Abstract
The Akt/mTOR signaling cascade is a critical pathway involved in various physiological and pathological conditions, including regulation of cell proliferation, survival, invasion, and angiogenesis. In the present study, we investigated the anti-neoplastic effects of casticin (CTC), identified from the plant Vitex rotundifolia L., alone and/or in combination with BEZ-235, a dual Akt/mTOR inhibitor in human tumor cells. We found that CTC exerted a significant dose-dependent cytotoxicity and reduced cell proliferation in a variety of human tumor cells. Also, CTC effectively blocked the phosphorylation levels of Akt (Ser473) and mTOR (Ser2448) proteins as well as induced substantial apoptosis. Additionally treatment with CTC and BEZ-235 in conjunction resulted in a greater apoptotic effect than caused by either agent alone thus implicating the anti-neoplastic effects of this novel combination. Overall, the findings suggest that CTC can interfere with Akt/mTOR signaling cascade involved in tumorigenesis and can be used together with pharmacological agents targeting Akt/mTOR pathway.
1. Introduction
Most people diagnosed with cancers are treated with chemotherapy, surgery, radiation therapy. They may also receive immunotherapy, targeted and hormonal therapies. Sometimes cancer can be controlled by surgery, but if the cancer has spread, the effect of surgical operations may be limited. In the case of radiation and chemotherapy, it is difficult to avoid side effects because these treatments can also affect normal rapidly growing cells [1,2]. Many novel cancer therapies are currently being developed worldwide and the goal is to achieve optimum treatment for the patients with relatively lower side effects. Natural compounds can be obtained from a variety of sources, including plants [3], animals [4], microorganisms [5], and can be employed as pharmacological agents for cancer treatment [6,7,8]. Thus, natural compounds with broad modes of action are being explored for their potential to treat various malignancies [4,9,10,11,12,13,14,15,16].
Casticin (CTC), also known as vitexicarpin or 3′,5-dihydroxy-3,4′,6,7-tetramethoxyflavone, is a member of the class of compounds known as 7-O-methylated flavonoids [17,18]. It has been found to be practically insoluble in water and a weak acidic compound [19]. CTC is one of the components of the plant Vitex agnus-castus L. and can be found in fruits, herbs and spices [20]. Prior studies have shown that CTC can suppress the proliferation in human myeloid leukemia cells [21,22], and induce substantial apoptosis in human gall bladder cancer cells [23], ovarian cancer cells [24], cervical cancer cells through the induction of Jun N-terminal kinase [25], as well as lung cancer cells via mitochondrial pathway. CTC can also enhance tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL). apoptosis in human colon cancer cells [26]. In addition, CTC can exert anti-inflammatory effects in preclinical models [27,28], and can abrogate cellular migration in mouse melanoma cells [29,30]. Here, this study was designed to explore the anti-cancer activities of CTC on a variety of human cancer cells and investigate the potential mechanisms underlying its actions.
The Akt/mTOR is an intracellular signaling pathway that is crucial for regulating both the cell cycle and tumorigenesis. It can also mediate many aspects of cellular functions, including nutrient uptake, cell proliferation and survival [31]. It has been demonstrated that frequent overactivation of Akt/mTOR is often encountered in several types of solid tumors and in hematological malignancies [32,33,34,35,36,37,38,39]. This pathway may be activated by number of receptor tyrosine kinases, including the epidermal cell growth factor receptor (EGFR) family and insulin-like growth factor receptor (IGFRs). AKT, also known as protein kinase B (PKB), is known to be the central node of this signaling pathway, and can be phosphorylated at Thr308 by PDK1 and at Ser473 by mTOR complex 2 (mTORC2), which increases its kinase activity [40]. Activated Akt can regulate cellular processes including cell survival, proliferation and growth and act downstream of PI3K [41]. mTOR (mammalian target of rapamycin) is a major protein in this pathway that acts both upstream and downstream of AKT [42]. It is active component of multi protein complex, target of rapamycin complex TORC1 and TORC2 [33], and regulates protein synthesis necessary for cellular growth, proliferation, angiogenesis and other cellular functions [43]. Since Akt/mTOR pathway can be involved in several important processes as described above, identification of active drugs targeting this pathway can be expected to have a major impact on various therapeutic strategies against cancer.
In this work we analyzed whether CTC can exert its anticancer effects against diverse human cancer cells and the potential molecular mechanisms involved in its action. We also sought to determine whether modulation of the Akt/mTOR signaling pathway, in particular by CTC, could mediate its anti-neoplastic actions against tumor cells. Also, the combinatorial anticancer potential of CTC along with pharmacological dual phosphatidylinositol 3-kinase (PI3K)-mTOR inhibitor, BEZ-235 was systematically examined in cancer cells.
2. Results
2.1. CTC Inhibits Cellular Growth in Several Human Cancer Cells
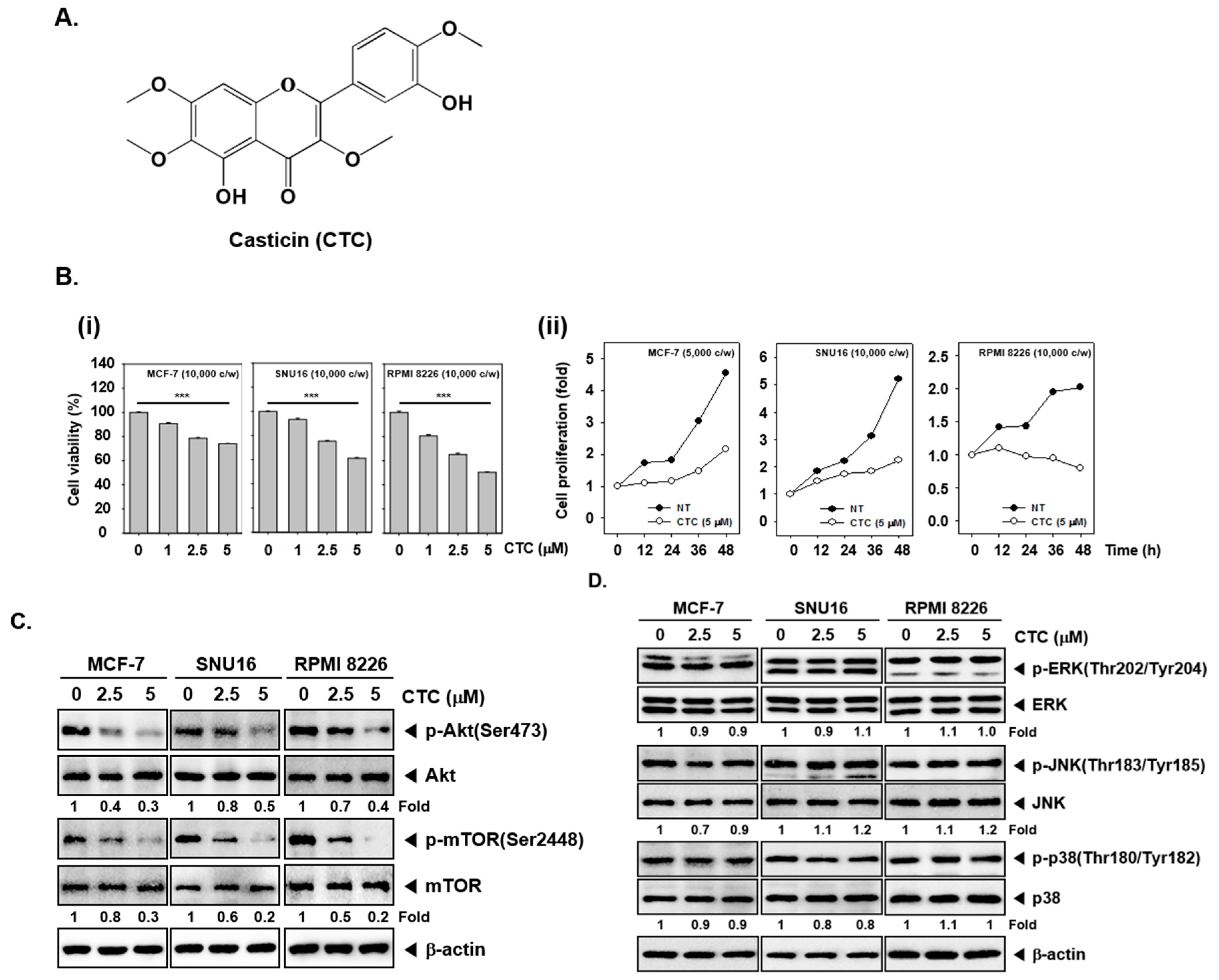
To evaluate the effects of these CTC on the growth of human different cell lines, the inhibitory potential of CTC on viability was determined in human breast cancer MCF-7 cells, gastric cancer SNU16, and myeloma RPMI 8226 cells. We found that the cell viability decreased in a dose-dependent manner in cells treated with CTC. The cytotoxicity was 26% in MCF-7 cells, 39% in SNU16 cells, and 49% in RPMI8226 cells respectively, after treated with 5 μM CTC compared to non-treated group. The IC50 values ranging from 6 to 8.5 µM (8. 5 µM for MCF-7, 7 µM for SNU16, 6 µM for RPMI8226) (Figure 1B-i). Interestingly, the data also showed that CTC inhibited cell proliferation in in a time-dependent manner in three cancer cell lines (Figure 1B-ii).
