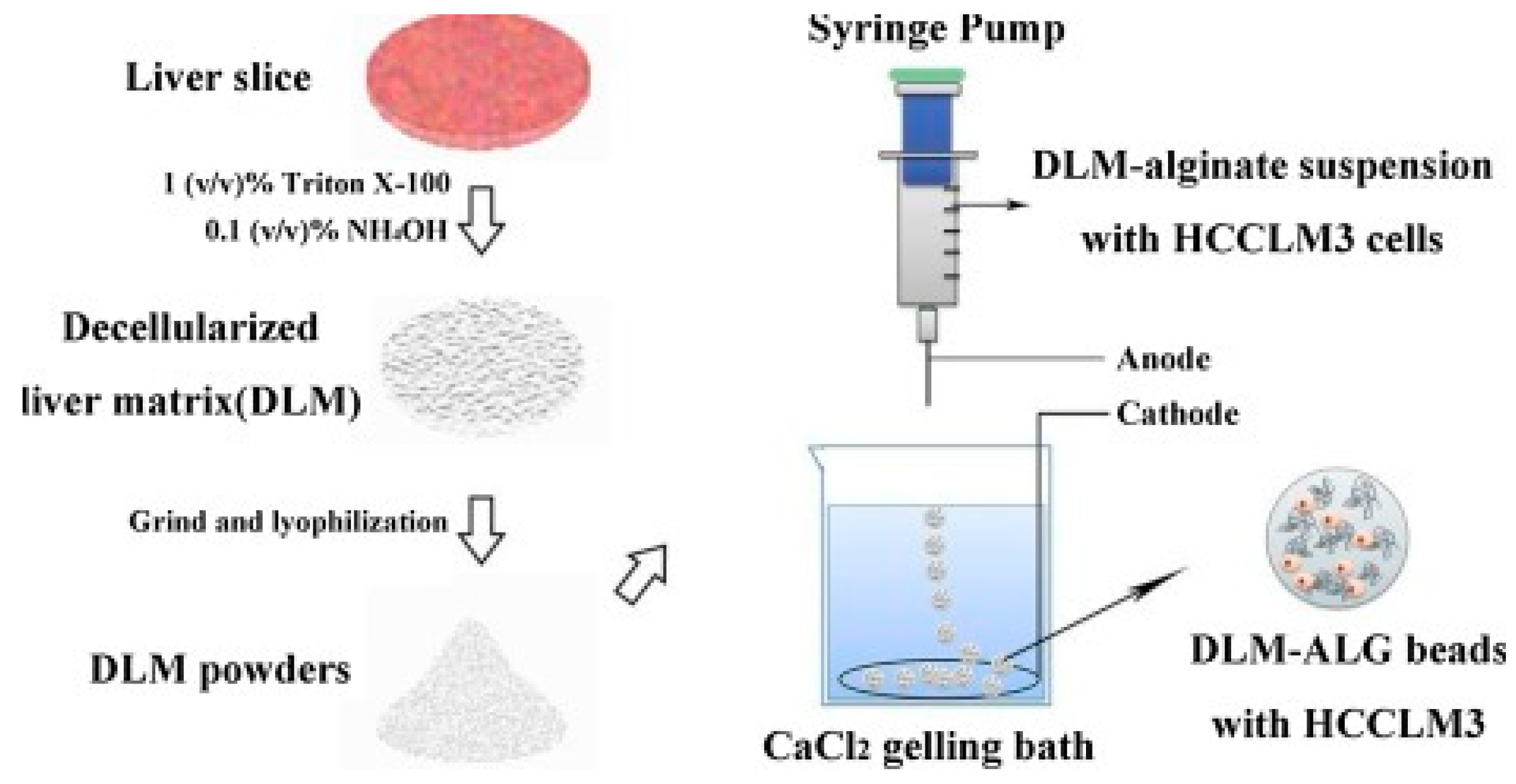
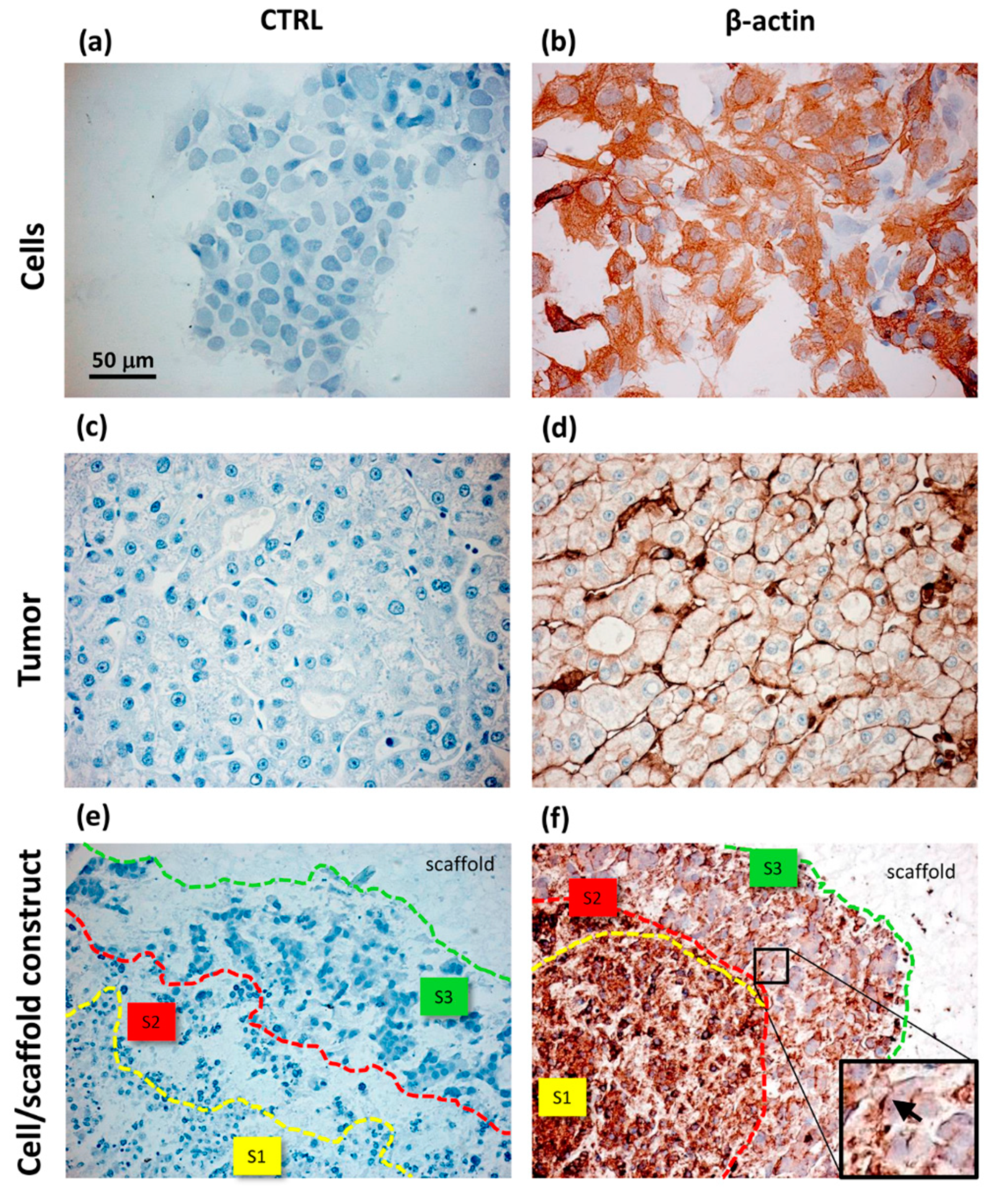
| Alginate | Spheroid | MHCC97L HCCLM3 | Metastatic mechanism study | TME bio-mimicry: increased cell maturity, in particular in metastatic cells MHCC97L | Xu et al., 2013 [143] | |
| Alginate/chitosan blend | Spheroid | PLC/PRF/5 HepG2 | Cytotoxicity study (DOX) | TME bio-mimicry: increased malignancy and drug resistance | Leung et al., 2010 [158] | |
| Silk fibroin | TE | HepR21 HepG2 | TME study | HepR21 showed irregular aggregation and higher proliferation capacity than HepG2 | Kundu et al., 2013 [177] | |
| Silk fibroin/chitosan | TE | HepG2 | Liver regeneration | Mechanical properties controllable, good cell proliferation | She et al., 2008 [181] | |
| Silk fibroin-lactose (Lac-CY-SF) | TE | FALC-4 | Liver regeneration | Functional gene expression not found using collagen | Gotoh et al., 2011 [175] | |
| Composite | ||||||
| Decellularized human liver tissue | TE | LX2 Sk-Hep-1 HepG2 | Liver regeneration | Bio-compatibility and ECM remodeling | Mazza et al., 2015 [179] | |
| Alginate/decellularized liver ECM | Bead | HCCLM3 | Metastatic mechanism study | TME bio-mimicry: Increased cell viability and metastatic potential due to liver ECM | Sun et al., 2018 [170] | |
| Alginate/gelatin microspheres | Spheroid | HepG2 | Formation of spheroids with defined size by microsphere dissolution with MMP-9 | Spheroids of 200 µm with no necrotic core | Lau et al., 2012 [157] | |
| Bioartificial | ||||||
| pNIPAAm-co-gelatin | Spheroid | Hepa/8F5 | Cytotoxicity study (tamoxifen and acetaminophen) | Increased cell function (albumin CYP3A4 activity, ammonia removal) and drug resistance | Sarkar et al., 2017 [160] | |
| Collagen-PEG/succinic acid | TE | HepG2 | Effect of material stiffness | Higher cell cluster size and infiltration capacity in softer hydrogels | Liang et al., 2011 [178] | |
| PVA/gelatin | TE | HepG2 | Cell migration | TME bio-mimicry: tissue-like cell organization, possibility to visualize migratory phenomena | Moscato et al., 2015 [180] |
References
- Dutta, R.; Mahato, R.I. Recent advances in hepatocellular carcinoma therapy. Pharmacol. Ther. 2017, 173, 106–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Wang, J.; Ling, D. Surface Engineering of Nanoparticles for Targeted Delivery to Hepatocellular Carcinoma. Small 2018, 14, 1702037. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.K.; Hamad, M.A.; Hafez, M.Z.; Wooley, K.L.; Elsabahy, M. Nanomedicine in management of hepatocellular carcinoma: Challenges and opportunities. Int. J. Cancer 2017, 140, 1475–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turato, C.; Balasso, A.; Carloni, V.; Tiribelli, C.; Mastrotto, F.; Mazzocca, A.; Pontisso, P. New molecular targets for functionalized nanosized drug delivery systems in personalized therapy for hepatocellular carcinoma. J. Control. Release 2017, 268, 184–197. [Google Scholar] [CrossRef]
- Usmani, A.; Mishra, A.; Ahmad, M. Nanomedicines: A theranostic approach for hepatocellular carcinoma. Artif. Cells Nanomed. Biotechnol. 2018, 46, 680–690. [Google Scholar] [CrossRef]
- Varshosaz, J.; Farzan, M. Nanoparticles for targeted delivery of therapeutics and small interfering RNAs in hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 12022–12041. [Google Scholar] [CrossRef]
- Limeres, M.J.; Moretton, M.A.; Bernabeu, E.; Chiappetta, D.A.; Cuestas, M.L. Thinking small, doing big: Current success and future trends in drug delivery systems for improving cancer therapy with special focus on liver cancer. Mater. Sci. Eng. CMater. Biol. Appl. 2019, 95, 328–341. [Google Scholar] [CrossRef]
