Targeting Receptor Kinases in Colorectal Cancer
Abstract
1. Introduction
1.1. Colorectal Cancer
1.2. Targeted Therapies for Colorectal Cancer Treatment
1.3. Molecular Classification of Colorectal Cancer
1.4. Protein Kinases
1.4.1. Protein Kinases as Key Regulators of Cell Function
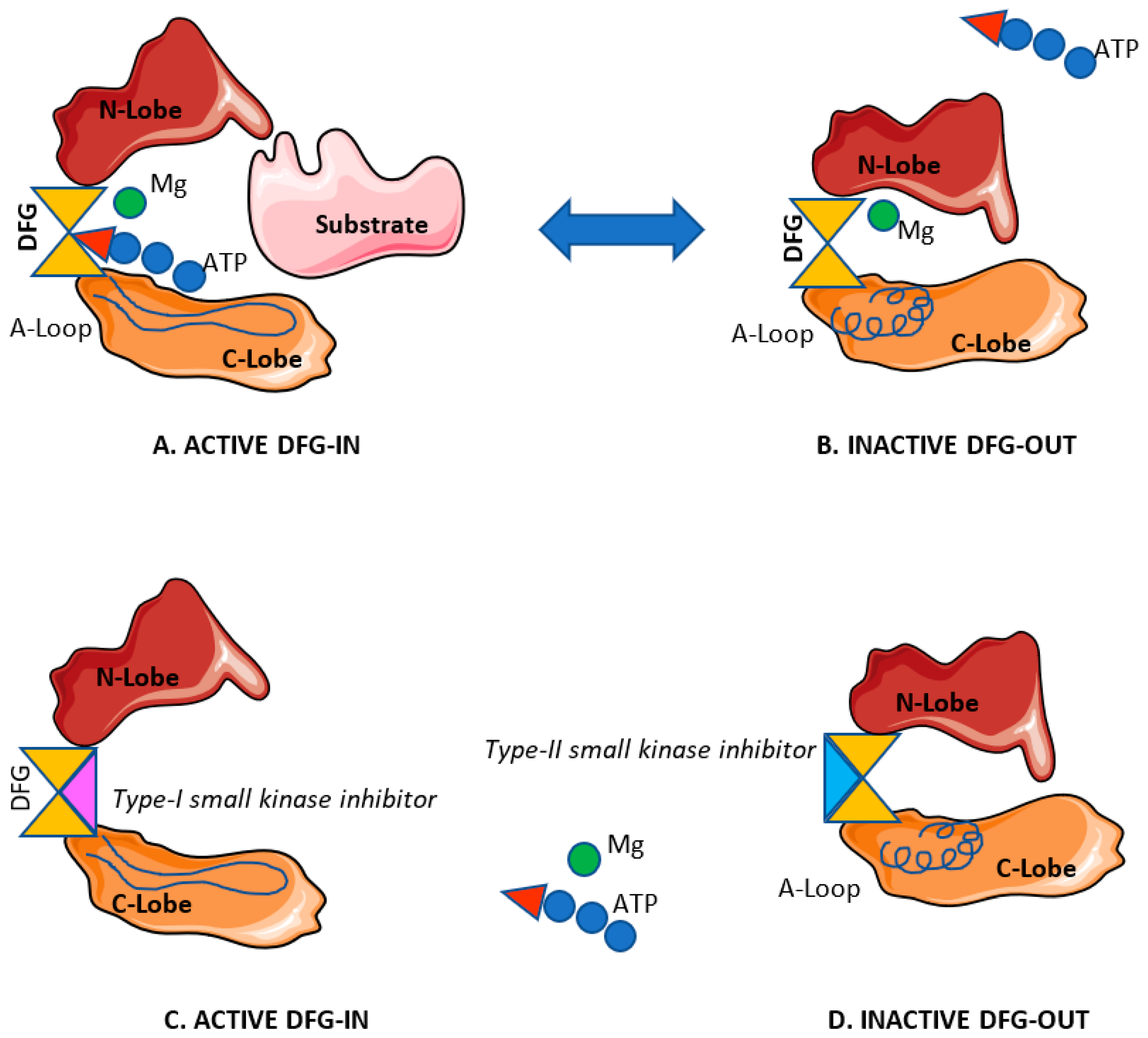
1.4.2. Small Molecule Kinase Inhibitors for Cancer Treatment
2. Altered Kinases in Colorectal Cancer
2.1. Main Altered Kinases in Colorectal Cancer
2.1.1. Receptor Kinases
2.1.2. Non-Receptor Kinases
MAPK Kinases
The PI3K/AKT Pathway
PTEN
3. Targeting Receptor Kinases in Colorectal Cancer
3.1. Targeting Receptor Kinases in Colorectal Cancer
3.2. Overcoming Resistance to Kinase Inhibitors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Vatandoust, S.; Price, T.J.; Karapetis, C.S. Colorectal cancer: Metastases to a single organ. World J. Gastroenterol. 2015, 21, 11767–11776. [Google Scholar] [CrossRef]
- Zarcos-Pedrinaci, I.; Tellez, T.; Rivas-Ruiz, F.; Padilla-Ruiz, M.D.C.; Alcaide, J.; Rueda, A.; Bare, M.L.; Suarez-Varela, M.M.M.; Briones, E.; Sarasqueta, C.; et al. Factors Associated with Prolonged Patient-Attributable Delay in the Diagnosis of Colorectal Cancer. Cancer Res. Treat. 2018, 50, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Zarcos-Pedrinaci, I.; Fernández-López, A.; Téllez, T.; Rivas-Ruiz, F.; Rueda, A.; Suarez-Varela, M.M.M.; Briones, E.; Baré, M.; Escobar, A.; Sarasqueta, C. Factors that influence treatment delay in patients with colorectal cancer. Oncotarget 2017, 8, 36728. [Google Scholar] [CrossRef] [PubMed]
- Byles, J.E.; Redman, S.; Hennrikus, D.; Sanson-Fisher, R.W.; Dickinson, J. Delay in consulting a medical practitioner about rectal bleeding. J. Epidemiol. Community Health 1992, 46, 241–244. [Google Scholar] [CrossRef]
- Stein, A.; Bokemeyer, C. How to select the optimal treatment for first line metastatic colorectal cancer. World J. Gastroenterol. 2014, 20, 899–907. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- American Cancer Society. Survival Rates for Colorectal Cancer, by Stage. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 15 December 2018).