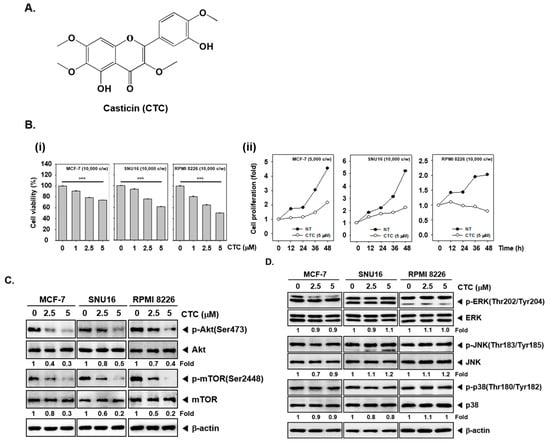
Figure 1.
CTC inhibits cell viability and proliferation through Akt/mTOR signaling pathway in several cancer cells. (A) The chemical structure of casticin (CTC). (B-i) Effect of CTC on cell viability. Several cancer cells MCF-7, SNU16, and RPMI 8226 (1 × 104 cells/well) were treated with the indicated concentrations of CTC for 24 h. Thereafter, cell viability was determined by MTT assay. (B-ii) Effect of CTC on cellular proliferation. MCF-7, SNU16 and RPMI 8226 cells (1 × 104 cells/well) were treated with 5 µM of CTC for the indicated times. The cell proliferation was measured using the MTT assay. Abbreviation: NT = non-treated and c/w = cells per wells. (C) Effect of CTC on Akt signaling cascade. The cells were treated with the indicated concentrations of CTC for 9 h. Whole-cell extracts were prepared, and subjected to western blot analysis using antibodies against p-Akt(Ser473), Akt, p-mTOR(Ser2448), mTOR. (D) Equal amounts of lysates were analyzed by western blot analysis as described in panel C above.
2.2. CTC Suppresses Activation of Akt/mTOR Signaling Pathway
We investigated the effect of CTC on the Akt/mTOR and MAPKs signaling pathways, which are closely associated with cell proliferation and survival in tumor cell lines. Interestingly, the phosphorylation levels of Akt and mTOR were markedly decreased by CTC in MCF-7, SNU16, and RPMI 8226 cells (Figure 1C); however, phosphorylation level of members of mitogen activated protein kinases (MAPKs) signaling cascade, such as ERK, JNK, and p38 remained unchanged (Figure 1D).
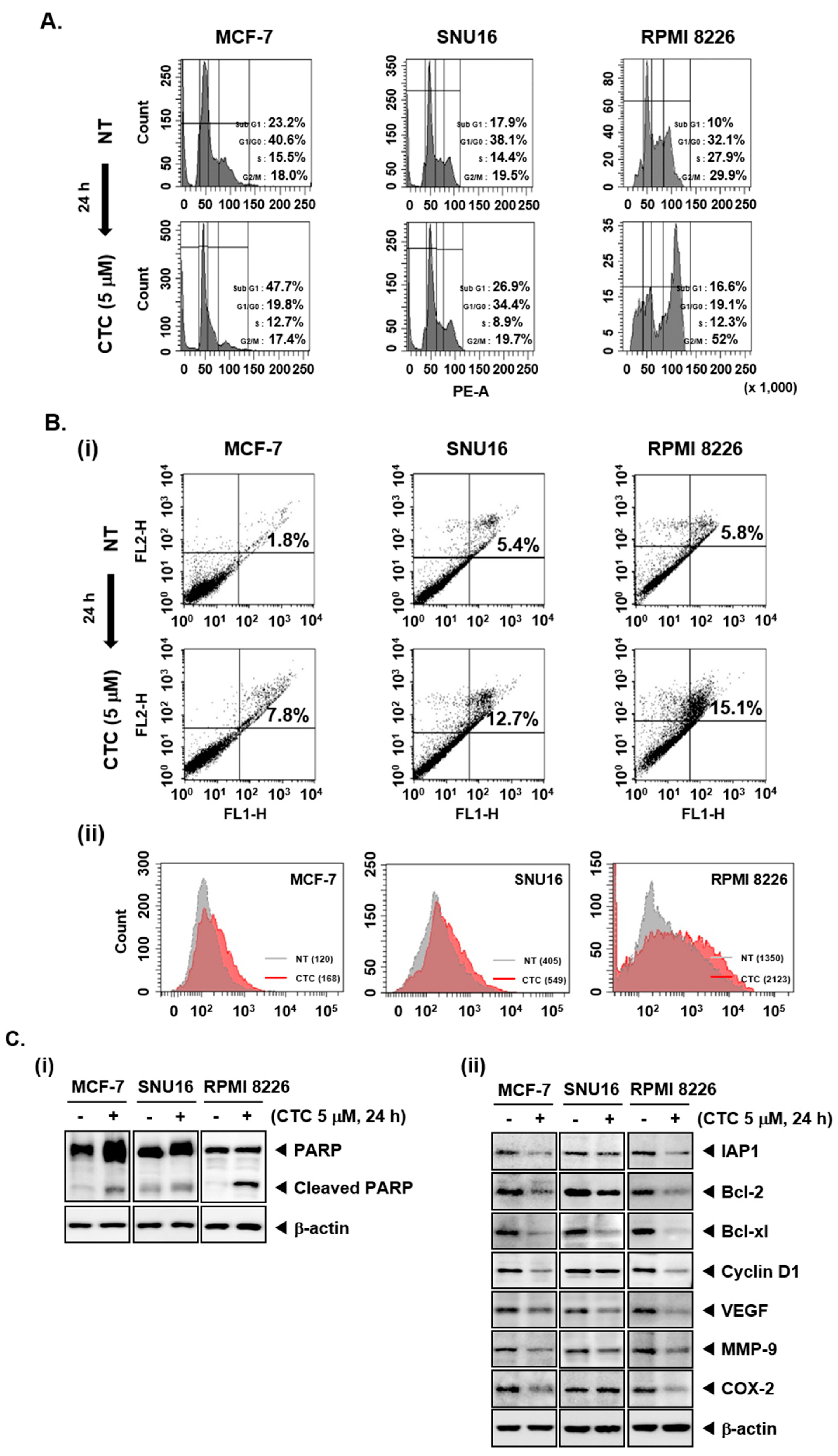
2.3. CTC Increases Accumulation of Cells in Sub-G1 Phase and Induces Apoptosis
As the cells accumulated in the Sub-G1 phase represent the apoptotic population [44], flow cytometry was first performed to study the pro-apoptotic effect of CTC in MCF-7, SNU16, and RPMI 8226 cells. An increase in the percentage of the cells in sub-G1 phase compared with controls was noted, thereby suggesting that these cells may undergo apoptosis (Figure 2A). In addition, after treatment with 5 µM of CTC, annexin V staining was carried out by flow cytometry. As shown in Figure 2B-i, the percentage of apoptotic cells were increased compared with non-treated population in MCF-7, SNU16, and RPMI 8226 cells. Moreover, as shown in Figure 2B-ii, highly significant differences in mean values between NT and CTC treated groups for TUNEL positive cell population was noted. The mean value was found to increase from 120 to 168, 405 to 549, and 1350 to 2123 in MCF-7, SNU16, and RPMI 8226 cells, respectively. These results confirm that CTC treatment caused substantial apoptosis in tumor cell lines.