- Baig, B.; Halim, S.A.; Farrukh, A.; Greish, Y.; Amin, A. Current status of nanomaterial-based treatment for hepatocellular carcinoma. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 116, 108852. [Google Scholar] [CrossRef]
- Hou, X.Y.; Jiang, G.; Yang, C.S.; Tang, J.Q.; Wei, Z.P.; Liu, Y.Q. Application of Nanotechnology in the Diagnosis and Therapy of Hepatocellular Carcinoma. Recent Pat. Anti-Cancer Drug Discov. 2016, 11, 322–331. [Google Scholar] [CrossRef]
- Kang, J.H.; Toita, R.; Murata, M. Liver cell-targeted delivery of therapeutic molecules. Crit. Rev. Biotechnol. 2016, 36, 132–143. [Google Scholar] [CrossRef]
- Ozougwu, J.C. Physiology of the liver. Int. J. Res. Pharm. Biosci. 2017, 4, 13–24. [Google Scholar]
- Liver Diseases: An Essential Guide for Nurses and Health Care Professionals; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2009.
- Abdellatif, H. Oval Cells: Potential Role in Liver Regeneration. Biochem. J. Sci. Tech. Res. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Fausto, N.; Campbell, J.S. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 2003, 120, 117–130. [Google Scholar] [CrossRef]
- Adams, D.H.; Eksteen, B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat. Rev. Immunol. 2006, 6, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Ribes, J.; Diaz, M.; Cleries, R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Turati, F.; Carioli, G.; Rodriguez, T.; La Vecchia, C.; Malvezzi, M.; Negri, E. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 2017, 67, 302–309. [Google Scholar] [CrossRef]
- Clark, T.; Maximin, S.; Meier, J.; Pokharel, S.; Bhargava, P. Hepatocellular Carcinoma: Review of Epidemiology, Screening, Imaging Diagnosis, Response Assessment, and Treatment. Curr. Probl. Diagn. Radiol. 2015, 44, 479–486. [Google Scholar] [CrossRef]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239 e1224. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Reig, M.; Sherman, M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016, 150, 835–853. [Google Scholar] [CrossRef] [Green Version]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; Castro de Moura, M.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Fuster, J.; Bruix, J.; Barcelona-Clinic Liver Cancer, G. The Barcelona approach: Diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004, 10, S115–S120. [Google Scholar] [CrossRef] [PubMed]
- Armengol, C.; Sarrias, M.R.; Sala, M. Hepatocellular carcinoma: Present and future. Med. Clin. Barc. 2018, 150, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, N.; Cillo, U.; Cucchetti, A.; Donadon, M.; Pinna, A.D.; Torzilli, G.; Kokudo, N. Surgery and Hepatocellular Carcinoma. Liver Cancer 2016, 6, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Romito, R.; Schiavo, M.; Mariani, L.; Camerini, T.; Bhoori, S.; Capussotti, L.; Calise, F.; Pellicci, R.; Belli, G.; et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 2006, 44, 1543–1554. [Google Scholar] [CrossRef]
- Schwartz, J.D.; Schwartz, M.; Mandeli, J.; Sung, M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: Review of the randomised clinical trials. Lancet Oncol. 2002, 3, 593–603. [Google Scholar] [CrossRef]
- Xia, Y.; Qiu, Y.; Li, J.; Shi, L.; Wang, K.; Xi, T.; Shen, F.; Yan, Z.; Wu, M. Adjuvant therapy with capecitabine postpones recurrence of hepatocellular carcinoma after curative resection: A randomized controlled trial. Ann. Surg. Oncol. 2010, 17, 3137–3144. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef]
- Eisai, I. Positive Topline Results of Large Phase 3 Trial Show Eisai’s Lenvatinib Meets Primary Endpoint in Unresectable Hepatocellular Carcinoma. Available online: http://eisai.mediaroom.com/2017-01-25-Positive-Topline-Results-of-Large-Phase-3-Trial-Show-Eisais-Lenvatinib-Meets-Primary-Endpoint-in-Unresectable-Hepatocellular-Carcinoma (accessed on 2 December 2019).