- Manning, A.; Garvin, J.; Shahbazi, R.; Miller, N.; McNeill, R.; Kerin, M. Molecular profiling techniques and bioinformatics in cancer research. Eur. J. Surg. Oncol. 2007, 33, 255–265. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Okita, A.; Takahashi, S.; Ouchi, K.; Inoue, M.; Watanabe, M.; Endo, M.; Honda, H.; Yamada, Y.; Ishioka, C. Consensus molecular subtypes classification of colorectal cancer as a predictive factor for chemotherapeutic efficacy against metastatic colorectal cancer. Oncotarget 2018, 9, 18698–18711. [Google Scholar] [CrossRef]
- Muller, M.F.; Ibrahim, A.E.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134. [Google Scholar] [CrossRef]
- Thanki, K.; Nicholls, M.E.; Gajjar, A.; Senagore, A.J.; Qiu, S.; Szabo, C.; Hellmich, M.R.; Chao, C. Consensus Molecular Subtypes of Colorectal Cancer and their Clinical Implications. Int. Biol. Biomed. J. 2017, 3, 105–111. [Google Scholar] [PubMed]
- McDonald, A.G.; Boyce, S.; Tipton, K.F. ExplorEnz: The primary source of the IUBMB enzyme list. Nucleic Acids Res. 2008, 37, D593–D597. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Swulius, M.T.; Waxham, M.N. Ca(2+)/calmodulin-dependent protein kinases. Cell. Mol. Life Sci. 2008, 65, 2637–2657. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy. Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhao, R.; Zhe, H. The emerging role of CaMKII in cancer. Oncotarget 2015, 6, 11725–11734. [Google Scholar] [CrossRef] [PubMed]
- Knippschild, U.; Kruger, M.; Richter, J.; Xu, P.; Garcia-Reyes, B.; Peifer, C.; Halekotte, J.; Bakulev, V.; Bischof, J. The CK1 Family: Contribution to Cellular Stress Response and Its Role in Carcinogenesis. Front. Oncol. 2014, 4, 96. [Google Scholar] [CrossRef]
- Oruganty, K.; Kannan, N. Design principles underpinning the regulatory diversity of protein kinases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2529–2539. [Google Scholar] [CrossRef]
- Saha, S.; Biswas, K.H.; Kondapalli, C.; Isloor, N.; Visweswariah, S.S. The linker region in receptor guanylyl cyclases is a key regulatory module: Mutational analysis of guanylyl cyclase C. J. Biol. Chem. 2009, 284, 27135–27145. [Google Scholar] [CrossRef]
- Duda, T.; Yadav, P.; Sharma, R.K. Allosteric modification, the primary ATP activation mechanism of atrial natriuretic factor receptor guanylate cyclase. Biochemistry 2011, 50, 1213–1225. [Google Scholar] [CrossRef]
- Kraatz, H.-B.; Martic, S. Kinomics: Approaches and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Duong-Ly, K.C.; Peterson, J.R. The human kinome and kinase inhibition. Curr. Protoc. Pharmacol. 2013. [Google Scholar] [CrossRef]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aranda, M.; Redondo, M. Protein Kinase Targets in Breast Cancer. Int. J. Mol. Sci. 2017, 18, 2543. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.; Punt, C.J. Monoclonal antibodies in the treatment of metastatic colorectal cancer: A review. Clin. Ther. 2010, 32, 437–453. [Google Scholar] [CrossRef]
- Rosa, B.; de Jesus, J.P.; de Mello, E.L.; Cesar, D.; Correia, M.M. Effectiveness and safety of monoclonal antibodies for metastatic colorectal cancer treatment: Systematic review and meta-analysis. Ecancermedicalscience 2015, 9, 582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smyth, L.A.; Collins, I. Measuring and interpreting the selectivity of protein kinase inhibitors. J. Chem. Biol. 2009, 2, 131–151. [Google Scholar] [CrossRef]
- Ung, P.M.; Schlessinger, A. DFGmodel: Predicting protein kinase structures in inactive states for structure-based discovery of type-II inhibitors. ACS Chem. Biol. 2015, 10, 269–278. [Google Scholar] [CrossRef]
- Stout, T.; Foster, P.; Matthews, D. High-throughput structural biology in drug discovery: Protein kinases. Curr. Pharm. Des. 2004, 10, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.I.; Hunt, J.P.; Herrgard, S.; Ciceri, P.; Wodicka, L.M.; Pallares, G.; Hocker, M.; Treiber, D.K.; Zarrinkar, P.P. Comprehensive analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2011, 29, 1046–1051. [Google Scholar] [CrossRef]
- De Roock, W.; De Vriendt, V.; Normanno, N.; Ciardiello, F.; Tejpar, S. KRAS, BRAF, PIK3CA, and PTEN mutations: Implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011, 12, 594–603. [Google Scholar] [CrossRef]
- Alexander, S.P.; Fabbro, D.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; Southan, C.; et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Catalytic receptors. Br. J. Pharmacol. 2017, 174 (Suppl. 1), S225–S271. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Zhou, Q.-Y.; Hu, Y.; Wen, Y.; Qiu, Z.-W.; Liang, M.-G.; Mo, J.-L.; Xu, J.-H.; Sun, C.; Liu, F.-B. Hepatocyte growth factor is a prognostic marker in patients with colorectal cancer: A meta-analysis. Oncotarget 2017, 8, 23459. [Google Scholar] [CrossRef]
- Spano, J.P.; Lagorce, C.; Atlan, D.; Milano, G.; Domont, J.; Benamouzig, R.; Attar, A.; Benichou, J.; Martin, A.; Morere, J.F.; et al. Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann. Oncol. 2005, 16, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, P.G.; Tirro, E.; Pennisi, M.S.; Massimino, M.; Stella, S.; Romano, C.; Manzella, L. The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front. Oncol. 2015, 5, 230. [Google Scholar] [CrossRef]
- De Meyts, P. The Insulin Receptor and Its Signal Transduction Network. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Manzat Saplacan, R.M.; Balacescu, L.; Gherman, C.; Chira, R.I.; Craiu, A.; Mircea, P.A.; Lisencu, C.; Balacescu, O. The Role of PDGFs and PDGFRs in Colorectal Cancer. Mediat. Inflamm. 2017, 2017, 4708076. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.R.; Mok, S.; Allison, J.P. Blocking colony stimulating factor 1 receptor (CSF-1R) and tropomyosin receptor kinase A (TrkA) improves the antitumor efficacy of immune checkpoint blockade. J. Immunol. 2018, 200, 122.15. [Google Scholar]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.M.; Ries, C.H.; Ruttinger, D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J. Immunother. Cancer 2017, 5, 53. [Google Scholar] [CrossRef]
- Shah, Y.M.; van den Brink, G.R. c-Kit as a Novel Potential Therapeutic Target in Colorectal Cancer. Gastroenterology 2015, 149, 534–537. [Google Scholar] [CrossRef]
- Heldin, C.H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. 2013, 11, 97. [Google Scholar] [CrossRef]
- Yang, X.; Liu, F.; Xu, Z.; Chen, C.; Wu, X.; Li, G.; Li, J. Expression of granulocyte colony stimulating factor receptor in human colorectal cancer. Postgrad. Med. J. 2005, 81, 333–337. [Google Scholar] [CrossRef]
- Abbaspour Babaei, M.; Kamalidehghan, B.; Saleem, M.; Huri, H.Z.; Ahmadipour, F. Receptor tyrosine kinase (c-Kit) inhibitors: A potential therapeutic target in cancer cells. Drug Des. Dev. Ther. 2016, 10, 2443–2459. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, S.Y.; Kim, K.; Jang, H.; Ahn, S.; Kim, K.M.; Kim, N.K.; Park, W.; Lee, S.J.; Kim, S.T.; et al. The implication of FLT3 amplification for FLT targeted therapeutics in solid tumors. Oncotarget 2017, 8, 3237–3245. [Google Scholar] [CrossRef]
- Smith, G.A.; Fearnley, G.W.; Tomlinson, D.C.; Harrison, M.A.; Ponnambalam, S. The cellular response to vascular endothelial growth factors requires co-ordinated signal transduction, trafficking and proteolysis. Biosci. Rep. 2015, 35. [Google Scholar] [CrossRef] [PubMed]
- D’Haene, N.; Koopmansch, C.; Van Eycke, Y.R.; Hulet, F.; Allard, J.; Bouri, S.; Rorive, S.; Remmelink, M.; Decaestecker, C.; Maris, C.; et al. The Prognostic Value of the Combination of Low VEGFR-1 and High VEGFR-2 Expression in Endothelial Cells of Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 3536. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qi, L.; Li, Y.; Zhao, X.; Sun, B. VEGFR2 regulates endothelial differentiation of colon cancer cells. BMC Cancer 2017, 17, 593. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.R.; Baker, D.; James, N.H.; Ratcliffe, K.; Jenkins, M.; Ashton, S.E.; Sproat, G.; Swann, R.; Gray, N.; Ryan, A.; et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin. Cancer Res. 2010, 16, 3548–3561. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ueda, J.; Ishiwata, T. Fibroblast growth factor receptor 2: Expression, roles, and potential as a novel molecular target for colorectal cancer. Pathol. Res. Int. 2012, 2012, 574768. [Google Scholar] [CrossRef]
- Kwak, Y.; Nam, S.K.; Seo, A.N.; Kim, D.W.; Kang, S.B.; Kim, W.H.; Lee, H.S. Fibroblast Growth Factor Receptor 1 Gene Copy Number and mRNA Expression in Primary Colorectal Cancer and Its Clinicopathologic Correlation. Pathobiology 2015, 82, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Antibody-drug conjugate targeting protein tyrosine kinase 7, a receptor tyrosine kinase-like molecule involved in WNT and vascular endothelial growth factor signaling: Effects on cancer stem cells, tumor microenvironment and whole-body homeostasis. Ann. Transl. Med. 2017, 5, 462. [Google Scholar] [CrossRef]
- FDA. FDA Approves Larotrectinib for Solid Tumors with NTRK Gene Fusions. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm626720.htm (accessed on 31 January 2019).
- Lange, A.M.; Lo, H.W. Inhibiting TRK Proteins in Clinical Cancer Therapy. Cancers 2018, 10, 105. [Google Scholar] [CrossRef]
- Madison, R.; Pietrantonio, F.; Juckett, L.; Cremolini, C.; Chung, J.; Albacker, L.; Miller, V.; Klempner, S.; Resnick, M.; Yakirevich, E. 457PD Kinase fusions in colorectal cancers: A unique biologic subset. Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Stricker, S.; Rauschenberger, V.; Schambony, A. ROR-Family Receptor Tyrosine Kinases. Curr. Top. Dev. Biol. 2017, 123, 105–142. [Google Scholar] [CrossRef]
- Zhou, J.-K.; Zheng, Y.-Z.; Liu, X.-S.; Gou, Q.; Ma, R.; Guo, C.-L.; Croce, C.M.; Liu, L.; Peng, Y. ROR1 expression as a biomarker for predicting prognosis in patients with colorectal cancer. Oncotarget 2017, 8, 32864. [Google Scholar] [CrossRef]
- Ford, C.E.; Qian Ma, S.S.; Quadir, A.; Ward, R.L. The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in human carcinogenesis. Int. J. Cancer 2013, 133, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.S.; Srivastava, S.; Llamosas, E.; Hawkins, N.J.; Hesson, L.B.; Ward, R.L.; Ford, C.E. ROR2 is epigenetically inactivated in the early stages of colorectal neoplasia and is associated with proliferation and migration. BMC Cancer 2016, 16, 508. [Google Scholar] [CrossRef]
- The Human Protein Atlas. MUSK. Available online: https://www.proteinatlas.org/ENSG00000030304-MUSK/tissue (accessed on 31 January 2019).
