Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Endpoints and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Postoperative Stage and Morbidity
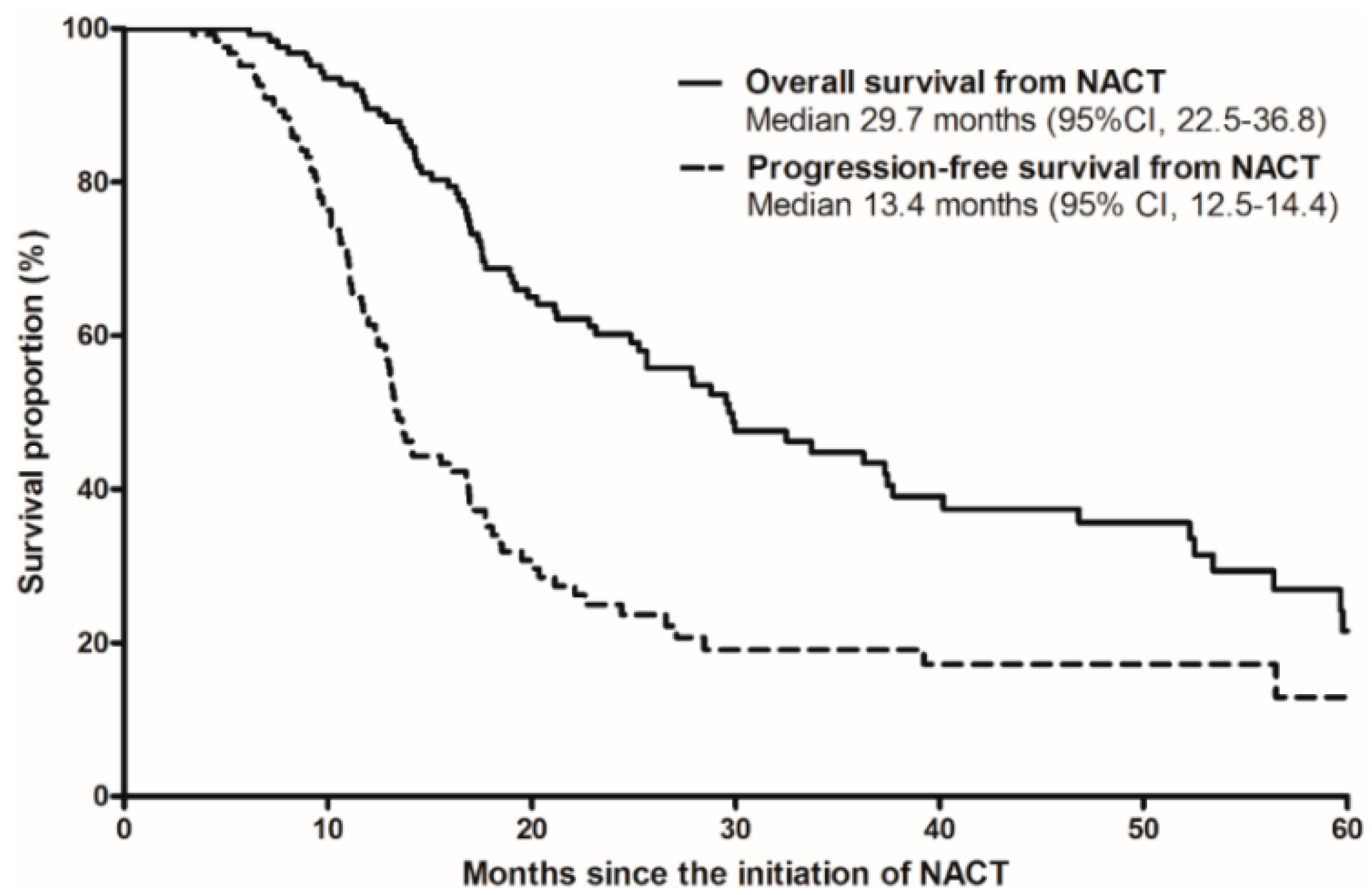
3.3. Survival Outcomes and Prognostic Factors with Conversion Surgery after NACT
3.4. Survival Outcomes with Conversion Surgery after NACT in Comparison with Upfront Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Won, Y.-J.; Oh, C.-M.; Kong, H.-J.; Lee, D.H.; Lee, K.H.; The Community of Population-Based Regional Cancer Registries. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2014. Cancer Res. Treat. 2017, 49, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Heestand, G.M.; Murphy, J.D.; Lowy, A.M. Approach to Patients with Pancreatic Cancer Without Detectable Metastases. J. Clin. Oncol. 2015, 33, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.G.; Marsh, R.; Herman, J.M.; Shi, Q.; Collison, E.; Venook, A.P.; Kindler, H.L.; Alberts, S.R.; Philip, P.; Lowy, A.M.; et al. Borderline Resectable Pancreatic Cancer: Need for Standardization and Methods for Optimal Clinical Trial Design. Ann. Surg. Oncol. 2013, 20, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 1). 2016. Available online: Http://www.Nccn.org/Professionals/Physician_Gls/Pdf/Pancreatic.Pdf (accessed on 11 July 2016).