- Gomaa, A.; Waked, I. Management of advanced hepatocellular carcinoma: Review of current and potential therapies. Hepatoma Res. 2017, 3, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.X.; Rosmorduc, O.; Evans, T.R.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 559–566. [Google Scholar] [CrossRef]
- Koeberle, D.; Dufour, J.F.; Demeter, G.; Li, Q.; Ribi, K.; Samaras, P.; Saletti, P.; Roth, A.D.; Horber, D.; Buehlmann, M.; et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): A randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann. Oncol. 2016, 27, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Heo, J.; Choi, H.J.; Lin, C.Y.; Yoon, J.H.; Hsu, C.; Rau, K.M.; Poon, R.T.; Yeo, W.; Park, J.W.; et al. A phase II study of the efficacy and safety of the combination therapy of the MEK inhibitor refametinib (BAY 86-9766) plus sorafenib for Asian patients with unresectable hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 5976–5985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Inarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Stein, S.; Pishvaian, M.J.; Lee, M.S.; Lee, K.H.; Hernandez, S.; Kwan, A.; Liu, B.; Grossman, W.; Iizuka, K.; Ryoo, B.Y. Safety and clinical activity of 1L atezolizumab plus bevacizumab in a phase Ib study in hepatocellular carcinoma (HCC). J. Clin. Oncol. 2018, 36, 4074. [Google Scholar] [CrossRef]
- Daher, S.; Massarwa, M.; Benson, A.A.; Khoury, T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J. Clin. Transl. Hepatol. 2018, 6, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, S.A. Progress in human tumour immunology and immunotherapy. Nature 2001, 411, 380–384. [Google Scholar] [CrossRef]
- Komori, H.; Nakatsura, T.; Senju, S.; Yoshitake, Y.; Motomura, Y.; Ikuta, Y.; Fukuma, D.; Yokomine, K.; Harao, M.; Beppu, T.; et al. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin. Cancer Res. 2006, 12, 2689–2697. [Google Scholar] [CrossRef] [Green Version]
- Iwama, T.; Uchida, T.; Sawada, Y.; Tsuchiya, N.; Sugai, S.; Fujinami, N.; Shimomura, M.; Yoshikawa, T.; Zhang, R.; Uemura, Y.; et al. Vaccination with liposome-coupled glypican-3-derived epitope peptide stimulates cytotoxic T lymphocytes and inhibits GPC3-expressing tumor growth in mice. Biochem. Biophys. Res. Commun. 2016, 469, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Mizukoshi, E.; Nakagawa, H.; Kitahara, M.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Iida, N.; Fushimi, K.; Kaneko, S. Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. Cancer Lett. 2015, 369, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Yutani, S.; Ueshima, K.; Abe, K.; Ishiguro, A.; Eguchi, J.; Matsueda, S.; Komatsu, N.; Shichijo, S.; Yamada, A.; Itoh, K.; et al. Phase II study of personalized peptide vaccination with both a hepatitis c virus-derived peptide and peptides from tumor-associated antigens for the treatment of hcv-positive advanced hepatocellular carcinoma patients. J. Immunol. Res. 2015, 2015, 473909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Farra, R.; Grassi, M.; Grassi, G.; Dapas, B. Therapeutic potential of small interfering RNAs/micro interfering RNA in hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 8994–9001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layzer, J.M.; McCaffrey, A.P.; Tanner, A.K.; Huang, Z.; Kay, M.A.; Sullenger, B.A. In vivo activity of nuclease-resistant siRNAs. RNA 2004, 10, 766–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, C.N.; Chayama, K. MicroRNAs as Biomarkers for Liver Disease and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2016, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.R.; Sahebkar, A.; Mohammadi, M.; Yari, R.; Salehi, H.; Jafari, M.H.; Namdar, A.; Khabazian, E.; Jaafari, M.R.; Mirzaei, H. Circulating microRNAs in Hepatocellular Carcinoma: Potential Diagnostic and Prognostic Biomarkers. Curr. Pharm. Des. 2016, 22, 5257–5269. [Google Scholar] [CrossRef]
- Tomimaru, Y.; Eguchi, H.; Nagano, H.; Wada, H.; Kobayashi, S.; Marubashi, S.; Tanemura, M.; Tomokuni, A.; Takemasa, I.; Umeshita, K.; et al. Circulating microRNA-21 as a novel biomarker for hepatocellular carcinoma. J. Hepatol. 2012, 56, 167–175. [Google Scholar] [CrossRef]
- Deng, X.; Yin, Z.; Zhou, Z.; Wang, Y.; Zhang, F.; Hu, Q.; Yang, Y.; Lu, J.; Wu, Y.; Sheng, W.; et al. Carboxymethyl Dextran-Stabilized Polyethylenimine-Poly(epsilon-caprolactone) Nanoparticles-Mediated Modulation of MicroRNA-34a Expression via Small-Molecule Modulator for Hepatocellular Carcinoma Therapy. ACS Appl. Mater. Interfaces 2016, 8, 17068–17079. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2011, 7, 11–23. [Google Scholar] [CrossRef]
- Mochalin, V.; Osswald, S.; Gogotsi, Y. Contribution of Functional Groups to the Raman Spectrum of Nanodiamond Powders. Chem. Mater. 2008, 21, 273–279. [Google Scholar]
- Wang, X.; Low, X.C.; Hou, W.; Abdullah, L.N.; Toh, T.B.; Mohd Abdul Rashid, M.; Ho, D.; Chow, E.K. Epirubicin-adsorbed nanodiamonds kill chemoresistant hepatic cancer stem cells. ACS Nano 2014, 8, 12151–12166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, J.H.; Emmett, C.; Shen, P.; Aswani, S.; Iwamoto, T.; Vaghefi, F.; Cain, G.; Sanders, L. Bioerosion and biocompatibility of poly(d,llactic-co-glycolic acid) implants in brain. J. Control. Release 1997, 43, 123–130. [Google Scholar] [CrossRef]
- Menei, P.; Daniel, V.; Montero-Menei, C.; Brouillard, M.; Pouplard-Barthelaix, A.; Benoit, J.P. Biodegradation and brain tissue reaction to poly(D,L-lactide-co-glycolide) microspheres. Biomaterials 1993, 14, 470–478. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, Z.; Jin, Y.; Liang, X.; Yang, X.; Dai, Z.; Tian, J. SM5-1-conjugated PLA nanoparticles loaded with 5-fluorouracil for targeted hepatocellular carcinoma imaging and therapy. Biomaterials 2014, 35, 2878–2889. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; An, T.; Wang, D.; Wan, G.; Zhang, M.; Wang, H.; Zhang, S.; Li, R.; Yang, X.; Wang, Y. Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly(beta-amino ester)/poly(lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. J. Control. Release 2016, 226, 193–204. [Google Scholar] [CrossRef]