- Otte, J.M.; Schmitz, F.; Kiehne, K.; Stechele, H.U.; Banasiewicz, T.; Krokowicz, P.; Nakamura, T.; Folsch, U.R.; Herzig, K. Functional expression of HGF and its receptor in human colorectal cancer. Digestion 2000, 61, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Liska, D.; Chen, C.T.; Bachleitner-Hofmann, T.; Christensen, J.G.; Weiser, M.R. HGF rescues colorectal cancer cells from EGFR inhibition via MET activation. Clin. Cancer Res. 2011, 17, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, D.; Ma, J.; Tan, X.; Kwon, Y.-K.; Muhammad, E.; Melhem, M.; DeFrances, M.C.; Zarnegar, R. Genomic instability causes HGF gene activation in colon cancer cells, promoting their resistance to necroptosis. Gastroenterology 2015, 148, 181–191. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kitagawa, Y.; Kim, J.; Bilchik, A.; Kuo, C.; Kitajima, M.; Hoon, D. HGF activation in colorectal cancer via c-Met receptor regulates IAP proteins. In Proceedings of the AACR Annual Meeting, Los Angeles, CA, USA, 14–18 April 2007. [Google Scholar]
- Kammula, U.S.; Kuntz, E.; Zeng, Z.; Shia, J.; Landmann, R.; Paty, P.; Weiser, M. MET and HGF predict outcome in colorectal cancer. J. Am. Coll. Surg. 2004, 199, 90. [Google Scholar] [CrossRef]
- Sun, Y.L.; Liu, W.D.; Ma, G.Y.; Gao, D.W.; Jiang, Y.Z.; Liu, Q.; Du, J.J. Expression of HGF and Met in human tissues of colorectal cancers: Biological and clinical implications for synchronous liver metastasis. Int. J. Med. Sci. 2013, 10, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.N.; Liu, P. Targeting MET in cancer therapy. Chronic Dis. Transl. Med. 2017, 3, 148–153. [Google Scholar] [CrossRef]
- Mira, A.; Morello, V.; Céspedes, M.V.; Perera, T.; Comoglio, P.M.; Mangues, R.; Michieli, P. Stroma-derived HGF drives metabolic adaptation of colorectal cancer to angiogenesis inhibitors. Oncotarget 2017, 8, 38193. [Google Scholar] [CrossRef]
- Rajput, A.; Rose, R.; Levea, C.; Beko, A.; Brattain, M.G.; Wang, J. RON tyrosine kinase: A potential target for colorectal cancer therapy. In Proceedings of the AACR Annual Meeting, Washington, DC, USA, 17 April 2010. [Google Scholar]
- Zhou, Y.-Q.; He, C.; Chen, Y.-Q.; Wang, D.; Wang, M.-H. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: Generation of different splicing RON variants and their oncogenic potential. Oncogene 2003, 22, 186. [Google Scholar] [CrossRef]
- Wang, M.H.; Kurtz, A.L.; Chen, Y. Identification of a novel splicing product of the RON receptor tyrosine kinase in human colorectal carcinoma cells. Carcinogenesis 2000, 21, 1507–1512. [Google Scholar] [CrossRef]
- Spidel, C.M. Pathological Role of the RON Receptor Tyrosine Kinase in Colorectal Cancer: Characterization of a Short-Form RON Variant. Ph.D. Thesis, Texas Tech University, Lubbock, TX, USA, 2006. [Google Scholar]
- Van der Meer, J.H.; van der Poll, T.; van‘t Veer, C. TAM receptors, Gas6, and protein S: Roles in inflammation and hemostasis. Blood 2014, 123, 2460–2469. [Google Scholar] [CrossRef] [PubMed]
- Uribe, D.J.; Mandell, E.K.; Watson, A.; Martinez, J.D.; Leighton, J.A.; Ghosh, S.; Rothlin, C.V. The receptor tyrosine kinase AXL promotes migration and invasion in colorectal cancer. PLoS ONE 2017, 12, e0179979. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Martini, G.; Cardone, C.; Troiani, T.; Liguori, G.; Vitagliano, D.; Napolitano, S.; Morgillo, F.; Rinaldi, B.; Melillo, R.M. AXL is an oncotarget in human colorectal cancer. Oncotarget 2015, 6, 23281. [Google Scholar] [CrossRef]
- Gay, C.M.; Balaji, K.; Byers, L.A. Giving AXL the axe: Targeting AXL in human malignancy. Br. J. Cancer 2017, 116, 415–423. [Google Scholar] [CrossRef]
- ESMO. AXL Has a Prognostic Role in Metastatic Colorectal Cancer (mCRC) and is a Predictive Biomarker of Lack of Efficacy of Chemotherapy (CT) + Cetuximab. Available online: https://oncologypro.esmo.org/Meeting-Resources/ESMO-2018-Congress/AXL-has-a-prognostic-role-in-metastatic-colorectal-cancer-mCRC-and-is-a-predictive-biomarker-of-lack-of-efficacy-of-chemotherapy-CT-cetuximab-in-RAS-wild-type-WT-patients-pts (accessed on 31 January 2019).
- Dunne, P.D.; McArt, D.G.; Blayney, J.K.; Kalimutho, M.; Greer, S.; Wang, T.; Srivastava, S.; Ong, C.W.; Arthur, K.; Loughrey, M. AXL is a key regulator of inherent and chemotherapy-induced invasion and predicts a poor clinical outcome in early-stage colon cancer. Clin. Cancer Res. 2014, 20, 164–175. [Google Scholar] [CrossRef]
- Schmitz, R.; Valls, A.F.; Yerbes, R.; von Richter, S.; Kahlert, C.; Loges, S.; Weitz, J.; Schneider, M.; de Almodovar, C.R.; Ulrich, A. TAM receptors Tyro3 and Mer as novel targets in colorectal cancer. Oncotarget 2016, 7, 56355. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Iljin, K.; Dumont, D.J.; Alitalo, K. Tie receptors: New modulators of angiogenic and lymphangiogenic responses. Nat. Rev. Mol. Cell Biol. 2001, 2, 257. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Y.; Wei, J.; Zhao, Y. Role of Ang-2, Tie-2 and VEGFR-2 in angiogenesis in colorectal carcinoma and their prognostic value. Nan Fang Yi Ke Da Xue Xue Bao= J. South. Med Univ. 2012, 32, 1658–1662. [Google Scholar]
- Jayson, G.C.; Zhou, C.; Backen, A.; Horsley, L.; Marti-Marti, K.; Shaw, D.; Mescallado, N.; Clamp, A.; Saunders, M.P.; Valle, J.W. Plasma Tie2 is a tumor vascular response biomarker for VEGF inhibitors in metastatic colorectal cancer. Nat. Commun. 2018, 9, 4672. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Jung, H.I.; Ahn, T.S.; Kim, H.J.; Lee, K.T.; Baek, M.J.; Bae, S.B. Expressions and Clinical Significances of Angiopoietin-1, Angiopoietin-2, and Tie-2 Receptor in Patients with Colorectal Cancer. Ann. Coloproctol. 2017, 33, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Herath, N.I.; Boyd, A.W. The role of Eph receptors and ephrin ligands in colorectal cancer. Int. J. Cancer 2010, 126, 2003–2011. [Google Scholar] [CrossRef]
- UniProt. UniProtKB - P07949 (RET_HUMAN). Available online: https://www.uniprot.org/uniprot/P07949 (accessed on 21 January 2019).
- Luo, Y.; Tsuchiya, K.D.; Park, D.I.; Fausel, R.; Kanngurn, S.; Welcsh, P.; Dzieciatkowski, S.; Wang, J.; Grady, W.M. RET is a potential tumor suppressor gene in colorectal cancer. Oncogene 2013, 32, 2037. [Google Scholar] [CrossRef] [PubMed]
- Le Rolle, A.-F.; Klempner, S.J.; Garrett, C.R.; Seery, T.; Sanford, E.M.; Balasubramanian, S.; Ross, J.S.; Stephens, P.J.; Miller, V.A.; Ali, S.M. Identification and characterization of RET fusions in advanced colorectal cancer. Oncotarget 2015, 6, 28929. [Google Scholar] [CrossRef]
- Green, J.; Nusse, R.; van Amerongen, R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb. Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef]
- Mohan, R.R.; Mohan, R.R.; Wilson, S.E. Discoidin domain receptor (DDR) 1 and 2: Collagen-activated tyrosine kinase receptors in the cornea. Exp. Eye Res. 2001, 72, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, A.; Lafitte, M.; Roche, S. DDR1 inhibition as a new therapeutic strategy for colorectal cancer. Mol. Cell. Oncol. 2018, 5, e1465882. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, I.M.; Yoo, K.H.; Lee, S.H. ROS receptor tyrosine kinase: A new potential target for anticancer drugs. Med. Res. Rev. 2011, 31, 794–818. [Google Scholar] [CrossRef] [PubMed]
- Aisner, D.L.; Nguyen, T.T.; Paskulin, D.D.; Le, A.T.; Haney, J.; Schulte, N.; Chionh, F.; Hardingham, J.; Mariadason, J.; Tebbutt, N.; et al. ROS1 and ALK fusions in colorectal cancer, with evidence of intratumoral heterogeneity for molecular drivers. Mol. Cancer Res. 2014, 12, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.D.; Doebele, R.C. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. 2013, 19, 4040–4045. [Google Scholar] [CrossRef]
- Shi, H.; Li, Q.; Ji, M.; Wu, J.; Li, Z.; Zheng, X.; Xu, B.; Chen, L.; Li, X.; Lu, C. Lemur tyrosine kinase-3 is a significant prognostic marker for patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 1101. [Google Scholar]
- UniProt. UniProtKB - P29376 (LTK_HUMAN). Available online: https://www.uniprot.org/uniprot/P29376 (accessed on 21 January 2019).