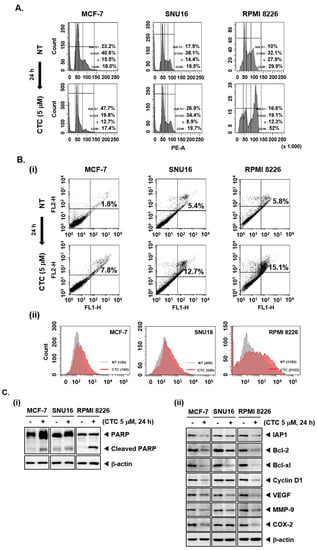
Figure 2.
CTC induces apoptosis and reduces expression of Akt/mTOR regulated gene products. (A) The cells were treated with 5 µM of CTC for 24 h, harvested, stained with propidium iodide (PI), and analyzed using a flow cytometer. (B-i) The cells were treated with 5 µM of CTC for 24 h, harvested, stained with FITC-conjugated anti-Annexin V, and analyzed using a flow cytometer. (B-ii) The cells were treated with 5 µM of CTC for 24 h, harvested, fixed, incubated with a TUNEL reaction solution, and analyzed using a flow cytometer. (C-i,ii) The cells were treated with 5 µM of CTC for 24 h and western blot analysis was performed as described above in panel 1C. Abbreviation: NT = non-treated.
2.4. CTC Induces Apoptosis via the Suppression of Various Oncogenic Proteins
We also examined the effect of CTC on the proteolytic cleavage of poly (ADP-ribose) polymerase (PARP) which is regarded as an important indicator of apoptosis [45]. CTC treatment resulted in substantial PARP cleavage in MCF-7, SNU16, and RPMI 8226 cells (Figure 2C-i). Additional western blot analysis showed that CTC also markedly suppressed the expression of anti-apoptotic proteins (IAP1, Bcl-2, and Bcl-xl), the cell cycle regulator protein (Cyclin D1), angiogenic gene product (VEGF), metastatic gene product (MMP-9), and the inflammatory protein (COX-2) (Figure 2C-ii).
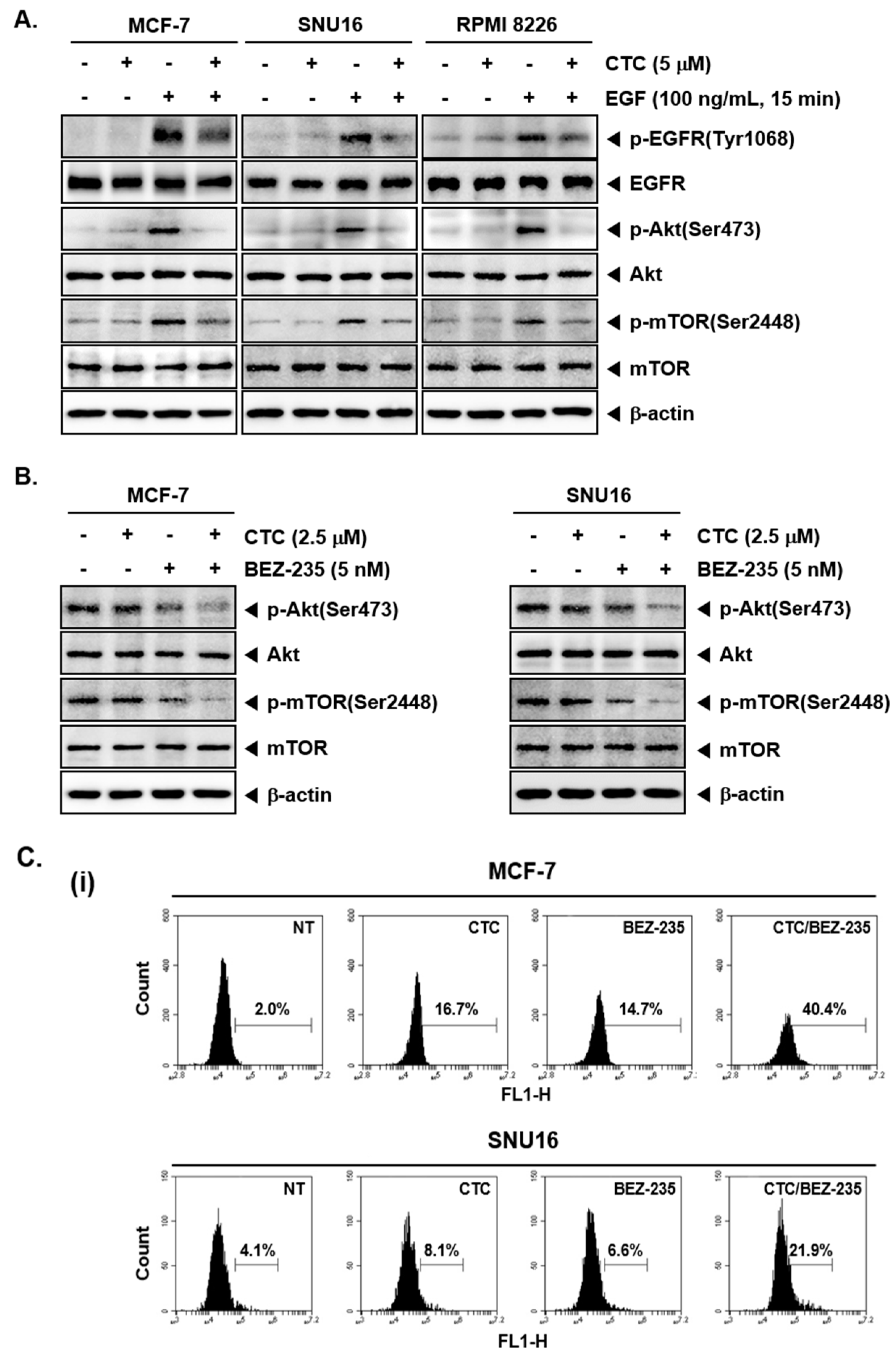
2.5. Pre-Treatment with CTC Abrogates EGF-Induced Oncogenic Signaling Cascade
Epidermal growth factor (EGF) stimulates cell growth and differentiation by binding to its receptor, EGFR [46,47,48] in order to induce the activation of downstream Akt and mTOR signaling pathways [49]. To further investigate the effect of CTC on the EGFR signaling pathway, MCF-7 and SNU16 cells were starved for 12 h with serum free culture and then treated with CTC in the presence or absence of EGF. Interestingly, in EGF-stimulated cells, the phosphorylation levels of EGFR, Akt, and mTOR proteins were significantly increased compared with the respective control group. However, this increase was substantially attenuated upon exposure to CTC (Figure 3A).