- Xu, Q.; Leong, J.; Chua, Q.Y.; Chi, Y.T.; Chow, P.K.; Pack, D.W.; Wang, C.H. Combined modality doxorubicin-based chemotherapy and chitosan-mediated p53 gene therapy using double-walled microspheres for treatment of human hepatocellular carcinoma. Biomaterials 2013, 34, 5149–5162. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.F.; Chen, C.T.; Chen, S.C.; Kulkarni, A.R.; Chiu, Y.L.; Chen, M.C.; Sung, H.W. Paclitaxel-loaded poly(gamma-glutamic acid)-poly(lactide) nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Biomaterials 2006, 27, 2051–2059. [Google Scholar] [CrossRef]
- Ni, W.; Li, Z.; Liu, Z.; Ji, Y.; Wu, L.; Sun, S.; Jian, X.; Gao, X. Dual-Targeting Nanoparticles: Codelivery of Curcumin and 5-Fluorouracil for Synergistic Treatment of Hepatocarcinoma. J. Pharm. Sci. 2019, 108, 1284–1295. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Xie, K.; Wu, J.; Song, P.; Xie, H.; Zhou, L.; Liu, J.; Xu, X.; Shen, Y.; et al. Polylactide-tethered prodrugs in polymeric nanoparticles as reliable nanomedicines for the efficient eradication of patient-derived hepatocellular carcinoma. Theranostics 2018, 8, 3949–3963. [Google Scholar] [CrossRef]
- Gupta, P.; Authimoolam, S.P.; Hilt, J.Z.; Dziubla, T.D. Quercetin conjugated poly(beta-amino esters) nanogels for the treatment of cellular oxidative stress. Acta Biomater. 2015, 27, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Ruan, S.; Zhang, L.; Chen, J.; Cao, T.; Yang, Y.; Liu, Y.; He, Q.; Gao, F.; Gao, H. Targeting delivery and deep penetration using multistage nanoparticles for triple-negative breast cancer. RSC Adv. 2015, 5, 64303–64317. [Google Scholar] [CrossRef]
- Zha, Z.; Zhang, S.; Deng, Z.; Li, Y.; Li, C.; Dai, Z. Enzyme-responsive copper sulphide nanoparticles for combined photoacoustic imaging, tumor-selective chemotherapy and photothermal therapy. Chem. Commun. 2013, 49, 3455–3457. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Li, L.; Zhou, Z.; Wang, F.; Xiong, X.; Zhou, R.; Huang, Y. Programmed drug delivery system based on optimized “size decrease and hydrophilicity/hydrophobicity transformation” for enhanced hepatocellular carcinoma therapy of doxorubicin. Nanomedicine 2018, 14, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Hanes, J.; Sills, A.; Zhao, Z.; Suh, K.W.; Tyler, B.; DiMeco, F.; Brat, D.J.; Choti, M.A.; Leong, K.W.; Pardoll, D.M.; et al. Controlled local delivery of interleukin-2 by biodegradable polymers protects animals from experimental brain tumors and liver tumors. Pharm. Res. 2001, 18, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, Z.; Xu, Z. Chitosan nanoparticles inhibit the growth of human hepatocellular carcinoma xenografts through an antiangiogenic mechanism. Anticancer. Res. 2009, 29, 5103–5109. [Google Scholar]
- Qi, L.; Xu, Z.; Chen, M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer 2007, 43, 184–193. [Google Scholar] [CrossRef]
- Bu, L.; Gan, L.C.; Guo, X.Q.; Chen, F.Z.; Song, Q.; Qi, Z.; Gou, X.J.; Hou, S.X.; Yao, Q. Trans-resveratrol loaded chitosan nanoparticles modified with biotin and avidin to target hepatic carcinoma. Int. J. Pharm. 2013, 452, 355–362. [Google Scholar] [CrossRef]
- Cheng, M.; Zhu, W.; Li, Q.; Dai, D.; Hou, Y. Anti-cancer efficacy of biotinylated chitosan nanoparticles in liver cancer. Oncotarget 2017, 8, 59068–59085. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.; Zhang, M. Multifunctional Magnetic Nanoparticles for Medical Imaging Applications. J. Mater. Chem. 2009, 19, 6258–6266. [Google Scholar] [CrossRef]
- Xue, W.J.; Feng, Y.; Wang, F.; Guo, Y.B.; Li, P.; Wang, L.; Liu, Y.F.; Wang, Z.W.; Yang, Y.M.; Mao, Q.S. Asialoglycoprotein receptor-magnetic dual targeting nanoparticles for delivery of RASSF1A to hepatocellular carcinoma. Sci. Rep. 2016, 6, 22149. [Google Scholar] [CrossRef] [Green Version]
- Kou, G.; Wang, S.; Cheng, C.; Gao, J.; Li, B.; Wang, H.; Qian, W.; Hou, S.; Zhang, D.; Dai, J.; et al. Development of SM5-1-conjugated ultrasmall superparamagnetic iron oxide nanoparticles for hepatoma detection. Biochem. Biophys. Res. Commun. 2008, 374, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Paino, I.M.; Marangoni, V.S.; de Oliveira Rde, C.; Antunes, L.M.; Zucolotto, V. Cyto and genotoxicity of gold nanoparticles in human hepatocellular carcinoma and peripheral blood mononuclear cells. Toxicol. Lett. 2012, 215, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Liu, Y.; Liao, J.Z.; Lin, J.S.; Li, B.; Yuan, W.G.; Lee, R.J.; Li, L.; Xu, C.R.; He, X.X. Gold nanoparticles delivered miR-375 for treatment of hepatocellular carcinoma. Oncotarget 2016, 7, 86675–86686. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic properties of gold nanoparticles. Clin. Cancer Res. 2005, 11, 3530–3534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomuleasa, C.; Soritau, O.; Orza, A.; Dudea, M.; Petrushev, B.; Mosteanu, O.; Susman, S.; Florea, A.; Pall, E.; Aldea, M.; et al. Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cells. J. Gastrointestin. Liver Dis. 2012, 21, 187–196. [Google Scholar] [PubMed]