- Webb, T.R.; Slavish, J.; George, R.E.; Look, A.T.; Xue, L.; Jiang, Q.; Cui, X.; Rentrop, W.B.; Morris, S.W. Anaplastic lymphoma kinase: Role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev. Anticancer Ther. 2009, 9, 331–356. [Google Scholar] [CrossRef]
- The Human Protein Atlas. TLK. Available online: https://www.proteinatlas.org/ENSG00000062524-LTK/pathology (accessed on 1 February 2019).
- NCBI. STYK1 serine/threonine/tyrosine kinase 1 [ Homo sapiens (human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/?term=PubMed+15150103 (accessed on 21 January 2019).
- Hu, L.; Chen, H.-Y.; Cai, J.; Zhang, Y.; Qi, C.-Y.; Gong, H.; Zhai, Y.-X.; Fu, H.; Yang, G.-Z.; Gao, C.-F. Serine threonine tyrosine kinase 1 is a potential prognostic marker in colorectal cancer. BMC Cancer 2015, 15, 246. [Google Scholar] [CrossRef]
- Moustakas, A.; Souchelnytskyi, S.; Heldin, C.H. Receptor Serine/Threonine Kinases. In Encyclopedic Reference of Genomics and Proteomics in Molecular Medicine; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1603–1608. [Google Scholar]
- Jung, B.; Staudacher, J.J.; Beauchamp, D. Transforming Growth Factor beta Superfamily Signaling in Development of Colorectal Cancer. Gastroenterology 2017, 152, 36–52. [Google Scholar] [CrossRef]
- Stransky, N.; Cerami, E.; Schalm, S.; Kim, J.L.; Lengauer, C. The landscape of kinase fusions in cancer. Nat. Commun. 2014, 5, 4846. [Google Scholar] [CrossRef]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Cespedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.; Evans, S.R.; Vidal-Vanaclocha, F.; Calvo, A. Role of TGF-beta in metastatic colon cancer: It is finally time for targeted therapy. Cell Tissue Res. 2017, 370, 29–39. [Google Scholar] [CrossRef]
- Eberle, J.; Hossini, A.M. Expression and function of bcl-2 proteins in melanoma. Curr. Genom. 2008, 9, 409–419. [Google Scholar] [CrossRef]
- Danielsen, S.A.; Eide, P.W.; Nesbakken, A.; Guren, T.; Leithe, E.; Lothe, R.A. Portrait of the PI3K/AKT pathway in colorectal cancer. Biochim. Biophys. Acta 2015, 1855, 104–121. [Google Scholar] [CrossRef]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef]
- Grossi, V.; Peserico, A.; Tezil, T.; Simone, C. p38alpha MAPK pathway: A key factor in colorectal cancer therapy and chemoresistance. World J. Gastroenterol. 2014, 20, 9744–9758. [Google Scholar] [CrossRef]
- Nishina, H.; Wada, T.; Katada, T. Physiological roles of SAPK/JNK signaling pathway. J. Biochem. 2004, 136, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Ameyar, M.; Wisniewska, M.; Weitzman, J. A role for AP-1 in apoptosis: The case for and against. Biochimie 2003, 85, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Burotto, M.; Chiou, V.L.; Lee, J.M.; Kohn, E.C. The MAPK pathway across different malignancies: A new perspective. Cancer 2014, 120, 3446–3456. [Google Scholar] [CrossRef]
- Aksamitiene, E.; Kiyatkin, A.; Kholodenko, B.N. Cross-Talk between Mitogenic Ras/MAPK and Survival PI3K/Akt Pathways: A Fine Balance; Portland Press Limited: London, UK, 2012. [Google Scholar]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Berg, M.; Danielsen, S.A.; Ahlquist, T.; Merok, M.A.; Ågesen, T.H.; Vatn, M.H.; Mala, T.; Sjo, O.H.; Bakka, A.; Moberg, I. DNA sequence profiles of the colorectal cancer critical gene set KRAS-BRAF-PIK3CA-PTEN-TP53 related to age at disease onset. PLoS ONE 2010, 5, e13978. [Google Scholar] [CrossRef]
- Salmena, L.; Carracedo, A.; Pandolfi, P.P. Tenets of PTEN tumor suppression. Cell 2008, 133, 403–414. [Google Scholar] [CrossRef]
- Laurent-Puig, P.; Cayre, A.; Manceau, G.; Buc, E.; Bachet, J.-B.; Lecomte, T.; Rougier, P.; Lievre, A.; Landi, B.; Boige, V. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J. Clin. Oncol. 2009, 27, 5924–5930. [Google Scholar] [CrossRef]
- Loupakis, F.; Pollina, L.; Stasi, I.; Ruzzo, A.; Scartozzi, M.; Santini, D.; Masi, G.; Graziano, F.; Cremolini, C.; Rulli, E. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J. Clin. Oncol. 2009, 27, 2622–2629. [Google Scholar] [CrossRef]
- Szwajda, A.; Gautam, P.; Karhinen, L.; Jha, S.K.; Saarela, J.; Shakyawar, S.; Turunen, L.; Yadav, B.; Tang, J.; Wennerberg, K.; et al. Systematic Mapping of Kinase Addiction Combinations in Breast Cancer Cells by Integrating Drug Sensitivity and Selectivity Profiles. Chem. Biol. 2015, 22, 1144–1155. [Google Scholar] [CrossRef]
- Garcia-Aranda, M.; Perez-Ruiz, E.; Redondo, M. Bcl-2 Inhibition to Overcome Resistance to Chemo- and Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3950. [Google Scholar] [CrossRef]
- Huang, C.W.; Chen, Y.T.; Tsai, H.L.; Yeh, Y.S.; Su, W.C.; Ma, C.J.; Tsai, T.N.; Wang, J.Y. EGFR expression in patients with stage III colorectal cancer after adjuvant chemotherapy and on cancer cell function. Oncotarget 2017, 8, 114663–114676. [Google Scholar] [CrossRef]
- FDA. Information on Cetuximab (Marketed as Erbitux). Available online: https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm113714.htm (accessed on 28 January 2019).
- Giusti, R.M.; Shastri, K.A.; Cohen, M.H.; Keegan, P.; Pazdur, R. FDA drug approval summary: Panitumumab (Vectibix). Oncologist 2007, 12, 577–583. [Google Scholar] [CrossRef]
- Fujino, S.; Miyoshi, N.; Ohue, M.; Takahashi, Y.; Yasui, M.; Hata, T.; Matsuda, C.; Mizushima, T.; Doki, Y.; Mori, M. Platelet-derived growth factor receptor-β gene expression relates to recurrence in colorectal cancer. Oncol. Rep. 2018, 39, 2178–2184. [Google Scholar] [CrossRef]
- FDA. FDA Approves Regorafenib for Advanced Colorectal Cancer. Available online: https://www.onclive.com/web-exclusives/fda-approves-regorafenib-for-advanced-colorectal-cancer (accessed on 8 March 2019).
- FDA. Crenolanib Approval Status. Available online: https://www.drugs.com/history/crenolanib.html (accessed on 7 February 2019).
- Attoub, S.; Rivat, C.; Rodrigues, S.; Van Bocxlaer, S.; Bedin, M.; Bruyneel, E.; Louvet, C.; Kornprobst, M.; Andre, T.; Mareel, M.; et al. The c-kit tyrosine kinase inhibitor STI571 for colorectal cancer therapy. Cancer Res. 2002, 62, 4879–4883. [Google Scholar]
- CenterWatch. Cyramza (ramucirumab). Available online: https://www.centerwatch.com/drug-information/fda-approved-drugs/drug/1320/cyramza-ramucirumab (accessed on 30 January 2019).