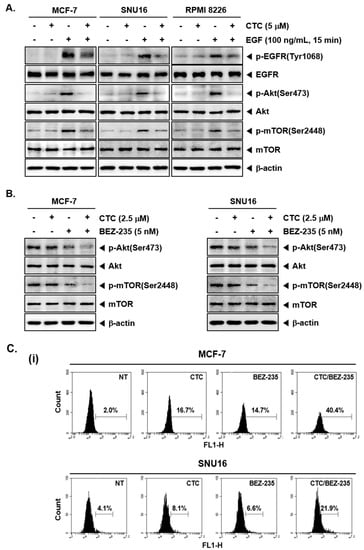
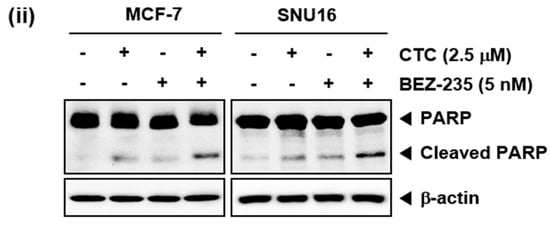
Figure 3.
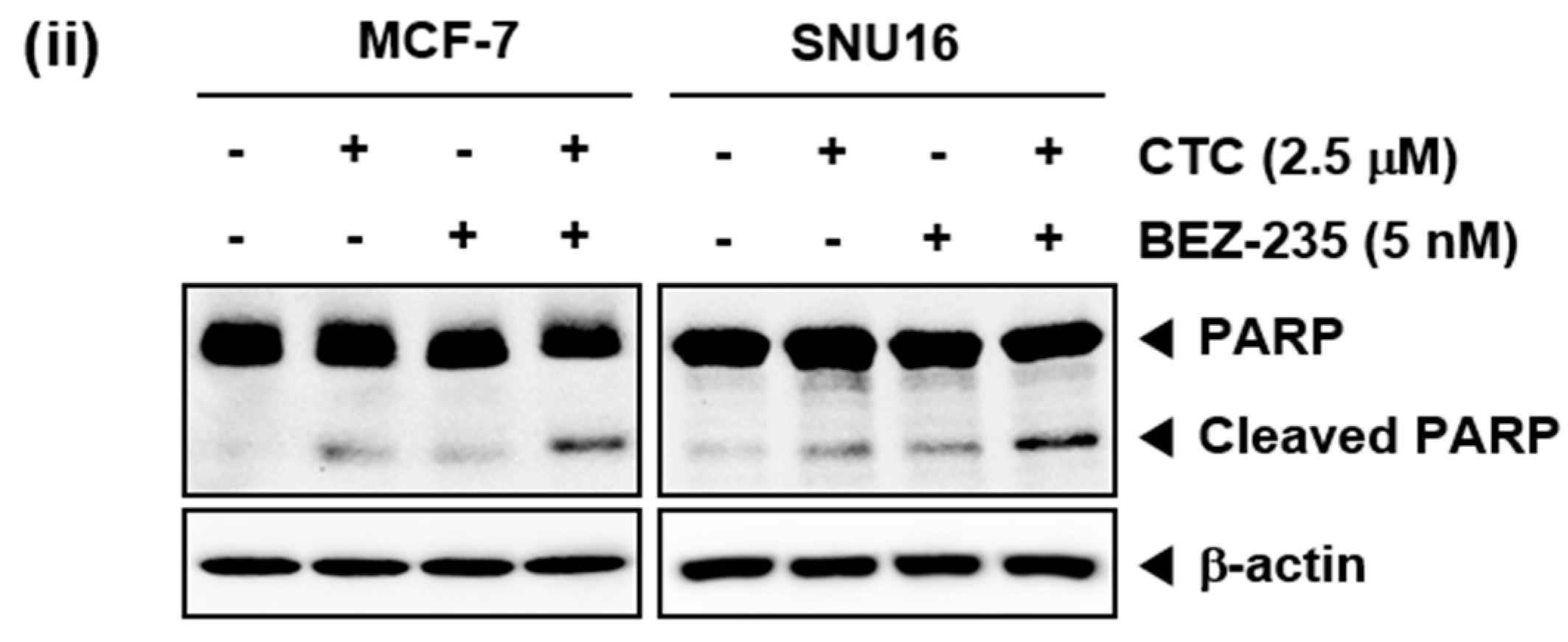
CTC suppresses EGF-stimulated Akt/mTOR signaling pathway. (A) MCF-7, SNU16, and RPMI 8226 cells were serum starved 12 h and treated with 5 µM of CTC for 9 h, followed by stimulation with 100 ng/mL of EGF for 15 min and western blot analysis was performed as described above in panel 1C. (B) The cells were treated with 2.5 µM of CTC alone or combined with 5 nM BEZ-235 for 9 h. Whole-cell extracts were prepared, and western blot analysis was performed as described above in panel 1C. (C-i) The cells were treated with 2.5 µM of CTC alone or in combination with 5 nM BEZ-235 for 24 h and TUNEL staining done as described in panel 2B-ii above. (C-ii) The cells were treated with 2.5 µM of CTC alone or combined with 5 nM BEZ-235 for 24 h and western blot analysis was performed as described above in panel 1C.
2.6. CTC and BEZ-235 Efficiently Inhibits Akt/mTOR Signaling Pathway
BEZ-235 has been reported to attenuate activation of PI3K/mTOR signaling proteins and induce apoptosis as a dual PI3K-mTOR inhibitor [50]. In our study, western blot analysis revealed that BEZ-235 at sub-optimal doses slightly blocked the expression of p-Akt and p-mTOR, whereas, BEZ-235 in combination with CTC substantially inhibited the expression of p-Akt and p-mTOR in these cells (Figure 3B).
2.7. CTC and BEZ-235 Can Exhibit Enhanced Apoptosis in MCF-7 and SNU16 Cells
We next determined the percentage of apoptotic cells by the well-established TUNEL assay, which enables the visualization of apoptotic cells using an in situ end-labelling technique that labels DNA breaks in apoptotic cells [51]. As shown in Figure 3C, CTC alone, at 2.5 µM, resulted in 16.7% apoptosis, while treatment with BEZ-235 alone, at 5 nM, led to 14.7% apoptosis. However the combinatorial application of both agents produced a greater effect, resulting in 40.4% apoptosis in MCF-7 cells (Figure 3C-i Top). In SNU16 cells, exposure to CTC alone resulted in 8.1% cell apoptosis, whereas treatment with BEZ-235 alone led to 6.6% cell apoptosis. However, exposure of cells to the combination of CTC and BEZ-235 resulted in 21.9% apoptosis (Figure 3C-i bottom). Furthermore, we observed that PARP cleavage were further increased by the combination treatment of two drugs together (Figure 3C-ii).