- Ma, X.; Hui, H.; Jin, Y.; Dong, D.; Liang, X.; Yang, X.; Tan, K.; Dai, Z.; Cheng, Z.; Tian, J. Enhanced immunotherapy of SM5-1 in hepatocellular carcinoma by conjugating with gold nanoparticles and its in vivo bioluminescence tomographic evaluation. Biomaterials 2016, 87, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Guo, X.; Tu, L.; Zou, Q.; Li, Q.; Tang, C.; Chen, B.; Xu, Y.; Wu, C. Lactoferrin-modified PEGylated liposomes loaded with doxorubicin for targeting delivery to hepatocellular carcinoma. Int. J. Nanomed. 2015, 10, 5123–5137. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug. Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Lo, A.; Lin, C.T.; Wu, H.C. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol. Cancer. 2008, 7, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, Z.; Pan, G.; Ni, J.; Xie, F.; Jiang, B.; Wei, L.; Gao, J.; Zhou, W. Enhanced doxorubicin delivery to hepatocellular carcinoma cells via CD147 antibody-conjugated immunoliposomes. Nanomedicine 2018, 14, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Su, W.; Liu, Z.; Zhou, M.; Chen, S.; Chen, Y.; Lu, D.; Liu, Y.; Fan, Y.; Zheng, Y.; et al. CD44 antibody-targeted liposomal nanoparticles for molecular imaging and therapy of hepatocellular carcinoma. Biomaterials 2012, 33, 5107–5114. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoneem, M.A.; Mahmoud, M.; Zaky, A.; Helmy, M.W.; Sallam, M.; Fang, J.Y.; Elkhodairy, K.A.; Elzoghby, A.O. Dual-targeted casein micelles as green nanomedicine for synergistic phytotherapy of hepatocellular carcinoma. J. Control. Release 2018, 287, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Bogorad, R.L.; Yin, H.; Zeigerer, A.; Nonaka, H.; Ruda, V.M.; Zerial, M.; Anderson, D.G.; Koteliansky, V. Nanoparticle-formulated siRNA targeting integrins inhibits hepatocellular carcinoma progression in mice. Nat. Commun. 2014, 5, 3869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Chen, L.; Gu, W.; Gao, Y.; Lin, L.; Zhang, Z.; Xi, Y.; Li, Y. The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials 2009, 30, 226–232. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H.; Zhang, L. Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2016, 140, 297–306. [Google Scholar]
- Bauer, I.W.; Li, S.P.; Han, Y.C.; Yuan, L.; Yin, M.Z. Internalization of hydroxyapatite nanoparticles in liver cancer cells. J. Mater. Sci. Mater. Med. 2008, 19, 1091–1095. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, C.; Qian, J.; Wang, J.; Zhang, Y. Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010, 31, 730–740. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Liu, Y.; Wu, J.; Zhou, P.; Wang, Y.; Li, R.; Yang, X.; Zhang, N. pH-sensitive pullulan-based nanoparticle carrier of methotrexate and combretastatin A4 for the combination therapy against hepatocellular carcinoma. Biomaterials 2013, 34, 7181–7190. [Google Scholar] [CrossRef]
- Zhao, P.; Li, M.; Wang, Y.; Chen, Y.; He, C.; Zhang, X.; Yang, T.; Lu, Y.; You, J.; Lee, R.J.; et al. Enhancing anti-tumor efficiency in hepatocellular carcinoma through the autophagy inhibition by miR-375/sorafenib in lipid-coated calcium carbonate nanoparticles. Acta Biomater. 2018, 72, 248–255. [Google Scholar] [CrossRef]
- Shen, Z.; Wei, W.; Tanaka, H.; Kohama, K.; Ma, G.; Dobashi, T.; Maki, Y.; Wang, H.; Bi, J.; Dai, S. A galactosamine-mediated drug delivery carrier for targeted liver cancer therapy. Pharm. Res. 2011, 64, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Barraud, L.; Merle, P.; Soma, E.; Lefrancois, L.; Guerret, S.; Chevallier, M.; Dubernet, C.; Couvreur, P.; Trepo, C.; Vitvitski, L. Increase of doxorubicin sensitivity by doxorubicin-loading into nanoparticles for hepatocellular carcinoma cells in vitro and in vivo. J. Hepatol. 2005, 42, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Nguyen, L.H.; Miller, J.B.; Yan, Y.; Kos, P.; Xiong, H.; Li, L.; Hao, J.; Minnig, J.T.; Zhu, H.; et al. Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proc. Natl. Acad. Sci. USA 2016, 113, 520–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibby, D.C.; Talmadge, J.E.; Dalal, M.K.; Kurz, S.G.; Chytil, K.M.; Barry, S.E.; Shand, D.G.; Steiert, M. Pharmacokinetics and biodistribution of RGD-targeted doxorubicin-loaded nanoparticles in tumor-bearing mice. Int. J. Pharm. 2005, 293, 281–290. [Google Scholar] [CrossRef]
- Wang, X.M.; Yin, Z.Y.; Yu, R.X.; Peng, Y.Y.; Liu, P.G.; Wu, G.Y. Preventive effect of regional radiotherapy with phosphorus-32 glass microspheres in hepatocellular carcinoma recurrence after hepatectomy. World J. Gastroenterol. 2008, 14, 518–523. [Google Scholar] [CrossRef]
- Che, H.L.; Lee, H.J.; Uto, K.; Ebara, M.; Kim, W.J.; Aoyagi, T.; Park, I.K. Simultaneous Drug and Gene Delivery from the Biodegradable Poly(epsilon-caprolactone) Nanofibers for the Treatment of Liver Cancer. J. Nanosci. Nanotechnol. 2015, 15, 7971–7975. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Ai, H.; Blanco, E.; Anderson, J.M.; Gao, J. Antitumor efficacy and local distribution of doxorubicin via intratumoral delivery from polymer millirods. J. Biomed. Mater. Res. Part A 2007, 81, 161–170. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Blanco, E.; Lempka, S.F.; Anderson, J.M.; Exner, A.A.; Gao, J. Combined radiofrequency ablation and doxorubicin-eluting polymer implants for liver cancer treatment. J. Biomed. Mater. Res. A 2007, 81, 205–213. [Google Scholar] [CrossRef]
- Qian, F.; Stowe, N.; Saidel, G.M.; Gao, J. Comparison of doxorubicin concentration profiles in radiofrequency-ablated rat livers from sustained- and dual-release PLGA millirods. Pharm. Res. 2004, 21, 394–399. [Google Scholar] [CrossRef]
- Szymanski-Exner, A.; Gallacher, A.; Stowe, N.T.; Weinberg, B.; Haaga, J.R.; Gao, J. Local carboplatin delivery and tissue distribution in livers after radiofrequency ablation. J. Biomed. Mater. Res. A 2003, 67, 510–516. [Google Scholar] [CrossRef]
- Qian, F.; Nasongkla, N.; Gao, J. Membrane-encased polymer millirods for sustained release of 5-fluorouracil. J. Biomed. Mater. Res. 2002, 61, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ruddon, R. Cancer Biology; Oxford Press: New York, NY, USA, 1995. [Google Scholar]
- Muller, H.; Hilger, R. Curative and palliative aspects of regional chemotherapy in combination with surgery. Support. Care Cancer 2003, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Gonzalez, M.V.; Lloyd, A.W.; Hall, B.; Tang, Y.; Willis, S.L.; Leppard, S.W.; Wolfenden, L.C.; Palmer, R.R.; Stratford, P.W. DC bead: In vitro characterization of a drug-delivery device for transarterial chemoembolization. J. Vasc. Interv. Radiol. 2006, 17, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Taylor, R.R.; Hall, B.; Gonzalez, M.V.; Willis, S.L.; Stratford, P.W. Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J. Vasc. Interv. Radiol. 2006, 17, 1335–1343. [Google Scholar] [CrossRef]