- Goel, G.; Sun, W. Ramucirumab, another anti-angiogenic agent for metastatic colorectal cancer in second-line setting--its impact on clinical practice. J. Hematol. Oncol. 2015, 8, 92. [Google Scholar] [CrossRef]
- Turkington, R.C.; Longley, D.B.; Allen, W.L.; Stevenson, L.; McLaughlin, K.; Dunne, P.D.; Blayney, J.K.; Salto-Tellez, M.; Van Schaeybroeck, S.; Johnston, P.G. Fibroblast growth factor receptor 4 (FGFR4): A targetable regulator of drug resistance in colorectal cancer. Cell Death Dis. 2014, 5, e1046. [Google Scholar] [CrossRef] [PubMed]
- Lhoumeau, A.-C.; Martinez, S.; Boher, J.-M.; Monges, G.; Castellano, R.; Goubard, A.; Doremus, M.; Poizat, F.; Lelong, B.; de Chaisemartin, C. Overexpression of the promigratory and prometastatic PTK7 receptor is associated with an adverse clinical outcome in colorectal cancer. PLoS ONE 2015, 10, e0123768. [Google Scholar] [CrossRef]
- Lee, S.J.; Li, G.G.; Kim, S.T.; Hong, M.E.; Jang, J.; Yoon, N.; Ahn, S.M.; Murphy, D.; Christiansen, J.; Wei, G. NTRK1 rearrangement in colorectal cancer patients: Evidence for actionable target using patient-derived tumor cell line. Oncotarget 2015, 6, 39028. [Google Scholar] [CrossRef] [PubMed]
- NIH. Study of Milciclib in Patients with Unresectable/Metastatic Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT03109886 (accessed on 7 February 2019).
- Saigusa, S.; Toiyama, Y.; Tanaka, K.; Yokoe, T.; Fujikawa, H.; Matsushita, K.; Okugawa, Y.; Inoue, Y.; Uchida, K.; Mohri, Y.; et al. Inhibition of HGF/cMET expression prevents distant recurrence of rectal cancer after preoperative chemoradiotherapy. Int. J. Oncol. 2012, 40, 583–591. [Google Scholar] [CrossRef]
- Grenga, I.; Kwilas, A.R.; Donahue, R.N.; Farsaci, B.; Hodge, J.W. Inhibition of the angiopoietin/Tie2 axis induces immunogenic modulation, which sensitizes human tumor cells to immune attack. J. Immunother. Cancer 2015, 3, 52. [Google Scholar] [CrossRef]
- Chiu, J.W.; Hotte, S.J.; Kollmannsberger, C.K.; Renouf, D.J.; Cescon, D.W.; Hedley, D.; Chow, S.; Moscow, J.; Chen, Z.; Perry, M. A phase I trial of ANG1/2-Tie2 inhibitor trebaninib (AMG386) and temsirolimus in advanced solid tumors (PJC008/NCI# 9041). Investig. New Drugs 2016, 34, 104–111. [Google Scholar]
- Hidalgo, M.; Martinez-Garcia, M.; Le Tourneau, C.; Massard, C.; Garralda, E.; Boni, V.; Taus, A.; Albanell, J.; Sablin, M.-P.; Alt, M. First-in-Human Phase I Study of Single-agent Vanucizumab, A First-in-Class Bispecific Anti-Angiopoietin-2/Anti-VEGF-A Antibody, in Adult Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Oh, S.O.; Kim, K.; Lee, J.; Kang, S.; Kim, K.-M.; Lee, W.; Kim, S.T.; Nam, D.N. NCOA4-RET fusion in colorectal cancer: Therapeutic challenge using patient-derived tumor cell lines. J. Cancer 2018, 9, 3032–3037. [Google Scholar] [CrossRef]
- Yuge, R.; Kitadai, Y.; Takigawa, H.; Tanaka, S.; Chayama, K.; Yasui, W. Inhibition of collagen receptor discoidin domain receptor-1 (DDR1) reduces gastric cancer cell motility and metastasis. In Proceedings of the AACR Annual Meeting, Washington, DC, USA, 1 April 2017. [Google Scholar]
- Jeitany, M.; Leroy, C.; Tosti, P.; Lafitte, M.; Le Guet, J.; Simon, V.; Bonenfant, D.; Robert, B.; Grillet, F.; Mollevi, C. Inhibition of DDR1-BCR signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol. Med. 2018, 10, e7918. [Google Scholar] [CrossRef] [PubMed]
- Yakirevich, E.; Resnick, M.B.; Mangray, S.; Wheeler, M.; Jackson, C.L.; Lombardo, K.A.; Lee, J.; Kim, K.-M.; Gill, A.J.; Wang, K. Oncogenic ALK fusion in rare and aggressive subtype of colorectal adenocarcinoma as a potential therapeutic target. Clin. Cancer Res. 2016, 22, 3831–3840. [Google Scholar] [CrossRef] [PubMed]
- Blue Ridge Institute for Medical Research in Horse Shoe, N.C. FDA-Approved Protein Kinase Inhibitors Compiled by Robert Roskoski Jr. Available online: http://www.brimr.org/PKI/PKIs.htm (accessed on 8 March 2019).
- Drugs.com. Stivarga Approval Story. Available online: https://www.drugs.com/history/stivarga.html (accessed on 11 March 2019).
- FDA. FDA Approves First Cancer Treatment for any Solid Tumor with a Specific Genetic Feature. Available online: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm (accessed on 1 February 2019).
- Ahronian, L.G.; Corcoran, R.B. Effective MAPK Inhibition is critical for therapeutic responses in colorectal cancer with BRAF mutations. Mol. Cell. Oncol. 2016, 3, e1048405. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ruiz, E.; Rueda, A.; Pereda, T.; Alcaide, J.; Bautista, D.; Rivas-Ruiz, F.; Villatoro, R.; Perez, D.; Redondo, M. Involvement of K-RAS mutations and amino acid substitutions in the survival of metastatic colorectal cancer patients. Tumour Biol. 2012, 33, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- OncologyPRO. BRAF in Colorectal Cancer: ESMO Biomarker Factsheet. Available online: https://oncologypro.esmo.org/Education-Library/Factsheets-on-Biomarkers/BRAF-in-Colorectal-Cancer (accessed on 13 February 2019).
- American Cancer Society. Targeted Therapy Drugs for Colorectal Cancer. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/treating/targeted-therapy.html (accessed on 7 March 2019).
| Criteria | Overview | Role and Significance | |
|---|---|---|---|
| Location in the cell | Transmembrane Receptor Kinases | Consist of a ligand-binding extracellular domain and a catalytic intracellular kinase domain. | Key initial step in the translation of an extracellular stimulus as down-stream signaling cascades within the cell. |
| Non-receptor Kinases | Lack transmembrane domains. Located in the cytosol, nucleus or associated to the inner surface of plasma membrane. | Signal transduction throughout the cytoplasm and the nucleus and gene transcription. | |
| Eukaryotic catalytic domain sequence | AGC | Subgroup of 60 Serine/Threonine kinases, including A, G and C protein kinases (PKA, PKG and PKC respectively), all of them with high homology of the catalytic kinase domain [15]. This subfamily also includes well-studied enzymes such as AKT (PKB), S6K, RSK, MSK, PDK1 and GRK as well as SGK, NDR, LATS, CRIK, SGK494, PRKX, PRKY and MAST [15]. | Most AGC kinases are activated by phosphorylation [15]. AGC kinases are involved in numerous cellular processes including metabolism control, protein synthesis (AKT, RSK), cell proliferation (AKT), mediation of growth factors and hormones effect (PKC, PKA) or sodium transport (SGK) [15] and their mutation and/or dysregulation has been related to multiple human diseases including cancer, diabetes and different inherited syndromes [15]. |
| CAMK | The Ca2+/Calmodulin-dependent protein kinases (CAMK I, CAMK II, CAMK III, CAMK IV, CAMK V) are Serine/Threonine kinases with a highly conserved architecture of their active pocket which contains a bi-lobed catalytic domain followed by a regulatory domain with both an autoinhibitory and a CaM-binding domain [16]. CAMK kinases are activated in response to an increase in the concentration of intracellular calcium ions [17]. | CAMK, whose functionality are regulated by Ca2+-binding protein calmodulin (CaM), play a key role in processes such as gene transcription, apoptosis or cytoskeletal reorganization [16] and are responsible for the phosphorylation of various transcription factors [17]. CAMPK dysregulation has been related to cancer progression and therapy response [18]. | |
| CK1 | Members of the Casein Kinase (CK) 1 family (alpha, beta 1, gamma 1, gamma 2, gamma 3, delta, epsilon) are monomeric Serine/Threonine kinases with highly conserved regions within the kinase domain but differing in length and sequence of the N-terminal and the C-terminal non-catalytic domain, the last with a crucial role in substrate specificity and in the regulation of kinase activity [19]. | Members of the CK1 family act as regulators of signal transduction pathways [17], playing a key role in the phosphorylation of regulatory molecules involved in DNA-transcription and repair, cell proliferation, cytoskeleton dynamics, vesicular trafficking, apoptosis and cell differentiation [19] among others. CK1 are also able to alter the activity of key proteins involved in signal transduction and signal integration molecules [19]. Aberrant expression of CK1 is usually detected in different malignancies including kidney, breast, pancreas and ovarian cancer [19]. | |
| CMGC | CMGC kinase family is named after the initials of its subfamily members: CDK (cyclin-dependent kinases), MAPK (mitogen activated protein kinases), GSK3 (glycose synthase kinase-3) and CLK (cdc2-like kinases). CMGC enzymes are Serine/Threonine kinases which preferentially phosphorylate substrates with proline at the P+1 position [20]. CMGC family members are also characterized by a unique regulatory mechanism that involves a phosphorylated tyrosine in the activation loop or a pre-phosphorylated residue in the substrate [20]. | CMGC family members are involved in the regulation of cell cycle (CDK), signal transduction, cell proliferation, differentiation and death (MAPK), glycogen metabolism and embryonic development (GSK3), gene transcription (CLK) [17]. CMGC dysregulations have been linked to oncogenic transformation [17]. | |