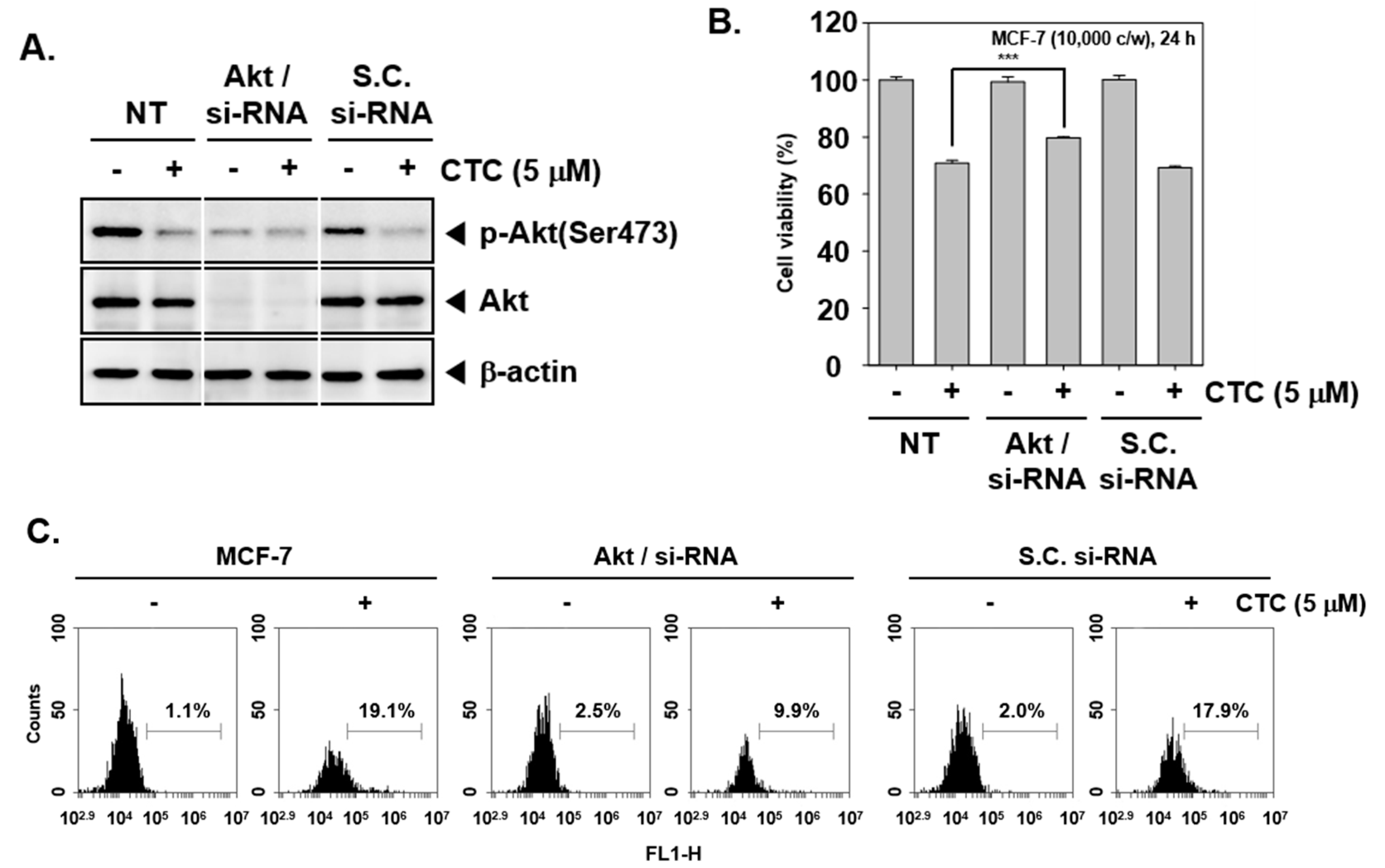
2.8. Knockdown of Akt by si-RNA Reverses the Growth Inhibitory and Apoptotic Effects of CTC
To provide a direct evidence that apoptotic effects induced by CTC are due to inhibition of Akt pathway, transient transfections were carried out using Akt/si-RNA and scrambled si-RNA (control) in MCF-7 cells. The data demonstrated that Akt expression was substantially blocked upon transfection with Akt/si-RNA (Figure 4A). Subsequently, When Akt/si-RNA and scrambled si-RNA transfected MCF-7 cells were treated with CTC for 24 h, cell viability were performed using MTT assay. As shown in Figure 4B, MCF-7 cells transfected with scrambled si-RNA, cell viability were reduced to 69% upon treatment with CTC. In contrast, transfection of CTC-treated cells with Akt/si-RNA slightly inhibited CTC-induced reduction in cell viability as compared to the control group.
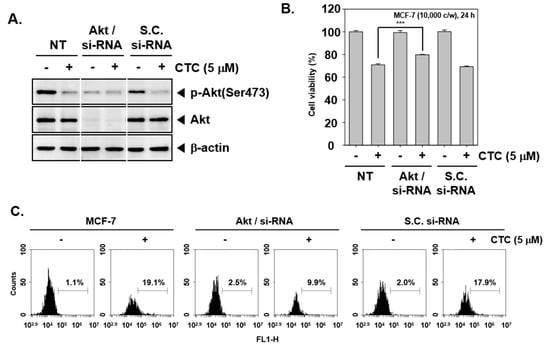
Figure 4.
The role of Akt in growth inhibitory effects of CTC. (A) MCF-7 cells were transiently transfected with Akt/si-RNA or scrambled si-RNA (control). Then, whole cell extracts were prepared and western blot analysis was performed as described above in panel 1C. (B) Akt/scrambled si-RNA transfected MCF-7 cells were treated with 5 µM of CTC for 24 h. Thereafter, cell viability was measured using MTT assay; (C) Cellular apoptosis was determined using TUNEL assay.
Also, as shown in Figure 4C, upon transfection with scrambled si-RNA drug treatment alone resulted in substantial apoptosis (19.1%) compared with the control group (1.1%), whereas only 9.9% of the cells in the knockdown group were found to be apoptotic.
3. Discussion
We report here that CTC can significantly inhibit the cell viability of three different tumor cell lines (breast, gastric, myeloma cancer cells). These findings are in agreement with prior reports where CTC was observed to suppress the proliferation of diverse tumor cells [52]. The Akt/mTOR pathway has been reported to be frequently deregulated in human cancers, regulating the apoptotic response through its ability to interact with a number of key players in the apoptotic process [37]. We found that CTC can effectively suppress the Akt activation at Ser residue 473 in a concentration-dependent manner in MCF-7, SNU16, and RPMI 8226 cells, which may contribute to its anticancer activity. A previous study has reported that CTC can also suppress self-renewal and invasion through the negative regulation of Akt signaling pathway in lung cancer cells. [53]. Our results also indicate that CTC abrogated the mTOR activation at Ser residue 2448 in tumor cells.
Mitogen-activated protein kinases (MAPKs) such as ERK, JNK and p38 can regulate tumorigenesis and associated processes of proliferation, migration and survival. The Akt/mTOR and the MAPKs signaling pathways can be concurrently constitutively activated in several human cancers [54] and possible cross talks between these two cascades can drive tumor progression [55]. Previous reports have shown that CTC can inhibit phosphorylation of Akt, PI3K and MAPK in lung epithelial cells [56]. Thus, we also examined the ability of CTC to modulate MAPK signaling cascades but noted that this agent did not affect activation of ERK, JNK, and p38 proteins in tumor cells analyzed.
The cell cycle is a conserved mechanism by which eukaryotic cells replicate themselves. This can be divided into three stages: interphase, mitotic stage (M) phase, and cytokinesis. During interphase (G1, S, G2), cells grows, accumulating nutrients needed for mitosis, and replicate DNA. In M phase, the chromosomes are separated and during the final stage, cytokinesis, the chromosomes and cytoplasm are separated into two new daughter cells. Cells that have stopped dividing are known to enter a quiescent state called the G0 phase [57]. According to the literature, CTC can induce apoptosis through causing cell cycle arrest in oral cancer cells [58]. We also noted that CTC can induce accumulation of the cells in the sub G1 phase of cell cycle in and induce apoptosis in MCF-7, SNU16, and RPMI 8226 cells as evident by positive annexin V and TUNEL staining. Previous studies have also reported that CTC can induce early cell death in a concentration-dependent manner in bladder cancer NOZ and SGC996 cells [23]. Moreover, CTC also inhibited the expression of Akt/mTOR-controlled gene products such as anti-apoptotic (IAP2, Bcl-2, and Bcl-xl), cell cycle regulator (Cyclin D1), angiogenetic (VEGF), metastatic (MMP-9), and inflammation (COX-2). Interestingly, we also noted that the deletion of Akt by si-RNA can effectively abrogate the observed apoptotic effects of CTC, thereby indicating that downregulation of various oncogenic proteins may be caused by direct modulation of Akt activation by CTC.