- Hong, K.; Khwaja, A.; Liapi, E.; Torbenson, M.S.; Georgiades, C.S.; Geschwind, J.F. New intra-arterial drug delivery system for the treatment of liver cancer: Preclinical assessment in a rabbit model of liver cancer. Clin. Cancer Res. 2006, 12, 2563–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlik, T.M.; Reyes, D.K.; Cosgrove, D.; Kamel, I.R.; Bhagat, N.; Geschwind, J.F. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J. Clin. Oncol. 2011, 29, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Truebenbach, J.; Graepler, F.; Pereira, P.; Huppert, P.; Eul, T.; Wiemann, G.; Claussen, C. Application of poly-lactide-co-glycolide-microspheres in the transarterial chemoembolization in an animal model of hepatocellular carcinoma. World J. Gastroenterol. 2003, 9, 94–98. [Google Scholar] [CrossRef]
- Poon, R.T.; Tso, W.K.; Pang, R.W.; Ng, K.K.; Woo, R.; Tai, K.S.; Fan, S.T. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin. Gastroenterol. Hepatol. 2007, 5, 1100–1108. [Google Scholar] [CrossRef]
- Kang, T.H.; Pae, H.O.; Yoo, J.C.; Kim, N.Y.; Kim, Y.C.; Ko, G.I.; Chung, H.T. Antiproliferative effects of alkaloids from Sedum sarmentosum on murine and human hepatoma cell lines. J. Ethnopharmacol. 2000, 70, 177–182. [Google Scholar] [CrossRef]
- Lin, H.L.; Liu, T.Y.; Chau, G.Y.; Lui, W.Y.; Chi, C.W. Comparison of 2-methoxyestradiol-induced, docetaxel-induced, and paclitaxel-induced apoptosis in hepatoma cells and its correlation with reactive oxygen species. Cancer 2000, 89, 983–994. [Google Scholar] [CrossRef]
- Gueritte-Voegelein, F.; Guenard, D.; Lavelle, F.; Le Goff, M.T.; Mangatal, L.; Potier, P. Relationships between the structure of taxol analogues and their antimitotic activity. J. Med. Chem. 1991, 34, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Ghose, R.; Ghose, A.K.; Sodhi, A.; Singh, S.M.; Singh, R.K. The effect of histidine on the structure and antitumor activity of metal-5-halouracil complexes. J. Inorg. Biochem. 1989, 37, 325–339. [Google Scholar] [CrossRef]
- Thomas, D.M.; Zalcberg, J.R. 5-fluorouracil: A pharmacological paradigm in the use of cytotoxics. Clin. Exp. Pharmacol. Physiol. 1998, 25, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. Cancer vaccines. Nat. Med. 1998, 4, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. Paracrine cytokine adjuvants in cancer immunotherapy. Annu. Rev. Immunol. 1995, 13, 399–415. [Google Scholar] [CrossRef]
- Gao, J.; Kou, G.; Chen, H.; Wang, H.; Li, B.; Lu, Y.; Zhang, D.; Wang, S.; Hou, S.; Qian, W.; et al. Treatment of hepatocellular carcinoma in mice with PE38KDEL type I mutant-loaded poly(lactic-co-glycolic acid) nanoparticles conjugated with humanized SM5-1 F(ab′) fragments. Mol. Cancer 2008, 7, 3399–3407. [Google Scholar] [CrossRef] [Green Version]
- Kou, G.; Gao, J.; Wang, H.; Chen, H.; Li, B.; Zhang, D.; Wang, S.; Hou, S.; Qian, W.; Dai, J.; et al. Preparation and Characterization of Paclitaxel-loaded PLGA nanoparticles coated with cationic SM5-1 single-chain antibody. J. Biochem. Mol. Biol. 2007, 40, 731–739. [Google Scholar] [CrossRef]
- Li, B.; Wang, H.; Zhang, D.; Qian, W.; Hou, S.; Shi, S.; Zhao, L.; Kou, G.; Cao, Z.; Dai, J.; et al. Construction and characterization of a high-affinity humanized SM5-1 monoclonal antibody. Biochem. Biophys. Res. Commun. 2007, 357, 951–956. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; An, F.F.; Liu, J.; Jin, S.; Zhang, J.C.; Wang, P.C.; Zhang, X.; Lee, C.S.; Liang, X.J. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale 2015, 7, 13503–13510. [Google Scholar] [CrossRef]
- Agathanggelou, A.; Cooper, W.N.; Latif, F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 2005, 65, 3497–3508. [Google Scholar] [CrossRef] [Green Version]
- Donninger, H.; Vos, M.D.; Clark, G.J. The RASSF1A tumor suppressor. J. Cell Sci. 2007, 120, 3163–3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Ahsan, H.; Chen, Y.; Lunn, R.M.; Wang, L.Y.; Chen, S.Y.; Lee, P.H.; Chen, C.J.; Santella, R.M. High frequency of promoter hypermethylation of RASSF1A and p16 and its relationship to aflatoxin B1-DNA adduct levels in human hepatocellular carcinoma. Mol. Carcinog. 2002, 35, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.S.; La, Z.; Yang, L.; He, Q.; Li, P. p53 gene in treatment of hepatic carcinoma: Status quo. World J. Gastroenterol. 2007, 13, 985–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Chen, H.; Yu, Y.; Song, J.; Song, H.; Su, X.; Li, W.; Tong, X.; Qian, W.; Wang, H.; et al. Inhibition of hepatocellular carcinoma growth using immunoliposomes for co-delivery of adriamycin and ribonucleotide reductase M2 siRNA. Biomaterials 2013, 34, 10084–10098. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.; Chow, M.Y.; Zhang, Y.; Leung, S.W. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [Green Version]
- Shan, S.W.; Fang, L.; Shatseva, T.; Rutnam, Z.J.; Yang, X.; Du, W.; Lu, W.Y.; Xuan, J.W.; Deng, Z.; Yang, B.B. Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J. Cell Sci. 2013, 126, 1517–1530. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Magnus, J.; Kaimal, V.; Karmali, P.; Li, J.; Walls, M.; Prudente, R.; Sung, E.; Sorourian, M.; Lee, R.; et al. Lipid Nanoparticle-Mediated Delivery of Anti-miR-17 Family Oligonucleotide Suppresses Hepatocellular Carcinoma Growth. Mol. Cancer 2017, 16, 905–913. [Google Scholar] [CrossRef] [Green Version]
- Harrington, K.J.; Mohammadtaghi, S.; Uster, P.S.; Glass, D.; Peters, A.M.; Vile, R.G.; Stewart, J.S. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001, 7, 243–254. [Google Scholar]
- Al-Batran, S.E.; Bischoff, J.; von Minckwitz, G.; Atmaca, A.; Kleeberg, U.; Meuthen, I.; Morack, G.; Lerbs, W.; Hecker, D.; Sehouli, J.; et al. The clinical benefit of pegylated liposomal doxorubicin in patients with metastatic breast cancer previously treated with conventional anthracyclines: A multicentre phase II trial. Br. J. Cancer 2006, 94, 1615–1620. [Google Scholar] [CrossRef]