| RGC | Members of the Receptor Guanylate Cyclase (RGC) subfamily have an N-terminal extracellular ligand binding domain, a single-pass transmembrane domain and a C-terminal intracellular domain [21] which catalyzes the synthesis of cyclic guanosine monophosphate (cGMP) from GTP. RGC intracellular domain contains a region with sequences homologous to protein kinase core [21], being usually classified as pseudo-kinases. The kinase domain can bind to ATP, causing a conformational change which is thought to regulate the guanylate cyclase domain [22]. RGC kinases can be activated by hormones, peptides and low calcium-induced guanylyl cyclase-activating proteins [23]. | RGC kinases play a key role as transducers of extracellular information to the interior of the cell, since intracellular cGMP is a second messenger that modulates the activity of different intracellular protein kinases. | |
| STE | Based on their homology to the yeast proteins [24], the homologues of yeast Sterile (STE) kinase group is classified into three main families (Ste7, Ste11 and Ste20) which sequentially activate each other to then activate the MAPK family [17]. | Members of the STE family are critical regulators of multiple signaling pathways and their aberrant expression is found in different malignancies [24]. | |
| TK | The Tyrosine Kinase (TK) group includes receptor and non-receptor (cytosolic) kinases [24] that specifically phosphorylate tyrosine residues [17]. This subfamily includes the human epidermal growth factor receptor (HER/EGFR) family, the insulin receptor (IR), the insulin-like growth factor 1-receptor (IGF1-R), the SRC, ABL and JAK kinases [24]. | TKs are important mediators in transmembrane signaling and signal transduction within the cell, being involved in cell proliferation, differentiation, migration, metabolism and apoptosis in response to internal and external stimuli [25]. Since multiple studies have identified TKs dysregulation during the pathophysiology of cancer [25], decreased apoptosis and increased cell proliferation, this group contains the majority of targets for kinase inhibitors that are currently in clinical use [24]. | |
| TKL | Tyrosine kinase-like (TKL) protein kinases are mostly serine/threonine kinases [24] with sequence similarity to TKs but lacking TK-specific motifs. This group, which contains both receptor and non-receptor kinases, includes the RAF (Rapidly Accelerated Fibrosarcoma) kinases and the transforming growth factor beta (TGF-β) receptors. | Members of the TKL family are involved in the MAPK pathways (RAF/MAPK), in cellular processes such as cell growth, differentiation and apoptosis (TGF-β) and angiogenesis and vascular development (TGF-β-I receptor activin receptor-like kinase, ALK1). | |
| RTK | Overview | Role in CRC | |
|---|---|---|---|
| I | Epidermal Growth Factor (EGF/ErbB) receptor family: EGFR, HER2, HER3, HER4 receptors | EGFR can respond to and be activated by protein hormones, cytokines or growth factors, acting as key regulators of decisive cellular processes such as proliferation, differentiation, survival, metabolism, migration and cell cycle control [34]. | Positive EGFR expression is a significant independent negative prognostic factor for CRC disease-free survival and overall survival [35]. Positive EGFR expression is also significantly associated with tumor-node-metastasis (TNM) stage T, with a predictive value for postoperative relapse in these patients [35]. EGFR is overexpressed in up to 97% of CRC patients and significantly associated with highly malignant behavior [35,36]. Indeed, as in the case of other malignancies, including breast or lung cancer, EGFR plays a crucial role in the tumorigenesis and tumor progression of CRC and has become a valuable target in the treatment of metastatic CRC. |
| II | Insulin Growth Factor/Insulin receptor family (IGFR/InsR): IGFR and IRR receptors | Both IGF1 and IGF2 bind and activate IGF1R transmembrane receptor kinase. IGF2R does not contain a kinase domain and binding with IGF2 does not result in downstream signaling [37]. IGFR responses, which include apoptosis and autophagy inhibition, DNA synthesis or amino acid uptake [38], are mediated through intracellular adaptor proteins [34]. | The InsR/IGF1R have a major role in the pathogenesis and progression of CRC, contributing to the transformation of normal colon epithelial cells and the development of resistance to both chemotherapeutic drugs and epidermal growth factor receptor targeted agents [37]. |
| III | Platelet Derived Growth Factor Receptor (PDGFR), Colony stimulating factor 1 receptor (CSF-1R) (Ems), KIT proto-oncogene receptor tyrosine kinase (KIT) and FMS related tyrosine kinase 3 (FLT3) receptors | PDGFs are important growth factors for normal tissue growth and division with a role in blood vessel formation [39]. Cancer cells can escape immune responses by secreting CSF to the tumor environment, which stimulates the proliferation and recruitment of immunosuppressive myeloid cells [40]. Accordingly, intratumoral presence of myeloid cells expressing CSFR correlates with poor survival in different malignancies [41]. In CRC, KIT activation by Stem Cell Factor (SCF) ligand induces signaling by different pathways including PI3K, RAS and JAK/STAT [42]. | PDFGs are often over-expressed or mutated in CRC stromal cells, pericytes and CRC cell lines [43]. In CRC, PDGF overexpression is associated with angiogenesis, invasion, metastasis, poor survival and resistance to targeted therapies in CRC patients [39], having been proposed as useful biomarker for both diagnosis and CRC treatment [39]. CF1R dependency by intestinal macrophages along with CSF1R overexpression in CRC tumors correlates with tumor stage and differentiation [44]. KIT mutations are usually found in different malignancies including CRC and associated with resistance to chemotherapy and malignant mesothelioma [45]. FLT3 amplification has been reported in approximately 3% of CRC samples associated with primary or acquired resistance to EGFR blockade [46]. Binding of TLT3 ligand to FLT3 triggers PI3K and RAS pathways, leading to increased cell proliferation and apoptosis inhibition [46]. |
| IV | Vascular Endothelial Growth Factor (VEGF) receptor family: VEGFR-1, VEGFR-2, VEGFR-3 receptors | Key regulators of metabolic homeostasis, cell proliferation, migration, tubulogenesis [47], angiogenesis and lymphangiogenesis [34]. | Due to its role in regulating endothelial cells differentiation, VEGFR2 is one of the major angiogenesis mediators in CRC [48]. VEGFR2 overexpression correlates with differentiation, metastasis, recurrence and poor prognosis of CRC patients [49]. Interestingly, it has been reported that CRC patients with good overall survival and/or good metastasis free survival are characterized by low VEGFR1 and high VEGFR2 expression [48]. Likewise, VEGFR3 is usually found to be overexpressed in CRC tumor vasculature [50]. |
| V | Fibroblast Growth Factor (FGF) receptor family: FGFR1, FGFR2, FGFR3, FGFR4 receptors | Mediate progenitor cells growth, differentiation, survival and patterning during embryonic development and organogenesis as well as metabolic functions, tissue repair and regeneration in adult tissues [51]. | All four FGFR and their ligands are expressed in CRC [52]. Among them, FGR1 is usually overexpressed in CRC patients, correlating with an aggressive clinical behavior [53]. FGFR2 regulates CRC cells migration, invasion and growth and plays an important role in cancer progression [52]. |
| VI | Protein tyrosine kinase-like 7 (PTK7)/ Colon Carcinoma Kinase 4 (CCK4) receptor | These receptors are associated with epithelial cells polarization and neural structures development [34]. Although sequence analysis suggests that the gene product is catalytically inactive as a protein kinase, it is involved in Wnt [34] and VEGF signaling [54]. | PTK7, which can promote survival, motility and invasion of cancer cells through non-canonical Wnt-signaling activation [54], is usually overexpressed in colon cancer [54]. However, as a result of complexity in the canonical and non-canonical Wnt signaling network [54], PTK7 upregulation can result in tumor promotion or suppression in a cell context-dependent manner [54], resulting in both favorable o poor prognosis in patients with CRC patients [54]. For these reasons, PTK7-targeted treatments might only be beneficial for CRC patients with oncogenic PTK7 upregulation [54]. |