BEZ-235 is a dual PI3K-mTOR inhibitor that can target activation of PI3K and mTOR kinases and has been actively used against various cancers [59]. It is well tolerated, exhibits disease arrest upon oral administration, and improves the efficacy of other anticancer drugs when used in combinatorial setting [60]. Moreover, it has been found that BEZ-235 can synergistically potentiate the antitumor effects of cisplatin in bladder cancer cells though the cell cycle progression [61]. We noted that CTC in combination with BEZ-235 can effectively down modulate the phosphorylation of AKT/mTOR proteins and induce substantial apoptosis in tumor cells. This finding is quite intriguing as combinatorial antineoplastic effects of various flavonoids have been previously reported with different anti-cancer agents commonly used in the clinic [62,63,64,65,66]. Our group has also reported that isorhamnetin, a methylated metabolite of dietary flavonoid quercetin, can abrogate the activation of master transcription factor NF-κB [67,68,69,70,71,72] and thus significantly enhance the anti-tumoral effects of capecitabine in gastric cancer xenograft mouse model [72]. Overall, our data suggested that CTC can be potentially employed in combination therapy against malignancies, however these results have to be further validated in preclinical studies.
4. Materials and Methods
4.1. Reagents
Casticin (CTC, Figure 1A) was purchased from Biopurify Phytochemicals Ltd. (Sichuan, China). Stock solution of CTC (100 mM) was prepared in dimethyl sulfoxide, stored at −80 °C, and diluted in cell culture medium for use. Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), sodium dodecyl sulfate (SDS), and ribonuclease A from bovine pancreas were purchased from Sigma–Aldrich (St. Louis, MO, USA). Bovine serum albumin was purchased from Biosesang (Sungnam, Korea). RPMI1640 media, fetal bovine serum (FBS), and antibiotic-antimycotic mixture were obtained from Thermo Scientific HyClone (Waltham, MA, USA). ApoScanTM Annexin V FITC apoptosis detection kit was purchased from bio-bud (Seoul, Korea). TUNEL enzyme and TUNEL label were purchased Roche (Basel, Switzerland). BEZ-235 obtained from Selleckchem (Houston, TX, USA). Acryl-bisacrylamide (29:1) was obtained from ELPIS Biotech (Daejeon, Korea).
4.2. Cell Lines and Culture Conditions
Human breast adenocarcinoma cell line MCF-7 cells and human myeloma cell line RPMI 8226 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Human gastric carcinoma SNU16 cells were purchased from the Korean Cell Line Bank (Seoul, Korea). All cells were cultured in RPMI 1640 medium containing 10% FBS and 1% penicillin-streptomycin (P/S). The cells were maintained at 37 °C in a humidified atmosphere 5% CO2. All the cultures were routinely tested and were mycoplasma-free.
4.3. Cell Viability Assays
To evaluate cell viability, MCF-7, SNU16, and RPMI 8226 cells were seeded (1 × 105 cells/well) in a 96-well plate, and incubated at 37 °C for 12 h. The cells were treated with various concentrations of CTC for 24 h. Thereafter, 20 µL of MTT solution (2 mg/mL in PBS) was added to each well and incubated at 37 °C in the dark. After 2 h incubation, a MTT lysis solution (20% SDS, 50% dimethylformamide) was added to each well and incubated at 37 °C for overnight, and the absorbance was then measured at 570 nm by a Varioskan LUX multimode microplate reader (Thermo Scientific). Cell viability was expressed relative to untreated control cells.
4.4. Western Blot Analysis
To assess the effects of Akt/mTOR inhibitors and apoptosis MCF-7, SNU16, and RPMI8226 cells were treated with 0, 2.5, and 5 µM of CTC for 9 h or 24 h. To determine the inhibitory effect of EGF-induced Akt/mTOR inhibitors, MCF-7 and SNU16 cells were treated with 100 ng/mL of EGF for 15 min after pretreatment with 5 µM of CTC for 9 h. To evaluate the combined effect of CTC and BEZ-235, MCF-7 and SNU16 cells were treated with 2.5 µM of CTC or 5 nM of BEZ-235 for 9 h. Whole-cell extracts were lysed in a lysis buffer (20 mM Tris (pH 7.4), 250 mM NaCl, 2 mM EDTA (pH 8.0), 0.1% Triton X-100, 0.01 mg/mL aprotinin, 0.005 mg/mL leupeptin, 0.4 mM phenyl methane sulfonyl fluoride (PMSF), and 4 mM NaVO4) for 1 h. The lysates were then spun at 14,000 rpm for 20 min to remove insoluble material, and supernatant was transferred to a fresh tube kept on ice. Protein concentrations was performed using a Bradford protein assay [73]. Bovine serum albumin (BSA) was used as a protein standard. Equal amounts of protein (10 µg) were separated by 8–12% SDS-PAGE and electro-transferred onto nitrocellulose membrane and western blot analysis was carried out as described before [74]. Antibodies against p-Akt(Ser473), Akt, p-mTOR(Ser2448), mTOR, p-ERK (Thr202/Tyr204), ERK, p-JNK(Thr183/Tyr185), JNK, p-p38(Thr180/Tyr182), p38, PARP, Caspase-3, IAP1, Bcl-2, Bcl-xl, Cyclin D1, VEGF, MMP-9, COX-2, p-EGFR(Tyr1068), EGFR, and β-actin were used for western blots. Repeated experiments were performed twice to obtain quantitative data. Quantification of band intensities for each represented blot was performed using Image J software (National Institutes of Health (NIH), Bethesda, MD, USA).
4.5. Cell Cycle Analysis
To determine apoptosis, cell cycle analysis was performed using propidium iodide (PI) staining. Briefly the MCF-7, SNU16, and RPMI8226 cells were treated with 5 µM of CTC for 24 h, and then the cells were harvested, washed with cold PBS. Cell pellets were fixed with 70% cold ethanol overnight at 4 °C. The fixed cells were resuspended in 1× PBS containing 1 mg/mL RNase A, incubated for 1 h at 37 °C incubation. Cells were then washed, resuspended, and stained in PBS containing 25 µg/mL of PI for 30 min at room temperature in the dark. Stained samples were analyzed by BD Accuri C6 plus flow cytometer (BD Biosciences, San Diego, CA, USA). Acquisition and analysis of the data were performed using BD Accuri C6 plus software (version 1.0.23.1).
4.6. Annexin V and TUNEL Assays
The MCF-7, SNU16, and RPMI 8226 cells were treated with 5 µM of CTC for 24 h. Apoptosis was evaluated by annexin V-FITC and propidium iodide (PI) stained cells using a FITC annexin V Apoptosis Detection Kit I according to the manufacturer’s protocols. Briefly, the cells were harvested using 1% trypsin in PBS. The cell pellet was resuspended in 1× binding buffer add 5 µL of FITC Annexin V and 5 µL of PI for 15 min at room temperature in the dark. Stained samples were analyzed by BD Accuri C6 plus flow cytometer (BD Biosciences). Acquisition and analysis of the data were performed using BD Accuri C6 plus software (version 1.0.23.1).
4.7. Drug Combination Analyses
MCF-7 and SNU16 cells were drug-treated for 24 h with CTC, BEZ-235 or their combination; cytotoxicity was measured by MTT assay. Synergy or antagonism were determined with computer software CalcuSyn for windows (Biosoft, Cambridge, UK). In this system, synergism, additivity, or antagonism is defined by the combination index; a CI value <1 indicates the synergistic effect, a CI value of 1 indicates an additive effect and a CI value >1 indicates an antagonistic effect.
4.8. siRNA Transfection
siRNA transfection was performed as described before [74].
4.9. Statistical Analysis
Data are expressed as the mean ± S.D. In all figures, vertical error bars denote the S.D. The significance of differences between groups was evaluated by Student’s t-test and one way analysis of variance, (ANOVA) test. The p value of less than 0.05 was considered statistically significant.