- Xue, W.J.; Li, C.; Zhou, X.J.; Guan, H.G.; Qin, L.; Li, P.; Wang, Z.W.; Qian, H.X. RASSF1A expression inhibits the growth of hepatocellular carcinoma from Qidong County. J. Gastroenterol. Hepatol. 2008, 23, 1448–1458. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Devarajan, P.V. Asialoglycoprotein receptor mediated hepatocyte targeting - strategies and applications. J. Control. Release 2015, 203, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, M.; Yung, B.; Li, H.; Zhou, C.; Lee, L.J.; Lee, R.J. Lactosylated liposomes for targeted delivery of doxorubicin to hepatocellular carcinoma. Int. J. Nanomed. 2012, 7, 5465–5474. [Google Scholar] [CrossRef] [Green Version]
- Kaneo, Y.; Tanaka, T.; Nakano, T.; Yamaguchi, Y. Evidence for receptor-mediated hepatic uptake of pullulan in rats. J. Control. Release 2001, 70, 365–373. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug. Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit. Rev. Drug. Carr. Syst. 1989, 6, 193–210. [Google Scholar]
- Litzinger, D.C.; Buiting, A.M.; van Rooijen, N.; Huang, L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. Biochim. Biophys. Acta 1994, 1190, 99–107. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [Green Version]
- Ricci, C.; Moroni, L.; Danti, S. Cancer tissue engineering—New perspectives in understanding the biology of solid tumours—A critical review. OA Tissue Eng 2013, 1, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.X.; Liu, C.; Liu, Y.; Li, N.; Guo, X.; Wang, S.J.; Sun, G.W.; Wang, W.; Ma, X.J. Encapsulated human hepatocellular carcinoma cells by alginate gel beads as an in vitro metastasis model. Exp. Cell Res. 2013, 319, 2135–2144. [Google Scholar] [CrossRef]
- Kulig, K.M.; Vacanti, J.P. Hepatic tissue engineering. Transpl. Immunol. 2004, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.J.; Okuda, K.; Ishak, K.G. (Eds.) Human hepatoma cell lines. In Neoplasms of the Liver; Springer: Berlin, Germany, 1987. [Google Scholar]
- Ramachandran, S.D.; Schirmer, K.; Munst, B.; Heinz, S.; Ghafoory, S.; Wolfl, S.; Simon-Keller, K.; Marx, A.; Oie, C.I.; Ebert, M.P.; et al. In Vitro Generation of Functional Liver Organoid-Like Structures Using Adult Human Cells. PLoS ONE 2015, 10, e0139345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuciforo, S.; Fofana, I.; Matter, M.S.; Blumer, T.; Calabrese, D.; Boldanova, T.; Piscuoglio, S.; Wieland, S.; Ringnalda, F.; Schwank, G.; et al. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep. 2018, 24, 1363–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasirci, V.; Berthiaume, F.; Bondre, S.P.; Gresser, J.D.; Trantolo, D.J.; Toner, M.; Wise, D.L. Expression of liver-specific functions by rat hepatocytes seeded in treated poly(lactic-co-glycolic) acid biodegradable foams. Tissue Eng. 2001, 7, 385–394. [Google Scholar] [CrossRef]
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacanti, J.P.; Langer, R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 1993, 14, 323–330. [Google Scholar] [CrossRef]
- Mooney, D.J.; Park, S.; Kaufmann, P.M.; Sano, K.; McNamara, K.; Vacanti, J.P.; Langer, R. Biodegradable sponges for hepatocyte transplantation. J. Biomed. Mater. Res. 1995, 29, 959–965. [Google Scholar] [CrossRef]
- Burdett, E.; Kasper, F.K.; Mikos, A.G.; Ludwig, J.A. Engineering tumors: A tissue engineering perspective in cancer biology. Tissue Eng. Part B Rev. 2010, 16, 351–359. [Google Scholar] [CrossRef]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855–860. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Horch, R.E.; Loessner, D.; Rizzi, S.; Sieh, S.; Reichert, J.C.; Clements, J.A.; Beier, J.P.; Arkudas, A.; Bleiziffer, O.; et al. Translating tissue engineering technology platforms into cancer research. J. Cell. Mol. Med. 2009, 13, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Hutmacher, D.W.; Loessner, D.; Rizzi, S.; Kaplan, D.L.; Mooney, D.J.; Clements, J.A. Can tissue engineering concepts advance tumor biology research? Trends Biotechnol. 2010, 28, 125–133. [Google Scholar] [CrossRef]
- Sutherland, R.M. Cell and environment interactions in tumor microregions: The multicell spheroid model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.F.; Safiejko-Mroczka, B.; Starly, B. Long-term cultivation of HepG2 liver cells encapsulated in alginate hydrogels: A study of cell viability, morphology and drug metabolism. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2010, 24, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.T.; Lee, L.Q.; Leong, W.; Wang, D.A. Formation of model hepatocellular aggregates in a hydrogel scaffold using degradable genipin crosslinked gelatin microspheres as cell carriers. Biomed. Mater. 2012, 7, 065003. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Kievit, F.M.; Florczyk, S.J.; Veiseh, O.; Wu, J.; Park, J.O.; Zhang, M. Chitosan-alginate scaffold culture system for hepatocellular carcinoma increases malignancy and drug resistance. Pharm. Res. 2010, 27, 1939–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
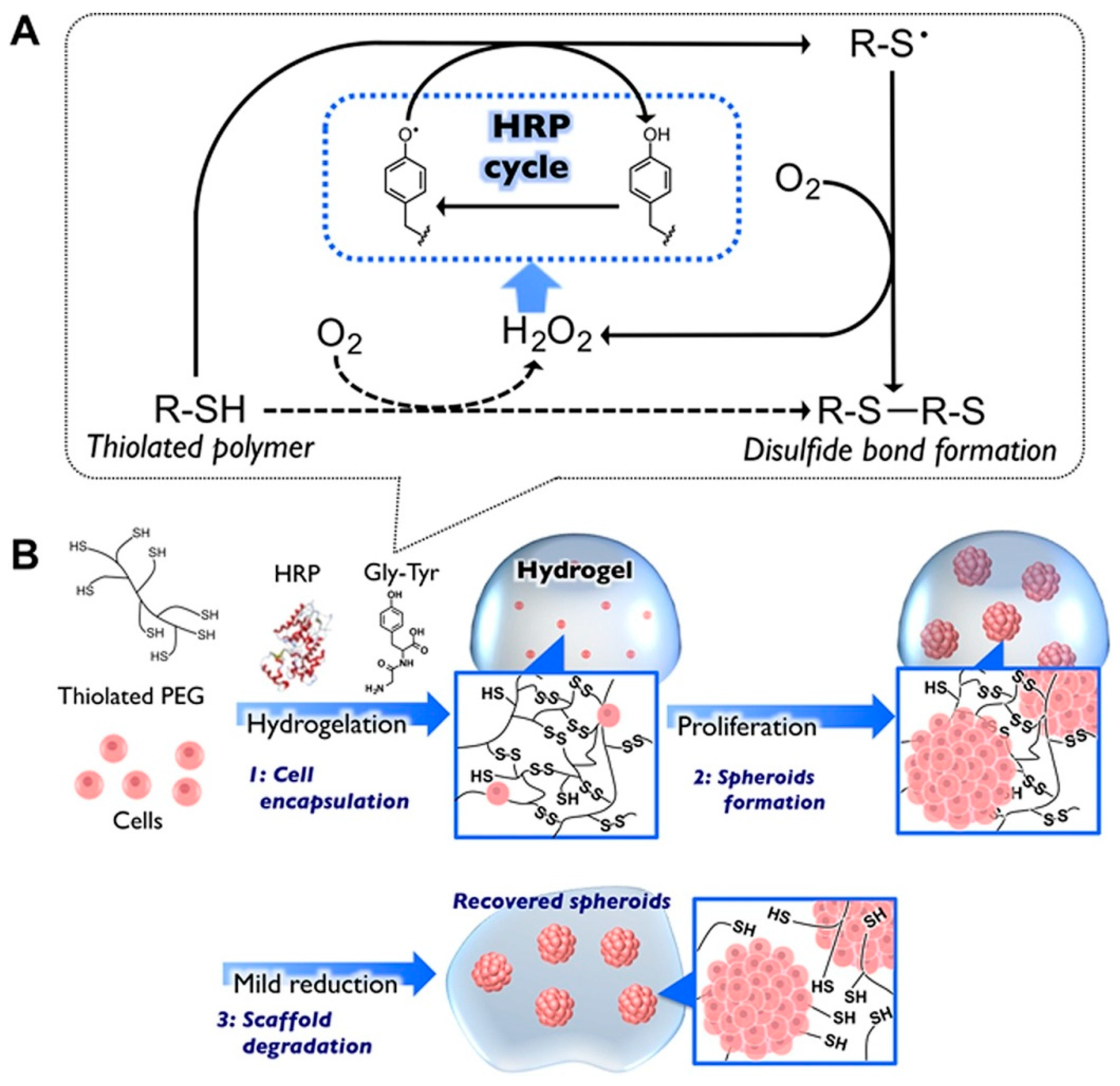
- Moriyama, K.; Naito, S.; Wakabayashi, R.; Goto, M.; Kamiya, N. Enzymatically prepared redox-responsive hydrogels as potent matrices for hepatocellular carcinoma cell spheroid formation. Biotechnol. J. 2016, 11, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Kumari, J.; Tonello, J.M.; Kamihira, M.; Kumar, A. Enhanced Hepatic Functions of Genetically Modified Mouse Hepatoma Cells by Spheroid Culture for Drug Toxicity Screening. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef]
- Yip, D.; Cho, C.H. A multicellular 3D heterospheroid model of liver tumor and stromal cells in collagen gel for anti-cancer drug testing. Biochem. Biophys. Res. Commun. 2013, 433, 327–332. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Menne, H.; Dittfeld, C.; West, J.; Mueller-Klieser, W.; Kunz-Schughart, L.A. Multicellular tumor spheroids: An underestimated tool is catching up again. J. Biotechnol. 2010, 148, 3–15. [Google Scholar] [CrossRef]