| VII | Neurotrophin receptor/Tropomyosin Receptor Kinase (TRK, NTRK) family: TRKA, TRKB, TRKC receptors | TRKA, TRKB and TRKC receptors respond to Nerve Growth Factor (NGF), Brain-derived Neurotrophic Factor (BDNF) and Neurtrophin-3, respectively [34] and mediate proliferative and migration processes in neural systems [34]. | Food and Drug Administration (FDA) has recently approved larotrectinib (Vitrakvi) for the treatment of patients with solid tumors affected by NTRK gene fusions [55]. In CRC patients, chromosomal rearrangements involving the NTRK1 gene (encoding the TRKA protein) are shown in a small subset of patients, associated with the constitutive activation of the TRKA kinase domain as well as with proliferation and survival in CRC tumors [56]. 16% of samples from CRC patients present NTRK gene rearrangements [57] along with high microsatellite instability [57], suggesting that these patients may benefit from both tyrosine kinase inhibitors and checkpoint inhibitors as either monotherapy or in combination [57]. |
| VIII | Receptor Tyrosine Kinase-like Orphan Receptors (ROR) family: ROR1 and ROR2 receptors | Act as alternative receptors and coreceptors of Wnt signals [58], regulating cell proliferation and polarity as well as tissue maintenance. | ROR1 is usually overexpressed in CRC cells when compared to the adjacent normal tissues and positively associated with the clinical stage and lymph-node metastasis, having been proposed as a novel prognostic marker and therapeutic target for CRC [59]. As non-canonical Wnt signaling mediator, ROR2 has a dual role as tumor suppressor or activator depending on tumor type [60] or stage. In CRC, ROR2 overexpression correlates to decreased tumor size [60] is frequently epigenetically inactivated by promoter hypermethylation in the early stages, contributing to CRC progression [61]. |
| IX | Muscle-Specific Kinase (MuSK) receptor | Associated with the formation and organization of the neuromuscular junction from the skeletal muscle side [34]. | MuSK receptor is usually expressed in rectum and colon tissues and has been proposed as a potential drug target in CRC [62]. |
| X | Hepatocyte Growth Factor Receptor (HGF) receptor family: mesenchymal-epithelial transition factor (MET) and (Recepteur d’Origine Nantais) RON receptors | HGF stimulates proliferation, migration and morphogenesis of epithelial cells by binding to and activating its receptor c-Met (MET) [63,64]. | Genomic instability causes HGF gene activation in colon cancer cells, promoting their resistance to necroptosis [65]. Since HGF induces proliferation, motility, adhesion and invasion of CRC cells [66] and is related to CRC development, progression and metastasis [35] high levels of HGF have been proposed as a valuable prognosis biomarker in CRC [35] as well as a marker of tumors with aggressive biology [67,68]. In CRC, high levels of HGF are usually accompanied by the overexpression of c-MET receptor, which is associated with CRC invasion and distant metastases [69] due to c-Met activation of different proteins like survivin, livin and X-linked inhibitor of apoptosis protein (XIAP), which inhibit apoptosis proteins (IAP), through AKT pathway [66]. In this regard, HGF has been proposed to protect CRC cells against EGFR inhibition via c-MET activation [64] and also against glucose starvation-induced apoptosis, promoting resistance to both anti-EGFR agents [64], anti-glycolytic agents and angiogenesis inhibitors [70]. Provided that RON kinase, which is overexpressed in 60% of human colon cancers [71] and altered in certain primary colon cancers, has been related to CRC progression and metastasis [72,73,74], it has recently been proposed as a novel target for advanced CRC patients [71]. |
| XI | TAM (TYRO3-, AXL- and MER-TK) receptor family: AXL, TYRO3, MERTK receptors | TAM receptors can be activated by the vitamin K-dependent proteins Growth arrest specific protein 6 (Gas6) and protein S, affecting cell proliferation, survival, adhesion and migration [75]. TAM act as potent inhibitors of inflammation and have an oncogenic role in a number of cancers [76]. | AXL tyrosine kinase receptor is overexpressed in CRC [77,78], having a role in epithelial to mesenchymal transition, tumor angiogenesis, resistance to chemotherapy and targeted agents and decreased antitumor immune response [78]. AXL has also been proposed as a negative prognostic biomarker for CRC patients [78] and as a predictive biomarker of lack of efficacy in RAS-wildtype metastatic CRC patients treated with chemotherapy and cetuximab [79]. As a result, AXL has been proposed as a novel therapeutic target for CRC treatment [77], in particular in those cases in which the adjuvant disease in which EGFR/VEGF-targeted therapies have failed [80]. Apart from AXL, TYRO3 and MER have also been proposed as potential targets in CRC [81]. |
| XII | Tyrosine Kinase with Immunoglobulin-like and EGF-like domains (TIE) or angiopoietin receptor family: TIE1 and TIE2 receptors | Modulators of angiogenic and lymphangiogenic responses [82]. | Angiopoietin 2 (Ang-2), TIE2 and VEGFR2 are involved in the development, invasion, angiogenesis, metastasis and prognosis of CRC [83]. In this regard, TIE2 expression has been validated as tumor vascular response biomarker for VEGF inhibitors in metastatic CRC [84] Provided the relation between Ang-2 and TIE2 expression, the Ang/TIE2 signaling pathway has been proposed to have an important role in the progression of CRC [85]. |
| XIII | Ephrin (Eph) receptor family: EphA1, EphA2, EphA3, EphA4, EphA5, EphA6, EphA7, EphA8, EphA10, EphB1, EphB2, EphB3, EphB4, EphB6 receptors | Implicated in the regulation of neuronal development, cell migration, patterning and angiogenesis [34]. | The role of the different members of Eph family, the largest one of RTK, is complex. In the early stages (I/II) of CRC malignant transformation, EphA1, EphA2, EphB1, EphB2 and EphB4 are upregulated and may play a role in tumor migration/invasion and metastatic behavior [86]. During CRC progression, Eph expression is gradually reduced until the loss of Eph expression in late stage CRC, which has been proposed as a potential valuable marker for these patients [86]. |
| XIV | Rearranged During Transfection (RET) receptor | After activation by glial cell derived neurotrophic factor family ligands, RET receptors mediate a wide range of responses such as cell proliferation, neuronal navigation, cell migration and cell differentiation [87]. | RET has been proposed as a tumor suppressor kinase in CRC [88]. RET inactivation, due to RET gene aberrant methylation or mutations, would be involved in the progression of colon adenomas to cancer [88,89]. Provided that rearrangements affecting RET is present in 22% of samples from CRC patients [57] along with high microsatellite instability [57], these patients may benefit from both tyrosine kinase inhibitors and checkpoint inhibitors as either monotherapy or in combination [57]. |
| XV | Related to Tyrosine Kinase (RYK) receptor | RYK contains functional extracellular Wnt-binding domains and is implicated in Wnt signaling [90]. | RYK role in CRC is under study. |
| XVI | Discoidin Domain Receptor Family (DDR) receptor family: DDR1, DDR2 receptors | DDR1 is activated by collagen, one of the major components of the extracellular matrix. After activation, DDR1 modulates cell adhesion, proliferation and metalloprotease expression [91]. | In a collagen rich environment, DDR1 can promote tumor cell invasion and cancer stem cell survival [92]. DDR1 has a role in invasive and metastatic abilities of CRC cells [92]. Interestingly, KRAS mutations induce DDR1 expression and sustains Notch oncogenic signaling and tumorigenesis [92]. |
| XVII | Reactive Oxygen Species (ROS) receptor family | Although ROS ligand and normal function have not been fully identified yet, aberrant expression of ROS has been reported in different malignancies [93], which has turned this protein in a potential target for anticancer drugs. | Genomic fusions causing ROS1 kinase constitutive activation and uncontrolled cellular proliferation are observed in CRC, which has been proposed as a potential therapeutic target in CRC [94,95]. |
| XVIII | Lemur receptor kinases (LMR/LMTK): 1, 2, 3 | The precise role of these receptors has not yet been defined [34]. | LMTK3 expression is significantly correlated with lymph node metastasis and overall survival in CRC patients, having been proposed as a prognostic marker for these patients [96]. |
| XIX | Leukocyte Tyrosine Kinase (LTK) receptor family: LTK (Leucocyte Receptor Tyrosine Kinase) and ALK (Anaplastic Lymphoma Kinase) | LTK endogenous ligands and precise roles are unknown [34]. Studies with chimeric proteins have shown LTK ability to promote growth and cell survival [97]. Genomic fusions causing ALK constitutive activation and uncontrolled cell proliferation are usually found in human cancer [98]. | Only a few cases of CRC show moderate immune-staining for TLK [99]. Genomic fusions affecting ALK are observed in CRC, which has been proposed as a potential therapeutic target in CRC [94,95]. |