5. Conclusions
CTC inhibited the survival and proliferation of diverse cancer cells as well as down-regulated Akt/mTOR signaling pathway and suppressed various proteins involved in anti-apoptosis, metastasis, and angiogenesis. In addition, the combinatorial treatment of CTC and BEZ-235 exhibited a significant apoptotic effects against neoplastic cells. Overall, our results conclusively demonstrate that CTC can function as a potential inhibitor of tumor cell survival and proliferation by negatively regulating Akt/mTOR activation.
Author Contributions
J.H.L., C.K.: Data Curation and formal analysis; J.-Y.U.: Formal analysis and supervision; G.S. and K.S.A.: Supervision and writing original draft.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2015R1A4A1042399, 2017R1A6A3A11031224 and 2018R1D1A1B07042969).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
MTT, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; ICC, Immunocytochemistry; RTCA, real-time cell analysis; SDS-PAGE, sodium dodecyl-polyacrylamide gel electrophoresis; HRP, horseradish peroxidase; ECL, enhanced chemiluminescence.
References
- Tolentino Ede, S.; Centurion, B.S.; Ferreira, L.H.; Souza, A.P.; Damante, J.H.; Rubira-Bullen, I.R. Oral adverse effects of head and neck radiotherapy: Literature review and suggestion of a clinical oral care guideline for irradiated patients. J. Appl. Oral Sci. 2011, 19, 448–454. [Google Scholar] [PubMed]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Christen, P.; Cuendet, M. Plants as a source of therapeutic and health products. Chimia (Aarau) 2012, 66, 320–323. [Google Scholar] [CrossRef]
- Zaffiri, L.; Gardner, J.; Toledo-Pereyra, L.H. History of antibiotics. From salvarsan to cephalosporins. J. Investig. Surg. 2012, 25, 67–77. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sethi, G.; Baladandayuthapani, V.; Krishnan, S.; Shishodia, S. Targeting cell signaling pathways for drug discovery: An old lock needs a new key. J. Cell. Biochem. 2007, 102, 580–592. [Google Scholar] [CrossRef]
- Deorukhkar, A.; Krishnan, S.; Sethi, G.; Aggarwal, B.B. Back to basics: How natural products can provide the basis for new therapeutics. Expert Opin. Investig. Drugs 2007, 16, 1753–1773. [Google Scholar] [CrossRef]
- Yang, S.F.; Weng, C.J.; Sethi, G.; Hu, D.N. Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid. Based Complement. Altern. Med. 2013, 2013, 698190. [Google Scholar] [CrossRef]
- Tang, C.H.; Sethi, G.; Kuo, P.L. Novel medicines and strategies in cancer treatment and prevention. Biomed. Res. Int. 2014, 2014, 474078. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Yang, S.F.; Sethi, G.; Hu, D.N. Natural bioactives in cancer treatment and prevention. Biomed. Res. Int. 2015, 2015, 182835. [Google Scholar] [CrossRef] [PubMed]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Hasanpourghadi, M.; Looi, C.Y.; Pandurangan, A.K.; Sethi, G.; Wong, W.F.; Mustafa, M.R. Phytometabolites Targeting the Warburg Effect in Cancer Cells: A Mechanistic Review. Curr. Drug Targets 2017, 18, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Warrier, S.; Kumar, A.P.; Sethi, G.; Arfuso, F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017, 15, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Zhao, B.J.; Liu, J.; Liu, B.; Sun, J.X.; Li, J.; Li, X.M. Molecular mechanisms of casticin action: An update on its antitumor functions. Asian Pac. J. Cancer Prev. 2014, 15, 9049–9058. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Ko, J.H.; Jung, Y.Y.; Jung, S.H.; Kim, E.; Kong, M.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; et al. Casticin inhibits growth and enhances ionizing radiation-induced apoptosis through the suppression of STAT3 signaling cascade. J. Cell. Biochem. 2018. [Google Scholar] [CrossRef]
- Mesaik, M.A.; Azizuddin; Murad, S.; Khan, K.M.; Tareen, R.B.; Ahmed, A.; Atta-ur, R.; Choudhary, M.I. Isolation and immunomodulatory properties of a flavonoid, casticin from Vitex agnus-castus. Phytother. Res. 2009, 23, 1516–1520. [Google Scholar] [CrossRef]
- You, K.M.; Son, K.H.; Chang, H.W.; Kang, S.S.; Kim, H.P. Vitexicarpin, a flavonoid from the fruits of Vitex rotundifolia, inhibits mouse lymphocyte proliferation and growth of cell lines in vitro. Planta Med. 1998, 64, 546–550. [Google Scholar] [CrossRef]
- Ko, W.G.; Kang, T.H.; Lee, S.J.; Kim, N.Y.; Kim, Y.C.; Sohn, D.H.; Lee, B.H. Polymethoxyflavonoids from Vitex rotundifolia inhibit proliferation by inducing apoptosis in human myeloid leukemia cells. Food Chem. Toxicol. 2000, 38, 861–865. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Nishimura, Y.; Imai, M.; Furutani, R.; Kamoi, S.; Seno, M.; Fukushima, S.; Hazama, S.; Hirobe, C.; et al. Cytotoxicity of Vitex agnus-castus fruit extract and its major component, casticin, correlates with differentiation status in leukemia cell lines. Int. J. Oncol. 2013, 43, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Song, X.L.; Zhang, Y.J.; Wang, X.F.; Zhang, W.J.; Wang, Z.; Zhang, F.; Zhang, Y.J.; Lu, J.H.; Mei, J.W.; Hu, Y.P.; et al. Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells. Cancer Cell Int. 2017, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Cao, X.C.; Cao, J.G.; Liu, F.; Quan, M.F.; Sheng, X.F.; Ren, K.Q. Casticin induces ovarian cancer cell apoptosis by repressing FoxM1 through the activation of FOXO3a. Oncol. Lett. 2013, 5, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Tian, L.; Liu, F.; Cao, J.; Quan, M.; Sheng, X. Induction of apoptosis by casticin in cervical cancer cells: Reactive oxygen species-dependent sustained activation of Jun N-terminal kinase. Acta Biochim. Biophys. Sin. (Shanghai) 2012, 44, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Zhong, M.Z.; Yuan, G.J.; Hou, S.P.; Yin, L.L.; Jiang, H.; Yu, Z. Casticin, a flavonoid, potentiates TRAIL-induced apoptosis through modulation of anti-apoptotic proteins and death receptor 5 in colon cancer cells. Oncol. Rep. 2013, 29, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, K.H.; Lee, H.; Park, S.; Choi, W.; Bae, H. Casticin, an active compound isolated from Vitex Fructus, ameliorates the cigarette smoke-induced acute lung inflammatory response in a murine model. Int. Immunopharmacol. 2015, 28, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, H.; Han, T.; Wu, J.Z.; Rahman, K.; Qin, L.P. In vivo effect of casticin on acute inflammation. Zhong Xi Yi Jie He Xue Bao 2007, 5, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.L.; Chou, H.M.; Chou, H.C.; Lu, H.F.; Chu, Y.L.; Shang, H.S.; Chung, J.G. Casticin impairs cell migration and invasion of mouse melanoma B16F10 cells via PI3K/AKT and NF-kappaB signaling pathways. Environ. Toxicol. 2017, 32, 2097–2112. [Google Scholar] [CrossRef] [PubMed]