- Callery, M.P.; Chang, K.J.; Fishman, E.K.; Talamonti, M.S.; William, T.L.; Linehan, D.C. Pretreatment Assessment of Resectable and Borderline Resectable Pancreatic Cancer: Expert Consensus Statement. Ann. Surg. Oncol. 2009, 16, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-L.; Kim, S.C.; Kim, J.-H.; Lee, S.S.; Kim, T.-W.; Park, D.H.; Seo, D.W.; Lee, S.K.; Kim, M.-H.; Kim, J.H.; et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery 2012, 152, 851–862. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.M.; Perelshteyn, A.; Jarnagin, W.R.; Schattner, M.; Gerdes, H.; Capanu, M.; Tang, L.H.; LaValle, J.; Winston, C.; DeMatteo, R.P.; et al. A Single-Arm, Nonrandomized Phase II Trial of Neoadjuvant Gemcitabine and Oxaliplatin in Patients with Resectable Pancreas Adenocarcinoma. Ann. Surg. 2014, 260, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Becouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Gemenetzis, G.; Groot, V.P.; Blair, A.B.; Laheru, D.A.; Zheng, L.; Narang, A.K.; Fishman, E.K.; Hruban, R.H.; Yu, J.; Burkhart, R.A.; et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; et al. Total Neoadjuvant Therapy with FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma. JAMA Oncol. 2018, 4, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef]
- Khushman, M.; Dempsey, N.; Maldonado, J.C.; Loaiza-Bonilla, A.; Velez, M.; Carcas, L.; Dammrich, D.; Hurtado-Cordovi, J.; Parajuli, R.; Pollack, T.; et al. Full dose neoadjuvant FOLFIRINOX is associated with prolonged survival in patients with locally advanced pancreatic adenocarcinoma. Pancreatology 2015, 15, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kang, J.; Kim, K.-P.; Lee, J.-L.; Ryoo, B.-Y.; Chang, H.-M.; Lee, S.S.; Park, D.H.; Song, T.J.; Seo, D.W.; et al. Efficacy and safety of neoadjuvant FOLFIRINOX for borderline resectable pancreatic adenocarcinoma: Improved efficacy compared with gemcitabine-based regimen. Oncotarget 2017, 8, 46337–46347. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.G.; Ou, F.-S.; Herman, J.M.; Ahmad, S.A.; Wolpin, B.; Marsh, R.; Behr, S.; Shi, Q.; Chuong, M.; Schwartz, L.H.; et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: Preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer 2017, 17, 505. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.R.; Marchegiani, G.; Hong, T.S.; Ryan, D.P.; Deshpande, V.; McDonnell, E.I.; Sabbatino, F.; Santos, D.D.; Allen, J.N.; Blaszkowsky, L.S.; et al. Radiological and Surgical Implications of Neoadjuvant Treatment with FOLFIRINOX for Locally Advanced and Borderline Resectable Pancreatic Cancer. Ann. Surg. 2015, 261, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.A.; Minter, R.M.; Zhu, H. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: A propensity score matched analysis. J. Clin. Oncol. 2016, 35, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.-C.; Raoul, J.-L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. Unicancer GI PRODIGE 24/CCTG PA.6 trial: A multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J. Clin. Oncol. 2018, 36, LBA4001. [Google Scholar] [CrossRef]