| XX | Serine/threonine/tyrosine kinase (STYK) receptor: STYK1 | Involved in different cellular and developmental processes such as cell proliferation, differentiation and survival [100]. | STYK1 overexpression may be involved in the progression of CRC [101]. Aberrant expression of STYK1 has been reported in colorectal cancer with a prognostic value [101]. |
| RSTK | Description | Overview |
|---|---|---|
| Type I | Activin Receptor-like kinases (ACVR/ALKs): Activin A Receptor Type 1L (ACVR1L, ALK1), Activin A Receptor Type 1 (ACVR1, ALK2), Bone Morphogenetic Protein Receptor Type IA (BMPR1A), Activin A Receptor Type 1B (ACVR1B, ALK4), Transforming Growth Factor β Receptor 1 (TGFBR1), Bone Morphogenetic Protein Receptor Type IB (BMPR1B), Activin A Receptor Type 1C (ACVR1C, ALK7). | Since TGF-β signaling reduces proliferation and promotes apoptosis and differentiation in colon epithelial cells, loss of TGF-β signaling is considered a feature of CRC cells [103]. ALK gene is rearranged, mutated, or amplified in different tumors [100]. In the particular case of CRC, ALK fusions are frequent [104]. Alterations affecting the expression and activity of TGF-β receptors and SMAD protein signal transducers determine if proliferation of CRC cell is inhibited [103]. |
| Type II | Activin A Receptor Type 2A (ACVR2A, ActR2), Activin A Receptor type 2B (ACVR2B, ActR2B), Anti-Mullerian Hormone Receptor type 2 (AMHR2, MISR2), Bone Morphogenetic Protein Receptor Type 2 (BMPR2), Transforming Growth Factor Beta Receptor 2 (TGFBR2) | Under study |
| Type III | Transforming Growth Factor Beta Receptor 3 (TGFBR3) | Under study |
| Targeted RTK | Knockdown Effect on CRC Cells | Current Status for CRC Patients | FDA Approved Multi-Kinase Inhibitors |
|---|---|---|---|
| EGFR | Significantly reduces CRC cell proliferation, colony formation and migration [122]. | FDA approved Cetuximab (Erbitux) and panitumumab (Vectibix) monoclonal antibodies for the treatment of patients with EGFR-expressing, metastatic colorectal carcinoma [123,124]. | Afatinib, Brigatinib, Dacomitinib, Dasatinib, Erlotinib, Gefitinib, Lapatinib, Osimertinib, Vandetanib. |
| IGF1R/IGF1-IGF2 | IGF1R knockdown inhibits human CRC cell growth and downstream PI3K/AKT pathway [37]. | Antibodies targeting IGF1, IGF2 and the extracellular portion of the IGF1R receptor are in clinical trials [34]. Despite the promising results of preclinical and clinical studies, phase II and III trials are showing disappointing conclusions, justifying additional studies for the validation of predictive biomarkers in CRC patients [37]. | Brigatinib, Ceritinib |
| PDGFR | Reduction in cell growth, proliferation an invasion [125]. | Multikinase inhibitor regorafenib FDA approved for the treatment of patients with metastatic CRC whose disease has progressed after prior therapy [126]. Crenolanib, a kinase inhibitor in development for the treatment of multiple malignancies, is under clinical trial for the treatment of patients with advanced gastrointestinal stromal tumors with PDGFRA mutations [127]. | Axitinib, Dasatinib, Imatinb, Lenvatinib, Nilotinib, Nintedanib, Pazopanib, Ponatinib, Sorafenib, Sunitinib |
| CSFR | Reduces intestinal macrophages in CRC patients, reducing epithelial-to-mesenchymal transition and matrix remodeling [41]. | To date, different CSF1 inhibitors are in clinical development both as monotherapy or in combination with conventional treatment or immunotherapy [41]. | Sunitinib |
| KIT | Decreases tumor growth and colony forming capacity [42]. | Different preclinical studies with KIT inhibitors are showing encouraging results for CRC prevention and treatment [128]. FDA approved multikinase inhibitor regorafenib for the treatment of patients with metastatic CRC whose disease has progressed after prior therapy [126]. | Cabozantinib, Dasatinib, Imatinib, Lenvatinib, Pazopanib, Ponatinib, Sorafenib, Sunitinib |
| FLT3 | Under study | FLT3 amplification in CRC seems to be a passenger alteration that occurs as a late event and might not be the most effective alteration for therapy [46]. | Brigatinib, Cabozantinib, Gilteritinib, Midostaurin, Nintedanib, Ponatinib, Sorafenib, Sunitinib |
| VEGFR | VEGFR1 inhibition decreases tumor growth and metastasis [129]. | Bevacizumab and Ramucirumab FDA approved for the treatment of locally advanced or metastatic gastric cancer [129,130]. Multikinase inhibitor regorafenib FDA approved for the treatment of patients with metastatic CRC whose disease has progressed after prior therapy [126]. | Axitinib, Cabozantinib, Nintedanib, Pazopanib, Ponatinib, Regorafenib, Sorafenib, Lenvatinib, Vandetanib |
| FGFR/FGF | Inhibits cell proliferation and tumor growth and enhances tumor cell sensitivity to chemotherapy [131] | Multikinase inhibitor regorafenib approved for the treatment of patients with metastatic CRC whose disease has progressed after prior therapy [126]. | Lenvatinib, Nintedanib, Pazopanib, Ponatinib |
| PTK7 | Decreases cell proliferation, drug-resistance and cell migration [132]. | PTK7 expression has been proposed as prognostic and predictive biomarker [132] and different PTK7-targeting agents are under development [54]. | Under development |
| TRK | Inactivation of TRKA and down-regulation of downstream signaling pathways followed along with cell proliferation inhibition [133]. | Entrectinib, Larotrectinib [56] and Milciclib has shown promising clinical responses in patients with colon cancer and are under clinical trial in patients with other different malignancies [134] | Cabozantinib, Larotrectinib, Milciclib |
| ROR | As non-canonical Wnt signaling mediator, ROR2 has a dual role as tumor suppressor or activator depending on tumor type [60] or stage. | Before ROR-selective inhibitors can truly be used as valuable targets in CRC a better understanding of Wnt signaling pathways in human carcinogenesis is needed. | Under study |
| HGFR/c-Met | Prevents distant recurrence of rectal cancer after preoperative chemoradiotherapy [135]. In combination with glucose metabolism inhibition, enhances the effect of angiogenesis inhibitors in CRC treatment [70]. | Different clinical trials have evaluated MET inhibitors alone or in combination with cytotoxic chemotherapy in patients with gastrointestinal cancer, most of them showing no efficacy [69] | Crizotinib |
| AXL | There is strong evidence for the potential utility of AXL inhibitors to decrease the metastatic potential of CRC as well as to overcome resistance to immune checkpoint inhibitors, conventional chemotherapy and targeted therapies [78]. | AXL represents a promising tool for CRC treatment. Evidence of this is the growing number of AXL inhibitors that are being developed and the ongoing clinical trials employing them [78]. | Cabozantinib |
| Ang/TIE2 | Tumor vasculature reduction [136] and enhanced tumor sensitivity to antigen-specific cytotoxic T lymphocytes killing [136]. | Multikinase inhibitor regorafenib FDA approved for the treatment of patients with metastatic CRC whose disease has progressed after prior therapy [126]. Phase I clinical trials with Trebaninib and Vanucizumab have showed no satisfactory results [137] or partial responses [138], respectively. | Cabozantinib, Ponatinib, Vandetanib |
| EPHR | Multikinase inhibitor regorafenib FDA approved for the treatment of patients with metastatic CRC whose disease has progressed after prior therapy [126]. | Asatinib, Ponatinib, Vandetanib | |
| RET | Vandetanib potently inhibits CRC cells proliferation and AKT and ERK phosphorylation [139]. | Multikinase inhibitor regorafenib FDA approved for the treatment of patients with metastatic CRC whose disease has progressed after prior therapy [126]. | Alectinib, Cabozantinib, Lenvatinib, Ponatenib, Sorafenib, Sunitinib, Vandetanib |
| DDR1 | Strongly inhibits human CRC cell invasion and reduces their metastatic potential [140,141]. | Promising pre-clinical studies [92,141]. | Nilotinib |
| ALK/ROS | Inhibition of cell proliferation and MAPK/PI3K downregulation [142] | Clinical evidence supports that patient with advanced metastatic CRC harboring ALK fusions may benefit from targeted monotherapy with ALK inhibitors [142]. | Alectinib, Brigatinib, Cabozantinib, Ceritinib, Crizotinib, Lorlatinib |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Aranda, M.; Redondo, M. Targeting Receptor Kinases in Colorectal Cancer. Cancers 2019, 11, 433. https://doi.org/10.3390/cancers11040433
García-Aranda M, Redondo M. Targeting Receptor Kinases in Colorectal Cancer. Cancers. 2019; 11(4):433. https://doi.org/10.3390/cancers11040433
Chicago/Turabian StyleGarcía-Aranda, Marilina, and Maximino Redondo. 2019. "Targeting Receptor Kinases in Colorectal Cancer" Cancers 11, no. 4: 433. https://doi.org/10.3390/cancers11040433
APA StyleGarcía-Aranda, M., & Redondo, M. (2019). Targeting Receptor Kinases in Colorectal Cancer. Cancers, 11(4), 433. https://doi.org/10.3390/cancers11040433