- Shiue, Y.W.; Lu, C.C.; Hsiao, Y.P.; Liao, C.L.; Lin, J.P.; Lai, K.C.; Yu, C.C.; Huang, Y.P.; Ho, H.C.; Chung, J.G. Casticin Induced Apoptosis in A375.S2 Human Melanoma Cells through the Inhibition of NF-[Formula: See text]B and Mitochondria-Dependent Pathways In Vitro and Inhibited Human Melanoma Xenografts in a Mouse Model In Vivo. Am. J. Chin. Med. 2016, 44, 637–661. [Google Scholar] [CrossRef] [PubMed]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Polivka, J., Jr.; Janku, F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol. Ther. 2014, 142, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-beta-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Ahn, K.S.; Ong, T.H.; Shanmugam, M.K.; Li, F.; Yap, W.N.; Kumar, A.P.; Fong, C.W.; Tergaonkar, V.; Hui, K.M.; et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget 2014, 5, 1897–1911. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.M.; Bae, H.; Nam, D.; Lee, J.H.; Lee, S.G.; Shim, B.S.; Kim, S.H.; Ahn, K.S.; Choi, S.H.; et al. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate 2013, 73, 296–305. [Google Scholar] [CrossRef]
- Park, K.R.; Nam, D.; Yun, H.M.; Lee, S.G.; Jang, H.J.; Sethi, G.; Cho, S.K.; Ahn, K.S. Beta-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Cui, W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 2016, 143, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.M.; Garcia-Haro, L.; Sparks, C.A.; Guertin, D.A. mTOR-dependent cell survival mechanisms. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, M.G.; Sun, X.M.; Brown, D.; Snowden, R.T.; Cohen, G.M. Quantification of apoptosis and necrosis by flow cytometry. Acta Oncol. 1993, 32, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Oliver, F.J.; de la Rubia, G.; Rolli, V.; Ruiz-Ruiz, M.C.; de Murcia, G.; Murcia, J.M. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J. Biol. Chem. 1998, 273, 33533–33539. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Chaturvedi, M.M.; Aggarwal, B.B. Epidermal growth factor (EGF) activates nuclear factor-kappaB through IkappaBalpha kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of IkappaBalpha. Oncogene 2007, 26, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Syn, N.L.; Subhash, V.V.; Any, Y.; Thuya, W.L.; Cheow, E.S.H.; Kong, L.; Yu, F.; Peethala, P.C.; Wong, A.L.; et al. Pan-HDAC inhibition by panobinostat mediates chemosensitization to carboplatin in non-small cell lung cancer via attenuation of EGFR signaling. Cancer Lett. 2018, 417, 152–160. [Google Scholar] [CrossRef]
- Guo, Y.H.; Zhang, C.; Shi, J.; Xu, M.H.; Liu, F.; Yuan, H.H.; Wang, J.Y.; Jiang, B.; Gao, F.H. Abnormal activation of the EGFR signaling pathway mediates the downregulation of miR145 through the ERK1/2 in non-small cell lung cancer. Oncol. Rep. 2014, 31, 1940–1946. [Google Scholar] [CrossRef]
- Ryu, N.H.; Park, K.R.; Kim, S.M.; Yun, H.M.; Nam, D.; Lee, S.G.; Jang, H.J.; Ahn, K.S.; Kim, S.H.; Shim, B.S.; et al. A hexane fraction of guava Leaves (Psidium guajava L.) induces anticancer activity by suppressing AKT/mammalian target of rapamycin/ribosomal p70 S6 kinase in human prostate cancer cells. J. Med. Food 2012, 15, 231–241. [Google Scholar] [CrossRef]
- Ben-Sasson, S.A.; Sherman, Y.; Gavrieli, Y. Identification of dying cells--in situ staining. Methods Cell Biol. 1995, 46, 29–39. [Google Scholar] [PubMed]
- Haidara, K.; Zamir, L.; Shi, Q.W.; Batist, G. The flavonoid Casticin has multiple mechanisms of tumor cytotoxicity action. Cancer Lett. 2006, 242, 180–190. [Google Scholar] [CrossRef]
- Liu, F.; Cao, X.; Liu, Z.; Guo, H.; Ren, K.; Quan, M.; Zhou, Y.; Xiang, H.; Cao, J. Casticin suppresses self-renewal and invasion of lung cancer stem-like cells from A549 cells through down-regulation of pAkt. Acta Biochim. Biophys. Sin. (Shanghai) 2014, 46, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Kempf, R.C.; Long, J.; Laidler, P.; Mijatovic, S.; Maksimovic-Ivanic, D.; Stivala, F.; Mazzarino, M.C.; et al. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011, 3, 192–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Liou, C.J.; Huang, W.C. Casticin inhibits interleukin-1beta-induced ICAM-1 and MUC5AC expression by blocking NF-kappaB, PI3K-Akt, and MAPK signaling in human lung epithelial cells. Oncotarget 2017, 8, 101175–101188. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001, 15, 2177–2196. [Google Scholar] [CrossRef]
- Chou, G.L.; Peng, S.F.; Liao, C.L.; Ho, H.C.; Lu, K.W.; Lien, J.C.; Fan, M.J.; La, K.C.; Chung, J.G. Casticin impairs cell growth and induces cell apoptosis via cell cycle arrest in human oral cancer SCC-4 cells. Environ. Toxicol. 2018, 33, 127–141. [Google Scholar] [CrossRef]
- Laroche, A.; Chaire, V.; Algeo, M.P.; Karanian, M.; Fourneaux, B.; Italiano, A. MDM2 antagonists synergize with PI3K/mTOR inhibition in well-differentiated/dedifferentiated liposarcomas. Oncotarget 2017, 8, 53968–53977. [Google Scholar] [CrossRef]
- Maira, S.M.; Stauffer, F.; Brueggen, J.; Furet, P.; Schnell, C.; Fritsch, C.; Brachmann, S.; Chene, P.; De Pover, A.; Schoemaker, K.; et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 2008, 7, 1851–1863. [Google Scholar] [CrossRef]
- Moon du, G.; Lee, S.E.; Oh, M.M.; Lee, S.C.; Jeong, S.J.; Hong, S.K.; Yoon, C.Y.; Byun, S.S.; Park, H.S.; Cheon, J. NVP-BEZ235, a dual PI3K/mTOR inhibitor synergistically potentiates the antitumor effects of cisplatin in bladder cancer cells. Int. J. Oncol. 2014, 45, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Narayanan, S.; Sethuraman, S.; Krishnan, U.M. Combinations of plant polyphenols & anti-cancer molecules: A novel treatment strategy for cancer chemotherapy. Anticancer Agents Med. Chem. 2013, 13, 281–295. [Google Scholar] [PubMed]
- Sak, K.; Everaus, H. Chemomodulating Effects of Flavonoids in Human Leukemia Cells. Anticancer Agents Med. Chem. 2015, 15, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Sak, K.; Everaus, H. Role of flavonoids in future anticancer therapy by eliminating the cancer stem cells. Curr. Stem Cell Res. Ther. 2015, 10, 271–282. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [PubMed]
- Yang, M.Y.; Wang, C.J.; Chen, N.F.; Ho, W.H.; Lu, F.J.; Tseng, T.H. Luteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3. Chem. Biol. Interact. 2014, 213, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-kappaB in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Fong, C.W.; Kumar, A.P.; Tan, P.; Sethi, G. First evidence that gamma-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-kappaB pathway. Clin. Cancer Res. 2012, 18, 2220–2229. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef]
- Li, F.; Sethi, G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta 2010, 1805, 167–180. [Google Scholar]
- Sethi, G.; Tergaonkar, V. Potential pharmacological control of the NF-kappaB pathway. Trends Pharmacol. Sci. 2009, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Arfuso, F.; Kumar, A.P.; et al. Isorhamnetin augments the anti-tumor effect of capeciatbine through the negative regulation of NF-kappaB signaling cascade in gastric cancer. Cancer Lett. 2015, 363, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.G.; Yang, W.M.; Arfuso, F.; Um, J.Y.; Kumar, A.P.; Bian, J.; Sethi, G.; Ahn, K.S. Formononetin-induced oxidative stress abrogates the activation of STAT3/5 signaling axis and suppresses the tumor growth in multiple myeloma preclinical model. Cancer Lett. 2018, 431, 123–141. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).