| Variable | Upfront Surgery (n = 359) | Conversion Surgery after NACT (n = 135) | p Value |
|---|---|---|---|
| Age, median (range) | 61 (30–82 years) | 60 (30–78 years) | 0.18 |
| Age ≥65 years | 132 (37%) | 33 (24%) | 0.01 |
| Gender, male/female | 202 (56%)/157 (44%) | 72 (53%)/63 (47%) | 0.18 |
| Resectability criteria at diagnosis | |||
| Borderline resectable | 281 (78%) | 65 (48%) | <0.001 |
| Locally advanced unresectable | 78 (22%) | 70 (52%) | |
| NACT regimens | |||
| Gemcitabine-based | 69 (51%) | ||
| FOLFIRINOX | 66 (49%) | ||
| Response to chemotherapy | |||
| Partial response | 52 (39%) | ||
| Stable disease | 80 (59%) | ||
| Progressive disease | 3 (2%) | ||
| Surgical type | |||
| Pancreatoduodenectomy | 237 (66%) | 80 (59%) | <0.001 |
| Distal pancreatectomy | 45 (13%) | 40 (30%) | |
| Total pancreatectomy | 77 (21%) | 15 (11%) | |
| Major vascular resection | 351 (98%) | 99 (73%) | <0.001 |
| Vein resection | 324 (90%) | 79 (59%) | |
| Artery resection | 64 (18%) | 39 (29%) | |
| Combined resection | 37 (10%) | 21 (16%) | |
| CA 19-9 level at the time of surgery | N = 351 | N = 132 | 0.001 |
| Within normal range | 97 (28%) | 58 (44%) | |
| Elevated | 254 (72%) | 74 (56%) | |
| Hospital stay for surgery, median (interquartile range) | 17 days (11–24) | 13 days (10–17) | 0.14 |
| Postoperative adjuvant chemotherapy | 248 (69%) | 105 (78%) | 0.06 |
| Postoperative adjuvant radiotherapy | 76 (21%) | 18 (13%) | 0.05 |
| Variables | Upfront Surgery (n = 359) | Conversion Surgery after NACT (n = 135) | p Value |
|---|---|---|---|
| Pathological staging * | |||
| T1–2/T3–4 | 5 (1%)/354 (99%) | 10 (7%)/125 (93%) | 0.001 |
| T1 | 2 (0.6%) | 8 (5.9%) | |
| T2 | 3 (0.8%) | 2 (1.5%) | |
| T3 | 337 (93.9%) | 119 (88.1%) | |
| T4 | 17 (4.7%) | 6 (4.4%) | |
| N0/N1 | 104 (29%)/253 (71%) | 69 (51%)/66 (49%) | <0.001 |
| Resection margin status ** | 0.06 | ||
| R0 | 240 (67%) | 102 (76%) | |
| R1 | 119 (33%) | 33 (24%) | |
| Lymphovascular invasion | 209 (58%) | 48 (36%) | <0.001 |
| Perineural invasion | 338 (94%) | 108 (80%) | <0.001 |
| Variables | Upfront Surgery (n = 359) | Conversion Surgery after NACT (n = 135) | p Value |
|---|---|---|---|
| Surgical complication * | 136 (38%) | 37 (27%) | 0.03 |
| Grade I–II | 110 (31%) | 24 (18%) | |
| Grade III–IV | 23 (6%) | 12 (9%) | |
| Grade V | 3 (1%) | 1 (1%) | |
| Postoperative pancreatic fistula ** | 42 (12%) | 20 (15%) | 0.02 |
| Biochemical leakage | 27 (8%) | 6 (4%) | |
| Grade B or C | 15 (4%) | 14 (10%) |
| Variables | Overall Survival | Disease-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age (≥65 vs. <65 years) | 2.52 (1.24–5.13) | 0.01 | 2.70 (1.39–5.23) | 0.003 | 1.67 (0.69–3.13) | 0.11 | ||
| Gender (female vs. male) | 1.56 (0.86–2.66) | 0.15 | 1.30 (0.75–2.26) | 0.34 | ||||
| Pathological T stage (pT3–4 vs. pT1–2) | 0.65 (0.21–2.01) | 0.47 | 1.63 (0.53–5.00) | 1.63 | ||||
| Pathological N stage (pN0 vs. pN+) | 0.82 (0.43–1.56) | 0.55 | 0.87 (0.50–1.52) | 0.63 | ||||