- Achilli, T.M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.B. Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Daster, S.; Amatruda, N.; Calabrese, D.; Ivanek, R.; Turrini, E.; Droeser, R.A.; Zajac, P.; Fimognari, C.; Spagnoli, G.C.; Iezzi, G.; et al. Induction of hypoxia and necrosis in multicellular tumor spheroids is associated with resistance to chemotherapy treatment. Oncotarget 2017, 8, 1725–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef] [PubMed]
- Mitry, R.R.; Jitraruch, S.; Iansante, V.; Dhawan, A. Alginate Encapsulation of Human Hepatocytes and Assessment of Microbeads. Methods Mol. Biol. 2017, 1506, 273–281. [Google Scholar] [CrossRef] [PubMed]
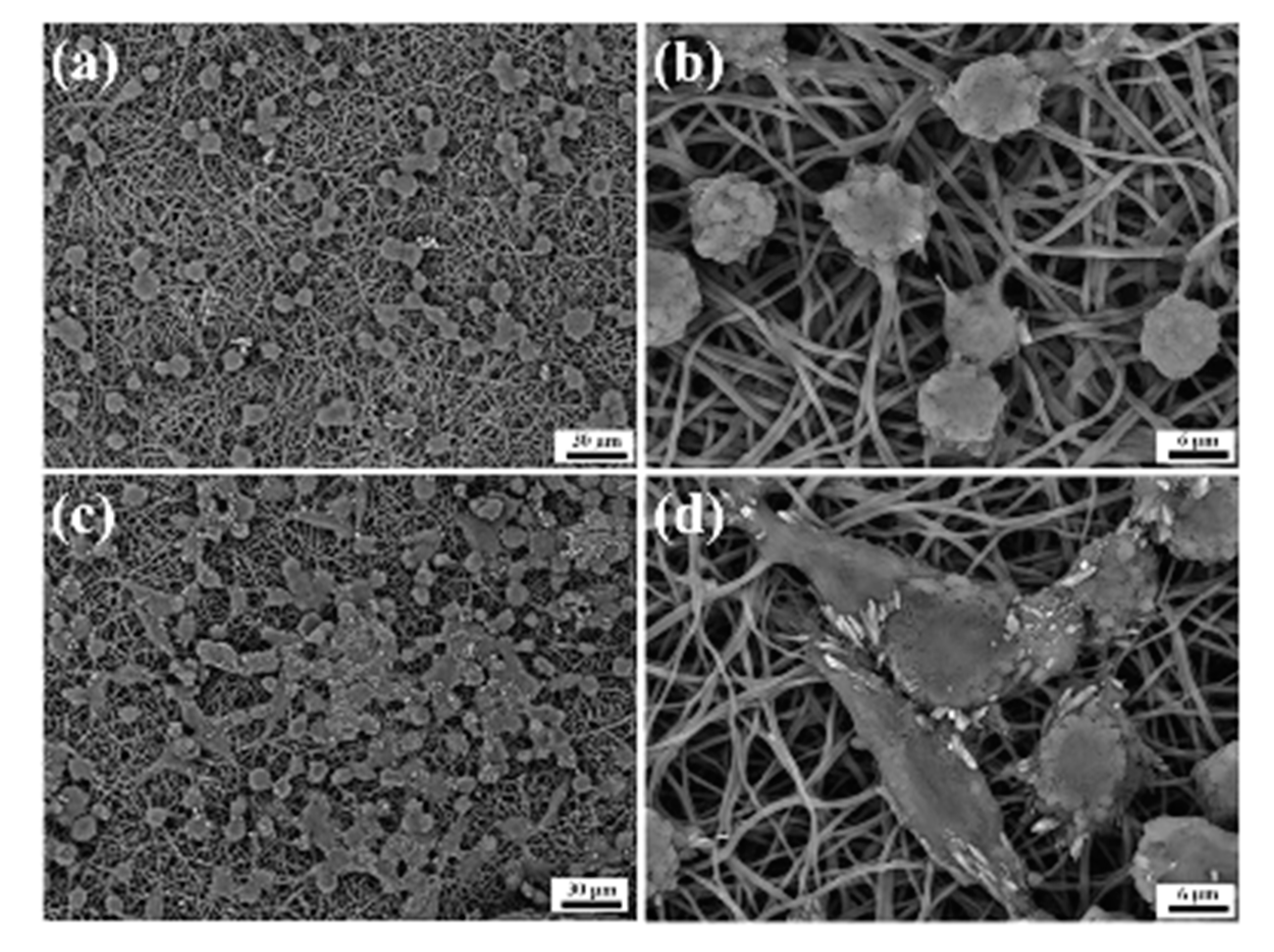
- Sun, D.; Liu, Y.; Wang, H.; Deng, F.; Zhang, Y.; Zhao, S.; Ma, X.; Wu, H.; Sun, G. Novel decellularized liver matrix-alginate hybrid gel beads for the 3D culture of hepatocellular carcinoma cells. Int. J. Biol. Macromol. 2018, 109, 1154–1163. [Google Scholar] [CrossRef]
- Benton, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int. J. Cancer 2011, 128, 1751–1757. [Google Scholar] [CrossRef]
- Bokhari, M.; Carnachan, R.J.; Cameron, N.R.; Przyborski, S.A. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge. J. Anat. 2007, 211, 567–576. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2008, 17, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Mikos, A.G.; Herring, S.W.; Ochareon, P.; Elisseeff, J.; Lu, H.H.; Kandel, R.; Schoen, F.J.; Toner, M.; Mooney, D.; Atala, A.; et al. Engineering complex tissues. Tissue Eng. 2006, 12, 3307–3339. [Google Scholar] [CrossRef]
- Gotoh, Y.; Ishizuka, Y.; Matsuura, T.; Niimi, S. Spheroid formation and expression of liver-specific functions of human hepatocellular carcinoma-derived FLC-4 cells cultured in lactose-silk fibroin conjugate sponges. Biomacromolecules 2011, 12, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Nagao, Y.; Nukui, T.; Akiyama, I.; Tsuru, K.; Hayakawa, S.; Osaka, A.; Huh, N.H. An organic-inorganic hybrid scaffold for the culture of HepG2 cells in a bioreactor. Biomaterials 2005, 26, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Saha, P.; Datta, K.; Kundu, S.C. A silk fibroin based hepatocarcinoma model and the assessment of the drug response in hyaluronan-binding protein 1 overexpressed HepG2 cells. Biomaterials 2013, 34, 9462–9474. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jeong, J.; DeVolder, R.J.; Cha, C.; Wang, F.; Tong, Y.W.; Kong, H. A cell-instructive hydrogel to regulate malignancy of 3D tumor spheroids with matrix rigidity. Biomaterials 2011, 32, 9308–9315. [Google Scholar] [CrossRef]
- Mazza, G.; Rombouts, K.; Rennie Hall, A.; Urbani, L.; Vinh Luong, T.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 13079. [Google Scholar] [CrossRef]
- Moscato, S.; Ronca, F.; Campani, D.; Danti, S. Poly(vinyl alcohol)/gelatin Hydrogels Cultured with HepG2 Cells as a 3D Model of Hepatocellular Carcinoma: A Morphological Study. J. Funct. Biomater. 2015, 6, 16–32. [Google Scholar] [CrossRef] [Green Version]
- She, Z.; Jin, C.; Huang, Z.; Zhang, B.; Feng, Q.; Xu, Y. Silk fibroin/chitosan scaffold: Preparation, characterization, and culture with HepG2 cell. J. Mater. Sci. Mater. Med. 2008, 19, 3545–3553. [Google Scholar] [CrossRef]
- Akay, G.; Birch, M.A.; Bokhari, M.A. Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 2004, 25, 3991–4000. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L. Tumor microenviroment and hepatocellular carcinoma metastasis. J. Gastroenterol. Hepatol. 2013, 28, 43–48. [Google Scholar] [CrossRef]
- Pinkse, G.G.; Jiawan-Lalai, R.; Bruijn, J.A.; de Heer, E. RGD peptides confer survival to hepatocytes via the beta1-integrin-ILK-pAkt pathway. J. Hepatol. 2005, 42, 87–93. [Google Scholar] [CrossRef]
- Price, Z.K.; Lokman, N.A.; Ricciardelli, C. Differing Roles of Hyaluronan Molecular Weight on Cancer Cell Behavior and Chemotherapy Resistance. Cancers 2018, 10, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, W.C.; Li, P.C.; Jeng, Y.M.; Hsu, H.C.; Kuo, P.L.; Li, M.L.; Yang, P.M.; Lee, P.H. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med. Biol. 2002, 28, 467–474. [Google Scholar] [CrossRef]
- Boudreau, N.; Weaver, V. Forcing the third dimension. Cell 2006, 125, 429–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Cascone, M.G.; Lazzeri, L.; Sparvoli, E.; Scatena, M.; Serino, L.P.; Danti, S. Morphological evaluation of bioartificial hydrogels as potential tissue engineering scaffolds. J. Mater. Sci. Mater. Med. 2004, 15, 1309–1313. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; Trombi, L.; D’Alessandro, D.; Coltelli, M.B.; Serino, L.P.; Pini, R.; Lazzeri, A.; Petrini, M.; Danti, S. Pore Size Distribution and Blend Composition Affect In Vitro Prevascularized Bone Matrix Formation on Poly(Vinyl Alcohol)/Gelatin Sponges. Macromol. Mater. Eng. 2017, 302, 1700300. [Google Scholar] [CrossRef]
- Lazzeri, L.; Cascone, M.G.; Danti, S.; Serino, L.P.; Moscato, S.; Bernardini, N. Gelatine/PLLA sponge-like scaffolds: Morphological and biological characterization. J. Mater. Sci. Mater. Med. 2007, 18, 1399–1405. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, Z.; Shen, M.; Shi, X. Capturing hepatocellular carcinoma cells using lactobionic acid-functionalized electrospun polyvinyl alcohol/polyethyleneimine nanofibers. RSC Adv. 2015, 5, 70439–70447. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.K.; Huch, M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chew, S.A.; Moscato, S.; George, S.; Azimi, B.; Danti, S. Liver Cancer: Current and Future Trends Using Biomaterials. Cancers 2019, 11, 2026. https://doi.org/10.3390/cancers11122026
Chew SA, Moscato S, George S, Azimi B, Danti S. Liver Cancer: Current and Future Trends Using Biomaterials. Cancers. 2019; 11(12):2026. https://doi.org/10.3390/cancers11122026
Chicago/Turabian StyleChew, Sue Anne, Stefania Moscato, Sachin George, Bahareh Azimi, and Serena Danti. 2019. "Liver Cancer: Current and Future Trends Using Biomaterials" Cancers 11, no. 12: 2026. https://doi.org/10.3390/cancers11122026
APA StyleChew, S. A., Moscato, S., George, S., Azimi, B., & Danti, S. (2019). Liver Cancer: Current and Future Trends Using Biomaterials. Cancers, 11(12), 2026. https://doi.org/10.3390/cancers11122026