| Surgery (vs. pancreatoduodenectomy) | ||||||||
| Distal pancreatectomy | 0.75 (0.37–1.52) | 0.43 | 0.81 (0.42–1.57) | 0.54 | ||||
| Total pancreatectomy | 1.93 (0.59–6.25) | 0.28 | 1.65 (0.52–5.30) | 0.40 | ||||
| NACT regimens (gemcitabine-based vs. FOLFIRINOX) | 1.22 (0.62–2.40) | 0.57 | 0.99 (0.57–1.72) | 0.97 | ||||
| CA 19-9 (elevated vs. WNL) | 1.70 (0.92–3.16) | 0.09 | 1.67 (0.96–2.92) | 0.07 | 1.76 (1.00–3.10) | 0.05 | 1.65 (1.00–2.73) | 0.049 |
| Response to NACT (vs. partial response) | ||||||||
| Stable disease | 2.09 (1.03–4.24) | 0.04 | 2.12 (1.16–3.87) | 0.02 | 1.38 (0.78–2.41) | 0.27 | ||
| Progressive disease | 6.59 (1.20–36.33) | 0.03 | 4.92 (1.05–23.04) | 0.04 | 2.59 (0.51–13.06) | 0.25 | ||
| Artery resection (yes vs. no) | 1.10 (0.58–2.03) | 0.80 | 1.18 (0.66–2.10) | 0.57 | ||||
| Vein resection (yes vs. no) | 1.20 (0.60–2.35) | 0.63 | 1.30 (0.69–2.47) | 0.42 | 1.67 (1.01–2.77) | 0.047 | ||
| Resection margin status (positive vs. negative) | 1.33 (0.66–2.66) | 0.43 | 1.01 (0.53–4.96) | 0.98 | ||||
| Variables | OS from Surgical Resection | DFS from Surgical Resection | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (≥ 65 vs. <65 years) | 1.17 | 0.94–1.46 | 0.15 | 1.08 | 0.87–1.34 | 0.50 |
| Gender (female vs. male) | 1.07 | 0.87–1.32 | 0.51 | 1.09 | 0.89–1.33 | 0.41 |
| NACT (yes vs. no) | 0.73 | 0.56–0.96 | 0.02 | 0.72 | 0.56–0.93 | 0.01 |
| CA 19-9 (elevated vs. WNL) | 1.22 | 0.97–1.53 | 0.09 | 1.19 | 0.96–1.49 | 0.12 |
| Surgery (vs. pancreatoduodenectomy) | ||||||
| Distal pancreatectomy | 0.99 | 0.74–1.33 | 0.96 | 1.07 | 0.81–1.41 | 0.66 |
| Total pancreatectomy | 1.47 | 1.13–1.91 | 0.004 | 1.39 | 1.07–1.80 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, C.; Shin, S.H.; Kim, K.-p.; Jeong, J.H.; Chang, H.-M.; Kang, J.H.; Lee, S.S.; Park, D.H.; Song, T.J.; Seo, D.W.; et al. Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis. Cancers 2019, 11, 278. https://doi.org/10.3390/cancers11030278
Yoo C, Shin SH, Kim K-p, Jeong JH, Chang H-M, Kang JH, Lee SS, Park DH, Song TJ, Seo DW, et al. Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis. Cancers. 2019; 11(3):278. https://doi.org/10.3390/cancers11030278
Chicago/Turabian StyleYoo, Changhoon, Sang Hyun Shin, Kyu-pyo Kim, Jae Ho Jeong, Heung-Moon Chang, Jun Ho Kang, Sang Soo Lee, Do Hyun Park, Tae Jun Song, Dong Wan Seo, and et al. 2019. "Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis" Cancers 11, no. 3: 278. https://doi.org/10.3390/cancers11030278
APA StyleYoo, C., Shin, S. H., Kim, K.-p., Jeong, J. H., Chang, H.-M., Kang, J. H., Lee, S. S., Park, D. H., Song, T. J., Seo, D. W., Lee, S. K., Kim, M.-H., Park, J.-h., Hwang, D. W., Song, K. B., Lee, J. H., Ryoo, B.-Y., & Kim, S. C. (2019). Clinical Outcomes of Conversion Surgery after Neoadjuvant Chemotherapy in Patients with Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer: A Single-Center, Retrospective Analysis. Cancers, 11(3), 278. https://doi.org/10.3390/cancers11030278









